Recent from talks
Nothing was collected or created yet.
Migraine
View on Wikipedia
| Migraine | |
|---|---|
 | |
| Woman during a migraine attack | |
| Specialty | Neurology |
| Symptoms | Headaches coupled with sensory disturbances such as nausea, sensitivity to light, sound, and smell |
| Usual onset | Around puberty |
| Duration | Recurrent, long term |
| Causes | Environmental and genetic |
| Risk factors | Family history, female sex |
| Differential diagnosis | Subarachnoid hemorrhage, venous thrombosis, idiopathic intracranial hypertension, brain tumor, tension headache, sinusitis, cluster headache |
| Prevention | Propranolol, amitriptyline, topiramate, calcitonin gene-related peptide receptor antagonists (CGRPs) |
| Medication | Ibuprofen, paracetamol (acetaminophen), triptans, ergotamines |
| Prevalence | ~15% |
Migraine (UK: /ˈmiːɡreɪn/, US: /ˈmaɪ-/)[1][2] is a complex neurological disorder characterized by episodes of moderate-to-severe headache, most often unilateral and generally associated with nausea, and light and sound sensitivity.[3][4] Other characterizing symptoms may include vomiting, cognitive dysfunction, allodynia, and dizziness.[3] Exacerbation or worsening of headache symptoms during physical activity is another distinguishing feature.[5]
Up to one-third of people with migraine experience aura, a premonitory period of sensory disturbance widely accepted to be caused by cortical spreading depression at the onset of a migraine attack.[4] Although primarily considered to be a headache disorder, migraine is highly heterogenous in its clinical presentation and is better thought of as a spectrum disease rather than a distinct clinical entity.[6] Disease burden can range from episodic discrete attacks to chronic disease.[6][7]
Migraine is believed to be caused by a mixture of environmental and genetic factors that influence the excitation and inhibition of nerve cells in the brain.[8] The accepted hypothesis suggests that multiple primary neuronal impairments lead to a series of intracranial and extracranial changes, triggering a physiological cascade that leads to migraine symptomatology.[9]
Initial recommended treatment for acute attacks is with over-the-counter (OTC) analgesics (pain medication) such as ibuprofen and paracetamol (acetaminophen) for headache, antiemetics (anti-nausea medication) for nausea, and the avoidance of migraine triggers.[10] Specific medications such as triptans, ergotamines, or calcitonin gene-related peptide receptor (CGRP) antagonists may be used in those experiencing headaches that do not respond to the OTC pain medications.[11] For people who experience four or more attacks per month, or could otherwise benefit from prevention, prophylactic medication is recommended.[12] Commonly prescribed prophylactic medications include beta blockers like propranolol, anticonvulsants like sodium valproate, antidepressants like amitriptyline, and other off-label classes of medications.[13] Preventive medications inhibit migraine pathophysiology through various mechanisms, such as blocking calcium and sodium channels, blocking gap junctions, and inhibiting matrix metalloproteinases, among other mechanisms.[14][15] Non-pharmacological preventive therapies include nutritional supplementation, dietary interventions, sleep improvement, and aerobic exercise.[16] In 2018, the first medication (Erenumab) of a new class of drugs specifically designed for migraine prevention called CGRPs was approved by the United States Food and Drug Administration (FDA).[17] As of July 2023, the FDA has approved eight drugs that act on the CGRP system for use in the treatment of migraine.[18]
Globally, approximately 15% of people are affected by migraine.[19] In the Global Burden of Disease Study, conducted in 2010, migraine ranked as the third-most prevalent disorder in the world.[20] It most often starts at puberty and is worst during middle age.[21] As of 2016[update], it is one of the most common causes of disability.[22]
Signs and symptoms
[edit]Migraine typically presents with self-limited, recurrent severe headaches associated with autonomic symptoms.[23][24] About 15–30% of people living with migraine experience episodes with aura,[10][25] and they also frequently experience episodes without aura.[26] The severity of the pain, duration of the headache, and frequency of attacks are variable.[23] A migraine attack lasting longer than 72 hours is termed status migrainosus.[27] There are four possible phases to a migraine attack, although not all the phases are necessarily experienced:[28]
- The prodrome, which occurs hours or days before the headache
- The aura, which immediately precedes the headache
- The pain phase, also known as the headache phase
- The postdrome, the effects experienced following the end of a migraine attack
Migraine is associated with major depression, bipolar disorder, anxiety disorders, and obsessive–compulsive disorder. These psychiatric disorders are approximately 2–5 times more common in people without aura, and 3–10 times more common in people with aura.[29]
Prodrome phase
[edit]Prodromal or premonitory symptoms occur in about 60% of those with migraine,[30][31] with an onset that can range from two hours to two days before the start of pain or the aura.[32] These symptoms may include a wide variety of phenomena,[33] including altered mood, irritability, depression or euphoria, fatigue, craving for certain food(s), stiff muscles (especially in the neck), constipation or diarrhea, and sensitivity to smells or noise.[31] This may occur in those with either migraine with aura or migraine without aura.[34] Neuroimaging indicates the limbic system and hypothalamus as the origin of prodromal symptoms in migraine.[35]
Aura phase
[edit] |

|
 |

|
Aura is a transient focal neurological phenomenon that occurs before or during the headache.[30] Aura appears gradually over a number of minutes (usually occurring over 5–60 minutes) and generally lasts less than 60 minutes.[36][37] Symptoms can be visual, sensory or motoric in nature, and many people experience more than one.[38] Visual effects occur most frequently: they occur in up to 99% of cases, and in more than 50% of cases are not accompanied by sensory or motor effects.[38] If any symptom remains after 60 minutes, the state is known as persistent aura.[39]
Visual disturbances often consist of a scintillating scotoma (an area of partial alteration in the field of vision, which flickers and may interfere with a person's ability to read or drive).[30] These typically start near the center of vision and then spread out to the sides with zigzagging lines, which have been described as looking like fortifications or walls of a castle.[38] Usually, the lines are in black and white, but some people also see colored lines.[38] Some people lose part of their field of vision known as hemianopsia while others experience blurring.[38]
Sensory auras are the second most common type; they occur in 30–40% of people with auras.[38] Often, a feeling of pins-and-needles begins on one side in the hand and arm and spreads to the nose–mouth area on the same side.[38] Numbness usually occurs after the tingling has passed with a loss of position sense.[38] Other symptoms of the aura phase can include speech or language disturbances, world spinning, and, less commonly, motor problems.[38] Motor symptoms indicate that this is a hemiplegic migraine, and weakness often lasts longer than one hour unlike other auras.[38] Auditory hallucinations or delusions have also been described.[40]
Pain phase
[edit]Classically the headache is unilateral, throbbing, and moderate to severe in intensity.[36] It usually comes on gradually[36] and is aggravated by physical activity during a migraine attack.[28] However, the effects of physical activity on migraine are complex, and some researchers have concluded that, while exercise can trigger migraine attacks, regular exercise may have a prophylactic effect and decrease frequency of attacks.[41] The feeling of pulsating pain is not in phase with the pulse.[42] In more than 40% of cases, however, the pain may be bilateral (both sides of the head), and neck pain is commonly associated with it.[43] Bilateral pain is particularly common in those who have migraine without aura.[30] Less commonly pain may occur primarily in the back or top of the head.[30] The pain usually lasts 4 to 72 hours in adults;[36] however, in young children frequently lasts less than 1 hour.[44] The frequency of attacks is variable, from a few in a lifetime to several a week, with the average being about one a month.[45][46]
The pain is frequently accompanied by nausea, vomiting, sensitivity to light, sensitivity to sound, sensitivity to smells, fatigue, and irritability.[30] Many thus seek a dark and quiet room.[47] In a basilar migraine, a migraine with neurological symptoms related to the brain stem or with neurological symptoms on both sides of the body,[48] common effects include a sense of the world spinning, light-headedness, and confusion.[30] Nausea occurs in almost 90% of people, and vomiting occurs in about one-third.[47] Other symptoms may include blurred vision, nasal stuffiness, diarrhea, frequent urination, pallor, or sweating.[49] Swelling or tenderness of the scalp may occur as can neck stiffness.[49] Associated symptoms are less common in the elderly.[50]
Silent migraine
[edit]Sometimes, aura occurs without a subsequent headache.[38] This is known in modern classification as a typical aura without headache, or acephalgic migraine in previous classification, or commonly as a silent migraine.[51][52] However, silent migraine can still produce debilitating symptoms, with visual disturbance, vision loss in half of both eyes, alterations in color perception, and other sensory problems, like sensitivity to light, sound, and odors.[53] It can last from 15 to 30 minutes, usually no longer than 60 minutes, and it can recur or appear as an isolated event.[54] Many report a sore feeling in the area where the migraine was, and some report impaired thinking for a few days after the headache has passed. The person may feel tired or "hung over" and have head pain, cognitive difficulties, gastrointestinal symptoms, mood changes, and weakness.[55] According to one summary, "Some people feel unusually refreshed or euphoric after an attack, whereas others note depression and malaise."[56][unreliable medical source?]
Cause
[edit]The underlying cause of migraine is unknown.[57] However, it is believed to be related to a mix of environmental and genetic factors.[8] Migraine runs in families in about two-thirds of cases[23] and rarely occur due to a single gene defect.[58] While migraine attacks were once believed to be more common in those of high intelligence, this does not appear to be true.[45] A number of psychological conditions are associated, including depression, anxiety, and bipolar disorder.[59]

Success of the surgical migraine treatment by decompression of extracranial sensory nerves adjacent to vessels[60] suggests that people with migraine may have anatomical predisposition for neurovascular compression[61] that may be caused by both intracranial and extracranial vasodilation due to migraine triggers.[62] This, along with the existence of numerous cranial neural interconnections,[63] may explain the multiple cranial nerve involvement and consequent diversity of migraine symptoms.[64]
Genetics
[edit]Studies of twins indicate a 34–51% genetic influence on the likelihood of developing migraine.[8] This genetic relationship is stronger for migraine with aura than for migraine without aura.[26] It is clear from family and populations studies that migraine is a complex disorder, where numerous genetic risk variants exist, and where each variant increases the risk of migraine marginally.[65][66] It is also known that having several of these risk variants increases the risk by a small to moderate amount.[58]
Single gene disorders that result in migraine are rare.[58] One of these is known as familial hemiplegic migraine, a type of migraine with aura, which is inherited in an autosomal dominant fashion.[67][68] Four genes have been shown to be involved in familial hemiplegic migraine.[69] Three of these genes are involved in ion transport.[69] The fourth is the axonal protein PRRT2, associated with the exocytosis complex.[69] Another genetic disorder associated with migraine is CADASIL syndrome or cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.[30] One meta-analysis found a protective effect from angiotensin converting enzyme polymorphisms on migraine.[70] The TRPM8 gene, which codes for a cation channel, has been linked to migraine.[71]
The common forms of migraine are polygenetic, where common variants of numerous genes contribute to the predisposition for migraine. These genes can be placed in three categories, increasing the risk of migraine in general, specifically migraine with aura, or migraine without aura.[72][73] Three of these genes, CALCA, CALCB, and HTR1F are already target for migraine specific treatments. Five genes are specific risk to migraine with aura, PALMD, ABO, LRRK2, CACNA1A and PRRT2, and 13 genes are specific to migraine without aura. Using the accumulated genetic risk of the common variations, into a so-called polygenetic risk, it is possible to assess e.g. the treatment response to triptans.[74][75]
Triggers
[edit]Migraine may be induced by triggers, with some reporting it as an influence in a minority of cases[23] and others, the majority.[76] Many things, such as fatigue, certain foods, alcohol, and weather, have been labeled as triggers; however, the strength and significance of these relationships are uncertain.[76][77] Most people with migraine report experiencing triggers.[78] Symptoms may start up to 24 hours after a trigger.[23]
Also, evidence shows a strong association between migraine and the quality of sleep, particularly poor subjective quality of sleep. The relationship seems to be bidirectional, as migraine frequency increases with low quality of sleep, yet the underlying mechanism of this correlation remains poorly understood.[79]
Physiological aspects
