Recent from talks
Nothing was collected or created yet.
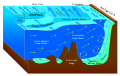
Benthic zone
View on Wikipedia
| Marine habitats |
|---|
| Coastal habitats |
| Ocean surface |
| Open ocean |
| Sea floor |
The benthic zone is the ecological region at the lowest level of a body of water such as an ocean, lake, or stream, including the sediment surface and some sub-surface layers. The name comes from the Ancient Greek word βένθος (bénthos), meaning "the depths".[1] Organisms living in this zone are called benthos and include microorganisms (e.g., bacteria and fungi)[2][3] as well as larger invertebrates, such as crustaceans and polychaetes.[4]
Organisms here, known as bottom dwellers, generally live in close relationship with the substrate and many are permanently attached to the bottom. The benthic boundary layer, which includes the bottom layer of water and the uppermost layer of sediment directly influenced by the overlying water, is an integral part of the benthic zone, as it greatly influences the biological activity that takes place there. Examples of contact soil layers include sand bottoms, rocky outcrops, coral, and bay mud.
Description
[edit]| Part of a series related to |
| Benthic life |
|---|
 |
Oceans
[edit]The benthic region of the ocean begins at the shore line (intertidal or littoral zone) and extends downward along the surface of the continental shelf out to sea. Thus, the region incorporates a great variety of physical conditions differing in: depth, light penetration and pressure.[5] Depending on the water-body, the benthic zone may include areas that are only a few inches below the surface.
The continental shelf is a gently sloping benthic region that extends away from the land mass. At the continental shelf edge, usually about 200 metres (660 ft) deep, the gradient greatly increases and is known as the continental slope. The continental slope drops down to the deep sea floor. The deep-sea floor is called the abyssal plain and is usually about 4,000 metres (13,000 ft) deep. The ocean floor is not all flat but has submarine ridges and deep ocean trenches known as the hadal zone.[6] For comparison, the pelagic zone is the descriptive term for the ecological region above the benthos, including the water column up to the surface. At the other end of the spectrum, benthos of the deep ocean includes the bottom levels of the oceanic abyssal zone.[7]
For information on animals that live in the deeper areas of the oceans see aphotic zone. Generally, these include life forms that tolerate cool temperatures and low oxygen levels, depending on the depth of the water.[8]
Lakes
[edit]As with oceans, the benthic zone is the floor of the lake, composed of accumulated sunken organic matter. The littoral zone is the zone bordering the shore; light penetrates easily and aquatic plants thrive. The pelagic zone represents the broad mass of water, down as far as the depth to which no light penetrates.[9]
Organisms
[edit]Benthos are the organisms that live in the benthic zone, and are different from those elsewhere in the water column; even within the benthic zone variations in such factors as light penetration, temperature and salinity give rise to distinct differences, delineated vertically, in the groups of organisms supported.[10] Many organisms adapted to deep-water pressure cannot survive in the upper parts of the water column: the pressure difference can be very significant (approximately one atmosphere for each 10 meters of water depth). Many have adapted to live on the substrate (bottom). In their habitats they can be considered as dominant creatures, but they are often a source of prey for Carcharhinidae such as the lemon shark.[11]
Because light does not penetrate very deep into ocean-water, the energy source for the benthic ecosystem is often marine snow. Marine snow is organic matter from higher up in the water column that drifts down to the depths.[12] This dead and decaying matter sustains the benthic food chain; most organisms in the benthic zone are scavengers or detritivores. Some microorganisms use chemosynthesis to produce biomass.
Benthic organisms can be divided into two categories based on whether they make their home on the ocean floor or a few centimeters into the ocean floor. Those living on the surface of the ocean floor are known as epifauna.[13] Those who live burrowed into the ocean floor are known as infauna.[10] Extremophiles, including piezophiles, which thrive in high pressures, may also live there. An example of benthos organism is Chorismus antarcticus.
Nutrient flux
[edit]Sources of food for benthic communities can derive from the water column above these habitats in the form of aggregations of detritus, inorganic matter, and living organisms.[14] These aggregations are commonly referred to as marine snow, and are important for the deposition of organic matter, and bacterial communities.[15] The amount of material sinking to the ocean floor can average 307,000 aggregates per m2 per day.[16] This amount will vary on the depth of the benthos, and the degree of benthic-pelagic coupling. The benthos in a shallow region will have more available food than the benthos in the deep sea. Because of their reliance on it, microbes may become spatially dependent on detritus in the benthic zone. The microbes found in the benthic zone, specifically dinoflagellates and foraminifera, colonize quite rapidly on detritus matter while forming a symbiotic relationship with each other.[17][18] In the deep sea, which covers 90–95% of the ocean floor, 90% of the total biomass is made up of prokaryotes. To release all the nutrients locked inside these microbes to the environment, viruses are important in making it available to other organisms.[19][20]
Habitats
[edit]Modern seafloor mapping technologies have revealed linkages between seafloor geomorphology and benthic habitats, in which suites of benthic communities are associated with specific geomorphic settings.[21] Examples include cold-water coral communities associated with seamounts and submarine canyons, kelp forests associated with inner shelf rocky reefs and rockfish associated with rocky escarpments on continental slopes.[22] In oceanic environments, benthic habitats can also be zoned by depth. From the shallowest to the deepest are: the epipelagic (less than 200 meters), the mesopelagic (200–1,000 meters), the bathyal (1,000–4,000 meters), the abyssal (4,000–6,000 meters) and the deepest, the hadal (below 6,000 meters).[23]
