Recent from talks
Nothing was collected or created yet.
Xylella fastidiosa
View on Wikipedia
| Xylella fastidiosa | |
|---|---|
| |
| Scientific classification | |
| Domain: | Bacteria |
| Kingdom: | Pseudomonadati |
| Phylum: | Pseudomonadota |
| Class: | Gammaproteobacteria |
| Order: | Lysobacterales |
| Family: | Lysobacteraceae |
| Genus: | Xylella |
| Species: | X. fastidiosa
|
| Binomial name | |
| Xylella fastidiosa Wells et al., 1987
| |
Xylella fastidiosa is an aerobic, Gram-negative bacterium of the genus Xylella.[1] It is a plant pathogen, that grows in the water transport tissues of plants (xylem vessels) and is transmitted exclusively by xylem sap-feeding insects such as sharpshooters and spittlebugs.[1][2][3][4] Many plant diseases are due to infections of X. fastidiosa, including bacterial leaf scorch, oleander leaf scorch, coffee leaf scorch (CLS), alfalfa dwarf, phony peach disease, and the economically important Pierce's disease of grapes (PD),[5] olive quick decline syndrome (OQDS),[6][7] and citrus variegated chlorosis (CVC).[8] While the largest outbreaks of X. fastidiosa–related diseases have occurred in the Americas[9] and Europe,[10] this pathogen has also been found in Taiwan,[11] Israel,[12] and a few other countries worldwide.[13][14]
Xylella fastidiosa can infect an extremely wide range of plants, many of which do not show any symptoms of disease.[15] Disease occurs in plant species that are susceptible due to blockage of water flow in the xylem vessels caused by several factors: bacterial obstruction, overreaction of the plant immune response (tylose formation), and formation of air embolisms.[16][17][18] A strain of X. fastidiosa responsible for citrus variegated chlorosis was the first bacterial plant pathogen to have its genome sequenced, in part because of its importance in agriculture.[19] Due to the significant impacts of this pathogen on agricultural crops around the world, there is substantial investment in scientific research related to X. fastidiosa and the diseases it causes.[20]
Taxonomy
[edit]Xylella fastidiosa is the first-proposed species in the genus Xylella and the only species until 2016. The genus Xyella Wells et al. 1987 currently consists of two species, Xylella fastidiosa Wells et al. 1987 and Xylella taiwanensis Su et al. 2016.[21] Xylella fastidiosa in turn consists of several subspecies, each with own preferred host plants and geographic origin:
- X. f. subsp. fastidiosa (Wells et al. 1987) Schaad et al. 2009 is defined around the original type strain and is the best-studied so far. It is mainly known for Pierce's disease of grapevines and leaf scorch of almond.[22] It also affects alfalfa and maple.[23] It is thought to have originated in southern Central America.[24]
- Identical to invalid name "X. f. subsp. piercei" Schaad et al. 2004.[25]
- X. f. subsp. multiplex Schaad et al. 2009 affects many trees, including stone-fruit ones such as peaches and plums, and is thought to originate in temperate and southern North America.[24] It also affects elm, grape, and sycamore.[23]
- "X. f. subsp. pauca" Schaad et al. 2004 is believed to have originated in South America. It is the causal agent of citrus variegated chlorosis (CVC) in Brazil.[26] It also affects South American coffee crops, causing coffee leaf scorch.[24]
- "X. f. subsp. sandyi" Schuenzel et al. 2005 is thought to have originated in the southern part of the United States, and is notable for causing oleander leaf scorch.[24]
- "X. f. subsp. tashke" Randell et al., 2009 is proposed to include isolates associated with Chitalpa tashkentensis leaf scorch in Southwestern USA. Whether it is causative is unknown. Whether it forms a distinct group that can be called a subspecies is also unknown.[24]
- "X. f. subsp. morus" Nunney et al. 2014 includes a strain associated with mulberry leaf scorch in the eastern USA and California. It is genetically distinct enough to be its own subspecies.[24]
The three X. fastidiosa subspecies by Schaad et al. were outlined in the same 2004 publication. Two of them were made valid according to the Prokaryotic Code in 2009. No reason was given for not validating the third subspecies, pauca.[27]
X. taiwanensis affects Pyrus pyrifolia in Taiwan, causing leaf scorch. It has not spread to the EU.[28] It was originally proposed to be a subspecies of X. f., but the large divergence lead to the proposal of a separate species.[24]
Pathogen anatomy and disease cycle
[edit]Xylella fastidiosa is rod-shaped, and at least one subspecies has two types of pili on only one pole; longer, type IV pili are used for locomotion, while shorter, type I pili assist in biofilm formation inside their hosts. As demonstrated using a PD-related strain, the bacterium has a characteristic twitching motion that enables groups of bacteria to travel upstream against heavy flow, such as that found in xylem vessels.[29] It is obligately insect-vector transmitted from xylem-feeding insects directly into xylem, but infected plant material for vegetative propagation (e.g. grafting) can produce mature plants that also have an X. fastidiosa disease.[30] In the wild, infections tend to occur during warmer seasons, when insect vector populations peak. The bacterium is not seed transmitted, but instead is transmitted through "xylem feed-ing, suctorial homopteran insects such as sharpshooter leafhoppers and spittle bugs"[31] and has been historically difficult to culture (fastidious),[32][33] as its specific epithet, fastidiosa, reflects.