[edit]Common triggers quoted are stress, hunger, and fatigue (these equally contribute to tension headaches).[76] Psychological stress has been reported as a factor by 50–80% of people.[80] Migraine has also been associated with post-traumatic stress disorder and abuse.[81] Migraine episodes are more likely to occur around menstruation.[80] Other hormonal influences, such as menarche, oral contraceptive use, pregnancy, perimenopause, and menopause, also play a role.[82] These hormonal influences seem to play a greater role in migraine without aura.[45] Migraine episodes typically do not occur during the second and third trimesters of pregnancy, or following menopause.[30]
Dietary aspects
[edit]Between 12% and 60% of people report foods as triggers.[83][84]
There are many reports[85][86][87][88][89] that tyramine – which is naturally present in chocolate, alcoholic beverages, most cheeses, processed meats, and other foods – can trigger migraine symptoms in some individuals. Monosodium glutamate (MSG) has been reported as a trigger for migraine,[90] but a systematic review concluded that "a causal relationship between MSG and headache has not been proven... It would seem premature to conclude that the MSG present in food causes headache".[91]
Environmental aspects
[edit]Migraines may be triggered by weather changes, including changes in temperature and barometric pressure.[92][93]
A 2009 review on potential triggers in the indoor and outdoor environment previously concluded that while there were insufficient studies to confirm environmental factors as causing migraine, "migraineurs worldwide consistently report similar environmental triggers ... such as barometric pressure change, bright sunlight, flickering lights, air quality and odors".[94]
Pathophysiology
[edit]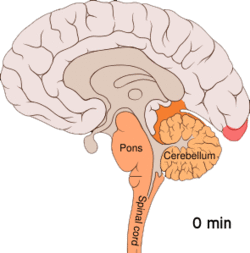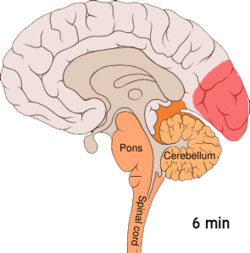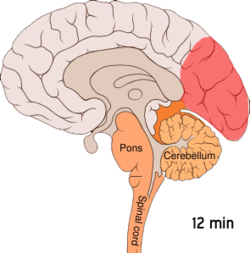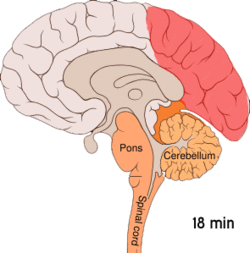
Migraine is believed to be primarily a neurological disorder,[95][96] while others believe it to be a neurovascular disorder with blood vessels playing the key role, although evidence does not support this completely.[97][98][99][100] Others believe both are likely important.[101][102][103][104] One theory is related to increased excitability of the cerebral cortex and abnormal control of pain neurons in the trigeminal nucleus of the brainstem.[105]
Sensitization of trigeminal pathways is a key pathophysiological phenomenon in migraine. It is debatable whether sensitization starts in the periphery or in the brain.[106][107]
Aura
[edit]Cortical spreading depression, or spreading depression according to Leão, is a burst of neuronal activity followed by a period of inactivity, which is seen in those with migraine with aura.[108] There are several explanations for its occurrence, including activation of NMDA receptors leading to calcium entering the cell.[108] After the burst of activity, the blood flow to the cerebral cortex in the affected area is decreased for two to six hours.[108] It is believed that when depolarization travels down the underside of the brain, nerves that sense pain in the head and neck are triggered.[108]
Pain
[edit]The exact mechanism of the head pain that occurs during a migraine episode is unknown.[109] Some evidence supports a primary role for central nervous system structures (such as the brainstem and diencephalon),[110] while other data support the role of peripheral activation (such as via the sensory nerves that surround blood vessels of the head and neck).[109] The potential candidate vessels include dural arteries, pial arteries and extracranial arteries such as those of the scalp.[109] The role of vasodilatation of the extracranial arteries, in particular, is believed to be significant.[111]
Neuromodulators
[edit]Adenosine, a neuromodulator, may be involved.[112] Released after the progressive cleavage of adenosine triphosphate (ATP), adenosine acts on adenosine receptors to put the body and brain in a low activity state by dilating blood vessels and slowing the heart rate, such as before and during the early stages of sleep. Adenosine levels are high during migraine attacks.[112][113] Caffeine's role as an inhibitor of adenosine may explain its effect in reducing migraine.[114] Low levels of the neurotransmitter serotonin, also known as 5-hydroxytryptamine (5-HT), are also believed to be involved.[115]
Calcitonin gene-related peptides (CGRPs) have been found to play a role in the pathogenesis of the pain associated with migraine, as levels of it become elevated during an attack.[10][42]
Diagnosis
[edit]The diagnosis of a migraine is based on signs and symptoms.[23] Neuroimaging tests are not necessary to diagnose migraine, but may be used to find other causes of headaches in those whose examination and history do not confirm a migraine diagnosis.[116] It is believed that a substantial number of people with the condition remain undiagnosed.[23]
The diagnosis of migraine without aura, according to the International Headache Society, can be made according to the "5, 4, 3, 2, 1 criteria", which is as follows:[28]
- Five or more attacks – for migraine with aura, two attacks are sufficient for diagnosis.
- Four hours to three days in duration
- Two or more of the following:
- Unilateral (affecting one side of the head)
- Pulsating
- Moderate or severe pain intensity
- Worsened by or causing avoidance of routine physical activity
- One or more of the following:
- Nausea and/or vomiting
- Sensitivity to both light (photophobia) and sound (phonophobia)
If someone experiences two of the following: photophobia, nausea, or inability to work or study for a day, the diagnosis is more likely.[117] In those with four out of five of the following: pulsating headache, duration of 4–72 hours, pain on one side of the head, nausea, or symptoms that interfere with the person's life, the probability that this is a migraine attack is 92%.[10] In those with fewer than three of these symptoms, the probability is 17%.[10]
Classification
[edit]Migraine was first comprehensively classified in 1988.[26]
The International Headache Society updated its classification of headaches in 2004.[28] A third version was published in 2018.[118] According to this classification, migraine is a primary headache disorder along with tension-type headaches and cluster headaches, among others.[119]
Migraine is divided into six subclasses (some of which include further subdivisions):[120]
- Migraine without aura, or "common migraine", involves migraine headaches that are not accompanied by aura.
- Migraine with aura, or "classic migraine", usually involves migraine headaches accompanied by aura. Less commonly, aura can occur without a headache or with a nonmigraine headache. Two other varieties are familial hemiplegic migraine and sporadic hemiplegic migraine, in which a person has migraine with aura and with accompanying motor weakness. If a close relative has had the same condition, it is called "familial"; otherwise, it is called "sporadic". Another variety is basilar-type migraine, where a headache and aura are accompanied by difficulty speaking, world spinning, ringing in ears, or several other brainstem-related symptoms, but not motor weakness. This type was initially believed to be due to spasms of the basilar artery, the artery that supplies the brainstem. Now that this mechanism is not believed to be primary, the symptomatic term migraine with brainstem aura (MBA) is preferred.[48] Retinal migraine (which is distinct from visual or optical migraine) involves migraine headaches accompanied by visual disturbances or even temporary blindness in one eye.
- Childhood periodic syndromes that are commonly precursors of migraine include cyclical vomiting (occasional intense periods of vomiting), abdominal migraine (abdominal pain, usually accompanied by nausea), and benign paroxysmal vertigo of childhood (occasional attacks of vertigo).
- Complications of migraine describe migraine headaches and/or auras that are unusually long or unusually frequent, or associated with a seizure or brain lesion.
- Probable migraine describes conditions that have some characteristics of migraine, but where there is not enough evidence to diagnose it as migraine with certainty (in the presence of concurrent medication overuse).
- Chronic migraine is a complication of migraine, and is a headache that fulfills diagnostic criteria for migraine headache and occurs for a greater time interval. Specifically, greater than or equal to 15 days/month for longer than 3 months.[121]
Abdominal migraine
[edit]The diagnosis of abdominal migraine is controversial.[122] Some evidence indicates that recurrent episodes of abdominal pain in the absence of a headache may be a type of migraine[122][123] or are at least a precursor to migraine attacks.[26] These episodes of pain may or may not follow a migraine-like prodrome and typically last minutes to hours.[122] They often occur in those with either a personal or family history of typical migraine.[122] Other syndromes that are believed to be precursors include cyclical vomiting syndrome and benign paroxysmal vertigo of childhood.[26]
Differential diagnosis
[edit]Other conditions that can cause similar symptoms to a migraine headache include temporal arteritis, cluster headaches, acute glaucoma, meningitis and subarachnoid hemorrhage.[10] Temporal arteritis typically occurs in people over 50 years old and presents with tenderness over the temple, cluster headache presents with one-sided nose stuffiness, tears and severe pain around the orbits, acute glaucoma is associated with vision problems, meningitis with fevers, and subarachnoid hemorrhage with a very fast onset.[10] Tension headaches typically occur on both sides, are not pounding, and are less disabling.[10]
Those with stable headaches that meet criteria for migraine should not receive neuroimaging to look for other intracranial disease.[124][125][126] This requires that other concerning findings such as papilledema (swelling of the optic disc) are not present. People with migraine are not at an increased risk of having another cause for severe headaches.[citation needed]
Management
[edit]Management of migraine includes prevention of migraine attacks and rescue treatment. There are three main aspects of treatment: trigger avoidance, acute (abortive), and preventive (prophylactic) control.[127]
Modern approaches to migraine management emphasize personalized care that considers individual patient needs. Lifestyle modifications, such as managing triggers and addressing comorbidities, form the foundation of treatment. Behavioral techniques and supplements like magnesium and riboflavin can serve as supportive options for some individuals.[128] Behavioral techniques that have been utilized in the treatment of migraines include Cognitive Behavioral Therapy (CBT), relaxation training, biofeedback, Acceptance and Commitment Therapy (ACT), as well as mindfulness-based therapies.[129] A 2024 systematic literature review and meta analysis found evidence that treatments such as CBT, relaxation training, ACT, and mindfulness-based therapies can reduce migraine frequency both on their own and in combination with other treatment options.[129] In addition, it was found that relaxation therapy aided in the lessening of migraine frequency when compared to education by itself.[129] Similarly, for children and adolescents, CBT and biofeedback strategies are effective in decreasing of frequency and intensity of migraines. These techniques often include relaxation methods and promotion of long-term management without medication side effects, which is emphasized for younger individuals.[129] Acute treatments, including NSAIDs and triptans, are most effective when administered early in an attack, while preventive medications are recommended for those experiencing frequent or severe migraines. Proven preventive options include beta blockers, topiramate, and calcitonin gene related peptides (CGRP) inhibitors like erenumab and galcanezumab, which have demonstrated significant efficacy in clinical studies.[130] The European Consensus Statement provides a framework for diagnosis and management, emphasizing the importance of accurate assessment, patient education, and consistent adherence to prescribed treatments. Innovative therapies of oral medications used to treat migraine symptoms, such as gepants and ditans, are emerging as alternatives for patients who cannot use traditional options.[131]
A 2024 systematic review and network meta analysis compared the effectiveness of medications for acute migraine attacks in adults. It found that triptans were the most effective class of drugs, followed by non-steroidal anti-inflammatories. Gepants were less effective than non-steroidal anti-inflammatory drugs.[132][133]
Calcitonin gene related peptide
[edit]Calcitonin gene-related peptide is a neuropeptide implicated in the pathophysiology of migraines. It is predominantly found in the trigeminal ganglion and central nervous system pathways associated with migraine mechanisms.[134] During migraine attacks, elevated levels of CGRP are detected, leading to vasodilation of cerebral and dural blood vessels and the release of inflammatory mediators from mast cells. These actions contribute to the transmission of nociceptive signals, culminating in migraine pain. Targeting CGRP has emerged as a promising therapeutic strategy for migraine management.[135][136]
Prognosis
[edit]"Migraine exists on a continuum of different attack frequencies and associated levels of disability."[137] For those with occasional, episodic migraine, a "proper combination of drugs for prevention and treatment of migraine attacks" can limit the disease's impact on patients' personal and professional lives.[138] But fewer than half of people with migraine seek medical care and more than half go undiagnosed and undertreated.[139] "Responsive prevention and treatment of migraine is incredibly important" because evidence shows "an increased sensitivity after each successive attack, eventually leading to chronic daily migraine in some individuals."[138] Repeated migraine results in "reorganization of brain circuitry", causing "profound functional as well as structural changes in the brain."[140] "One of the most important problems in clinical migraine is the progression from an intermittent, self-limited inconvenience to a life-changing disorder of chronic pain, sensory amplification, and autonomic and affective disruption. This progression, sometimes termed chronification in the migraine literature, is common, affecting 3% of migraineurs in a given year, such that 8% of migraineurs have chronic migraine in any given year." Brain imagery reveals that the electrophysiological changes seen during an attack become permanent in people with chronic migraine; "thus, from an electrophysiological point of view, chronic migraine indeed resembles a never-ending migraine attack."[140] Severe migraine ranks in the highest category of disability, according to the World Health Organization, which uses objective metrics to determine disability burden for the authoritative annual Global Burden of Disease report. The report classifies severe migraine alongside severe depression, active psychosis, quadriplegia, and terminal-stage cancer.[141]