The lower zones are in deep, pressurized areas of the ocean. Human impacts have occurred at all ocean depths, but are most significant on shallow continental shelf and slope habitats.[24] Many benthic organisms have retained their historic evolutionary characteristics. Some organisms are significantly larger than their relatives living in shallower zones, largely because of higher oxygen concentration in deep water.[25]
It is not easy to map or observe these organisms and their habitats, and most modern observations are made using remotely operated underwater vehicles (ROVs), and rarely submarines.[26][27]
Ecological research
[edit]Benthic macroinvertebrates have many important ecological functions, such as regulating the flow of materials and energy in river ecosystems through their food web linkages. Because of this correlation between flow of energy and nutrients, benthic macroinvertebrates have the ability to influence food resources on fish and other organisms in aquatic ecosystems. For example, the addition of a moderate amount of nutrients to a river over the course of several years resulted in increases in invertebrate richness, abundance, and biomass. These in turn resulted in increased food resources for native species of fish with insignificant alteration of the macroinvertebrate community structure and trophic pathways.[28] The presence of macroinvertebrates such as Amphipoda also affect the dominance of certain types of algae in Benthic ecosystems as well.[29] In addition, because benthic zones are influenced by the flow of dead organic material, there have been studies conducted on the relationship between stream and river water flows and the resulting effects on the benthic zone. Low flow events show a restriction in nutrient transport from benthic substrates to food webs, and caused a decrease in benthic macroinvertebrate biomass, which lead to the disappearance of food sources into the substrate.[30]
Because the benthic system regulates energy in aquatic ecosystems, studies have been made of the mechanisms of the benthic zone in order to better understand the ecosystem. Benthic diatoms have been used by the European Union's Water Framework Directive (WFD) to establish ecological quality ratios that determined the ecological status of lakes in the UK.[31] Beginning research is being made on benthic assemblages to see if they can be used as indicators of healthy aquatic ecosystems. Benthic assemblages in urbanized coastal regions are not functionally equivalent to benthic assemblages in untouched regions.[32]
Ecologists are attempting to understand the relationship between heterogeneity and maintaining biodiversity in aquatic ecosystems. Benthic algae has been used as an inherently good subject for studying short term changes and community responses to heterogeneous conditions in streams. Understanding the potential mechanisms involving benthic periphyton and the effects on heterogeneity within a stream may provide a better understanding of the structure and function of stream ecosystems.[33] Periphyton populations suffer from high natural spatial variability while difficult accessibility simultaneously limits the practicable number of samples that can be taken. Targeting periphyton locations which are known to provide reliable samples – especially hard surfaces – is recommended in the European Union benthic monitoring program (by Kelly 1998 for the United Kingdom then in the EU and for the EU as a whole by CEN 2003 and CEN 2004) and in some United States programs (by Moulton et al. 2002).[34]: 60 Benthic gross primary production (GPP) may be important in maintaining biodiversity hotspots in littoral zones in large lake ecosystems. However, the relative contributions of benthic habitats within specific ecosystems are poorly explored and more research is planned.[35]
See also
[edit]References
[edit]- ^
 The dictionary definition of benthos at Wiktionary
The dictionary definition of benthos at Wiktionary
- ^ Wetzel, Robert G. (2001). Limnology: Lake and River Ecosystems, 3rd edn. Academic Press, San Diego. pp. 635–637.
- ^ Fenchel, T.; King, G.; Blackburn, T. H. (2012). Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling, 3rd edn. Academic Press, London. pp. 121–122.
- ^ "What Are Benthos?". Baybenthos.versar.com. 2006-01-23. Retrieved 2013-11-24.
- ^ Walag, Angelo (2022). "Understanding the world of Benthos: an introduction to Benthology". In Godson, Prince; et al. (eds.). Ecology and Biodiversity of Benthos. Amsterdam, Netherlands: Elsevier. p. 1. ISBN 978-0-12-821161-8.
- ^ Nichols, C. Reid; Williams, Robert G. (2009). "hadal zone". Encyclopedia of marine science. New York: Infobase. ISBN 978-1-4381-1881-9.
- ^ Nichols, Williams (2009): "abyssal zone"
- ^ Nichols, Williams (2009): "aphotic zone"
- ^ Silk, Nicole; Ciruna, Kristine (2005). A practitioner's guide to freshwater biodiversity conservation. Washington, DC: Island Press. ISBN 978-1-59726-043-5.
- ^ a b Walag (2022) p.2
- ^ Bright, Michael (2000). The private life of sharks: the truth behind the myth. Mechanicsburg, Pennsylvania: Stackpole Books. ISBN 0-8117-2875-7.
- ^ Matthiessen, Berte (2018). "Ecological Organization of the Ocean". In Salomon, Markus; et al. (eds.). Handbook on Marine Environment Protection. Berlin: Springer. p. 53. ISBN 978-3-319-60154-0.
- ^ "Epifaunal - Definition and More from the Free Merriam-Webster Dictionary". Merriam-webster.com. 2012-08-31. Retrieved 2013-11-24.
- ^ Godson (2022) p.90
- ^ Alldredge, Alice; Silver, Mary W. (1988). "Characteristics, dynamics and significance of marine snow". Progress in Oceanography. 20 (1): 41–82. Bibcode:1988PrOce..20...41A. doi:10.1016/0079-6611(88)90053-5.
- ^ Shanks, Alan; Trent, Jonathan D. (1980). "Marine snow: sinking rates and potential role in vertical flux". Deep-Sea Research. 27A (2): 137–143. Bibcode:1980DSRA...27..137S. doi:10.1016/0198-0149(80)90092-8.
- ^ "Foraminifera". Retrieved 7 December 2014.
- ^ "foraminifera". Retrieved 7 December 2014.
- ^ Seaborg, David (30 June 2023). Organisms Amplify Diversity: An Autocatalytic Hypothesis. CRC Press. ISBN 978-1-000-82638-8.
- ^ Danovaro, R.; Molari, M.; Corinaldesi, C.; Dell'Anno, A. (2016). "Macroecological drivers of archaea and bacteria in benthic deep-sea ecosystems". Science Advances. 2 (4) e1500961. Bibcode:2016SciA....2E0961D. doi:10.1126/sciadv.1500961. PMC 4928989. PMID 27386507.
- ^ Harris, P. T.; Baker, E. K. 2012. "GEOHAB Atlas of seafloor geomorphic features and benthic habitats – synthesis and lessons learned", in: Harris, P. T.; Baker, E. K. (eds.), Seafloor Geomorphology as Benthic Habitat: GeoHab Atlas of seafloor geomorphic features and benthic habitats. Elsevier, Amsterdam, pp. 871–890.
- ^ Harris, P. T.; Baker, E. K.; 2012. Seafloor Geomorphology as Benthic Habitat: GeoHab Atlas of seafloor geomorphic features and benthic habitats. Elsevier, Amsterdam, p. 947.