X. fastidiosa has a two-part lifecycle, which occurs inside an insect vector and inside a susceptible plant. While the bacterium has been found across the globe, only once the bacterium reaches systemic levels do symptoms present themselves. Once established in a new region, X. fastidiosa spread is dependent on the obligate transmission by xylem-sap feeding insect.[34] Within susceptible plant hosts, X. fastidiosa forms a biofilm-like layer within xylem cells and tracheary elements that can completely block the water transport in affected vessels.[35]
Strains
[edit]EB92-1 is a significantly less pathogenic strain of X. fastidiosa which is used for biocontrol against its relatives.[36] There is very little genomic distance between pathogenic and EB92-1 strains. However, 10 genes believed to be responsible for causing diseases in plants are missing.[36] EB92-1 not only protects against X. fastidiosa infection; it also protects against Citrus Huanglongbing, which is caused by Liberibacter.[37]
Symptoms
[edit]Significant variation in symptoms is seen between diseases, though some symptoms are expressed across species. On a macroscopic scale, plants infected with a X. fastidiosa-related disease exhibit symptoms of water, zinc, and iron deficiencies,[38] manifesting as leaf scorching and stunting in leaves turning them yellowish-brown, gummy substance around leaves,[38] fruit reduction in size and quality,[38] and overall plant height. As the bacterium progressively colonizes xylem tissues, affected plants often block off their xylem tissue, which can limit the spread of this pathogen; blocking can occur in the form of polysaccharide-rich gels, tyloses, or both. These plant defenses do not seem to hinder the movement of X. fastidiosa. Occlusion of vascular tissue, while a normal plant response to infection, makes symptoms significantly worse; as the bacterium itself also reduces vascular function, a 90% reduction of vascular hydraulic function was seen in susceptible Vitis vinifera.[39] This bacterium rarely completely blocks vascular tissue. There usually is a slight amount of vascular function that keeps the plant alive, but makes its fruit or branches die, making the specific plant economically nonproductive. This can cause a massive drop on supply of quality fruit.[38] Smaller colonies usually occur throughout a high proportion of xylem vessels of a symptomatic plant.[citation needed]
X. fastidiosa is a Gram-negative, xylem-limited illness that is spread by insects. It can damage a variety of broadleaved tree species that are commonly grown in the United States. X. fastidiosa can be found in about 600 different plant species.[citation needed]
- Withering and desiccation of branches
- Leaf chlorosis
- Dwarfing or lack of growth of the plant
- Drooping appearance and shorter internodes
- Shriveled fruits on infected plants
- Premature fruit abscission
- gum-like substance on leaves
- hardening and size reduction of fruit
Pierce's disease
[edit](X. f. subsp. fastidiosa) Severe PD symptoms include shriveled fruit, leaf scorching, and premature abscission of leaves, with bare petioles remaining on stems.[40]
Citrus variegated chlorosis
[edit](X. f. subsp. pauca) This disease is named after the characteristic spotty chlorosis on upper sides of citrus leaves. Fruits of infected plants are small and hard.[8]
Leaf scorches
[edit]Some isolates cause Almond leaf scorch, in California that includes CFBP8071 (fastidiosa), M23 (fastidiosa), and M12 (multiplex).[41][42]
(X. f. subsp. pauca) Coffee Leaf Scorch (CLS) is a disease caused by the causal agent Xylella fastidiosa that is economically significant in Brazil.[43] Citrus variegated chlorosis (CVC), another significant disease in this region caused by a strain of X. fastidiosa has been shown to infect coffee plants with CLS. The disease has also been found in Costa Rica's Central Valley where it is referred to as 'crespera' disease by coffee growers.[44] Symptoms of the bacterial infection in coffee plants feature curling leaf margins, chlorosis and irregularly shaped leaves, stunting and reduced plant growth, and branch atrophy.[44] The disease reduced coffee production by up to 30% in plantations across Brazil.[43]
X. fastidiosa was discovered in Apulia, Italy in 2013 for the first time as a destructive disease agent of olive trees and likely came from strains present in asymptomatic plant material imported from Costa Rica. The strains were of a single origin in subspecies pauca.[45]
Environment
[edit]X. fastidiosa occurs worldwide, though its diseases are most prominent in riparian habitats including the southeastern United States, California, and South America.
Symptoms of X. fastidiosa diseases worsen during hot, dry periods in the summer; lack of water and maximum demand from a full canopy of leaves, combined with symptoms due to disease, stress infected plants to a breaking point. Cold winters can limit the spread of the disease,[33] as it occurs in California, but not in regions with milder winters such as Brazil. Additionally, dry summers seem to delay symptom development of PD in California.[30]
Any conditions that increase vector populations can increase disease incidence, such as seasonal rainfall and forests or tree cover adjacent to crops, which serve as alternate food sources and overwintering locations for leafhoppers.[30]
Alexander Purcell, an expert on X. fastidiosa, hypothesized that plants foreign to X. fastidiosa's area of origin, the neotropical regions, are more susceptible to symptom development. Thus, plants from warmer climates are more resistant to X. fastidiosa disease development, while plants from areas with harsher winters, such as grapes, are more severely affected by this disease.[33]
Host species
[edit]X. fastidiosa has a very wide host range; as of 2020, its known host range was 595 plant species, with 343 species confirmed by two different detection methods, in 85 botanical families.[46] Most X. fastidiosa host plants are dicots, but it has also been reported in monocots and ginkgo, a gymnosperm. However, the vast majority of host plants remain asymptomatic, making them reservoirs for infection.[citation needed]
Due to the temperate climates of South America and the southeastern and west coast of the United States, X. fastidiosa can be a limiting factor in fruit crop production, particularly for stone fruits in northern Florida and grapes in California.[35] In South America, X. fastidiosa can cause significant losses in the citrus and coffee industries; a third of today's citrus crops in Brazil has CVC symptoms.[40]
X. fastidiosa also colonizes the foreguts of insect vectors,[47] which can be any xylem-feeding insects, often sharpshooters in the Cicadellidae subfamily Cicadellinae.[3][33] After an insect acquires X. fastidiosa, it has a short latent period around 2 hours, then the bacterium is transmissible for a period of a few months or as long as the insect is alive.[48] The bacterium multiplies within its vectors, forming a "bacterial carpet" within the foregut of its host. If the host sheds its foregut during molting, the vector is no longer infected, but can reacquire the pathogen. At present, no evidence shows that the bacterium has any detrimental effect on its insect hosts.
The EFSA maintains a list of plants known to be susceptible to Xylella and updates it regularly. Classification is performed down to the subspecies level if possible. As of May 2025[update], the latest report is from July 2024, containing information from literature published before 31 December 2023. The lists provided in EFSA's journal article about the update is only a summary; full data is available from the Microstrategy platform of the EFSA.[49][50]
Oleander
[edit]Oleander leaf scorch is a disease of landscape oleanders (Nerium oleander) caused by a X. fastidiosa strain that has become prevalent in California and Arizona, starting in the mid-1990s. This disease is transmitted by a type of leafhopper (insect) called the glassy-winged sharpshooter (Homalodisca coagulata). Oleander is commonly used in decorative landscaping in California, so the plants serve as widely distributed reservoirs for Xylella.[51]
Both almond and oleander plants in the Italian region of Apulia have also tested positive for the pathogen.[52]
Grape vines
[edit]Pierce's disease (PD) was discovered in 1892[53] by Newton B. Pierce (1856–1916; California's first professional plant pathologist) on grapes in California near Anaheim,[26] where it was known as "Anaheim disease".[54] The disease is endemic in Northern California, being spread by the blue-green sharpshooter, which attacks only grapevines adjacent to riparian habitats. It became a real threat to California's wine industry when the glassy-winged sharpshooter, native to the Southeast United States, was discovered in the Temecula Valley in California in 1996; it spreads PD much more extensively than other vectors.[55]