Migraine with aura appears to be a risk factor for ischemic stroke[142] doubling the risk.[143] Being a young adult, being female, using hormonal birth control, and smoking further increases this risk.[142] There also appears to be an association with cervical artery dissection.[144] Migraine without aura does not appear to be a factor.[145] The relationship with heart problems is inconclusive with a single study supporting an association.[142] Migraine does not appear to increase the risk of death from stroke or heart disease.[146] Preventative therapy of migraine in those with migraine with aura may prevent associated strokes.[147] People with migraine, particularly women, may develop higher than average numbers of white matter brain lesions of unclear significance.[148]
Epidemiology
[edit]
Migraine is common, with around 33% of women and 18% of men affected at some point in their lifetime.[149] Onset can be at any age, but prevalence rises sharply around puberty, and remains high until declining after age 50.[149] Before puberty, boys and girls are equally impacted, with around 5% of children experiencing migraine attacks. From puberty onwards, women experience migraine attacks at greater rates than men. From age 30 to 50, up to 4 times as many women experience migraine attacks as men;[149] this is most pronounced in migraine without aura.[150]
Worldwide, migraine affects nearly 15% or approximately one billion people.[19] In the United States, about 6% of men and 18% of women experience a migraine attack in a given year, with a lifetime risk of about 18% and 43%, respectively.[23] In Europe, migraine affects 12–28% of people at some point in their lives, with about 6–15% of adult men and 14–35% of adult women getting at least one attack yearly.[151] Rates of migraine are slightly lower in Asia and Africa than in Western countries.[45][152] Chronic migraine occurs in approximately 1.4–2.2% of the population.[153]
During perimenopause symptoms often get worse before decreasing in severity.[154] While symptoms resolve in about two-thirds of the elderly, in 3–10% they persist.[50]
History
[edit]
An early description consistent with migraine is contained in the Ebers Papyrus, written around 1500 BCE in ancient Egypt.[155]
The word migraine is from the Greek ἡμικρᾱνίᾱ (hēmikrāníā), 'pain in half of the head',[156] from ἡμι- (hēmi-), 'half' and κρᾱνίον (krāníon), 'skull'.[157]
In 200 BCE, writings from the Hippocratic school of medicine described the visual aura that can precede the headache and a partial relief occurring through vomiting.[158]
A second-century description by Aretaeus of Cappadocia divided headaches into three types: cephalalgia, cephalea, and heterocrania.[159] Galen of Pergamon used the term hemicrania (half-head), from which the word migraine was eventually derived.[159] Galen also proposed that the pain arose from the meninges and blood vessels of the head.[158] Migraine was first divided into the two now used types – migraine with aura (migraine ophthalmique) and migraine without aura (migraine vulgaire) in 1887 by Louis Hyacinthe Thomas, a French librarian.[158] The mystical visions of Hildegard von Bingen, which she described as "reflections of the living light", are consistent with the visual aura experienced during migraine attacks.[160]

Trepanation, the deliberate drilling of holes into a skull, was practiced as early as 7,000 BCE.[155] While sometimes people survived, many would have died from the procedure due to infection.[161] It was believed to work via "letting evil spirits escape".[162] William Harvey recommended trepanation as a treatment for migraine in the 17th century.[163] The association between trepanation and headaches in ancient history may simply be a myth or unfounded speculation that originated several centuries later. In 1913, the world-famous American physician William Osler misinterpreted the French anthropologist and physician Paul Broca's words about a set of children's skulls from the Neolithic age that he found during the 1870s. These skulls presented no evident signs of fractures that could justify this complex surgery for mere medical reasons. Trepanation was probably born of superstitions, to remove "confined demons" inside the head, or to create healing or fortune talismans with the bone fragments removed from the skulls of the patients. However, Osler wanted to make Broca's theory more palatable to his modern audiences, and explained that trepanation procedures were used for mild conditions such as "infantile convulsions headache and various cerebral diseases believed to be caused by confined demons."[164]
While many treatments for migraine have been attempted, it was not until 1868 that use of a substance that eventually turned out to be effective began.[158] This substance was the fungus ergot from which ergotamine was isolated in 1918[165] and first used to treat migraine in 1925.[166] Methysergide was developed in 1959 and the first triptan, sumatriptan, was developed in 1988.[165] During the 20th century, with better study design, effective preventive measures were found and confirmed.[158]
Society and culture
[edit]Migraine is a significant source of both medical costs and lost productivity. It has been estimated that migraine is the most costly neurological disorder in the European Community, costing more than €27 billion per year.[167] In the United States, direct costs have been estimated at US$17 billion, while indirect costs – such as missed or decreased ability to work – is estimated at US$15 billion.[168] Nearly a tenth of the direct cost is due to the cost of triptans.[168] In those who do attend work during a migraine attack, effectiveness is decreased by around a third.[167] Negative impacts also frequently occur for a person's family.[167]
Research
[edit]Prevention mechanisms
[edit]Transcranial magnetic stimulation shows promise,[10][169] as does transcutaneous supraorbital nerve stimulation.[170] There is preliminary evidence that a ketogenic diet may help prevent episodic and long-term migraine.[171][172]
Sex dependency
[edit]Statistical data indicates that women may be more prone to having migraine, showing migraine incidence three times higher among women than men.[173][174] The Society for Women's Health Research has also mentioned hormonal influences, mainly estrogen, as having a considerable role in provoking migraine pain. Studies and research related to the sex dependencies of migraine are ongoing, and conclusions have yet to be achieved.[175]
See also
[edit]References
[edit]- ^ Wells JC (2008). Longman Pronunciation Dictionary (3rd ed.). Longman. ISBN 978-1-4058-8118-0.
- ^ Jones D (2011). Roach P, Setter J, Esling J (eds.). Cambridge English Pronouncing Dictionary (18th ed.). Cambridge University Press. ISBN 978-0-521-15255-6.
- ^ a b Pescador Ruschel MA, De Jesus O (2024). "Migraine Headache". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 32809622. Retrieved 13 September 2024.
- ^ a b "Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition". Cephalalgia: An International Journal of Headache. 38 (1): 1–211. January 2018. doi:10.1177/0333102417738202. ISSN 1468-2982. PMID 29368949.
- ^ Martins IP, Gouveia RG, Parreira E (June 2006). "Kinesiophobia in migraine". The Journal of Pain. 7 (6): 445–451. doi:10.1016/j.jpain.2006.01.449. PMID 16750801.
- ^ a b Katsarava Z, Buse DC, Manack AN, Lipton RB (February 2012). "Defining the differences between episodic migraine and chronic migraine". Current Pain and Headache Reports. 16 (1): 86–92. doi:10.1007/s11916-011-0233-z. PMC 3258393. PMID 22083262.
- ^ Shankar Kikkeri N, Nagalli S (December 2022). "Migraine With Aura". StatPearls Publishing. PMID 32119498. Bookshelf ID: NBK554611. Archived from the original on 8 June 2023. Retrieved 23 August 2023.
- ^ a b c Piane M, Lulli P, Farinelli I, Simeoni S, De Filippis S, Patacchioli FR, et al. (December 2007). "Genetics of migraine and pharmacogenomics: some considerations". The Journal of Headache and Pain. 8 (6): 334–339. doi:10.1007/s10194-007-0427-2. PMC 2779399. PMID 18058067.
- ^ Burstein R, Noseda R, Borsook D (April 2015). "Migraine: multiple processes, complex pathophysiology". The Journal of Neuroscience. 35 (17): 6619–6629. doi:10.1523/JNEUROSCI.0373-15.2015. PMC 4412887. PMID 25926442.
- ^ a b c d e f g h i Gilmore B, Michael M (February 2011). "Treatment of acute migraine headache". American Family Physician. 83 (3): 271–280. PMID 21302868.
- ^ Tzankova V, Becker WJ, Chan TL (January 2023). "Diagnosis and acute management of migraine". CMAJ. 195 (4): E153 – E158. doi:10.1503/cmaj.211969. PMC 9888545. PMID 36717129. Archived from the original on 4 July 2023. Retrieved 22 August 2023.
- ^ Silberstein SD (August 2015). "Preventive Migraine Treatment". Continuum. 21 (4 Headache): 973–989. doi:10.1212/CON.0000000000000199. PMC 4640499. PMID 26252585.
- ^ Kumar A, Kadian R (September 2022). "Migraine Prophylaxis". StatPearls Publishing. PMID 29939650. Bookshelf ID: NBK507873. Archived from the original on 8 March 2023. Retrieved 22 August 2023.
- ^ Noseda R, Burstein R (December 2013). "Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain". Pain. 154 (Suppl 1): S44 – S53. doi:10.1016/j.pain.2013.07.021. PMC 3858400. PMID 24347803.
- ^ Spierings EL (July 2001). "Mechanism of migraine and action of antimigraine medications". The Medical Clinics of North America. 85 (4): 943–958, vi–vii. doi:10.1016/s0025-7125(05)70352-7. PMID 11480266.
- ^ Haghdoost F, Togha M (1 January 2022). "Migraine management: Non-pharmacological points for patients and health care professionals". Open Medicine. 17 (1): 1869–1882. doi:10.1515/med-2022-0598. PMC 9691984. PMID 36475060.
- ^ Office of the Commissioner (24 March 2020). "FDA approves novel preventive treatment for migraine". FDA. Archived from the original on 11 June 2019. Retrieved 13 September 2024.
- ^ Rashid A, Manghi A (2024). "Calcitonin Gene-Related Peptide Receptor". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 32809483. Retrieved 13 September 2024.
- ^ a b Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. (December 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2163–96. doi:10.1016/S0140-6736(12)61729-2. PMC 6350784. PMID 23245607.
- ^ Gobel H. "1. Migraine". ICHD-3 The International Classification of Headache Disorders 3rd edition. Archived from the original on 24 October 2020. Retrieved 22 October 2020.
- ^ "Headache disorders Fact sheet N°277". October 2012. Archived from the original on 16 February 2016. Retrieved 15 February 2016.
- ^ Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators) (September 2017). "Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016". Lancet. 390 (10100): 1211–1259. doi:10.1016/S0140-6736(17)32154-2. PMC 5605509. PMID 28919117.
- ^ a b c d e f g h Bartleson JD, Cutrer FM (May 2010). "Migraine update. Diagnosis and treatment". Minnesota Medicine. 93 (5): 36–41. PMID 20572569.
- ^ Bigal ME, Lipton RB (June 2008). "The prognosis of migraine". Current Opinion in Neurology. 21 (3): 301–8. doi:10.1097/WCO.0b013e328300c6f5. PMID 18451714. S2CID 34805084.
- ^ Gutman SA (2008). Quick reference neuroscience for rehabilitation professionals: the essential neurologic principles underlying rehabilitation practice (2nd ed.). Thorofare, NJ: SLACK. p. 231. ISBN 978-1-55642-800-5. Archived from the original on 12 March 2017.
- ^ a b c d e Olesen J, Goadsby PJ (2006). "The Migraines: Introduction". In Olesen J (ed.). The Headaches (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 232–233. ISBN 978-0-7817-5400-2. Archived from the original on 13 March 2017.
- ^ Tfelt-Hansen P, Young WB, Silberstein (2006). "Antiemetic, Prokinetic, Neuroleptic and Miscellaneous Drugs in the Acute Treatment of Migraine". In Olesen J (ed.). The Headaches (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 512. ISBN 978-0-7817-5400-2. Archived from the original on 22 December 2016.
- ^ a b c d Headache Classification Subcommittee of the International Headache Society (2013). "The International Classification of Headache Disorders: 3rd edition". Cephalalgia. 33 (9): 644–648. doi:10.1177/0333102413485658. PMID 23771276.
- ^ Baskin SM, Lipchik GL, Smitherman TA (October 2006). "Mood and anxiety disorders in chronic headache". Headache. 46 (Suppl 3): S76-87. doi:10.1111/j.1526-4610.2006.00559.x. PMID 17034402. S2CID 35451906.
- ^ a b c d e f g h i Simon RP, Aminoff MJ, Greenberg DA (2009). Clinical neurology (7 ed.). New York, N.Y: Lange Medical Books/McGraw-Hill. pp. 85–88. ISBN 978-0-07-166433-2.
- ^ a b Lynn DJ, Newton HB, Rae-Grant A (2004). The 5-minute neurology consult. Philadelphia: Lippincott Williams & Wilkins. p. 26. ISBN 978-0-683-30723-8. Archived from the original on 13 March 2017.
- ^ Buzzi MG, Cologno D, Formisano R, Rossi P (October–December 2005). "Prodromes and the early phase of the migraine attack: therapeutic relevance". Functional Neurology. 20 (4): 179–83. PMID 16483458.
- ^ Rossi P, Ambrosini A, Buzzi MG (October–December 2005). "Prodromes and predictors of migraine attack". Functional Neurology. 20 (4): 185–91. PMID 16483459.
- ^ Ropper AH, Adams RD, Victor M, Samuels MA (2009). Adams and Victor's principles of neurology (9 ed.). New York: McGraw-Hill Medical. pp. Chapter 10. ISBN 978-0-07-149992-7.
- ^ May A, Burstein R (November 2019). "Hypothalamic regulation of headache and migraine". Cephalalgia. 39 (13): 1710–1719. doi:10.1177/0333102419867280. PMC 7164212. PMID 31466456.
- ^ a b c d Tintinalli JE (2010). Emergency Medicine: A Comprehensive Study Guide (Emergency Medicine (Tintinalli)). New York: McGraw-Hill Companies. pp. 1116–1117. ISBN 978-0-07-148480-0.
- ^ Ashina M (November 2020). "Migraine". The New England Journal of Medicine. 383 (19): 1866–1876. doi:10.1056/NEJMra1915327. PMID 33211930. S2CID 227078662.