- ^ "Coastal and Marine Ecological Classification Standard (CMECS)". 2012.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Harris, P. T., 2012. "Anthropogenic threats to benthic habitats", in: Harris, P. T.; Baker, E. K. (eds.), Seafloor Geomorphology as Benthic Habitat: GeoHab Atlas of seafloor geomorphic features and benthic habitats. Elsevier, Amsterdam, pp. 39–60.
- ^ Royal Belgian Institute of Natural Sciences, news item March 2005 Archived September 28, 2011, at the Wayback Machine
- ^ Clark, Malcolm; et al. (2016). Biological sampling in the deep sea. Hoboken, New Jersey: Wiley. p. 30. ISBN 978-1-118-33255-9.
- ^ Tillin, H. M.; et al. "Marine Monitoring Platform Guidelines: Remotely Operated Vehicles for use in marine benthic monitoring" (PDF). Peterborough, UK: Joint Nature Conservation Committee. p. 1. Retrieved 15 June 2022.
- ^ Minshall, Wayne; Shafii, Bahman; Price, William J.; Holderman, Charlie; Anders, Paul J.; Lester, Gary; Barrett, Pat (2014). "Effects of nutrient replacement on benthic macroinvertebrates in an ultraoligotrophic reach of the Kootenai River, 2003–2010". Freshwater Science. 33 (4): 1009–1023. Bibcode:2014FWSci..33.1009M. doi:10.1086/677900. JSTOR 10.1086/677900. S2CID 84495019.
- ^ Duffy, J. Emmett; Hay, Mark E. (2000-05-01). "Strong impacts of grazing amphipods on the organization of a benthic community". Ecological Monographs. 70 (2): 237–263. CiteSeerX 10.1.1.473.4746. doi:10.1890/0012-9615(2000)070[0237:SIOGAO]2.0.CO;2. ISSN 0012-9615. S2CID 54598097.
- ^ Rolls, Robert; Leigh, Catherine; Sheldon, Fran (2012). "Mechanistic effects of low-flow hydrology on riverine ecosystems: ecological principles and consequences of alteration". Freshwater Science. 31 (4): 1163–1186. Bibcode:2012FWSci..31.1163R. doi:10.1899/12-002.1. hdl:10072/48539. JSTOR 10.1899/12-002.1. S2CID 55593045.
- ^ Bennion, Helen; Kelly, Martyn G.; Juggins, Steve; Yallop, Marian L.; Burgess, Amy; Jamieson, Jane; Krokowski, Jan (2014). "Assessment of Ecological Status in UK lakes using benthic diatoms" (PDF). Freshwater Science. 33 (2): 639–654. Bibcode:2014FWSci..33..639B. doi:10.1086/675447. hdl:1983/d42210cd-45e2-48a6-87ed-e2d3cbd3f23b. JSTOR 10.1086/675447. S2CID 33631675.
- ^ Lowe, Michael; Peterson, Mark S. (2014). "Effects of Coastal Urbanization on Salt-Marsh Faunal Assemblages in the Northern Gulf of Mexico". Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science. 6 (1): 89–107. Bibcode:2014MCFis...6...89L. doi:10.1080/19425120.2014.893467. hdl:1912/6981.
- ^ Wellnitz, Todd; Rader, Russell B. (2003). "Mechanisms influencing community composition and succession in mountain stream periphyton: interactions between scouring history, grazing, and irradiance". Journal of the North American Benthological Society. 22 (4): 528–541. doi:10.2307/1468350. JSTOR 1468350. S2CID 85061936.
- ^ Smol, John P. (2010). The Diatoms: Applications for the Environmental and Earth Sciences. Cambridge New York City: Cambridge University Press (CUP). ISBN 978-0-521-50996-1. OCLC 671782244.
- ^ Althouse, Bryan; Higgins, Scott; Vander Zanden, Jake M. (2014). "Benthic and Planktonic primary production along a nutrient gradient in Green Bay, Lake Michigan, USA". Freshwater Science. 33 (2): 487–498. Bibcode:2014FWSci..33..487A. doi:10.1086/676314. JSTOR 10.1086/676314. S2CID 84535584.
External links
[edit]- Data Archive for Seabed Species and Habitats from the UK Marine Data Archive Centre
Benthic zone
View on GrokipediaDefinition and Characteristics
Physical Features
The benthic zone is the ecological region at the lowest level of a body of water, such as an ocean, lake, or stream, encompassing the sediment surface and some sub-surface layers.[4] This zone forms the interface between the water column and the underlying substrate, where physical conditions are influenced by overlying waters and geological features.[5] In marine environments, the benthic zone is subdivided into depth-based classifications that reflect varying physical conditions. The littoral zone is the intertidal region between high and low tide marks, typically 0-2 meters depth. The sublittoral zone extends from the low tide line across the continental shelf to about 200 meters depth, while the bathyal zone spans 200 to 4,000 meters along continental slopes. The abyssal zone occupies the deep ocean floor from 4,000 to 6,000 meters, and the hadal zone encompasses trenches deeper than 6,000 meters, reaching up to 11,000 meters in places like the Mariana Trench.[6][7][8] Sediments in the benthic zone vary by type and distribution, primarily categorized by grain size using the Wentworth scale: gravel (≥2 mm), sand (62.5 µm to 2 mm), silt (4 to 62.5 µm), and clay (<4 µm), with mud comprising silt and clay fractions. Rock substrates, including bedrock outcrops, occur in high-energy coastal areas or seamounts, while finer muds dominate low-energy deep-sea basins due to settling of suspended particles. Sediment distribution depends on grain size, with coarser gravel and sand concentrated in shallow, wave-exposed areas and finer silts and clays in deeper, calmer settings; sorting— the uniformity of grain sizes—improves in high-energy environments through winnowing, whereas poor sorting prevails in low-energy deposition. Porosity, the void space in sediments, ranges from 35-45% in well-sorted sands to higher values (up to 80%) in unconsolidated muds, influencing water and particle flow through the substrate.[9][10][11] Physical processes such as currents, tides, wave action, and sedimentation rates actively shape the benthic environment and substrate stability. Ocean currents and tidal flows transport and redistribute sediments, eroding fine particles in high-velocity areas while depositing them in slower zones, with tidal currents particularly dominant in coastal and shelf regions. Wave action, most intense in the littoral zone, generates shear stress that mobilizes sand and gravel, promoting substrate instability through scour and burial, though deeper bathyal areas experience reduced wave influence. Sedimentation rates vary widely, from millimeters per year in active coastal shelves to centimeters per thousand years in abyssal plains, stabilizing substrates by accumulating layers that resist erosion but can smother existing features during rapid events. These processes collectively determine substrate firmness, with stable rocky or gravelly bottoms in energetic settings contrasting unstable muddy expanses in quiescent deeps.[12][13][14] Temperature and pressure in the benthic zone exhibit pronounced gradients with increasing depth, creating extreme conditions in deeper realms. In the littoral and sublittoral zones, temperatures fluctuate seasonally from 0°C to over 20°C due to surface influences, but they stabilize near 4°C in the bathyal zone and approach 0°C to -1°C in the abyssal and hadal zones, with near-freezing conditions prevalent in deep-sea trenches like the Mariana. Pressure increases linearly with depth at about 1 atmosphere per 10 meters, reaching 200-400 atmospheres in the bathyal zone and over 1,000 atmospheres in hadal trenches, compressing sediments and influencing their physical properties. These gradients contribute to a stratified physical environment, with shallower zones experiencing greater variability than the uniform cold and high-pressure deep benthos.[15][6][16]Chemical and Biological Properties