Symptoms of infection on grape vines
[edit]When a grape vine becomes infected, the bacterium causes a gel to form in the xylem tissue of the vine, preventing water from being drawn through the vine.[56] Leaves on vines with Pierce's disease turn yellow and brown, and eventually drop off the vine. Shoots also die. After one to five years, the vine itself dies. The proximity of vineyards to citrus groves compounds the threat, because citrus is not only a host of sharpshooter eggs, but also is a popular overwintering site for this insect.[57]
Collaborative efforts for solutions
[edit]In a unique effort, growers, administrators, policy makers, and researchers are working on a solution for this immense X. fastidiosa threat.[10] No cure has been found,[58] but the understanding of X. fastidiosa and glassy-winged sharpshooter biology has markedly increased since 2000, when the California Department of Food and Agriculture, in collaboration with different universities, such as University of California, Davis; University of California, Berkeley; University of California, Riverside, and University of Houston–Downtown started to focus their research on this pest. The research explores the different aspects of the disease propagation from the vector to the host plant and within the host plant, to the impact of the disease on California's economy. All researchers working on Pierce's disease meet annually in San Diego in mid-December to discuss the progress in their field. All proceedings from this symposium can be found on the Pierce's disease website,[59] developed and managed by the Public Intellectual Property Resource for Agriculture (PIPRA).[60]
Few resistant Vitis vinifera varieties are known, and Chardonnay and Pinot noir are especially susceptible, but muscadine grapes (V. rotundifolia) have a natural resistance.[58] Pierce's disease is found in the Southeastern United States and Mexico. Also, it was reported by Luis G. Jiménez-Arias in Costa Rica, and Venezuela,[61] and possibly in other parts of Central and South America. In 2010, X. fastidiosa became apparent in Europe, posing a serious, real threat.[62] There are isolated hot spots of the disease near creeks in Napa and Sonoma in Northern California.[58] Work is underway at UC Davis to breed PD resistance from V. rotundifolia into V. vinifera. The first generation was 50% high-quality V. vinifera genes, the next 75%, the third 87% and the fourth 94%. In the spring of 2007, seedlings that are 94% V. vinifera were planted.[63]
A resistant variety, 'Victoria Red', was released for use especially in Coastal Texas.[64]
The management of X. fastidiosa, a dangerous plant pathogen, requires a multidisciplinary approach that includes genetic and spatial ecology perspectives.[65][10] Such an approach will improve knowledge on invasive processes and resource allocation, optimizing diagnostic and management efforts. Effective communication with stakeholders is crucial for successful and sustainable management. X. fastidiosa is a great way to study how pathogenicity changes at different levels of biological complexity. This will help scientists come up with better ways to find and control invasive species.[10]

Olive trees
[edit]
In October 2013, the bacterium was found infecting olive trees in the region of Apulia in southern Italy.[52] The disease caused rapid decline in olive grove yields, and by April 2015, was affecting the whole Province of Lecce and other zones of Apulia,[7][66] though it had not previously been confirmed in Europe.[67] The subspecies involved in Italy is X. f. subsp. pauca, which shows a marked preference for olive trees and warm conditions and is thought to be unlikely to spread to Northern Europe.[68]
The cycle in olives has been called olive quick decline syndrome (in Italian: complesso del disseccamento rapido dell'olivo).[67][69] The disease causes withering and desiccation of terminal shoots, distributed randomly at first but then expanding to the rest of the canopy[69] resulting in the collapse and death of trees.[69] In affected groves, all plants normally show symptoms.[69] The most severely affected olives are the century-old trees of local cultivars Cellina di Nardò and Ogliarola salentina.[70]
By 2015, the disease had infected up to a million olive trees in Apulia[71] and Xylella fastidiosa had reached Corsica,[72] By October 2015, it had reached Mainland France, near Nice, in Provence-Alpes-Côte d'Azur, affecting the non-native myrtle-leaf milkwort (Polygala myrtifolia). This is the subspecies X. fastidiosa subsp. multiplex which is considered to be a different genetic variant of the bacterium to that found in Italy.[73][74] On 18 August 2016 in Corsica, 279 foci of the infection have been detected, concentrated mostly in the south and the west of the island.[75] In August 2016, the bacterium was detected in Germany in an oleander plant.[76] In January 2017 it was detected in Mallorca and Ibiza.[77]
Notably, in 2016, olive leaf scorch was first detected in X. fastidiosa's native range, in Brazil.[26]
In June 2017, it was detected in the Iberian peninsula, specifically in Guadalest, Alicante.[78] In 2018, it was detected elsewhere in Spain[79] and Portugal,[80] and in Israel in 2019.[81]
Citrus
[edit]Xylella infection was detected in South American citrus in the 1980s and subsequently in the USA but had limited spread beyond the Americas until the detection in citrus groves in Portugal in 2023.[82]
Genome sequencing
[edit]The genome of X. fastidiosa was sequenced in 2000 by a pool of over 30 research laboratories in the state of São Paulo, Brazil, funded by the São Paulo Research Foundation.[83]
See also
[edit]References
[edit]- ^ a b Rapicavoli, Jeannette; Ingel, Brian; Blanco-Ulate, Barbara; Cantu, Dario; Roper, Caroline (April 2018). "Xylella fastidiosa: an examination of a re-emerging plant pathogen". Molecular Plant Pathology. 19 (4): 786–800. Bibcode:2018MolPP..19..786R. doi:10.1111/mpp.12585. PMC 6637975. PMID 28742234.
- ^ Krugner, Rodrigo; Sisterson, Mark S; Backus, Elaine A; Burbank, Lindsey P; Redak, Richard A (May 2019). "Sharpshooters: a review of what moves Xylella fastidiosa". Austral Entomology. 58 (2): 248–267. doi:10.1111/aen.12397. ISSN 2052-174X. S2CID 182504242.
- ^ a b Redak, Richard A.; Purcell, Alexander H.; Lopes, João R.S.; Blua, Matthew J.; Mizell III, Russell F.; Andersen, Peter C. (3 December 2003). "The biology of xylem fluid–feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology". Annual Review of Entomology. 49 (1): 243–270. doi:10.1146/annurev.ento.49.061802.123403. ISSN 0066-4170. PMID 14651464.
- ^ Cornara, Daniele; Saponari, Maria; Zeilinger, Adam R.; de Stradis, Angelo; Boscia, Donato; Loconsole, Giuliana; Bosco, Domenico; Martelli, Giovanni P.; Almeida, Rodrigo P. P.; Porcelli, Francesco (1 March 2017). "Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy". Journal of Pest Science. 90 (2): 521–530. Bibcode:2017JPesS..90..521C. doi:10.1007/s10340-016-0793-0. ISSN 1612-4766. PMC 5320020. PMID 28275326.
- ^ "Pierce's Disease". UC IPM. Retrieved 4 December 2022.
- ^ Martelli, G. P.; Boscia, D.; Porcelli, F.; Saponari, M. (1 February 2016). "The olive quick decline syndrome in south-east Italy: a threatening phytosanitary emergency". European Journal of Plant Pathology. 144 (2): 235–243. Bibcode:2016EJPP..144..235M. doi:10.1007/s10658-015-0784-7. ISSN 1573-8469. S2CID 254475576.
- ^ a b "Minimizing the Spread of Disease in Italy's Famous Olive Trees". Our Environment at Berkeley. University of California, Berkeley, Department of Environmental Science, Policy, and Management (ESPM). 9 February 2015. Retrieved 5 May 2015.
- ^ a b Coletta-Filho, Helvecio Della; Castillo, Andreina I.; Laranjeira, Francisco Ferraz; de Andrade, Eduardo Chumbinho; Silva, Natalia Teixeira; de Souza, Alessandra Alves; Bossi, Mariana Esteves; Almeida, Rodrigo P. P.; Lopes, João R. S. (1 June 2020). "Citrus Variegated Chlorosis: an Overview of 30 Years of Research and Disease Management". Tropical Plant Pathology. 45 (3): 175–191. Bibcode:2020TroPP..45..175C. doi:10.1007/s40858-020-00358-5. ISSN 1983-2052. S2CID 218652922.
- ^ Castro, Claudia; DiSalvo, Biagio; Roper, M. Caroline (9 September 2021). "Xylella fastidiosa: A reemerging plant pathogen that threatens crops globally". PLOS Pathogens. 17 (9) e1009813. doi:10.1371/journal.ppat.1009813. ISSN 1553-7374. PMC 8428566. PMID 34499674.
- ^ a b c d Raffini, Francesca; Bertorelle, Giorgio; Biello, Roberto; D'Urso, Guido; Russo, Danilo; Bosso, Luciano (2020). "From Nucleotides to Satellite Imagery: Approaches to Identify and Manage the Invasive Pathogen Xylella fastidiosa and Its Insect Vectors in Europe". Sustainability. 12 (11): 4508. Bibcode:2020Sust...12.4508R. doi:10.3390/su12114508. hdl:11392/2425360. ISSN 2071-1050.