- ^ a b c d e f g h i j k Cutrer FM, Olesen J (2006). "Migraines with Aura and Their Subforms". In Olesen J (ed.). The Headaches (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 238–240. ISBN 978-0-7817-5400-2. Archived from the original on 13 March 2017.
- ^ Viana M, Sances G, Linde M, Nappi G, Khaliq F, Goadsby PJ, et al. (August 2018). "Prolonged migraine aura: new insights from a prospective diary-aided study". The Journal of Headache and Pain. 19 (1) 77. doi:10.1186/s10194-018-0910-y. PMC 6119171. PMID 30171359.
- ^ Slap, GB (2008). Adolescent medicine. Philadelphia, PA: Mosby/Elsevier. p. 105. ISBN 978-0-323-04073-0. Archived from the original on 13 March 2017.
- ^ Amin FM, Aristeidou S, Baraldi C, Czapinska-Ciepiela EK, Ariadni DD, Di Lenola D, et al. (September 2018). "The association between migraine and physical exercise". The Journal of Headache and Pain. 19 (1) 83. doi:10.1186/s10194-018-0902-y. PMC 6134860. PMID 30203180.
- ^ a b Qubty W, Patniyot I (June 2020). "Migraine Pathophysiology". Pediatric Neurology. 107: 1–6. doi:10.1016/j.pediatrneurol.2019.12.014. PMID 32192818. S2CID 213191464.
- ^ Tepper SJ, Tepper DE (1 January 2011). The Cleveland Clinic manual of headache therapy. New York: Springer. p. 6. ISBN 978-1-4614-0178-0. Archived from the original on 22 December 2016.
- ^ Bigal ME, Arruda MA (July 2010). "Migraine in the pediatric population--evolving concepts". Headache. 50 (7): 1130–43. doi:10.1111/j.1526-4610.2010.01717.x. PMID 20572878. S2CID 23256755.
- ^ a b c d Rasmussen BK (2006). "Epidemiology of Migraine". In Olesen J (ed.). The Headaches (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 238–240. ISBN 978-0-7817-5400-2. Archived from the original on 13 March 2017.
- ^ Dalessio DJ (2001). Silberstein SD, Lipton RB, Dalessio DJ (eds.). Wolff's headache and other head pain (7 ed.). Oxford: Oxford University Press. p. 122. ISBN 978-0-19-513518-3.
- ^ a b Lisak RP, Truong DD, Carroll W, Bhidayasiri R (2009). International neurology: a clinical approach. Chichester, UK: Wiley-Blackwell. p. 670. ISBN 978-1-4051-5738-4.
- ^ a b Kaniecki RG (June 2009). "Basilar-type migraine". Current Pain and Headache Reports. 13 (3): 217–20. doi:10.1007/s11916-009-0036-7. PMID 19457282. S2CID 22242504.
- ^ a b Glaser JS (1999). Neuro-ophthalmology (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 555. ISBN 978-0-7817-1729-8. Archived from the original on 13 March 2017.
- ^ a b Dodick DW, Capobianco DJ (2008). "Chapter 14: Headaches". In Sirven JI, Malamut BL (eds.). Clinical neurology of the older adult (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 197. ISBN 978-0-7817-6947-1. Archived from the original on 12 March 2017.
- ^ Robblee J (21 August 2019). "Silent Migraine: A Guide". American Migraine Foundation. Archived from the original on 28 January 2021. Retrieved 22 January 2021.
- ^ He Y, Li Y, Nie Z (February 2015). "Typical aura without headache: a case report and review of the literature". Journal of Medical Case Reports. 9 (1) 40. doi:10.1186/s13256-014-0510-7. PMC 4344793. PMID 25884682.
- ^ Leonard J (7 September 2018). Han S (ed.). "Silent migraine: Symptoms, causes, treatment, prevention". Medical News Today. Archived from the original on 19 January 2021. Retrieved 22 January 2021.
- ^ Bose P, Goadsby PJ (June 2016). "The migraine postdrome". Current Opinion in Neurology. 29 (3): 299–301. doi:10.1097/WCO.0000000000000310. PMID 26886356. S2CID 22445093.
- ^ Kelman L (February 2006). "The postdrome of the acute migraine attack". Cephalalgia. 26 (2): 214–20. doi:10.1111/j.1468-2982.2005.01026.x. PMID 16426278. S2CID 21519111.
- ^ Halpern AL, Silberstein SD (2005). "Ch. 9: The Migraine Attack—A Clinical Description". In Kaplan PW, Fisher RS (eds.). Imitators of Epilepsy (2 ed.). New York: Demos Medical. ISBN 978-1-888799-83-5. NBK7326. Archived from the original on 27 August 2011. Retrieved 5 September 2017.
- ^ Robbins MS, Lipton RB (April 2010). "The epidemiology of primary headache disorders". Seminars in Neurology. 30 (2): 107–19. doi:10.1055/s-0030-1249220. PMID 20352581. S2CID 260317083.
- ^ a b c Schürks M (January 2012). "Genetics of migraine in the age of genome-wide association studies". The Journal of Headache and Pain. 13 (1): 1–9. doi:10.1007/s10194-011-0399-0. PMC 3253157. PMID 22072275.
- ^ The Headaches, pp. 246–247
- ^ Bink T, Duraku LS, Ter Louw RP, Zuidam JM, Mathijssen IM, Driessen C (December 2019). "The Cutting Edge of Headache Surgery: A Systematic Review on the Value of Extracranial Surgery in the Treatment of Chronic Headache". Plastic and Reconstructive Surgery. 144 (6): 1431–1448. doi:10.1097/PRS.0000000000006270. PMID 31764666. S2CID 208273535.
- ^ Szmyd B, Sołek J, Błaszczyk M, Jankowski J, Liberski PP, Jaskólski DJ, et al. (4 July 2022). "The Underlying Pathogenesis of Neurovascular Compression Syndromes: A Systematic Review". Frontiers in Molecular Neuroscience. 15 923089. doi:10.3389/fnmol.2022.923089. PMC 9289473. PMID 35860499.
- ^ Macionis V (November 2023). "Neurovascular Compression-Induced Intracranial Allodynia May Be the True Nature of Migraine Headache: an Interpretative Review". Current Pain and Headache Reports. 27 (11): 775–791. doi:10.1007/s11916-023-01174-7. PMID 37837483.
- ^ Adair D, Truong D, Esmaeilpour Z, Gebodh N, Borges H, Ho L, et al. (May 2020). "Electrical stimulation of cranial nerves in cognition and disease". Brain Stimulation. 13 (3): 717–750. doi:10.1016/j.brs.2020.02.019. PMC 7196013. PMID 32289703.
- ^ Villar-Martinez MD, Goadsby PJ (September 2022). "Pathophysiology and Therapy of Associated Features of Migraine". Cells. 11 (17): 2767. doi:10.3390/cells11172767. PMC 9455236. PMID 36078174.
- ^ Gormley P, Kurki MI, Hiekkala ME, Veerapen K, Häppölä P, Mitchell AA, et al. (May 2018). "Common Variant Burden Contributes to the Familial Aggregation of Migraine in 1,589 Families". Neuron. 98 (4): 743–753.e4. doi:10.1016/j.neuron.2018.04.014. PMC 5967411. PMID 29731251.
- ^ Harder AV, Terwindt GM, Nyholt DR, van den Maagdenberg AM (February 2023). "Migraine genetics: Status and road forward". Cephalalgia. 43 (2) 03331024221145962: 3331024221145962. doi:10.1177/03331024221145962. PMID 36759319.
{{cite journal}}: CS1 maint: article number as page number (link) - ^ de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM (July 2009). "Molecular genetics of migraine". Human Genetics. 126 (1): 115–32. doi:10.1007/s00439-009-0684-z. PMID 19455354. S2CID 20119237.
- ^ Montagna P (September 2008). "Migraine genetics". Expert Review of Neurotherapeutics. 8 (9): 1321–30. doi:10.1586/14737175.8.9.1321. PMID 18759544. S2CID 207195127.
- ^ a b c Ducros A (May 2013). "[Genetics of migraine]". Revue Neurologique. 169 (5): 360–71. doi:10.1016/j.neurol.2012.11.010. PMID 23618705.
- ^ Wan D, Wang C, Zhang X, Tang W, Chen M, Dong Z, et al. (1 January 2016). "Association between angiotensin-converting enzyme insertion/deletion polymorphism and migraine: a meta-analysis". The International Journal of Neuroscience. 126 (5): 393–9. doi:10.3109/00207454.2015.1025395. PMID 26000817. S2CID 34902092.
- ^ Dussor G, Cao YQ (October 2016). "TRPM8 and Migraine". Headache. 56 (9): 1406–1417. doi:10.1111/head.12948. PMC 5335856. PMID 27634619.
- ^ Bjornsdottir G, Chalmer MA, Stefansdottir L, Skuladottir AT, Einarsson G, Andresdottir M, et al. (November 2023). "Rare variants with large effects provide functional insights into the pathology of migraine subtypes, with and without aura". Nature Genetics. 55 (11): 1843–1853. doi:10.1038/s41588-023-01538-0. PMC 10632135. PMID 37884687.
- ^ Hautakangas H, Winsvold BS, Ruotsalainen SE, Bjornsdottir G, Harder AV, Kogelman LJ, et al. (February 2022). "Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles". Nature Genetics. 54 (2): 152–160. doi:10.1038/s41588-021-00990-0. PMC 8837554. PMID 35115687.
- ^ Mikol DD, Picard H, Klatt J, Wang A, Peng C, Stefansson K (14 April 2020). "Migraine Polygenic Risk Score Is Associated with Severity of Migraine – Analysis of Genotypic Data from Four Placebo-controlled Trials of Erenumab (1214)". Neurology. 94 (15_supplement) 1214. doi:10.1212/WNL.94.15_supplement.1214. ISSN 0028-3878.
- ^ Kogelman LJ, Esserlind AL, Francke Christensen A, Awasthi S, Ripke S, Ingason A, et al. (December 2019). "Migraine polygenic risk score associates with efficacy of migraine-specific drugs". Neurology. Genetics. 5 (6) e364. doi:10.1212/NXG.0000000000000364. PMC 6878840. PMID 31872049.
- ^ a b c Levy D, Strassman AM, Burstein R (June 2009). "A critical view on the role of migraine triggers in the genesis of migraine pain". Headache. 49 (6): 953–7. doi:10.1111/j.1526-4610.2009.01444.x. PMID 19545256. S2CID 31707887.
- ^ Martin PR (June 2010). "Behavioral management of migraine headache triggers: learning to cope with triggers". Current Pain and Headache Reports. 14 (3): 221–7. doi:10.1007/s11916-010-0112-z. PMID 20425190. S2CID 5511782.
- ^ Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB (2014). "Trigger factors and premonitory features of migraine attacks: summary of studies". Headache. 54 (10): 1670–9. doi:10.1111/head.12468. PMID 25399858. S2CID 25016889.
- ^ Stanyer EC, Creeney H, Nesbitt AD, Holland PR, Hoffmann J (October 2021). "Subjective Sleep Quality and Sleep Architecture in Patients With Migraine: A Meta-analysis". Neurology. 97 (16): e1620 – e1631. doi:10.1212/WNL.0000000000012701. PMC 8548957. PMID 34551985.
- ^ a b Radat F (May 2013). "[Stress and migraine]". Revue Neurologique. 169 (5): 406–12. doi:10.1016/j.neurol.2012.11.008. PMID 23608071.
- ^ Peterlin BL, Katsnelson MJ, Calhoun AH (October 2009). "The associations between migraine, unipolar psychiatric comorbidities, and stress-related disorders and the role of estrogen". Current Pain and Headache Reports. 13 (5): 404–12. doi:10.1007/s11916-009-0066-1. PMC 3972495. PMID 19728969.
- ^ Chai NC, Peterlin BL, Calhoun AH (June 2014). "Migraine and estrogen". Current Opinion in Neurology. 27 (3): 315–24. doi:10.1097/WCO.0000000000000091. PMC 4102139. PMID 24792340.
- ^ Finocchi C, Sivori G (May 2012). "Food as trigger and aggravating factor of migraine". Neurological Sciences. 33 (Suppl 1): S77-80. doi:10.1007/s10072-012-1046-5. PMID 22644176. S2CID 19582697.
- ^ Rockett FC, de Oliveira VR, Castro K, Chaves ML, Perla A, Perry ID (June 2012). "Dietary aspects of migraine trigger factors". Nutrition Reviews. 70 (6): 337–56. doi:10.1111/j.1753-4887.2012.00468.x. PMID 22646127.
- ^ Ghose K, Carroll JD (1984). "Mechanism of tyramine-induced migraine: similarity with dopamine and interactions with disulfiram and propranolol in migraine patients". Neuropsychobiology. 12 (2–3): 122–126. doi:10.1159/000118123. PMID 6527752.
- ^ Moffett A, Swash M, Scott DF (August 1972). "Effect of tyramine in migraine: a double-blind study". Journal of Neurology, Neurosurgery, and Psychiatry. 35 (4): 496–499. doi:10.1136/jnnp.35.4.496. PMC 494110. PMID 4559027.