The benthic zone is characterized by sharp chemical gradients, most notably in oxygen distribution, where surface sediments remain oxic due to molecular diffusion from the overlying water column, while deeper layers transition to anoxic conditions. This shift is delineated by the redox potential discontinuity (RPD), a boundary where oxidized surface materials give way to reduced deeper sediments, typically occurring at depths of 1–10 mm in fine-grained muds but extending to 1–5 cm in coarser or bioturbated sediments. Deeper RPD depths, often exceeding 1 cm, indicate higher oxygen availability and healthier benthic habitat quality, whereas shallower RPDs signal stress from organic enrichment or hypoxia.[17][18] Nutrient profiles in benthic sediments are profoundly shaped by the decomposition of settled organic matter, which elevates concentrations of carbon, nitrogen, phosphorus, and sulfur compounds, particularly in the anoxic subsurface where microbial processes dominate. Organic carbon accumulates as recalcitrant humic substances, while nitrogen appears as ammonium from ammonification, phosphorus as inorganic phosphates released during mineralization, and sulfur as reduced sulfides from sulfate reduction. These profiles vary with organic input rates; for instance, in coastal sediments receiving high particulate loads, nutrient levels can reach several millimoles per kilogram of dry sediment, fostering intense remineralization. Fine-grained sediments, by retaining these compounds more effectively than sandy ones, amplify local nutrient enrichment.[19][20] pH and salinity in the benthic zone exhibit regional variations, with coastal areas displaying greater fluctuations—pH ranging from 7.5 to 8.5 due to respiratory CO₂ production and photosynthetic uptake—compared to the more stable deep-sea environment, where pH hovers around 7.8–8.0 and salinity remains near 35 practical salinity units (psu). Ocean acidification, driven by anthropogenic CO₂ influx, exacerbates pH declines, with projections indicating a 0.29–0.37 unit drop by 2100 in bathyal depths (200–3,000 m), potentially dissolving carbonate sediments and altering chemical equilibria. In coastal settings, salinity can dip below 30 psu from freshwater runoff, influencing ion exchange and acidification sensitivity.[21][22] Early biological properties emerge through biofilm formation on sediment surfaces, where microbial communities embed in extracellular polymeric substances to create a matrix that stabilizes particles and initiates chemical cycling. These biofilms facilitate the initial oxidation of organic matter and reduction of electron acceptors like nitrate and sulfate, mediating fluxes of key elements without deeper community involvement. Biofilm development is rapid, often within days of sediment deposition, and enhances sediment cohesion while influencing local pH through metabolic byproducts.[23][24] The chemistry of benthic sediments is further modulated by the influence of the overlying water column, through which dissolved gases such as oxygen and carbon dioxide diffuse downward via Fickian processes, penetrating only the uppermost millimeters unless enhanced by bioturbation. Particulate matter, including organic detritus and minerals, settles from the water column, delivering nutrients and altering redox conditions upon burial. This exchange establishes the oxic-anoxic interface and sustains the chemical gradients essential to benthic habitat formation.[25][26]Environmental Contexts
Marine Settings
The marine benthic zone is divided into distinct depth-based subzones that reflect variations in light penetration, pressure, and substrate characteristics, influencing ecological adaptations across ocean environments. The neritic zone spans depths from 0 to 200 meters over the continental shelf, where sediments are often terrigenous and influenced by coastal processes.[27] The bathyal zone extends from 200 to 4,000 meters along the continental slope, featuring steeper topography and finer sediments derived from pelagic sources.[27] Deeper still, the abyssal zone occupies 4,000 to 6,000 meters across vast abyssal plains, which cover more than 50% of the global seafloor and consist of flat, sediment-blanketed expanses far from continental margins.[28] The hadal zone, exceeding 6,000 meters and confined to oceanic trenches, represents less than 1% of the seafloor but hosts the most extreme conditions.[29] Hydrostatic pressure in marine benthic environments escalates dramatically with depth, reaching over 1,000 atmospheres in the hadal zone—equivalent to about 100 megapascals at 10,000 meters—far surpassing surface levels and profoundly affecting sediment properties.[30] This intense pressure compacts sediments, reducing porosity and facilitating the accumulation of organic material in trench depocenters, where fine-grained particles settle and undergo diagenetic alterations under minimal bioturbation.[31] Seafloor topography significantly shapes benthic habitats in marine settings, with features like mid-ocean ridges, subduction zones, and hydrothermal vents creating localized hotspots of geological and biological activity. Mid-ocean ridges, formed at divergent plate boundaries, elevate the seafloor to 2,000–3,000 meters and support chemosynthetic communities around hydrothermal vents, where superheated fluids emerge and sustain dense biomass independent of surface productivity.[32] Subduction zones, conversely, generate deep trenches that channel sediments into hadal basins, enhancing organic deposition while exposing the benthos to seismic disturbances.[33] These dynamic structures contrast with the stable, expansive abyssal plains, where subtle variations in relief influence current flows and particle settling. Variations in organic input to the marine benthos drive spatial heterogeneity in productivity and community structure, with carbon flux rates typically ranging from 1 to 10 grams of carbon per square meter per year across different oceanic provinces. Coastal upwelling regions, such as those off Peru or California, receive elevated fluxes—often exceeding 10 gC/m²/year—due to nutrient-driven surface blooms that export labile organic matter to the seafloor.[34] In contrast, oligotrophic gyres in the open ocean, like the North Pacific Gyre, exhibit low fluxes below 1 gC/m²/year, resulting from limited primary production and efficient remineralization in the water column, which starves benthic ecosystems of food.[35] Illustrative examples highlight these marine benthic variations: the Clarion-Clipperton Zone in the central Pacific, an abyssal plain at 4,000–6,000 meters, is renowned for its dense fields of polymetallic nodules—potato-sized concretions rich in manganese and rare earth elements—that cover up to 30% of the seafloor and provide microhabitats for scavenging communities amid otherwise sparse sediments.[36] At the hadal extreme, the Mariana Trench plunges to over 10,900 meters, enduring pressures near 1,100 atmospheres, near-freezing temperatures around 1–4°C, and episodic sediment slides, yet harbors resilient chemosynthetic assemblages adapted to these conditions.[37]Freshwater and Estuarine Settings
