Recent from talks
Nothing was collected or created yet.
Bone fracture
View on Wikipedia| Bone fracture | |
|---|---|
| Other names | broken bone, bone break |
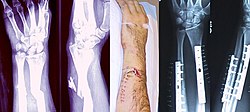 | |
| Internal and external views of an arm with a compound fracture, both before and after surgery | |
| Specialty | Orthopedics, emergency medicine |
| Diagnostic method | X-ray, computed tomography, MRI |
A bone fracture (abbreviated FRX or Fx, Fx, or #) is a medical condition in which there is a partial or complete break in the continuity of any bone in the body. In more severe cases, the bone may be broken into several fragments, known as a comminuted fracture.[1] An open fracture (or compound fracture) is a bone fracture where the broken bone breaks through the skin.[2]
A bone fracture may be the result of high force impact or stress, or a minimal trauma injury as a result of certain medical conditions that weaken the bones, such as osteoporosis, osteopenia, bone cancer, or osteogenesis imperfecta, where the fracture is then properly termed a pathologic fracture.[3] Most bone fractures require urgent medical attention to prevent further injury.
Signs and symptoms
[edit]Although bone tissue contains no pain receptors, a bone fracture is painful for several reasons:[4]
- Breaking in the continuity of the periosteum, with or without similar discontinuity in endosteum, as both contain multiple pain receptors.
- Edema and hematoma of nearby soft tissues caused by ruptured bone marrow evokes pressure pain.
- Involuntary muscle spasms trying to hold bone fragments in place.
Damage to adjacent structures such as nerves, muscles or blood vessels, spinal cord, and nerve roots (for spine fractures), or cranial contents (for skull fractures) may cause other specific signs and symptoms.[5]
Complications
[edit]
Some fractures may lead to serious complications, including a condition known as compartment syndrome. If not treated, eventually, compartment syndrome may require amputation of the affected limb. Other complications may include non-union, where the fractured bone fails to heal, or malunion, where the fractured bone heals in a deformed manner. One form of malunion is the malrotation of a bone, which is especially common after femoral and tibial fractures.[6] Complications of fractures may be classified into three broad groups, depending upon their time of occurrence. These are as follows –
- Immediate complications – occurs at the time of the fracture.
- Early complications – occurring in the initial few days after the fracture.
- Late complications – occurring a long time after the fracture.
| Immediate | Early | Late |
|---|---|---|
Systemic
|
Systemic
|
Imperfect union of the fracture |
Local
|
Local
|
Others
|
Pathophysiology
[edit]
The natural process of healing a fracture starts when the injured bone and surrounding tissues bleed, forming a fracture hematoma. The blood coagulates to form a blood clot situated between the broken fragments.[7] Within a few days, blood vessels grow into the jelly-like matrix of the blood clot. The new blood vessels bring phagocytes to the area, which gradually removes the non-viable material. The blood vessels also bring fibroblasts in the walls of the vessels and these multiply and produce collagen fibres. In this way, the blood clot is replaced by a matrix of collagen. Collagen's rubbery consistency allows bone fragments to move only a small amount unless severe or persistent force is applied.[citation needed]
At this stage, some of the fibroblasts begin to lay down bone matrix in the form of collagen monomers. These monomers spontaneously assemble to form the bone matrix, for which bone crystals (calcium hydroxyapatite) are deposited in amongst, in the form of insoluble crystals. This mineralization of the collagen matrix stiffens it and transforms it into bone. In fact, bone is a mineralized collagen matrix; if the mineral is dissolved out of bone, it becomes rubbery. Healing bone callus on average is sufficiently mineralized to show up on X-ray within 6 weeks in adults and less in children. This initial "woven" bone does not have the strong mechanical properties of mature bone. By a process of remodelling, the woven bone is replaced by mature "lamellar" bone. The whole process may take up to 18 months, but in adults, the strength of the healing bone is usually 80% of normal by 3 months after the injury.[citation needed]
Several factors may help or hinder the bone healing process. For example, tobacco smoking hinders the process of bone healing,[8] and adequate nutrition (including calcium intake) will help the bone healing process. Weight-bearing stress on bone, after the bone has healed sufficiently to bear the weight, also builds bone strength.
Although there are theoretical concerns about NSAIDs slowing the rate of healing, there is not enough evidence to warrant withholding the use of this type analgesic in simple fractures.[9]
Effects of smoking
[edit]Smokers generally have lower bone density than non-smokers, so they have a much higher risk of fractures. There is also evidence that smoking delays bone healing.[10]
Diagnosis
[edit]
A bone fracture may be diagnosed based on the history given and the physical examination performed. Radiographic imaging is often performed to confirm the diagnosis. Under certain circumstances, radiographic examination of the nearby joints is indicated to exclude dislocations and fracture-dislocations. In situations where projectional radiography alone is insufficient, Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) may be indicated.[citation needed]
Classification
[edit]
(a) closed fracture
(b) open fracture
(c) transverse fracture
(d) spiral fracture
(e) comminuted fracture
(f) impacted fracture
(g) greenstick fracture
(h) oblique fracture


In orthopedic medicine, fractures are classified in various ways. Historically, they are named after the physician who first described the fracture conditions; however, there are more systematic classifications as well.[citation needed]
They may be divided into stable versus unstable depending on the likelihood that they may shift further.[citation needed]
Mechanism
[edit]- Traumatic fracture – a fracture due to sustained trauma. e.g., fractures caused by a fall, road traffic accident, fight, etc.
- Pathologic fracture – A fracture through a bone that has been made weak by some underlying disease is called a pathological fracture. e.g., a fracture through a bone weakened by metastasis. Osteoporosis is the most common cause of pathological fracture.