- ^ Castillo, Andreina I.; Tsai, Chi-Wei; Su, Chiou-Chu; Weng, Ling-Wei; Lin, Yu-Chen; Cho, Shu-Ting; Almeida, Rodrigo P. P.; Kuo, Chih-Horng (2021). "Genetic differentiation of Xylella fastidiosa following the introduction into Taiwan". Microbial Genomics. 7 (12): 000727. doi:10.1099/mgen.0.000727. ISSN 2057-5858. PMC 8767338. PMID 34898423.
- ^ Zecharia, Noa; Krasnov, Helena; Vanunu, Miri; Siri, Andreina Castillo; Haberman, Ami; Dror, Orit; Vakal, Lera; Almeida, Rodrigo P. P.; Blank, Lior; Shtienberg, Dani; Bahar, Ofir (November 2022). "Xylella fastidiosa Outbreak in Israel: Population Genetics, Host Range, and Temporal and Spatial Distribution Analysis". Phytopathology. 112 (11): 2296–2309. Bibcode:2022PhPat.112.2296Z. doi:10.1094/PHYTO-03-22-0105-R. ISSN 0031-949X. PMID 35778787.
- ^ Su, Chiou-Chu; Chang, Chung Jan; Chang, Che-Ming; Shih, Hsien-Tzung; Tzeng, Kuo-Ching; Jan, Fuh-Jyh; Kao, Chin-Wen; Deng, Wen-Ling (June 2013). "Pierce's Disease of Grapevines in Taiwan: Isolation, Cultivation and Pathogenicity of Xylella fastidiosa". Journal of Phytopathology. 161 (6): 389–396. Bibcode:2013JPhyt.161..389S. doi:10.1111/jph.12075.
- ^ Zecharia, Noa; Krasnov, Helena; Vanunu, Miri; Siri, Andreina Castillo; Haberman, Ami; Dror, Orit; Vakal, Lera; Almeida, Rodrigo P. P.; Blank, Lior; Shtienberg, Dani; Bahar, Ofir (November 2022). "Xylella fastidiosa Outbreak in Israel: Population Genetics, Host Range, and Temporal and Spatial Distribution Analysis". Phytopathology. 112 (11): 2296–2309. Bibcode:2022PhPat.112.2296Z. doi:10.1094/PHYTO-03-22-0105-R. ISSN 0031-949X. PMID 35778787. S2CID 250218193.
- ^ "Update of the Xylella spp. host plant database – systematic literature search up to 30 June 2021". www.efsa.europa.eu. 12 January 2022. Retrieved 4 December 2022.
- ^ Petit, Giai; Bleve, Gianluca; Gallo, Antonia; Mita, Giovanni; Montanaro, Giuseppe; Nuzzo, Vitale; Zambonini, Dario; Pitacco, Andrea (2021). "Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europaea: revisiting a tale of plant–pathogen interaction". AoB Plants. 13 (4) plab027. doi:10.1093/aobpla/plab027. PMC 8300559. PMID 34316336.
- ^ Ingel, Brian; Reyes, Clarissa; Massonnet, Mélanie; Boudreau, Bailey; Sun, Yuling; Sun, Qiang; McElrone, Andrew J.; Cantu, Dario; Roper, M. Caroline (February 2021). "Xylella fastidiosa causes transcriptional shifts that precede tylose formation and starch depletion in xylem". Molecular Plant Pathology. 22 (2): 175–188. Bibcode:2021MolPP..22..175I. doi:10.1111/mpp.13016. ISSN 1464-6722. PMC 7814960. PMID 33216451.
- ^ Roper, M. Caroline; Greve, L. Carl; Labavitch, John M.; Kirkpatrick, Bruce C. (15 November 2007). "Detection and Visualization of an Exopolysaccharide Produced by Xylella fastidiosa In Vitro and In Planta". Applied and Environmental Microbiology. 73 (22): 7252–7258. Bibcode:2007ApEnM..73.7252R. doi:10.1128/AEM.00895-07. ISSN 0099-2240. PMC 2168192. PMID 17827325.
- ^ Simpson, A. J. G.; Reinach, F. C.; Arruda, P.; Abreu, F. A.; Acencio, M.; Alvarenga, R.; Alves, L. M. C.; Araya, J. E.; Baia, G. S.; Baptista, C. S.; Barros, M. H.; Bonaccorsi, E. D.; Bordin, S.; Bové, J. M.; Briones, M. R. S. (July 2000). "The genome sequence of the plant pathogen Xylella fastidiosa". Nature. 406 (6792): 151–157. Bibcode:2000Natur.406..151S. doi:10.1038/35018003. ISSN 1476-4687. PMID 10910347. S2CID 17344899.
- ^ Lindow, Steven (1 February 2019). "Money Matters: Fueling Rapid Recent Insight Into Xylella fastidiosa—An Important and Expanding Global Pathogen". Phytopathology. 109 (2): 210–212. Bibcode:2019PhPat.109..210L. doi:10.1094/PHYTO-09-18-0325-PER. ISSN 0031-949X. PMID 30644806. S2CID 58611726.
- ^ genus/xyella entry in LPSN; Parte, Aidan C.; Sardà Carbasse, Joaquim; Meier-Kolthoff, Jan P.; Reimer, Lorenz C.; Göker, Markus (1 November 2020). "List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ". International Journal of Systematic and Evolutionary Microbiology. 70 (11): 5607–5612. doi:10.1099/ijsem.0.004332.
- ^ Badel, J. L.; Zambolim, L. (April 2019). "Coffee bacterial diseases: a plethora of scientific opportunities". Plant Pathology. 68 (3): 411–425. Bibcode:2019PPath..68..411B. doi:10.1111/ppa.12966.
- ^ a b Schaad, Norman W.; Postnikova, Elena; Lacy, George; Fatmi, M'Barek; Chang, Chung-Jan (January 2004). "Xylella fastidiosa subspecies: X. fastidiosa subsp piercei, subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov". Systematic and Applied Microbiology. 27 (3): 290–300. Bibcode:2004SyApM..27..290S. doi:10.1078/0723-2020-00263. PMID 15214634.
- ^ a b c d e f g Robinson, R. (2016). "Xylella fastidiosa (Pierce's disease of grapevines)". www.cabi.org. CABI Compendium. doi:10.1079/cabicompendium.57195. Retrieved 6 November 2017.
- ^ Schaad, Norman W.; Postnikova, Elena; Lacy, George; Fatmi, M'Barek; Chang, Chung-Jan (December 2004). "Xylella fastidiosa subspecies: X. fastidiosa subsp piercei, subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov". Systematic and Applied Microbiology. 27 (6): 763. Bibcode:2004SyApM..27..763S. doi:10.1078/0723202042369848.
- ^ a b c Sicard, Anne; Zeilinger, Adam R.; Vanhove, Mathieu; Schartel, Tyler E.; Beal, Dylan J.; Daugherty, Matthew P.; Almeida, Rodrigo P.P. (25 August 2018). "Xylella fastidiosa: Insights into an Emerging Plant Pathogen" (PDF). Annual Review of Phytopathology. 56 (1). Annual Reviews: 181–202. Bibcode:2018AnRvP..56..181S. doi:10.1146/annurev-phyto-080417-045849. ISSN 0066-4286. PMID 29889627. S2CID 48353386. (AS ORCID: 0000-0002-0575-195X).
- ^ "List of new names and new combinations previously effectively, but not validly, published". International Journal of Systematic and Evolutionary Microbiology. 59 (5): 923–925. 1 May 2009. doi:10.1099/ijs.0.013961-0.