In freshwater lakes, the benthic zone is stratified into the littoral and profundal regions, with the former encompassing nearshore areas where light penetrates to the sediment surface, supporting rooted macrophytes and higher primary production, while the latter lies below the thermocline in deeper waters where the hypolimnion's isolation limits oxygen exchange and promotes sediment anoxia during stratification.[38] The thermocline acts as a barrier separating the warmer epilimnion from the colder hypolimnion, influencing benthic oxygen levels and nutrient release from profundal sediments through reduced mixing.[38] In temperate lakes like those in the Great Lakes system, profundal sediments consist of fine-grained, organic-rich silts that host assemblages dominated by malacostracans (such as amphipods) and oligochaetes, which tolerate low-oxygen conditions but exhibit reduced diversity compared to littoral zones.[39] Riverine benthic zones feature high-energy substrates shaped by current velocity, with riffle-pool dynamics creating alternating fast-flowing riffles that scour coarse gravel beds and slower pools that accumulate finer sediments, thereby influencing habitat heterogeneity and organism distribution.[40] Sediment transport during high flows erodes and redistributes bed materials, preventing long-term burial of benthic communities while promoting colonization by rheophilic invertebrates adapted to shifting substrates.[41] Seasonal flood pulses, as described in the flood pulse concept for large rivers, periodically inundate floodplains and redistribute sediments, enhancing connectivity between river channels and benthic habitats while flushing organic matter to support detritivore populations.[42] Estuarine benthic environments exhibit sharp salinity gradients from 0 to 35 parts per thousand (ppt), transitioning from freshwater to marine conditions and forming brackish mosaics that support diverse assemblages across tidal flats and vegetated substrates.[43] Brackish zones (0.2–30 ppt) foster heterogeneous habitats like intertidal mudflats, where periodic exposure to air and water drives cyclic benthic recolonization, and mangrove root systems, which stabilize sediments and create microhabitats for epibenthic organisms along elevation-driven salinity gradients.[43] In the Amazon River delta, these transitions manifest in macrobenthic assemblages featuring coralline algae rhodolith beds and sponge aggregations, which thrive amid high sediment inputs and fluctuating salinities, contributing to elevated biodiversity in proximal estuarine areas.[44] Seasonal variations profoundly affect these settings; in temperate lakes, winter ice cover isolates the hypolimnion, reducing oxygen replenishment and leading to benthic anoxia in profundal zones due to ongoing sediment respiration, with depletion risks heightened in shallow lakes with high sediment-to-volume ratios.[45] Conversely, in rivers, annual flood pulses from snowmelt or monsoons redistribute sediments and nutrients, temporarily disrupting but ultimately rejuvenating benthic communities by increasing habitat availability in floodplains.[42] Estuaries experience amplified tidal influences during wet seasons, which intensify salinity fluctuations and sediment resuspension, altering benthic mosaic stability.[43]Benthic Biota
Microbial Communities
Microbial communities in the benthic zone, comprising bacteria, archaea, and protists, form the foundational layer of ecosystem function, serving as primary decomposers and key mediators of biogeochemical cycles in sediments. These microorganisms thrive in the low-oxygen, nutrient-rich environments of the seafloor, where they process organic matter sinking from the water column, facilitating the breakdown of complex compounds into simpler forms. Protists, including ciliates and foraminifera, contribute to this dynamic by grazing on bacteria and recycling nutrients, enhancing overall community resilience. Their collective activities underpin the health of benthic ecosystems, influencing everything from local redox conditions to global elemental fluxes.[46][47] Among the dominant groups, sulfate-reducing bacteria (SRB) such as Desulfovibrio species are prevalent in marine sediments, where they utilize sulfate as an electron acceptor for anaerobic respiration of organic matter. Methanogenic archaea, exemplified by Methanococcus genera, become prominent in deeper, sulfate-depleted layers, converting simple substrates like hydrogen and carbon dioxide into methane. Nitrifying archaea, part of the Thaumarchaeota phylum, also play a crucial role by oxidizing ammonia to nitrite, often in oxic microzones within otherwise anoxic sediments. These groups exhibit zonation with depth: SRB dominate the upper sediment layers where sulfate is abundant, while methanogens prevail below the sulfate-methane transition zone, typically at depths of several centimeters to meters depending on organic input and sedimentation rates.[48][49][50][51] Key processes driven by these microbes include anaerobic respiration pathways, with sulfate reduction being a primary mechanism in sulfidic environments. This process can be represented as: where sulfate is reduced to hydrogen sulfide, releasing bicarbonate that buffers sediment pH. Such reactions occur preferentially in the uppermost sediment horizons, creating distinct redox gradients that stratify microbial activities and limit methanogenesis until sulfate is exhausted. Metagenomic studies reveal high functional diversity, with guilds specialized in these pathways comprising up to 10-20% of total community genes in coastal sediments. Microbial biomass in benthic environments is substantial, reaching up to cells per gram of dry sediment in organic-rich areas, underscoring their outsized role despite their microscopic scale.[52][53][54] Adaptations enable these communities to persist in extreme benthic conditions, such as anoxic zones with fluctuating temperatures and pressures. Extremophilic bacteria and archaea tolerate high sulfide levels through specialized enzymes, while vent-associated chemolithoautotrophs—often epsilonproteobacteria—harness hydrogen sulfide (HS) or methane (CH) as energy sources for carbon fixation via the Calvin-Benson-Bassham cycle. In hydrothermal settings, these microbes form dense mats, oxidizing reduced compounds to support higher trophic levels. These adaptations not only ensure survival but also drive rapid turnover of energy in otherwise energy-limited habitats.[55][56] Benthic microbes significantly influence global biogeochemical cycles, particularly nitrogen, with denitrification processes removing fixed nitrogen as N gas. Approximately 50% of oceanic denitrification occurs in benthic sediments, primarily through microbial consortia coupling nitrate reduction to organic matter oxidation or anaerobic ammonium oxidation. This removal helps regulate marine productivity by preventing nitrogen accumulation, with rates highest in shelf and margin sediments receiving substantial terrestrial inputs. Such contributions highlight the benthic zone's role as a critical sink in the ocean's nitrogen budget.[57][58]Invertebrate Assemblages