- ^ "Tyramine and Migraines: What You Need to Know". excedrin.com. Retrieved 4 March 2022.
- ^ Encyclopedia of Food Sciences and Nutrition (Second ed.). Academic Press. 2003. ISBN 978-0-12-227055-0.
- ^ Özturan A, Şanlıer N, Coşkun Ö (2016). "The Relationship Between Migraine and Nutrition" (PDF). Turk J Neurol. 22 (2): 44–50. doi:10.4274/tnd.37132. Archived (PDF) from the original on 23 August 2023. Retrieved 4 March 2022.
- ^ Sun-Edelstein C, Mauskop A (June 2009). "Foods and supplements in the management of migraine headaches" (PDF). The Clinical Journal of Pain. 25 (5): 446–452. CiteSeerX 10.1.1.530.1223. doi:10.1097/AJP.0b013e31819a6f65. PMID 19454881. S2CID 3042635. Archived from the original (PDF) on 13 August 2017.
- ^ Obayashi Y, Nagamura Y (17 May 2016). "Does monosodium glutamate really cause headache?: a systematic review of human studies". The Journal of Headache and Pain. 17 (1) 54. doi:10.1186/s10194-016-0639-4. PMC 4870486. PMID 27189588.
- ^ "Why Climate Change Might Be Affecting Your Headaches". www.pennmedicine.org.
- ^ Cioffi I, Farella M, Chiodini P, Ammendola L, Capuozzo R, Klain C, et al. (May 2017). "Effect of weather on temporal pain patterns in patients with temporomandibular disorders and migraine". Journal of Oral Rehabilitation. 44 (5): 333–339. doi:10.1111/joor.12498. PMID 28244179.
- ^ Friedman DI, De ver Dye T (June 2009). "Migraine and the environment". Headache. 49 (6): 941–52. doi:10.1111/j.1526-4610.2009.01443.x. PMID 19545255. S2CID 29764274.
- ^ Andreou AP, Edvinsson L (December 2019). "Mechanisms of migraine as a chronic evolutive condition". The Journal of Headache and Pain. 20 (1) 117. doi:10.1186/s10194-019-1066-0. PMC 6929435. PMID 31870279.
- ^ "Migraine". National Institute of Neurological Disorders and Stroke. 11 July 2023. Retrieved 25 August 2023.
- ^ Hoffmann J, Baca SM, Akerman S (April 2019). "Neurovascular mechanisms of migraine and cluster headache". Journal of Cerebral Blood Flow and Metabolism. 39 (4): 573–594. doi:10.1177/0271678x17733655. PMC 6446418. PMID 28948863.
- ^ Brennan KC, Charles A (June 2010). "An update on the blood vessel in migraine". Current Opinion in Neurology. 23 (3): 266–74. doi:10.1097/WCO.0b013e32833821c1. PMC 5500293. PMID 20216215.
- ^ Spiri D, Titomanlio L, Pogliani L, Zuccotti G (January 2012). "Pathophysiology of migraine: The neurovascular theory". Headaches: Causes, Treatment and Prevention: 51–64.
- ^ Goadsby PJ (January 2009). "The vascular theory of migraine--a great story wrecked by the facts". Brain. 132 (Pt 1): 6–7. doi:10.1093/brain/awn321. PMID 19098031.
- ^ Dodick DW (April 2008). "Examining the essence of migraine--is it the blood vessel or the brain? A debate". Headache. 48 (4): 661–7. doi:10.1111/j.1526-4610.2008.01079.x. PMID 18377395. S2CID 6272233.
- ^ Chen D, Willis-Parker M, Lundberg GP (October 2020). "Migraine headache: Is it only a neurological disorder? Links between migraine and cardiovascular disorders". Trends in Cardiovascular Medicine. 30 (7): 424–430. doi:10.1016/j.tcm.2019.10.005. PMID 31679956.
- ^ Jacobs B, Dussor G (December 2016). "Neurovascular contributions to migraine: Moving beyond vasodilation". Neuroscience. 338: 130–144. doi:10.1016/j.neuroscience.2016.06.012. PMC 5083225. PMID 27312704.
- ^ Mason BN, Russo AF (2018). "Vascular Contributions to Migraine: Time to Revisit?". Frontiers in Cellular Neuroscience. 12 233. doi:10.3389/fncel.2018.00233. PMC 6088188. PMID 30127722.
- ^ Dodick DW, Gargus JJ (August 2008). "Why migraines strike". Scientific American. 299 (2): 56–63. Bibcode:2008SciAm.299b..56D. doi:10.1038/scientificamerican0808-56. PMID 18666680.
- ^ Edvinsson L, Haanes KA (May 2020). "Views on migraine pathophysiology: Where does it start?". Neurology and Clinical Neuroscience. 8 (3): 120–127. doi:10.1111/ncn3.12356. ISSN 2049-4173. S2CID 214320892.
- ^ Do TP, Hougaard A, Dussor G, Brennan KC, Amin FM (January 2023). "Migraine attacks are of peripheral origin: the debate goes on". The Journal of Headache and Pain. 24 (1) 3. doi:10.1186/s10194-022-01538-1. PMC 9830833. PMID 36627561.
- ^ a b c d The Headaches, Chp. 28, pp. 269–72
- ^ a b c Olesen J, Burstein R, Ashina M, Tfelt-Hansen P (July 2009). "Origin of pain in migraine: evidence for peripheral sensitisation". The Lancet. Neurology. 8 (7): 679–90. doi:10.1016/S1474-4422(09)70090-0. PMID 19539239. S2CID 20452008.
- ^ Akerman S, Holland PR, Goadsby PJ (September 2011). "Diencephalic and brainstem mechanisms in migraine". Nature Reviews. Neuroscience. 12 (10): 570–84. doi:10.1038/nrn3057. PMID 21931334. S2CID 8472711.
- ^ Shevel E (March 2011). "The extracranial vascular theory of migraine--a great story confirmed by the facts". Headache. 51 (3): 409–417. doi:10.1111/j.1526-4610.2011.01844.x. PMID 21352215. S2CID 6939786.
- ^ a b Burnstock G (January 2016). "Purinergic Mechanisms and Pain". In Barrett JE (ed.). Pharmacological Mechanisms and the Modulation of Pain. Advances in Pharmacology. Vol. 75. pp. 91–137. doi:10.1016/bs.apha.2015.09.001. ISBN 978-0-12-803883-3. PMID 26920010.
- ^ Davidoff R (14 February 2002). Migraine: Manifestations, Pathogenesis, and Management. Oxford University Press. ISBN 978-0-19-803135-2. Archived from the original on 23 August 2023. Retrieved 12 November 2020.
- ^ Lipton RB, Diener HC, Robbins MS, Garas SY, Patel K (October 2017). "Caffeine in the management of patients with headache". The Journal of Headache and Pain. 18 (1) 107. doi:10.1186/s10194-017-0806-2. PMC 5655397. PMID 29067618.
- ^ Hamel E (November 2007). "Serotonin and migraine: biology and clinical implications". Cephalalgia. 27 (11): 1293–300. doi:10.1111/j.1468-2982.2007.01476.x. PMID 17970989. S2CID 26543041.
- ^
- Lewis DW, Dorbad D (September 2000). "The utility of neuroimaging in the evaluation of children with migraine or chronic daily headache who have normal neurological examinations". Headache. 40 (8): 629–32. doi:10.1046/j.1526-4610.2000.040008629.x. PMID 10971658. S2CID 14443890.
- Silberstein SD (September 2000). "Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology. 55 (6): 754–62. doi:10.1212/WNL.55.6.754. PMID 10993991.
- Medical Advisory Secretariat (2010). "Neuroimaging for the evaluation of chronic headaches: an evidence-based analysis". Ontario Health Technology Assessment Series. 10 (26): 1–57. PMC 3377587. PMID 23074404.
- ^ Cousins G, Hijazze S, Van de Laar FA, Fahey T (July–August 2011). "Diagnostic accuracy of the ID Migraine: a systematic review and meta-analysis". Headache. 51 (7): 1140–8. doi:10.1111/j.1526-4610.2011.01916.x. PMID 21649653. S2CID 205684294.
- ^ "Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition". Cephalalgia. 38 (1): 1–211. January 2018. doi:10.1177/0333102417738202. PMID 29368949.
- ^ Nappi G (September 2005). "Introduction to the new International Classification of Headache Disorders". The Journal of Headache and Pain. 6 (4): 203–4. doi:10.1007/s10194-005-0185-y. PMC 3452009. PMID 16362664.
- ^ "Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition". Cephalalgia. 38 (1): 1–211. January 2018. doi:10.1177/0333102417738202. ISSN 0333-1024. PMID 29368949.
- ^ Negro A, Rocchietti-March M, Fiorillo M, Martelletti P (December 2011). "Chronic migraine: current concepts and ongoing treatments". European Review for Medical and Pharmacological Sciences. 15 (12): 1401–20. PMID 22288302.
- ^ a b c d Davidoff RA (2002). Migraine: manifestations, pathogenesis, and management (2 ed.). Oxford [u.a.]: Oxford Univ. Press. p. 81. ISBN 978-0-19-513705-7. Archived from the original on 22 December 2016.
- ^ Russell G, Abu-Arafeh I, Symon DN (2002). "Abdominal migraine: evidence for existence and treatment options". Paediatric Drugs. 4 (1): 1–8. doi:10.2165/00128072-200204010-00001. PMID 11817981. S2CID 12289726.
- ^ Lewis DW, Dorbad D (September 2000). "The utility of neuroimaging in the evaluation of children with migraine or chronic daily headache who have normal neurological examinations". Headache. 40 (8): 629–32. doi:10.1046/j.1526-4610.2000.040008629.x. PMID 10971658. S2CID 14443890.
- ^ Silberstein SD (September 2000). "Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology. 55 (6): 754–62. doi:10.1212/WNL.55.6.754. PMID 10993991.
- ^ Medical Advisory Secretariat (2010). "Neuroimaging for the evaluation of chronic headaches: an evidence-based analysis". Ontario Health Technology Assessment Series. 10 (26): 1–57. PMC 3377587. PMID 23074404.
- ^ Chawla J, Lutsep HL (13 June 2023). "Migraine Headache Treatment & Management". Medscape. Retrieved 3 May 2024.
- ^ Jenkins, B. Migraine management. Australian Prescriber. 2020; 43(5):148–151. https://doi.org/10.18773/austprescr.2020.047
- ^ a b c d Treadwell JR, Tsou AY, Rouse B, Ivlev I, Fricke J, Buse D, et al. (2024). Behavioral Interventions for Migraine Prevention (Report). Agency for Healthcare Research and Quality (AHRQ). doi:10.23970/ahrqepccer270. PMID 39471258.
- ^ Mohanty D, Lippmann S (April 2020). "CGRP Inhibitors for Migraine". Innovations in Clinical Neuroscience. 17 (4–6): 39–40. PMC 7413335. PMID 32802591.
- ^ Eigenbrodt AK, Ashina H, Khan S, Diener HC, Mitsikostas DD, Sinclair AJ, et al. (August 2021). "Diagnosis and management of migraine in ten steps". Nature Reviews. Neurology. 17 (8): 501–514. doi:10.1038/s41582-021-00509-5. PMC 8321897. PMID 34145431.
- ^ Karlsson WK, Ostinelli EG, Zhuang ZA, Kokoti L, Christensen RH, Al-Khazali HM, et al. (18 September 2024). "Comparative effects of drug interventions for the acute management of migraine episodes in adults: systematic review and network meta-analysis". BMJ. 386 e080107. doi:10.1136/bmj-2024-080107. ISSN 1756-1833. PMC 11409395. PMID 39293828.
- ^ "Which drugs are best for migraine attacks?". NIHR Evidence. 4 March 2025.
- ^ Wattiez AS, Sowers LP, Russo AF (February 2020). "Calcitonin gene-related peptide (CGRP): role in migraine pathophysiology and therapeutic targeting". Expert Opinion on Therapeutic Targets. 24 (2): 91–100. doi:10.1080/14728222.2020.1724285. PMC 7050542. PMID 32003253.
- ^ Tanaka M, Szabó A, Körtési T, Szok D, Tajti J, Vécsei L (17 November 2023). "From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment". Cells. 12 (22): 2649. doi:10.3390/cells12222649. PMC 10670698. PMID 37998384.
- ^ Gawde P, Shah H, Patel H, Bharathi KS, Patel N, Sethi Y, et al. (2 February 2023). "Revisiting Migraine: The Evolving Pathophysiology and the Expanding Management Armamentarium". Cureus. 2 (15) e34553. doi:10.7759/cureus.34553. PMC 9985459. PMID 36879707.
- ^ Silberstein SD, Lee L, Gandhi K, Fitzgerald T, Bell J, Cohen JM (November 2018). "Health care Resource Utilization and Migraine Disability Along the Migraine Continuum Among Patients Treated for Migraine". Headache. 58 (10): 1579–1592. doi:10.1111/head.13421. PMID 30375650. S2CID 53114546.
- ^ a b "Migraine Information Page: Prognosis". National Institute for Neurological Disorders and Stroke (NINDS). National Institutes of Health (US). Archived from the original on 10 June 2020.