- ^ Bragard, Claude; Baptista, Paula; Chatzivassiliou, Elisavet; Di Serio, Francesco; Gonthier, Paolo; Jaques Miret, Josep Anton; Justesen, Annemarie Fejer; MacLeod, Alan; Magnusson, Christer Sven; Milonas, Panagiotis; Navas-Cortes, Juan A; Parnell, Stephen; Potting, Roel; Stefani, Emilio; Thulke, Hans-Hermann; Van der Werf, Wopke; Civera, Antonio Vicent; Yuen, Jonathan; Zappalà, Lucia; Chen, Jianchi; Migheli, Quirico; Vloutoglou, Irene; Streissl, Franz; Reignault, Philippe Lucien (January 2023). "Pest categorisation of Xylella taiwanensis". EFSA Journal. 21 (1): e07736. doi:10.2903/j.efsa.2023.7736. hdl:11380/1302450. PMC 9854164. PMID 36698497.
- ^ Meng, Yizhi; Li, Yaxin; Galvani, Cheryl D.; Hao, Guixia; Turner, James N.; Burr, Thomas J.; Hoch, H. C. (15 August 2005). "Upstream Migration of Xylella fastidiosa via Pilus-Driven Twitching Motility". Journal of Bacteriology. 187 (16): 5560–5567. doi:10.1128/jb.187.16.5560-5567.2005. ISSN 0021-9193. PMC 1196070. PMID 16077100.
- ^ a b c Mizell, Russell F.; Andersen; Tipping (January 2003). "Xylella Fastidiosa Diseases and Their Leafhopper Vectors" (PDF). University of Florida IFAS Extension. Archived from the original (PDF) on 25 February 2021. Retrieved 30 November 2017.
- ^ Hill, B. L.; Purcell, A. H. (1997). "Populations of Xylella fastidiosa in Plants Required for Transmission by an Efficient Vector". Phytopathology. 87 (12): 1197–1201. Bibcode:1997PhPat..87.1197H. doi:10.1094/phyto.1997.87.12.1197. PMID 18945018.
- ^ Coletta-Filho, Helvécio Della; Carvalho, Sérgio Alves; Silva, Luis Fernando Carvalho; Machado, Marcos Antonio (1 July 2014). "Seven years of negative detection results confirm that Xylella fastidiosa, the causal agent of CVC, is not transmitted from seeds to seedlings". European Journal of Plant Pathology. 139 (3): 593–596. Bibcode:2014EJPP..139..593C. doi:10.1007/s10658-014-0415-8. ISSN 0929-1873. S2CID 15561623.
- ^ a b c d Purcell, Alexander (4 August 2013). "Paradigms: Examples from the Bacterium Xylella fastidiosa". Annual Review of Phytopathology. 51 (1): 339–356. Bibcode:2013AnRvP..51..339P. doi:10.1146/annurev-phyto-082712-102325. ISSN 0066-4286. PMID 23682911.
- ^ Cornara, Daniele; Morente, Marina; Markheiser, Anna; Bodino, Nicola; et al. (2019). "An overview on the worldwide vectors of Xylella fastidiosa". Entomologia Generalis. 39 (3–4): 157–181. doi:10.1127/entomologia/2019/0811.
- ^ a b Chatterjee, Subhadeep; Almeida, Rodrigo P. P.; Lindow, Steven (4 August 2008). "Living in two Worlds: The Plant and Insect Lifestyles of Xylella fastidiosa". Annual Review of Phytopathology. 46 (1): 243–271. Bibcode:2008AnRvP..46..243C. doi:10.1146/annurev.phyto.45.062806.094342. ISSN 0066-4286. PMID 18422428.
- ^ a b
- Gross, Dennis C.; Lichens-Park, Ann; Kole, Chittaranjan, eds. (2014). Genomics of plant-associated bacteria. Heidelberg. pp. x+278. ISBN 978-3-642-55378-3. OCLC 881817015. ISBN 978-3-642-55377-6.
{{cite book}}: CS1 maint: location missing publisher (link): 177–202 - Zhang, Shujian; Flores-Cruz, Zomary; Kumar, Dibyendu; Chakrabarty, Pranjib; Hopkins, Donald L.; Gabriel, Dean W. (2011). "The Xylella fastidiosa Biocontrol Strain EB92-1 Genome Is Very Similar and Syntenic to Pierce's Disease Strains". Journal of Bacteriology. 193 (19). American Society for Microbiology: 5576–5577. doi:10.1128/jb.05430-11. ISSN 0021-9193. PMC 3187439. PMID 21914886. S2CID 7068164.
- Gross, Dennis C.; Lichens-Park, Ann; Kole, Chittaranjan, eds. (2014). Genomics of plant-associated bacteria. Heidelberg. pp. x+278. ISBN 978-3-642-55378-3. OCLC 881817015. ISBN 978-3-642-55377-6.
- ^ Hopkins, D. L.; Ager, K. L. (1 October 2021). "Biological Control of Citrus Huanglongbing with EB92-1, a Benign Strain of Xylella fastidiosa". Plant Disease. 105 (10): 2914–2918. Bibcode:2021PlDis.105.2914H. doi:10.1094/PDIS-02-21-0362-RE. PMID 33822659.
- ^ a b c d Almeida, Rodrigo P.P.; Coletta-Filho, Helvécio D.; Lopes, João R.S. (2014). "Xylella fastidiosa". In Liu, Dongyou (ed.). Manual of Security Sensitive Microbes and Toxins (1 ed.). CRC Press. ISBN 978-0-367-37874-5.
- ^ Sun, Qiang; Sun, Yuliang; Walker, M. Andrew; Labavitch, John M. (1 March 2013). "Vascular Occlusions in Grapevines with Pierce's Disease Make Disease Symptom Development Worse". Plant Physiology. 161 (3): 1529–1541. doi:10.1104/pp.112.208157. ISSN 0032-0889. PMC 3585614. PMID 23292789.
- ^ a b Hopkins, D. L.; Purcell, A. H. (1 October 2002). "Xylella fastidiosa: Cause of Pierce's Disease of Grapevine and Other Emergent Diseases". Plant Disease. 86 (10): 1056–1066. Bibcode:2002PlDis..86.1056H. doi:10.1094/pdis.2002.86.10.1056. ISSN 0191-2917. PMID 30818496.
- ^ Moralejo, E.; Borràs, D.; Gomila, M.; Montesinos, M.; Adrover, F.; Juan, A.; Nieto, A.; Olmo, D.; Seguí, G.; Landa, B. B. (7 August 2019). "Insights into the epidemiology of Pierce's disease in vineyards of Mallorca, Spain". Plant Pathology. 68 (8). British Society for Plant Pathology (Wiley): 1458–1471. Bibcode:2019PPath..68.1458M. doi:10.1111/ppa.13076. ISSN 0032-0862. S2CID 199641165.
- ^ Chen, J.; Xie, G.; Han, S.; Chertkov, O.; Sims, D.; Civerolo, E. L. (September 2010). "Whole genome sequences of two Xylella fastidiosa strains (M12 and M23) causing almond leaf scorch disease in California". Journal of Bacteriology. 192 (17): 4534. doi:10.1128/JB.00651-10. PMC 2937377. PMID 20601474.