The benthic zone hosts a rich diversity of invertebrate assemblages, spanning multiple phyla and size classes that play critical roles in sediment processing and ecosystem dynamics. Invertebrates are broadly categorized into meiofauna (organisms smaller than 1 mm, such as nematodes and copepods) and macrofauna (larger than 1 mm, including polychaetes and bivalves), with meiofauna often dominating in abundance, reaching densities of 10^5 to 10^6 individuals per square meter in coastal sediments.[59] Nematodes, for instance, can comprise up to 90% of meiofaunal individuals in these environments, facilitating nutrient turnover through their high densities and rapid reproduction.[60] Macrofauna, though less numerous, contribute disproportionately to bioturbation and organic matter decomposition due to their larger size and mobility.[61] Major phyla represented in benthic invertebrate assemblages include Annelida, Mollusca, Arthropoda, and Echinodermata, each exhibiting specialized adaptations to the sediment interface. Annelids, particularly polychaetes such as Nereis species (e.g., the clam worm Nereis virens), are abundant burrowers in soft sediments, where they engage in deposit feeding by ingesting organic-rich particles from the substrate.[62] Mollusks, dominated by bivalves like clams (e.g., Macoma balthica), often function as deposit or suspension feeders, using siphons to access surface detritus or suspended particles while burrowing to depths of several centimeters.[63] Arthropods, primarily crustaceans such as amphipods (e.g., Corophium spp.), scavenge detritus and algae on or within sediments, contributing to the breakdown of organic matter in intertidal and subtidal zones.[61] Echinoderms, including sea urchins (Strongylocentrotus spp.) and starfish (Asterias spp.), are typically epifaunal grazers or predators that influence macroalgal and invertebrate communities on hard or mixed substrates.[64] Adaptations among benthic invertebrates enable survival in low-oxygen, particle-laden environments, including specialized feeding strategies and physiological tolerances. Many species alternate between suspension feeding (filtering particles from the water column) and deposit feeding (consuming sediment-bound organics), as seen in bivalves that adjust siphon extension based on food availability.[65] Burrowing behaviors, exemplified by thalassinid shrimp (e.g., Upogebia spp.), promote bioturbation by excavating complex burrow networks up to 1-3 meters deep, enhancing sediment oxygenation and nutrient exchange.[66] Tolerance to hypoxia is achieved through hemoglobin-like pigments with high oxygen affinity, as in nereid polychaetes, allowing them to extract oxygen from low concentrations in burrow waters or anoxic sediments.[67] Zonation patterns divide assemblages into infaunal (burrow-dwelling, e.g., polychaetes and clams within sediments) and epifaunal (surface-attached or mobile, e.g., starfish and amphipods) groups, reflecting gradients in oxygen, food, and predation pressure. Infaunal species predominate in soft, muddy bottoms, where they exploit vertical strata for resource partitioning, while epifaunal forms thrive on rocky or shelly substrates, often forming visible aggregations.[68] In deep-sea settings, holothurians (sea cucumbers) exemplify macrofaunal dominance, processing substantial portions of surface detritus—assimilating up to 52% of organic phosphorus—through deposit feeding that recycles phytodetritus into the food web.[69] These assemblages underpin benthic productivity, linking detrital inputs to higher trophic levels.[70]Vertebrate and Macroalgal Components
The benthic zone hosts a variety of vertebrates, primarily demersal fish such as flatfish (e.g., flounder and halibut), which exhibit specialized adaptations for life on or near the seafloor. These include dorsoventrally flattened bodies that reduce hydrodynamic drag and enable camouflage against sediments, as well as both eyes positioned on the upper side of the head to monitor prey and predators while lying in wait.[71] Many such fish lack a swim bladder, relying instead on negative buoyancy or oily tissues for stability on the bottom, which facilitates energy-efficient station-holding and sediment interaction.[72] Rays, another key group, possess similarly flattened bodies and enlarged pectoral fins that allow them to glide closely over benthic substrates in search of invertebrates.[73] Marine mammals like walruses occasionally engage in benthic feeding, using their vibrissae (whiskers) for prey detection, powerful suction from the mouth to extract bivalves, and flippers to probe and clear sediments.[74] These adaptations enable walruses to target buried prey in shallow coastal benthos, though their presence is sporadic compared to more resident fish species. In deeper benthic environments beyond 1000 m, vertebrates become exceedingly rare, constituting a minor fraction of overall biomass—often less than 1%—as they depend on sporadic vertical migrations of pelagic food sources rather than sustained bottom dwelling.[75] Examples include Antarctic icefish (family Channichthyidae), which inhabit shelf benthos and have evolved cold-adapted traits like antifreeze glycoproteins and modified cardiovascular systems to exploit nutrient-rich sediments.[76] Macroalgae, as primary producers in the shallow benthic zone, form expansive kelp forests dominated by species in the order Laminariales, such as Laminaria spp., which thrive in temperate and polar coastal waters requiring light penetration.[77] These forests achieve substantial biomass, with standing crops reaching up to 5.5 kg fresh weight per m² in productive offshore sites, supporting rapid growth rates that can exceed 0.5 m per day under optimal conditions.[78] By providing three-dimensional structure, macroalgae create foundational habitats that shelter juvenile fish and invertebrates, while also stabilizing sediments and enhancing local biodiversity.[79] Demersal fish and rays often forage within these kelp stands, preying on associated macroinvertebrates and thereby influencing sediment turnover and nutrient availability. In estuarine settings, seagrass beds (e.g., Zostera spp.), though rooted in sediments, contribute similarly as benthic-associated producers, offering refuge and food resources in dynamic tidal environments.[80]Ecological Dynamics