- ^ "Key facts and figures about migraine". The Migraine Trust. 2017. Archived from the original on 12 March 2017. Retrieved 13 June 2021.
- ^ a b Brennan KC, Pietrobon D (March 2018). "A Systems Neuroscience Approach to Migraine". Neuron. 97 (5): 1004–1021. doi:10.1016/j.neuron.2018.01.029. PMC 6402597. PMID 29518355.
- ^ "The Global Burden of Disease: 2004 Update" (PDF). World Health Organization. 2008. p. 33. Archived from the original (PDF) on 13 June 2021.
Disability classes for the Global Burden of Disease study (table 8)
- ^ a b c Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T (October 2009). "Migraine and cardiovascular disease: systematic review and meta-analysis". BMJ. 339 b3914. doi:10.1136/bmj.b3914. PMC 2768778. PMID 19861375.
- ^ Kurth T, Chabriat H, Bousser MG (January 2012). "Migraine and stroke: a complex association with clinical implications". The Lancet. Neurology. 11 (1): 92–100. doi:10.1016/S1474-4422(11)70266-6. PMID 22172624. S2CID 31939284.
- ^ Rist PM, Diener HC, Kurth T, Schürks M (June 2011). "Migraine, migraine aura, and cervical artery dissection: a systematic review and meta-analysis". Cephalalgia. 31 (8): 886–96. doi:10.1177/0333102411401634. PMC 3303220. PMID 21511950.
- ^ Kurth T (March 2010). "The association of migraine with ischemic stroke". Current Neurology and Neuroscience Reports. 10 (2): 133–9. doi:10.1007/s11910-010-0098-2. PMID 20425238. S2CID 27227332.
- ^ Schürks M, Rist PM, Shapiro RE, Kurth T (September 2011). "Migraine and mortality: a systematic review and meta-analysis". Cephalalgia. 31 (12): 1301–14. doi:10.1177/0333102411415879. PMC 3175288. PMID 21803936.
- ^ Weinberger J (March 2007). "Stroke and migraine". Current Cardiology Reports. 9 (1): 13–9. doi:10.1007/s11886-007-0004-y. PMID 17362679. S2CID 46681674.
- ^ Hougaard A, Amin FM, Ashina M (June 2014). "Migraine and structural abnormalities in the brain". Current Opinion in Neurology. 27 (3): 309–14. doi:10.1097/wco.0000000000000086. PMID 24751961.
- ^ a b c Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, et al. (January 2022). "Migraine". Nature Reviews. Disease Primers. 8 (1) 2. doi:10.1038/s41572-021-00328-4. PMID 35027572. S2CID 245883895.
- ^ Chalmer MA, Kogelman LJ, Callesen I, Christensen CG, Techlo TR, Møller PL, et al. (June 2023). "Sex differences in clinical characteristics of migraine and its burden: a population-based study". European Journal of Neurology. 30 (6): 1774–1784. doi:10.1111/ene.15778. PMID 36905094.
- ^ Stovner LJ, Zwart JA, Hagen K, Terwindt GM, Pascual J (April 2006). "Epidemiology of headache in Europe". European Journal of Neurology. 13 (4): 333–45. doi:10.1111/j.1468-1331.2006.01184.x. PMID 16643310. S2CID 7490176.
- ^ Wang SJ (March 2003). "Epidemiology of migraine and other types of headache in Asia". Current Neurology and Neuroscience Reports. 3 (2): 104–8. doi:10.1007/s11910-003-0060-7. PMID 12583837. S2CID 24939546.
- ^ Natoli JL, Manack A, Dean B, Butler Q, Turkel CC, Stovner L, et al. (May 2010). "Global prevalence of chronic migraine: a systematic review". Cephalalgia. 30 (5): 599–609. doi:10.1111/j.1468-2982.2009.01941.x. PMID 19614702. S2CID 5328642.
- ^ Nappi RE, Sances G, Detaddei S, Ornati A, Chiovato L, Polatti F (June 2009). "Hormonal management of migraine at menopause". Menopause International. 15 (2): 82–6. doi:10.1258/mi.2009.009022. PMID 19465675. S2CID 23204921.
- ^ a b Miller N (2005). Walsh and Hoyt's clinical neuro-ophthalmology (6 ed.). Philadelphia, Pa.: Lippincott Williams & Wilkins. p. 1275. ISBN 978-0-7817-4811-7. Archived from the original on 12 March 2017.
- ^ Liddell HG, Scott R. "ἡμικρανία". A Greek-English Lexicon. Archived from the original on 8 November 2013. on Perseus
- ^ Anderson K, Anderson LE, Glanze WD (1994). Mosby's Medical, Nursing & Allied Health Dictionary (4 ed.). Mosby. p. 998. ISBN 978-0-8151-6111-0.
- ^ a b c d e Borsook D (2012). The migraine brain: imaging, structure, and function. New York: Oxford University Press. pp. 3–11. ISBN 978-0-19-975456-4. Archived from the original on 13 March 2017.
- ^ a b Waldman SD (2011). Pain management (2 ed.). Philadelphia, PA: Elsevier/Saunders. pp. 2122–2124. ISBN 978-1-4377-3603-8. Archived from the original on 23 August 2023. Retrieved 24 September 2016.
- ^ "Sex(ism), Drugs, and Migraines". Distillations. Science History Institute. 15 January 2019. Archived from the original on 14 March 2021. Retrieved 6 February 2020.
- ^ Margaret C, Simon M (2002). Human osteology: in archaeology and forensic science (Repr. ed.). Cambridge [etc.]: Cambridge University Press. p. 345. ISBN 978-0-521-69146-8. Archived from the original on 17 June 2013.
- ^ Colen C (2008). Neurosurgery. Colen Publishing. p. 1. ISBN 978-1-935345-03-9.
- ^ Daniel BT (2010). Migraine. Bloomington, IN: AuthorHouse. p. 101. ISBN 978-1-4490-6962-9. Archived from the original on 13 March 2017.
- ^ Butticè C (April 2022). What you need to know about headaches. Santa Barbara, California: Greenwood Publishing Group. pp. 29–30. ISBN 978-1-4408-7531-1. OCLC 1259297708. Archived from the original on 28 November 2022. Retrieved 2 November 2022.
{{cite book}}:|work=ignored (help) - ^ a b Tfelt-Hansen PC, Koehler PJ (May 2011). "One hundred years of migraine research: major clinical and scientific observations from 1910 to 2010". Headache. 51 (5): 752–78. doi:10.1111/j.1526-4610.2011.01892.x. PMID 21521208. S2CID 31940152.
- ^ Tfelt-Hansen P, Koehler P (2008). "History of the Use of Ergotamine and Dihydroergotamine in Migraine from 1906 and Onward". Cephalalgia. 28 (8): 877–886. doi:10.1111/j.1468-2982.2008.01578.x. PMID 18460007.
- ^ a b c Stovner LJ, Andrée C (June 2008). "Impact of headache in Europe: a review for the Eurolight project". The Journal of Headache and Pain. 9 (3): 139–46. doi:10.1007/s10194-008-0038-6. PMC 2386850. PMID 18418547.
- ^ a b Mennini FS, Gitto L, Martelletti P (August 2008). "Improving care through health economics analyses: cost of illness and headache". The Journal of Headache and Pain. 9 (4): 199–206. doi:10.1007/s10194-008-0051-9. PMC 3451939. PMID 18604472.
- ^ Magis D, Jensen R, Schoenen J (June 2012). "Neurostimulation therapies for primary headache disorders: present and future". Current Opinion in Neurology. 25 (3): 269–76. doi:10.1097/WCO.0b013e3283532023. PMID 22543428.
- ^ Jürgens TP, Leone M (June 2013). "Pearls and pitfalls: neurostimulation in headache". Cephalalgia. 33 (8): 512–25. doi:10.1177/0333102413483933. PMID 23671249. S2CID 42537455.
- ^ Barbanti P, Fofi L, Aurilia C, Egeo G, Caprio M (May 2017). "Ketogenic diet in migraine: rationale, findings and perspectives". Neurological Sciences (Review). 38 (Suppl 1): 111–115. doi:10.1007/s10072-017-2889-6. PMID 28527061. S2CID 3805337.
- ^ Gross EC, Klement RJ, Schoenen J, D'Agostino DP, Fischer D (April 2019). "Potential Protective Mechanisms of Ketone Bodies in Migraine Prevention". Nutrients. 11 (4): 811. doi:10.3390/nu11040811. PMC 6520671. PMID 30974836.
- ^ Artero-Morales M, González-Rodríguez S, Ferrer-Montiel A (2018). "TRP Channels as Potential Targets for Sex-Related Differences in Migraine Pain". Frontiers in Molecular Biosciences. 5 73. doi:10.3389/fmolb.2018.00073. PMC 6102492. PMID 30155469.
- ^ Stewart WF, Lipton RB, Celentano DD, Reed ML (January 1992). "Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors". JAMA. 267 (1): 64–69. doi:10.1001/jama.1992.03480010072027. PMID 1727198.
- ^ "Speeding Progress in Migraine Requires Unraveling Sex Differences". Society for Women's Health Research. 28 August 2018. Archived from the original on 18 June 2020. Retrieved 17 March 2021.
Further reading
[edit]- Ashina M (November 2020). Ropper AH (ed.). "Migraine". The New England Journal of Medicine. 383 (19): 1866–1876. doi:10.1056/nejmra1915327. PMID 33211930. S2CID 227078662.
- Oskoui M, Pringsheim T, Billinghurst L, Potrebic S, Gersz EM, Gloss D, et al. (September 2019). "Practice guideline update summary: Pharmacologic treatment for pediatric migraine prevention: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society". Neurology. 93 (11): 500–509. doi:10.1212/WNL.0000000000008105. PMC 6746206. PMID 31413170.
- Oskoui M, Pringsheim T, Holler-Managan Y, Potrebic S, Billinghurst L, Gloss D, et al. (September 2019). "Practice guideline update summary: Acute treatment of migraine in children and adolescents: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society". Neurology. 93 (11): 487–499. doi:10.1212/WNL.0000000000008095. PMID 31413171. S2CID 199662718.