- ^ a b Li, W.-B.; Pria, W. D.; Teixeira, D. C.; Miranda, V. S.; Ayres, A. J.; Franco, C. F.; Costa, M. G.; He, C.-X.; Costa, P. I.; Hartung, J. S. (1 May 2001). "Coffee Leaf Scorch Caused by a Strain of Xylella fastidiosa from Citrus". Plant Disease. 85 (5): 501–505. Bibcode:2001PlDis..85..501L. doi:10.1094/PDIS.2001.85.5.501. hdl:11449/38427. ISSN 0191-2917. PMID 30823125.
- ^ a b Montero-Astúa, Mauricio; Chacón-Díaz, Carlos; Aguilar, Estela; Rodríguez, Carlos Mario; Garita, Laura; Villalobos, William; Moreira, Lisela; Hartung, John S.; Rivera, Carmen (October 2008). "Isolation and molecular characterization of Xylella fastidiosa from coffee plants in Costa Rica". The Journal of Microbiology. 46 (5): 482–490. doi:10.1007/s12275-008-0072-8. hdl:10669/76372. ISSN 1225-8873. PMID 18974947. S2CID 40097733.
- ^ Sicard, Anne; Saponari, Maria; Vanhove, Mathieu; Castillo, Andreina I.; Giampetruzzi, Annalisa; Loconsole, Giuliana; Saldarelli, Pasquale; Boscia, Donato; Neema, Claire; Almeida, Rodrigo P. P. (2021). "Introduction and adaptation of an emerging pathogen to olive trees in Italy". Microbial Genomics. 7 (12): 000735. doi:10.1099/mgen.0.000735. ISSN 2057-5858. PMC 8767334. PMID 34904938.
- ^ a b European Food Safety Authority (2020). "Scientific report on the update of the Xylella spp. host plant database – systematic literature search up to 30 June 2019". EFSA Journal. 18 (4): 61. doi:10.2903/j.efsa.2020.6114. ISSN 1831-4732. PMC 7448098. PMID 32874307.
- ^ Cornara, Daniele; Morente, Marina; Markheiser, Anna; Bodino, Nicola; Tsai, Chi-Wei; Fereres, Alberto; Redak, Richard A.; Perring, Thomas M.; Lopes, Joao Roberto Spotti (23 December 2019). "An overview on the worldwide vectors of Xylella fastidiosa". Entomologia Generalis. 39 (3–4): 157–181. doi:10.1127/entomologia/2019/0811. ISSN 0171-8177.
- ^ Chatterjee, Subhadeep; Almeida, Rodrigo P. P.; Lindow, Steven (8 September 2008). "Living in two Worlds: The Plant and Insect Lifestyles of Xylella fastidiosa". Annual Review of Phytopathology. 46 (1): 243–271. Bibcode:2008AnRvP..46..243C. doi:10.1146/annurev.phyto.45.062806.094342. ISSN 0066-4286. PMID 18422428.
- ^ Cavalieri, Vincenzo; Fasanelli, Elisa; Gibin, Davide; Gutierrez Linares, Alicia; La Notte, Pierfederico; Pasinato, Luca; Delbianco, Alice (July 2024). "Update of the Xylella spp. host plant database – Systematic literature search up to 31 December 2023". EFSA Journal. 22 (7) e8898. doi:10.2903/j.efsa.2024.8898. PMC 11247332. PMID 39010863.
- ^ Delbianco, Alice; Gibin, Davide; Pasinato, Luca; Morelli, Massimiliano (2021). "Update of the Xylella spp. host plant database – systematic literature search up to 31 December 2020". EFSA Journal. 19 (6). European Food Safety Authority (Wiley): e06674. doi:10.2903/j.efsa.2021.6674. ISSN 1831-4732. PMC 8220458. PMID 34188716. S2CID 235671792.
- ^ Hernandez-Martinez, Rufina; de la Cerda, Karla A.; Costa, Heather S.; Cooksey, Donald A.; Wong, Francis P. (July 2007). "Phylogenetic Relationships of Xylella fastidiosa Strains Isolated from Landscape Ornamentals in Southern California". Phytopathology. 97 (7): 857–864. Bibcode:2007PhPat..97..857H. doi:10.1094/PHYTO-97-7-0857. ISSN 0031-949X. PMID 18943935.
- ^ a b Kinver, Mark (9 January 2015). "'Major consequences' if olive disease spreads across EU". BBC News. Retrieved 1 March 2015.
- ^ Pierce, Newton Barris (1892). The California Vine Disease: A Preliminary Report of Investigations. Bulletins. Government Printing Office, Washington, DC US: United States Department of Agriculture, Division of Vegetable Pathology. ISBN 978-1-331-97095-8. OCLC 4512989.
{{cite book}}: ISBN / Date incompatibility (help) ISBN 1331970954. - ^ Pinney, Thomas (1989). A History of Wine in America from the Beginnings to Prohibition. University of California Press. pp. 27. ISBN 978-0-520-06224-5.
- ^ Haviland, David R; Stone-Smith, Beth; Gonzalez, Minerva (1 January 2021). "Control of Pierce's Disease Through Areawide Management of Glassy-Winged Sharpshooter (Hemiptera: Cicadellidae) and Roguing of Infected Grapevines". Journal of Integrated Pest Management. 12 (1). doi:10.1093/jipm/pmab008. ISSN 2155-7470.
- ^ Meyer, Brittnay (2018). "Pierce's Disease on grapes". Texas Plant Disease Diagnostic Lab. Retrieved 5 May 2023.
- ^ Pollard, Herschel N.; Kaloostian, George H. (1 August 1961). "Overwintering Habits of Homalodisca coagulata, the Principal Natural Vector of Phony Peach Disease Virus". Journal of Economic Entomology. 54 (4): 810–811. doi:10.1093/jee/54.4.810. ISSN 1938-291X.
- ^ a b c winepros.com.au. Oxford Companion to Wine. "Pierce's disease". Archived from the original on 8 August 2008. Retrieved 7 May 2008.
- ^ PIPRA Pierce's Disease website. "Pierce's disease".
- ^ "Public Intellectual Property Resource for Agriculture (PIPRA)". Archived from the original on 5 March 2016.
- ^ Jiménez A., L.G. (July–September 1985). "Evidencia inmunológica del mal de pierce de la vid en Venezuela". Turrialba. 35 (3): 243–247.
- ^ Janse, J.D.; Obradovic, A. (2010). "Xylella Fastidiosa: ITS Biology, Diagnosis, Control and Risks". Journal of Plant Pathology. 92: S35 – S48. ISSN 1125-4653. JSTOR 41998754.
- ^ PD/GWSS Board bulletin Archived 2015-05-18 at the Wayback Machine, California Department of Food & Agriculture, Spring 2007 (p. 2)
- ^ Moore, James N.; Clark, John R.; Kamas, James; Stein, Larry; Tarkington, Friench; Tarkington, Martha (1 May 2011). "'Victoria Red' Grape". HortScience. 46 (5): 817–820. doi:10.21273/HORTSCI.46.5.817. ISSN 0018-5345.
- ^ De La Fuente, Leonardo; Navas-Cortés, Juan A.; Landa, Blanca B. (May 2024). "Ten Challenges to Understanding and Managing the Insect-Transmitted, Xylem-Limited Bacterial Pathogen Xylella fastidiosa". Phytopathology. 114 (5): 869–884. Bibcode:2024PhPat.114..869D. doi:10.1094/PHYTO-12-23-0476-KC. hdl:10261/362843. ISSN 0031-949X. PMID 38557216.