Nutrient Flux and Cycling
The benthic zone plays a pivotal role in nutrient flux and cycling through benthic-pelagic coupling, where organic matter sinking from the water column is remineralized in sediments, releasing nutrients that diffuse or advect back to the overlying water to fuel primary production.[81] Diffusive transport follows Fick's first law, expressed as , where is the flux, is the diffusion coefficient, and is the concentration gradient across the sediment-water interface.[82] Advective transport, driven by pressure gradients or bioirrigation, can dominate in permeable sediments, enhancing nutrient exchange beyond diffusion alone.[83] Key cycling processes include the remineralization of organic matter, primarily through aerobic respiration represented by the equation , which regenerates carbon dioxide and other nutrients under oxic conditions.[84] In anoxic deeper sediment layers, denitrification converts nitrate to dinitrogen gas via , removing fixed nitrogen from the system, while methanogenesis produces methane through , influencing carbon and hydrogen cycling.[85] These processes transform sinking organic detritus into bioavailable forms, with rates varying by oxygen availability and organic input. Of the organic matter derived from primary production reaching the benthos, burial efficiency (the fraction buried long-term in sediments, preserving it against remineralization) is typically less than 1% in the deep sea but can reach 10–20% or higher in coastal environments with high sedimentation rates, while the remainder is recycled to the water column.[86] Bioturbation by benthic invertebrates mixes sediments, increasing nutrient fluxes by 10-100 times relative to diffusion alone through enhanced ventilation and solute transport. Globally, benthic processes regenerate 10-50% of oceanic nutrients, sustaining much of the water-column productivity.[81]Trophic Interactions and Biodiversity
Benthic food webs are predominantly detritus-based, relying on organic detritus as the primary energy source that fuels successive trophic levels. Bacteria initiate the chain by decomposing this detritus, serving as a food source for protozoa and small invertebrates such as nematodes and copepods, which are then consumed by larger invertebrates like polychaetes and amphipods, ultimately supporting higher-level predators including demersal fish.[87] In deep-sea environments, these chains derive over 90% of their energy from allochthonous inputs, mainly particulate organic matter sinking from surface waters, which underscores the dependence on external productivity to sustain benthic communities.[88] This structure contrasts with pelagic systems, emphasizing the role of sedimentation in energy transfer and limiting the efficiency of trophic progression to approximately 10% per level due to respiratory losses and incomplete consumption.[89] Biodiversity patterns in benthic zones reveal pronounced gradients influenced by depth, latitude, and habitat type, with coastal regions supporting far greater species richness than deep-sea areas. Coastal meiofaunal assemblages, for instance, can exhibit high diversity with over 100 species per sample and abundances exceeding 1000 individuals per square meter in productive sediments, driven by nutrient-rich inputs and heterogeneous substrates.[90] In contrast, deep-sea benthic sites typically host 10–50 macrofaunal species per standard sample (0.1 m²), with cumulative richness reaching 100–200 over larger sampling areas, reflecting lower energy availability and more uniform conditions that constrain speciation.[91] These gradients follow a latitudinal decline, where tropical and subtropical coastal benthos display peak diversity, decreasing poleward, while bathymetric patterns show richness peaking at shelf breaks before declining into the abyss due to diminishing organic flux and temperature gradients.[92] Keystone interactions shape benthic community structure through critical predator-prey dynamics and symbiotic partnerships. Predatory sea stars, such as Asterias rubens, exert top-down control by selectively consuming bivalves like mussels, preventing dominance by these filter feeders and maintaining assemblage diversity in intertidal and subtidal habitats.[93] Symbiotic relationships further enhance trophic complexity, exemplified by the mutualism between chemosynthetic sulfur-oxidizing bacteria and the giant tubeworm Riftia pachyptila at hydrothermal vents, where the host provides a protected environment and chemicals, while the symbionts fix carbon via hydrogen sulfide oxidation to support the worm's nutrition in dark, aphotic conditions.[94] Benthic communities demonstrate resilience through elevated beta diversity, which captures turnover in species composition across habitat mosaics, fostering adaptability to environmental variability. Hydrothermal vent hotspots exemplify this, harboring endemic species like Riftia pachyptila that contribute to unique assemblages with high functional redundancy in chemosynthetic niches, enhancing overall ecosystem stability.[95] Disturbances such as bottom trawling disrupt these networks by reducing infaunal biomass and altering trophic linkages, yet recovery in soft-sediment systems often occurs within 1-5 years, depending on sediment type and larval recruitment rates, allowing gradual restoration of biodiversity and energy flow.[96]Human Influences and Research
Anthropogenic Impacts