External links
[edit]| External audio | |
|---|---|
Migraine
View on GrokipediaClinical Presentation
Prodromal and Aura Phases
The prodromal phase, also referred to as the premonitory phase, precedes the aura or headache in a significant proportion of migraine episodes, occurring in up to 66% of clinic-based cases.[8] This phase typically begins up to 48 hours before headache onset, with symptoms often emerging 6 to 11 hours prior on average.[8] Common manifestations include fatigue (reported in 49% of cases), mood changes such as irritability or depression (37%), neck stiffness (46%), yawning (22%), food cravings (11%), and increased urination.[8] These nonspecific symptoms arise from hypothalamic and brainstem dysregulation, providing early indicators of an impending attack without causing permanent neurological changes.[9] The aura phase, experienced in approximately 25–30% of migraine cases, follows the prodrome and lasts 5 to 60 minutes.[10] It represents a focal neurological disturbance that is fully reversible and does not result in lasting damage.[10] The underlying mechanism involves cortical spreading depression, a wave of neuronal and glial depolarization that propagates across the cerebral cortex at 2 to 5 mm per minute.[10] Visual auras are the most prevalent, occurring in over 90% of aura cases and featuring phenomena such as blurred or decreased eyesight, scintillating scotoma (a shimmering blind spot), zigzag lines, or temporary blind spots.[10] A representative example is the fortification spectrum, a dynamic pattern of flickering, crenellated lines that begins centrally in the visual field and expands outward, mimicking the battlements of a fortified wall.[11] Sensory auras, affecting about 30–35% of those with aura, manifest as unilateral numbness or tingling that typically starts in the fingers or hand, migrates gradually over 5 to 20 minutes, and lasts 5 to 60 minutes before resolving.[10] Speech disturbances, including transient dysphasia or difficulty finding words, occur in roughly 10–30% of aura episodes.[10] In rarer instances, auras include motor symptoms, as seen in hemiplegic migraine, where temporary unilateral weakness or paralysis develops over 20–30 minutes, often spreading from the hand to the arm and face before fully resolving.[12] These phases commonly transition into the headache stage, where pain intensifies.[10]Headache and Associated Symptoms
The headache phase of a migraine attack is characterized by unilateral throbbing or pulsating pain that is moderate to severe in intensity, may localize to various regions including the occipital area at the back of the head, and is typically aggravated by routine physical activity, such as walking or climbing stairs.[13][14] This pain often begins unilaterally but may become bilateral in approximately one-third of attacks and is frequently accompanied by autonomic symptoms.[14] Common associated symptoms include nausea, vomiting, photophobia (sensitivity to light), phonophobia (sensitivity to sound), and osmophobia (aversion to smells), with at least one of these sensory sensitivities present during most attacks.[13][15] Cutaneous allodynia, a heightened sensitivity to touch where non-painful stimuli like brushing hair or wearing glasses cause discomfort, develops in up to 70% of patients during this phase and reflects central sensitization.[16][17] Untreated or unsuccessfully treated, the headache typically lasts 4 to 72 hours, with pain building over 1 to 2 hours, often progressing from the frontal region posteriorly to become more diffuse, and peaking early in the course before gradually subsiding.[13][18] Cutaneous hypersensitivity may intensify as the attack progresses, contributing to overall debilitation.[16] Acephalgic migraine, also known as silent migraine, is characterized by typical aura symptoms without subsequent headache.[13][19] In this form, reversible neurological disturbances like visual scintillations or sensory changes last 5 to 60 minutes without evolving into head pain.[19]Postdromal Phase
The postdrome, often referred to as the "migraine hangover," represents the recovery phase following the resolution of the headache and associated symptoms in a migraine attack. This phase typically lasts from a few hours to up to 48 hours, with most individuals experiencing symptoms that persist for 24 hours or less. Common manifestations include profound exhaustion or weakness, affecting up to 88% of those in postdrome, alongside cognitive impairments such as difficulty concentrating or mental fog in about 56% of cases. Mood alterations, ranging from irritability or depression to occasional elation, are also reported, as are residual sensitivities to light (photophobia) and sound (phonophobia), along with mild neck stiffness or head discomfort.[20][21][1] These symptoms contribute to significant functional impairment during the postdrome, disrupting daily activities and productivity. Individuals often describe a reduced quality of life, with scores averaging 57 out of 100 compared to 81 between attacks, due to challenges in focusing on tasks, physical weakness, and overall malaise that can extend work or social engagements for hours to days. In some instances, a sense of relief or mild euphoria emerges as the acute phase subsides, though this is less common and varies by individual. Such impairments highlight the postdrome's role in prolonging the overall burden of a migraine episode beyond the headache itself.[20][22][23] Physiologically, the postdrome involves the gradual normalization of brain excitability following the heightened neuronal activity of the earlier phases, with evidence suggesting persistent alterations in diencephalic and brainstem function that contribute to lingering symptoms. Recovery also encompasses restoration of hydration status, as dehydration from nausea, vomiting, or reduced intake during the attack can exacerbate fatigue and cognitive issues, necessitating rehydration for full resolution. These processes typically complete within 24 to 48 hours, returning most individuals to baseline functioning, independent of acute treatment use or attack severity.[20][1][24]Migraine Variants
Migraine variants encompass less common subtypes that diverge from the typical episodic headache pattern, often involving atypical symptoms or chronicity, and primarily affect a subset of individuals with migraine disorders.[25] Abdominal migraine is characterized by recurrent episodes of moderate to severe, midline abdominal pain lasting 1–72 hours, accompanied by nausea, vomiting, pallor, or anorexia, and it predominantly affects children aged 4–15 years without prominent headache during attacks.[26] These episodes interfere with daily activities and are interspersed with asymptomatic periods, often serving as a precursor to classic migraine in adulthood, with up to 70% of affected children developing migraine headaches later in life.[26] Abdominal migraine is more prevalent in pediatric populations, occurring in about 1–4% of children experiencing recurrent abdominal pain.[26][25] Hemiplegic migraine features migraine aura accompanied by reversible motor weakness, typically unilateral and affecting the face, arm, or leg, which may last from minutes to days and can mimic stroke symptoms.[27] It includes other aura elements such as visual disturbances, sensory changes, or speech difficulties, and is linked to genetic mutations, notably in the CACNA1A gene encoding a calcium channel subunit, which heightens neuronal excitability.[27] This variant occurs in familial (autosomal dominant inheritance with affected relatives) and sporadic forms (no family history, often due to de novo mutations), with the latter comprising a significant portion of cases.[27] Chronic migraine represents a progression from episodic migraine, defined by the International Classification of Headache Disorders, third edition (ICHD-3) as headaches occurring on ≥15 days per month for >3 months, with at least 8 of those days fulfilling criteria for migraine (such as unilateral throbbing pain with nausea or photophobia).[28] It evolves gradually from lower-frequency attacks, influenced by factors like medication overuse, and affects daily functioning more severely than episodic forms.[29] Retinal migraine involves transient monocular visual disturbances, including partial vision loss or scotomas in one eye due to involvement of the retinal vasculature, typically lasting 5–60 minutes and often preceding or accompanying a headache.[30][31] These episodes are fully reversible but require differentiation from more serious ocular or vascular conditions.[30] Vestibular migraine is defined by episodes of vertigo or dizziness lasting 5 minutes to 72 hours, with features of migraine such as headache, photophobia, or phonophobia, and a history of migraine.[32] It affects approximately 10% of migraine patients in clinical settings and is more common among those with a personal or family history of migraine.[32]Etiology and Risk Factors
Genetic Influences
Migraine is a polygenic disorder with heritability estimates ranging from 40% to 60%, indicating a substantial genetic contribution to susceptibility.[33] Genome-wide association studies (GWAS) have identified over 120 genetic loci associated with migraine risk, highlighting its complex genetic architecture.[33] Notable examples include variants in PRDM16 and TRPM8, which influence neuronal excitability and pain pathways. These GWAS findings reveal shared genetic influences with vascular traits such as blood pressure, though the primary mechanisms appear neuronal, involving ion channels and synaptic function.[33] In contrast to the common polygenic forms, rare monogenic subtypes exist, most prominently familial hemiplegic migraine (FHM), which follows an autosomal dominant inheritance pattern.[34] FHM type 1 (FHM1) results from mutations in CACNA1A, encoding a voltage-gated calcium channel subunit that regulates neuronal signaling.[34] FHM type 2 (FHM2) is caused by mutations in ATP1A2, which encodes a sodium-potassium ATPase involved in ion homeostasis across neuronal membranes.[34] FHM type 3 (FHM3) arises from mutations in SCN1A, affecting a voltage-gated sodium channel critical for action potential generation.[34] These mutations disrupt cortical excitability, leading to hemiplegic aura symptoms. Family-based studies underscore the hereditary component, showing that first-degree relatives of individuals with migraine face a 1.5- to 3-fold increased risk compared to the general population.[35] This aggregation is influenced by sex-specific patterns, with GWAS identifying loci that differentially affect migraine susceptibility in males and females, such as those linked to hormonal interactions.[36]Physiological Triggers
Hormonal fluctuations, particularly involving estrogen, play a significant role in triggering migraine attacks, especially in women. Drops in estrogen levels during the menstrual cycle are associated with menstrual migraine, where attacks occur in relation to menstruation due to the withdrawal of this hormone.[37] Approximately 60% of women with migraine experience attacks linked to their menstrual period, highlighting the influence of these cyclical changes.[38] In contrast, pregnancy often leads to a reduction in migraine frequency, with 50% to 80% of affected women reporting fewer or less severe attacks, attributed to the sustained high levels of estrogen during this period.[39] Sleep disturbances are common physiological triggers for migraine, disrupting the body's internal rhythms and exacerbating susceptibility to attacks. Conditions such as insomnia, oversleeping, and jet lag can precipitate migraines by altering sleep quality and duration, with poor sleep patterns identified as a risk factor for increased attack frequency and intensity.[40] Severe sleep deprivation, in particular, disrupts brain excitability and lowers the threshold for cortical spreading depression, the neural phenomenon implicated in migraine aura (see Pathophysiology).[41] These disruptions are linked to dysregulation of the circadian rhythm, including abnormal melatonin secretion, which normally modulates pain pathways and sleep-wake cycles; in migraineurs, melatonin levels may fail to peak appropriately, contributing to vulnerability.[42] Stress and subsequent relaxation phases also act as internal triggers through their effects on the body's regulatory systems. Acute stress activates the autonomic nervous system, leading to physiological changes that can initiate a migraine attack in susceptible individuals.[43] Paradoxically, the "letdown" effect following a period of high stress—such as during weekends or vacations—can trigger attacks the next day, as a reduction in perceived stress from one day to the next is associated with onset, possibly due to shifts in neuroendocrine and autonomic balance.[44][45] Physical exertion represents another endogenous trigger, where intense bodily activity strains physiological systems and provokes attacks. Overexertion during exercise is reported as a lifetime trigger in approximately 38% of migraineurs, regardless of migraine type or gender, due to increased intracranial pressure and vascular changes.[46] Similarly, sexual activity can precipitate migraines in a subset of patients, with about 15% of exercise-related headaches manifesting as migrainous, often involving autonomic responses similar to those in primary headaches.[47] Genetic susceptibility may amplify the impact of these physiological triggers, making certain individuals more prone to attacks under such conditions.[48] Emerging evidence indicates that alterations in the gut microbiome contribute to migraine risk, with patients showing reduced abundance of butyrate-producing bacteria and lower levels of butyrate, a short-chain fatty acid with anti-inflammatory effects that may modulate neuroinflammation.[49][50]Dietary and Environmental Triggers
Dietary factors are among the most commonly reported precipitants of migraine attacks, with certain foods and beverages implicated due to their vasoactive compounds or effects on cerebral blood flow. Alcohol, particularly red wine, is frequently cited as a trigger, potentially owing to its histamine, tyramine, and sulfite content. Aged cheeses, chocolate, and processed meats containing nitrites or nitrates can also provoke episodes by influencing neurotransmitter release and vascular tone. Monosodium glutamate (MSG), found in many processed foods, has been associated with headache onset in susceptible individuals through excitotoxic mechanisms. Additionally, caffeine withdrawal can induce migraines by causing vasodilation and altered adenosine signaling in the brain. Environmental changes play a significant role in triggering migraines for many patients. Drops in barometric pressure, often linked to weather fronts, are reported to initiate attacks, possibly via effects on cerebral vasculature and oxygenation. Bright lights, strong odors such as perfumes or chemicals, and high altitude exposure—associated with hypoxia—further contribute by sensitizing trigeminal nerve pathways. At elevations above 8,500 feet, reduced oxygen levels may exacerbate susceptibility, leading to increased migraine frequency. Sensory stimuli often act as precipitants through overload of neural processing centers. Loud noises and flickering screens, including those from fluorescent lights or digital devices, can trigger attacks by heightening cortical excitability. Studies indicate that 50–70% of patients identify such personal triggers, with prevalence varying by type; for instance, weather changes affect about 53% and odors around 44%. Dehydration and skipping meals are common modifiable triggers, primarily through inducing hypoglycemia and reduced cerebral glucose availability. Low blood sugar from irregular eating patterns, such as fasting or missing breakfast, has been linked to migraine onset in over 57% of cases in some cohorts. These factors may interact with physiological states like stress to lower the threshold for attacks.Pathophysiology
Cortical Spreading Depression
Cortical spreading depression (CSD) is a slowly propagating wave of neuronal and glial depolarization that sweeps across the cerebral cortex, characterized by a speed of 2–5 mm/min, followed by a prolonged period of suppressed brain activity.[51] This phenomenon, first described in 1944, involves a massive influx of ions and neurotransmitters that disrupt normal cortical function temporarily.[52] The depolarization phase leads to a breakdown in ionic homeostasis, while the subsequent depression phase reflects recovery and inhibition of electrical activity.[53] Imaging studies provide direct evidence linking CSD to migraine aura. Functional magnetic resonance imaging (fMRI) has captured blood oxygenation level-dependent (BOLD) signals consistent with CSD propagation in the visual cortex during aura episodes, showing a slow march of hyperactivity followed by oligemia.[54] Electroencephalography (EEG), including intracranial recordings, reveals characteristic negative shifts in direct current potential and slowed rhythms during aura, aligning with CSD's electrophysiological signature.[55] These events typically endure for 20–60 minutes, mirroring the temporal profile of aura symptoms.[56] At the ionic level, CSD is driven by extracellular potassium efflux, which depolarizes adjacent cells and triggers further propagation.[57] This is accompanied by glutamate release from neurons, amplifying the wave through excitotoxic mechanisms, while both neuronal and glial cells contribute via gap junctions and ion pumps.[53] Glial involvement, particularly astrocytes, facilitates spatial buffering of ions and modulates the wave's intensity.[52] The spatial and temporal dynamics of CSD closely match the perceptual disturbances of migraine aura, such as the gradual expansion of visual field defects from central to peripheral regions.[54] CSD is primarily associated with the perceptual disturbances of migraine aura, though evidence suggests it may occur subclinically in migraine without aura.[58][59] Severe sleep deprivation disrupts brain excitability, reducing GABAergic inhibition and lowering the threshold for CSD initiation, thereby increasing susceptibility to migraine attacks.[60] CSD may also indirectly contribute to trigeminovascular activation, bridging aura to subsequent headache.[61]Trigeminovascular System Activation