- ^ Spagnolo, Chiara (29 April 2015). "Xylella, allarme nuovi focolai, per la Ue interessata tutta la Puglia" [Xylella, new outbreak alarm, for the EU all of Puglia affected]. La Repubblica (in Italian). Retrieved 8 May 2015.
- ^ a b "First report of Xylella fastidiosa in the EPPO region". European and Mediterranean Plant Protection Organization (EPPO). Retrieved 1 March 2015.
- ^ "Xylella fastidiosa". Plant Health Portal. Department for Environment, Food and Rural Affairs. Retrieved 24 June 2017.
- ^ a b c d Butler, Julie (29 March 2014). "Expert Says Eradication of New Olive Tree Disease in Europe Unlikely". Olive Oil Times. Retrieved 1 March 2015.
- ^ Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. (2019). "Xylella fastidiosa in Olive in Apulia: Where We Stand". Phytopathology. 109 (2). American Phytopathological Society: 175–186. Bibcode:2019PhPat.109..175S. doi:10.1094/PHYTO-08-18-0319-FI. hdl:11586/226511. PMID 30376439.
- ^ Squires, Nick (27 February 2015). "Italy warns deadly olive tree bacteria could spread across Europe". The Telegraph. Retrieved 1 March 2015.
- ^ "Olive oil dries up". The Economist. 31 July 2015. Retrieved 31 July 2015.
- ^ "Xylella fastidiosa". Plants. European Commission. 17 October 2016. Retrieved 24 June 2017.
- ^ "Un premier cas de la bactérie tueuse de végétaux découvert à Nice" [A first case of the plant killer bacterium discovered in Nice]. Nice Matin (in French). 9 October 2015. Retrieved 9 October 2015.
- ^ "Xylella: carte et liste des communes en zones délimitées en Corse au 18 août 2016". Direction régionale de l'alimentation, de l'agriculture et de la forêt de Corse (in French). Archived from the original on 23 August 2016. Retrieved 23 August 2016.
- ^ Schröder, Elke. "Pflanzen-Killerbakterium: Teile von Zeulenroda-Triebes zur Sperrzone erklärt" [Plant killer bacteria: Parts of Zeulenroda rails declared a restrict zone]. Antenne Thueringen (in German). Archived from the original on 27 August 2016. Retrieved 23 August 2016.
- ^ Elcacho, Joaquim. "La plaga vegetal más peligrosa de Europa invade las Baleares" [Europe's most dangerous plant pest invades the Balearic Islands]. La Vanguardia (in Spanish). Retrieved 24 January 2017.
- ^ "Tala preventiva de árboles ante el primer caso de 'Xylella fastidiosa' en la península" [Preventive tree lysing in the first case of Xylella fastidiosa on the peninsula]. La Vanguardia (in Spanish). Retrieved 4 July 2017.
- ^ "Detectada en Madrid la presencia de una bacteria que obligó a arrancar un millón de olivos en Italia". 20 Minutos (in Spanish). Retrieved 12 April 2018.
- ^ "Xylella fastidiosa (XYLEFA) [Portugal]| EPPO Global Database". gd.eppo.int. Retrieved 3 August 2020.
- ^ "Xylella fastidiosa (XYLEFA) [Israel]| EPPO Global Database". gd.eppo.int. Retrieved 3 August 2020.
- ^ NTC, À Punt (11 January 2023). "Portugal detecta la 'Xylella fastidiosa' en cítrics per primera vegada en la UE" [Portugal detects 'Xylella fastidiosa' in citrus fruits for the first time in the EU]. À Punt (in Catalan). Retrieved 20 January 2023.
- ^ Simpson, A. J. G.; Reinach, F. C.; Arruda, P.; Abreu, F. A.; Acencio, M.; Alvarenga, R.; Alves, L. M. C.; Araya, J. E.; Baia, G. S.; Baptista, C. S.; Barros, M. H.; Bonaccorsi, E. D.; Bordin, S.; Bové, J. M.; Briones, M. R. S. (2000). "The genome sequence of the plant pathogen Xylella fastidiosa". Nature. 406 (6792): 151–157. Bibcode:2000Natur.406..151S. doi:10.1038/35018003. ISSN 1476-4687. PMID 10910347.
Further reading
[edit]- "CDFA - Pierce's Disease Research Updates". piercesdisease.cdfa.ca.gov. Retrieved 15 February 2020.
- "Glassy-winged Sharpshooter and Pierce's Disease in California". December 2002. Archived from the original on 9 May 2006. Retrieved 15 February 2020.
- CDFA PD/GWSS Board Website PD/GWSS Interactive Forum
- "Oleander leaf scorch". 10 July 2006. Archived from the original on 18 September 2006. Retrieved 15 February 2020.
- Wells, J. M.; Raju, B. C.; Hung, H.-Y.; Weisburg, W. G.; Mandelco-Paul, L.; Brenner, D. J. (1 April 1987). "Xylella fastidiosa gen. nov., sp. nov: Gram-Negative, Xylem-Limited, Fastidious Plant Bacteria Related to Xanthomonas spp". International Journal of Systematic Bacteriology. 37 (2): 136–143. doi:10.1099/00207713-37-2-136. ISSN 0020-7713.
- Catalano, Luigi (2015). "Xylella fastidiosa la più grave minaccia dell'olivicoltura italiana" (PDF). L'Informatore Agrario (in Italian) (16): 36–42. Archived from the original (PDF) on 18 June 2018. Retrieved 15 February 2020.
External links
[edit]- Type strain of Xylella fastidiosa at BacDive - the Bacterial Diversity Metadatabase
- "XF-ACTORS". XF-ACTORS (Xylella Fastidiosa Active Containment Through a multidisciplinary-Oriented Research Strategy). 2 February 2021. Retrieved 30 March 2021.
- "XF-ACTORS "Xylella Fastidiosa Active Containment Through a multidisciplinary-Oriented Research"". EIP-AGRI - European Commission. 30 August 2017. Retrieved 30 March 2021.