Human activities have profoundly altered the benthic zone through various forms of exploitation and pollution, leading to habitat degradation, biodiversity loss, and disrupted ecological functions. Bottom trawling, a widespread fishing practice, physically disturbs seafloor sediments by dragging heavy nets across the bottom, causing resuspension of sediments and destruction of benthic habitats. Globally, commercial bottom trawling sweeps approximately 1.1 million km² of the seafloor each year (as of 2021 estimates), primarily on continental shelves, which cover roughly 28 million km² worldwide.[97][98] This disturbance can reduce long-term carbon storage in shelf sediments by up to 10% in heavily trawled areas and impairs deep-sea biodiversity by killing or displacing benthic communities.[99][100] Pollution from anthropogenic sources has resulted in the accumulation of contaminants in benthic sediments, posing risks to sediment-dwelling organisms. Heavy metals such as cadmium, mercury, lead, and arsenic, originating from industrial discharges, mining runoff, and urban wastewater, bind to fine sediments and persist in the benthic environment, exerting toxic effects on invertebrates even at low concentrations. Microplastics and pharmaceuticals also accumulate in these sediments; for instance, microplastic particles are ingested by over 72% of deep-sea amphipod specimens examined in hadal zones, leading to bioaccumulation and potential trophic transfer. Pharmaceuticals, including antibiotics and analgesics from treated effluents, serve as reservoirs in marine sediments, with residues detected in benthic flora and fauna, potentially disrupting microbial communities and physiological processes in benthic species.[101][102][103][104][105] Climate change exacerbates these pressures through ocean acidification and warming, which directly affect benthic carbonate structures and species distributions. Ocean acidification, driven by increased CO₂ absorption, lowers seawater pH—currently around 8.1 and projected to drop below 7.8 in some regions by 2100—leading to the dissolution of carbonate sediments and shells in calcifying benthic organisms like foraminifera and mollusks. Concurrently, warming ocean temperatures, rising by 0.11°C per decade since 1971, drive poleward shifts in benthic species assemblages, with temperature identified as the primary driver of distributional changes in foraminiferal communities from tropical to higher latitudes. These shifts can alter community composition and reduce habitat suitability for acid-sensitive species in shelf and deep-sea environments.[106][107][108][107] Resource extraction, particularly deep-sea mining for polymetallic nodules rich in metals like manganese and cobalt, threatens vast benthic areas in the Clarion-Clipperton Zone. As of 2025, exploration licenses cover approximately 1.3 million km² of the international seabed, amid debates at the International Seabed Authority over environmental regulations and potential moratoriums on commercial mining, with projections indicating that commercial mining could directly impact over 1 million km² by the 2030s through sediment plume generation and habitat removal.[109][110] The process risks releasing toxic metals from crushed nodules into the water column and sediments, potentially causing metal toxicity and bioaccumulation in benthic fauna, with oxidation of sulfides exacerbating contamination in nearby ecosystems.[111][112][113] Eutrophication from agricultural and urban nutrient runoff further degrades benthic zones by promoting algal blooms that deplete oxygen upon decay, creating anoxic conditions. In the Gulf of Mexico, excess nitrogen and phosphorus inputs have led to seasonal benthic hypoxia, with historical peaks exceeding 22,000 km² (e.g., 22,700 km² in 2017) and recent measurements (2024: ~17,400 km²; 2025: ~11,400 km²) averaging around 15,000 km², suffocating benthic communities and forming expansive "dead zones" that persist for months. These hypoxic events, driven by anthropogenic nutrient loading, disrupt nutrient cycling and lead to shifts toward tolerant, low-diversity assemblages in affected sediments.[114][115][116][117][118]Monitoring and Scientific Approaches
Sampling the benthic zone relies on a variety of physical and technological methods to collect sediment, biota, and environmental data from seafloor habitats. Traditional sampling techniques include box corers, which preserve intact sediment columns up to several meters deep for analyzing vertical stratification and infaunal communities, and grab samplers such as the Van Veen grab, which capture surface sediments over an area of approximately 0.1 square meters to assess macrofaunal abundance and sediment properties in soft-bottom environments.[119][120] For deeper or more complex terrains, remotely operated vehicles (ROVs) and submersibles enable in situ observation and targeted sampling, allowing high-resolution imaging and collection of samples from hard substrates or steep slopes without disturbing surrounding areas.[121][122] Remote sensing technologies have advanced benthic monitoring by providing non-invasive, large-scale mapping and assessment capabilities. Multibeam sonar systems, deployed from ships or autonomous underwater vehicles (AUVs), generate high-resolution bathymetric maps and backscatter data to delineate habitat types, such as seagrass beds or coral reefs, over expansive areas.[123] AUVs equipped with side-scan sonar and sensors facilitate time-series data collection on sediment dynamics and water column interactions, enabling repeated surveys in remote or hazardous locations.[124] Additionally, environmental DNA (eDNA) analysis from water or sediment samples offers a molecular approach to biodiversity assessment, detecting microbial and metazoan communities without direct observation, though it requires ground-truthing for species identification.[125] Experimental approaches in benthic research often employ controlled simulations and tracers to quantify processes like nutrient cycling. Mesocosm experiments replicate natural benthic conditions in enclosed systems to study flux rates and community responses under manipulated variables, such as temperature or oxygen levels.[126] Stable isotope tracing, particularly with 15N, tracks denitrification pathways in sediments by measuring the production of 15N-labeled dinitrogen gas, revealing rates of nitrogen removal in hypoxic zones or eutrophic systems. Major initiatives have synthesized benthic knowledge through coordinated global efforts. The Census of Marine Life (2000-2010) documented over 6,000 potentially new species and mapped biodiversity hotspots in benthic realms, including deep-sea plains and continental margins, via expeditions involving 2,700 scientists from 80 countries. Building on this, the UN Decade of Ocean Science for Sustainable Development (2021-2030) supports benthic-focused projects under its challenges for ecosystem restoration and digital ocean representation, emphasizing integrated monitoring of seafloor biodiversity amid climate pressures.[127][128] Despite these advances, significant knowledge gaps persist in benthic science as of 2025, particularly in the hadal zone beyond 6,000 meters depth, where extreme pressures limit sampling and only about 27% of the seafloor is mapped at high resolution.[129] Climate tipping points, such as abrupt shifts in benthic community structure due to ocean acidification or deoxygenation, remain poorly understood, complicating predictive models.[130] Emerging calls advocate for AI-integrated monitoring, including machine learning for analyzing sonar imagery and eDNA datasets, to enhance real-time detection and fill these voids efficiently.[131]References
- https://www.coastalwiki.org/wiki/Deep_sea_habitat
- https://www.coastalwiki.org/wiki/Kelp_forests
- https://www.coastalwiki.org/wiki/Benthos