The trigeminovascular system plays a central role in the generation of migraine headache pain by transmitting nociceptive signals from the meninges to the central nervous system. During a migraine attack, activation of this pathway begins with peripheral sensitization of meningeal nociceptors, leading to the throbbing pain typically felt in the forehead and temples. This system involves the trigeminal nerve and its connections to vascular structures, where inflammatory and neural processes amplify pain signals, contributing to the characteristic unilateral or bilateral headache phase.[62] The trigeminal nerve, particularly its ophthalmic division (V1), provides dense innervation to the dura mater, pial vessels, and large cerebral arteries via thinly myelinated Aδ fibers and unmyelinated C-fibers originating from the trigeminal ganglion and upper cervical ganglia (C1-C3). These meningeal nociceptors, which are pseudounipolar neurons, become activated by mechanical distortion, inflammatory mediators, or osmotic changes in the dura, resulting in referred pain to the craniofacial region due to convergence of trigeminal and cervical afferents onto second-order neurons in the spinal trigeminal nucleus. This activation is thought to underlie the localized pain distribution in migraine, with hypersensitivity developing rapidly during attacks.[63][64] Upon activation, trigeminal afferents release neuropeptides such as substance P and calcitonin gene-related peptide (CGRP) from their peripheral terminals in the meninges and from the trigeminal ganglia, initiating neurogenic inflammation. This process causes plasma protein extravasation, mast cell degranulation, and vasodilation in meningeal vessels, further sensitizing nociceptors and perpetuating the pain cycle. The release of these peptides can be triggered by peripheral stimuli and is a key mechanism linking vascular changes to nociceptive signaling, though it is modulated by antimigraine therapies like triptans that inhibit neuropeptide release.[62][64] Central sensitization occurs as prolonged input from activated meningeal nociceptors leads to enhanced excitability of second-order neurons in the trigeminocervical complex, located in the caudal portion of the spinal trigeminal nucleus (medullary dorsal horn) and upper cervical spinal cord. This amplification results in cutaneous allodynia, where innocuous stimuli like touch or hair brushing become painful, often in the periorbital and temporal regions, reflecting widespread central hyperexcitability during the headache phase. Such sensitization can persist and contribute to treatment resistance if the attack progresses.[64][63] Vasoactive changes in the trigeminovascular system involve dilation of meningeal and extracranial arteries, primarily mediated by CGRP, which promotes sustained vasodilation without being the direct cause of pain. While intracranial vessels show modest dilation, extracranial arteries like the temporal artery exhibit more pronounced changes, correlating with the pulsating quality of migraine pain; however, pain persists even after vessel constriction, indicating that neural activation is paramount. These vascular shifts are secondary to the primary nociceptive drive but exacerbate the sensory experience.[62][63]Neurotransmitter and Inflammatory Roles
Calcitonin gene-related peptide (CGRP) serves as a pivotal neuropeptide in migraine pathophysiology, functioning as both a potent vasodilator and a key pain transmitter. Released from trigeminal sensory neurons, CGRP promotes neurogenic inflammation by dilating cranial blood vessels and sensitizing nociceptors in the trigeminovascular system. During migraine attacks, CGRP concentrations are markedly elevated in jugular venous blood, saliva, and tear fluid, correlating with headache intensity and returning to baseline after effective treatment.[65][66] These elevations persist interictally in some chronic migraine patients, suggesting a role in attack predisposition and central sensitization.[66] Serotonin (5-hydroxytryptamine, 5-HT) modulates vascular tone and nociceptive signaling in migraine, with fluctuations contributing to the disorder's vascular and sensory components. Interictal brain 5-HT synthesis is reduced in migraineurs, particularly in the dorsal brainstem, while levels increase during attacks, potentially reflecting compensatory release to counteract pain.[67] These changes induce vasoconstriction via 5-HT1B receptors on cerebral arteries and inhibit trigeminovascular nociception through 5-HT1D receptors on sensory neurons.[68] Peripheral 5-HT reactivity is heightened in migraine patients, with reduced habituation to stimuli, exacerbating vascular instability during episodes.[67] Glutamate, the primary excitatory neurotransmitter, drives central sensitization in migraine via activation of N-methyl-D-aspartate (NMDA) receptors on trigeminal and thalamic neurons. Elevated glutamate levels during attacks promote excitotoxicity, cortical spreading depression, and enhanced pain transmission, amplifying hypersensitivity to sensory stimuli.[69] This process intersects with inflammation, as glutamate release from trigeminal ganglion neurons stimulates satellite glial cells to produce proinflammatory cytokines, including interleukin-6 (IL-6). IL-6, which rises rapidly within hours of attack onset, sustains neurogenic inflammation and nociceptor sensitization, particularly in chronic cases where it correlates with attack frequency and severity.[70][69] Recent investigations as of 2025 highlight the neuromodulatory roles of pituitary adenylate cyclase-activating polypeptide (PACAP) and orexins in migraine. PACAP, a vasodilatory neuropeptide structurally related to VIP, induces migraine-like attacks upon infusion and contributes to trigeminovascular activation, with ongoing trials of anti-PACAP antibodies showing partial reductions in monthly migraine days.[71] Orexins, hypothalamic neuropeptides regulating arousal and nociception, exhibit dysregulated levels in migraineurs—elevated in cerebrospinal fluid of chronic patients and reduced in episodic cases—modulating pain via OX1 receptors to inhibit trigeminal firing, though with sex-specific effects observed in preclinical models.[72] These mediators integrate with the trigeminovascular system to influence attack initiation and chronification.[73]Diagnosis and Classification
Diagnostic Criteria
The diagnosis of migraine relies on standardized clinical criteria outlined in the International Classification of Headache Disorders, third edition (ICHD-3), developed by the International Headache Society, which provides an algorithmic framework for classifying primary headache disorders based on patient-reported symptoms.[74] These criteria emphasize the recurrent nature of attacks and specific associated features, enabling clinicians to distinguish migraine from other headache types without relying on laboratory tests in typical cases.[75] For the most prevalent subtype, migraine without aura, ICHD-3 requires at least five attacks meeting the following specifications:- Headache attacks lasting 4–72 hours when untreated or unsuccessfully treated.
- Headache exhibiting at least two of these characteristics: unilateral location, pulsating quality, moderate or severe pain intensity, or aggravation by (or avoidance of) routine physical activity.
- Accompanying at least one of: nausea and/or vomiting, or photophobia and phonophobia.
- Not better accounted for by another ICHD-3 diagnosis.
Migraine Subtypes
Migraine is classified into subtypes primarily based on the presence or absence of aura, frequency of attacks, and specific modifiers such as hormonal or vestibular associations, according to the International Classification of Headache Disorders, 3rd edition (ICHD-3).[85] These classifications aid in diagnosis and management by delineating distinct clinical presentations within the broader migraine spectrum. The most common subtype is migraine without aura, also known as common migraine, which accounts for approximately 70-75% of cases.[14] It is characterized as a recurrent headache disorder with attacks lasting 4-72 hours when untreated or unsuccessfully treated. Diagnostic criteria require at least five attacks fulfilling specific features: headache with at least two of unilateral location, pulsating quality, moderate or severe pain intensity, and aggravation by routine physical activity; during the headache, at least one of nausea/vomiting or photophobia/phonophobia; and not better accounted for by another ICHD-3 diagnosis.[85] This subtype lacks preceding neurological symptoms, distinguishing it from aura-associated forms. Migraine with aura represents 25-30% of cases and involves transient focal neurological symptoms that typically precede or accompany the headache phase.[86] The aura consists of fully reversible visual, sensory, speech/language, or other central nervous system symptoms that develop gradually over at least 5 minutes and last 5-60 minutes.[85] Typical aura, the most frequent variant, includes visual disturbances such as scintillating scotoma or zigzag lines, or sensory symptoms like unilateral tingling, without motor weakness. At least two attacks are required, with aura symptoms meeting at least three characteristics: spreading gradually, occurring in succession with two or more symptoms, each lasting 5-60 minutes, at least one being unilateral, at least one being positive (e.g., flickering lights), and headache following within 60 minutes.[85] This subtype highlights the neurological component of migraine, often resolving before the headache intensifies. Migraines are further categorized by frequency into episodic and chronic forms. Episodic migraine involves fewer than 15 headache days per month, aligning with the acute attack patterns of subtypes like those with or without aura.[85] In contrast, chronic migraine is defined as headache occurring on 15 or more days per month for more than 3 months, with at least 8 days per month featuring migraine characteristics (e.g., fulfilling criteria for migraine without aura or responding to migraine-specific treatments).[85] This distinction underscores the progression potential of the disorder, with chronic forms often requiring preventive strategies. Other recognized subtypes include those influenced by hormonal cycles, vestibular symptoms, or incomplete diagnostic fulfillment. Pure menstrual migraine without aura occurs exclusively on days -2 to +3 of menstruation (day 1 being the first day of menstruation) in at least two out of three cycles, with no attacks at other times, fulfilling criteria for migraine without aura.[85] Menstrually related migraine without aura extends this pattern, with attacks during menstruation in at least two out of three cycles but also occurring at other times. Vestibular migraine, classified in the ICHD-3 appendix, features at least five episodes of moderate or severe vestibular symptoms (e.g., vertigo or dizziness) lasting 5 minutes to 72 hours, accompanied by a current or past history of migraine, and at least one migrainous feature (such as headache or photophobia) in at least 50% of episodes; it is not better explained by another diagnosis.[85] Probable migraine applies to attacks that meet all but one criterion for migraine without aura or with aura, without fulfilling criteria for another headache disorder.[85] These subtypes accommodate variations in presentation while maintaining alignment with core ICHD-3 standards.Differential Diagnosis
Differentiating migraine from other headache disorders is essential, as it ensures timely identification of potentially life-threatening secondary causes while avoiding misdiagnosis of primary conditions. Clinicians rely on patient history, symptom patterns, and red-flag assessments to distinguish migraine, which typically features recurrent, unilateral throbbing pain with associated nausea or photophobia, from mimics that may share some features like nausea but differ in onset, duration, or accompanying signs.[87][88] Persistent or chronic headache accompanied by nausea is commonly attributable to migraine, particularly chronic migraine, which is characterized by headaches occurring on 15 or more days per month for more than three months, with at least eight days exhibiting migrainous features such as throbbing pain (often unilateral), nausea, vomiting, and sensitivity to light and sound. However, other causes must be considered and excluded, including medication overuse headache resulting from frequent use of acute pain relievers, chronic tension-type headaches (which less commonly involve nausea), dehydration, infections (such as influenza or meningitis), low blood sugar, hormonal changes, or serious secondary conditions including increased intracranial pressure, brain tumors, or stroke. Persistent symptoms warrant prompt medical evaluation to rule out underlying pathology.[89][90] Secondary headaches, arising from underlying pathology, require exclusion before confirming a primary diagnosis like migraine. Subarachnoid hemorrhage often presents as a thunderclap headache with abrupt, maximal-intensity onset within seconds, unlike the gradual build-up in migraine; it may be accompanied by neck stiffness or altered consciousness, necessitating urgent neuroimaging.[87][91] Temporal arteritis, more common in individuals over 50, manifests as a new or worsening headache with scalp tenderness, jaw claudication during chewing, and elevated inflammatory markers, distinguishing it from migraine through systemic symptoms and vascular involvement.[92] Sinusitis can mimic sinus-related migraine pain with facial pressure and congestion, but it typically involves purulent nasal discharge, fever, or tenderness over the sinuses, often confirmed by imaging or response to antibiotics.[93][94] Among primary headache mimics, cluster headache features excruciating, unilateral pain lasting 15-180 minutes, occurring in bouts with prominent autonomic symptoms such as ipsilateral lacrimation, nasal congestion, or ptosis, contrasting with migraine's longer duration (4-72 hours) and less intense autonomic features; it predominantly affects males and has a circadian pattern.[95][96] Tension-type headache presents as bilateral, pressing or tightening pain of mild to moderate intensity without significant nausea, vomiting, or aura, differing from migraine's throbbing quality and associated symptoms; it often lacks the disabling impact of migraine attacks.[97][98] Neurological conditions can closely resemble migraine, particularly those involving aura-like phenomena. Seizure aura may produce transient visual or sensory disturbances similar to migraine aura, but it often evolves into altered awareness, motor convulsions, or postictal confusion, unlike the fully reversible nature of migrainous aura without infarction.[99] Stroke-related headaches, especially in cases of ischemic or hemorrhagic events, may include focal neurological deficits that persist beyond the typical 60-minute migraine aura resolution, with risk factors like sudden onset or vascular history prompting immediate evaluation.[99] To identify red flags suggestive of secondary headaches, the SNNOOP10 criteria are widely used, encompassing systemic symptoms (fever, weight loss), neurologic deficits, new-onset or progressive headache, sudden onset, onset after age 50, pattern change, positional worsening, and other factors like immunosuppression or prior malignancy.[88][100]| Criterion | Description | Example Implications |
|---|---|---|
| S (Systemic symptoms) | Fever, weight loss | Suggests infection or malignancy |
| N (Neurologic deficit) | Focal weakness, confusion | Indicates stroke or mass lesion |
| N (New headache) | First or atypical onset | Warrants imaging |
| O (Onset sudden) | Thunderclap pattern | Raises concern for subarachnoid hemorrhage |
| O (Onset after age 50) | New headache in elderly | Temporal arteritis risk |
| P (Pattern change) | Worsening or different from prior | Possible progression of underlying disease |
| 1 (Positional headache) | Worse lying down/standing | Consider intracranial pressure issues |
| 0 (Other) | Immunosuppression, cancer history | Higher secondary headache likelihood |