Xylella fastidiosa
View on GrokipediaTaxonomy and Classification
Discovery and Etymology
The bacterium Xylella fastidiosa was first linked to plant disease in 1892, when American plant pathologist Newton B. Pierce described a destructive condition in grapevines (Vitis vinifera) in southern California, later termed Pierce's disease, characterized by leaf scorching, dieback, and vine decline.[12] Although microscopic observations of xylem-inhabiting bacteria were reported in affected tissues as early as the late 19th century, the causal agent was initially misattributed to viral or fungal origins during the 1930s.[13] Pure cultures of the bacterium were not successfully isolated until 1978 from diseased grapevines using a specialized medium (JDI), enabling definitive identification as a xylem-limited pathogen.[14] [15] In 1987, researchers J.M. Wells, B.W. Raju, H.-Y. Chen, L.D. Schulz, R.J. Copeman, and C.L. Chang formally proposed the name Xylella fastidiosa for the species based on strains isolated from grapevines and other hosts, classifying it within the family Xanthomonadaceae.[16] This taxonomic description followed genomic and phenotypic analyses confirming its distinctiveness, marking the first solid cultivation and characterization of the organism despite prior challenges in axenic growth.[17] The genus name Xylella derives from the Greek xýlon (wood) combined with the diminutive suffix -ella, reflecting its exclusive colonization of plant xylem vessels.[16] The specific epithet fastidiosa is a Latin feminine adjective meaning "fastidious" or "delicate," alluding to the bacterium's nutritional exigency and resistance to laboratory cultivation, which necessitated enriched media with supplements like bovine hemin and yeast extract for propagation.[16] [18] This nomenclature underscores the organism's biological constraints, observed consistently across strains since its initial isolation.Subspecies and Strain Diversity
Xylella fastidiosa is classified into five genetically distinct subspecies—fastidiosa, multiplex, pauca, sandyi, and morus—differentiated primarily through multilocus sequence typing (MLST), whole-genome sequencing, and host specificity patterns.[19] These subspecies exhibit allopatric origins, with genomic analyses of over 70 strains revealing varying degrees of recombination and divergence that correlate with geographic distribution and pathogenicity.[20] Strain diversity within subspecies is extensive, encompassing dozens of sequence types (STs); for instance, more than 50 STs have been identified globally, each potentially linked to specific pathosystems.[21]| Subspecies | Primary Hosts | Associated Diseases |
|---|---|---|
| fastidiosa | Grapevines (Vitis spp.), almonds | Pierce's disease, almond leaf scorch |
| multiplex | Broadleaf plants, including blueberries, maples | Leaf scorch diseases in ornamentals and forest trees |
| pauca | Olives, coffee, citrus | Olive quick decline syndrome, coffee leaf scorch, citrus variegated chlorosis |
| sandyi | Oleander, coffee | Oleander leaf scorch |
| morus | Mulberry trees | Mulberry leaf scorch |
Biology and Pathogenesis
Cellular Structure and Anatomy
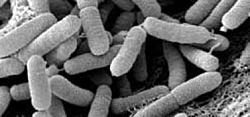
Xylella fastidiosa is a Gram-negative, rod-shaped prokaryote with cells typically measuring 0.25–0.35 μm in diameter and 0.9–3.5 μm in length.[3][25] As a member of the family Xanthomonadaceae, it possesses a standard Gram-negative cell envelope comprising an inner cytoplasmic membrane, a thin peptidoglycan layer in the periplasmic space, and an outer membrane embedded with lipopolysaccharides (LPS).[25] The bacterium is non-flagellate and catalase-positive but oxidase-negative, reflecting adaptations for its aerobic, xylem-limited lifestyle.[26] Motility in X. fastidiosa occurs via twitching, driven by type IV pili that extend and retract to propel cells across surfaces at speeds up to 5 μm per minute against fluid flow, facilitating upstream migration within plant xylem vessels.[27][3] Type I pili also contribute to this process, alongside roles in cell-cell aggregation and biofilm initiation.[28] The outer membrane features a rhamnose-rich O-antigen in its LPS, which modulates surface charge, adhesion to host tissues, and evasion of plant immune recognition by delaying innate responses.[25][29] Additional ultrastructural elements include outer membrane vesicles (OMVs), which are released to influence adhesion dynamics and biofilm maturation, and exopolysaccharides such as fastidian gum that stabilize aggregates.[3][25] Afimbrial adhesins, including hemagglutinins, complement pili in mediating attachment to xylem walls without fimbrial structures.[25] These features collectively enable persistent colonization in the nutrient-poor, flow-resistant xylem environment, where the bacterium forms rippled cell walls observable in electron microscopy of infected tissues.[30]Lifecycle, Transmission, and Disease Mechanism
Xylella fastidiosa is a gram-negative, xylem-limited bacterium that reproduces asexually via binary fission within the xylem vessels of susceptible host plants and the foregut of its insect vectors.[25] In the plant host, cells adhere to xylem walls, multiply to high densities, and form biofilms embedded in a matrix of extracellular polymeric substances, enabling persistent colonization from roots to shoots.[25] Bacterial populations can reach 10^6 to 10^9 cells per gram of xylem sap in advanced infections, with growth rates influenced by host genotype and environmental factors such as temperature optima around 25-30°C.[25] Within vectors, the bacterium colonizes the cuticular surface of the precibarium without invading hemolymph, multiplying locally to sustain transmission competence for weeks to months.[31] Transmission occurs exclusively through xylem sap-feeding insects, primarily sharpshooter leafhoppers (Cicadellinae subfamily) in the Americas and spittlebugs (Aphrophoridae) in Europe, in a manner that is propagative, persistent, and non-circulative.[32] Acquisition happens during vector feeding on infected plants, where bacteria adhere to and colonize the vector's foregut within hours, with no evidence of multiplication beyond the mouthparts or systemic spread in the insect.[33] Inoculation into a new host requires mechanical injection via the stylet during xylem probing, often within minutes of acquisition, as there is no extrinsic incubation period; efficiency varies by vector species, with some achieving over 50% transmission rates after brief acquisition access.[34] The pathogen does not persist in soil, seeds, or propagate vegetatively without vectors, limiting natural spread to insect-mediated dispersal over distances of tens to hundreds of meters per generation.[32] The disease mechanism centers on xylem dysfunction induced by bacterial colonization, leading to hydraulic failure rather than direct toxin production.[25] Biofilm formation and bacterial aggregates, augmented by host-derived polymers like pectin and gums, occlude vessels, reducing hydraulic conductivity by up to 80% in symptomatic tissues; this triggers cavitation, embolism, and reduced water transport, manifesting as leaf marginal scorch and canopy dieback.[35] Incompatible interactions may involve plant defenses such as reactive oxygen species or antimicrobial peptides that limit bacterial titers below pathogenic thresholds, while susceptible hosts exhibit minimal resistance, allowing unchecked proliferation.[25] Secondary effects include altered gene expression in hosts, promoting tylose and gel formation that exacerbates blockage, with symptom onset typically 3-12 months post-inoculation depending on strain virulence and environmental stress.[36]Hosts and Vectors
Susceptible Host Plants
Xylella fastidiosa possesses an exceptionally wide host range, infecting more than 600 plant species across over 80 families, including both agricultural crops and wild or ornamental plants.[37] While many hosts remain asymptomatic and serve as reservoirs for the bacterium, facilitating its spread without overt disease symptoms, certain species exhibit severe pathology upon infection, particularly under favorable environmental conditions.[35] The European Food Safety Authority's host plant database, updated through systematic literature reviews, lists 696 species as of December 2023, with 439 confirmed by at least two independent detection methods such as PCR and culture isolation; subsequent updates in 2025 added 14 more naturally infected species.[38][39] Susceptibility varies by bacterial subspecies and strain, with experimental inoculations demonstrating potential infection in additional species beyond those naturally observed. For example, subsp. fastidiosa predominantly affects North American hosts like grapevines, whereas subsp. pauca shows preference for olives in Mediterranean and South American contexts. Key symptomatic hosts include:- Grapevines (Vitis spp.): Primary host for Pierce's disease in California and other U.S. regions, leading to vine decline and reduced yields.[40]
- Olives (Olea europaea): Causes olive quick decline syndrome (OQDS) in Italy and Brazil, characterized by leaf scorching and tree dieback, with infections confirmed in over 100 cultivars.[15]
- Citrus (Citrus spp.): Associated with citrus variegated chlorosis (CVC) in Brazil and citrus leaf scorch in Florida, impacting fruit quality and tree longevity.[25]
- Almonds (Prunus dulcis): Induces almond leaf scorch in California, contributing to premature defoliation and nut crop losses.[15]
- Oleander (Nerium oleander): Develops oleander leaf scorch, a notable ornamental host with visible wilting and marginal necrosis.[41]