- Periprosthetic fracture – a fracture at the point of mechanical weakness at the end of an implant.
Soft-tissue involvement
[edit]- Closed/simple fractures are those in which the overlying skin is intact[11]
- Open/compound fractures involve wounds that communicate with the fracture, or where fracture hematoma is exposed, and may thus expose bone to contamination. Open injuries carry a higher risk of infection. Reports indicate an incidence of infection after internal fixation of closed fractures of 1-2%, rising to 30% in open fractures.[12]
- Clean fracture
- Contaminated fracture
Displacement
[edit]- Non-displaced
- Displaced
- Translated, or ad latus, with sideways displacement.[13]
- Angulated
- Rotated
- Shortened, a reduction in overall bone length when displaced fracture fragments overlap
Fracture pattern
[edit]- Linear fracture – a fracture that is parallel to the bone's long axis
- Transverse fracture – a fracture that is at a right angle to the bone's long axis
- Oblique fracture – a fracture that is diagonal to a bone's long axis (more than 30°)
- Spiral fracture – a fracture where at least one part of the bone has been twisted
- Compression fracture/wedge fracture – usually occurs in the vertebrae, for example when the front portion of a vertebra in the spine collapses due to osteoporosis (a medical condition which causes bones to become brittle and susceptible to fracture, with or without trauma)
- Impacted fracture – a fracture caused when bone fragments are driven into each other
- Avulsion fracture – a fracture where a fragment of bone is separated from the main mass
Fragments
[edit]- Incomplete fracture – a fracture in which the bone fragments are still partially joined; in such cases, there is a crack in the osseous tissue that does not completely traverse the width of the bone.
- Complete fracture – a fracture in which bone fragments separate completely.
- Comminuted fracture – a fracture in which the bone has broken into several pieces.
Anatomical location
[edit]An anatomical classification may begin with specifying the involved body part, such as the head or arm, followed by more specific localization. Fractures that have additional definition criteria than merely localization often may be classified as subtypes of fractures, such as a Holstein-Lewis fracture being a subtype of a humerus fracture. Most typical examples in an orthopaedic classification given in the previous section cannot be classified appropriately into any specific part of an anatomical classification, however, as they may apply to multiple anatomical fracture sites.
- Skull fracture
- Basilar skull fracture
- Blowout fracture – a fracture of the walls or floor of the orbit
- Mandibular fracture
- Nasal fracture
- Le Fort fracture of skull – facial fractures involving the maxillary bone and surrounding structures in a usually bilateral and either horizontal, pyramidal, or transverse way.
- Spinal fracture
- Cervical fracture
- Fracture of C1, including Jefferson fracture
- Fracture of C2, including Hangman's fracture
- Flexion teardrop fracture – a fracture of the anteroinferior aspect of a cervical vertebral
- Clay-shoveler fracture – fracture through the spinous process of a vertebra occurring at any of the lower cervical or upper thoracic vertebrae
- Burst fracture – in which a vertebra breaks from a high-energy axial load
- Compression fracture – a collapse of a vertebra, often in the form of wedge fractures due to larger compression anteriorly
- Chance fracture – compression injury to the anterior portion of a vertebral body with concomitant distraction injury to the posterior elements
- Holdsworth fracture – an unstable fracture dislocation of the thoraco lumbar junction of the spine
- Cervical fracture
- Rib fracture
- Sternal fracture
- Shoulder fracture
- Arm fracture
- Humerus fracture (fracture of upper arm)
- Supracondylar fracture
- Holstein-Lewis fracture – a fracture of the distal third of the humerus resulting in entrapment of the radial nerve
- Forearm fracture
- Ulnar fracture
- Monteggia fracture – a fracture of the proximal third of the ulna with the dislocation of the head of the radius
- Hume fracture – a fracture of the olecranon with an associated anterior dislocation of the radial head
- Radius fracture
- Essex-Lopresti fracture – a fracture of the radial head with concomitant dislocation of the distal radio-ulnar joint with disruption of the interosseous membrane[14]
- Distal radius fracture
- Galeazzi fracture – a fracture of the radius with dislocation of the distal radioulnar joint
- Colles' fracture – a distal fracture of the radius with dorsal (posterior) displacement of the wrist and hand
- Smith's fracture – a distal fracture of the radius with volar (ventral) displacement of the wrist and hand
- Barton's fracture – an intra-articular fracture of the distal radius with dislocation of the radiocarpal joint
- Ulnar fracture
- Humerus fracture (fracture of upper arm)
- Hand fracture
- Scaphoid fracture
- Rolando fracture – a comminuted intra-articular fracture through the base of the first metacarpal bone
- Bennett's fracture – a fracture of the base of the first metacarpal bone which extends into the carpometacarpal (CMC) joint [15]
- Boxer's fracture – a fracture at the neck of a metacarpal
- Broken finger – a fracture of the carpal phalanges
- Pelvic fracture
- Fracture of the hip bone
- Duverney fracture – an isolated pelvic fracture involving only the iliac wing
- Femoral fracture
- Hip fracture (anatomically a fracture of the femur bone and not the hip bone)
- Patella fracture
- Crus fracture
- Tibia fracture
- Pilon fracture
- Tibial plateau fracture
- Bumper fracture – a fracture of the lateral tibial plateau caused by a forced valgus applied to the knee
- Segond fracture – an avulsion fracture of the lateral tibial condyle
- Gosselin fracture – a fractures of the tibial plafond into anterior and posterior fragments [16]
- Toddler's fracture – an undisplaced and spiral fracture of the distal third to distal half of the tibia [17]
- Fibular fracture
- Maisonneuve fracture – a spiral fracture of the proximal third of the fibula associated with a tear of the distal tibiofibular syndesmosis and the interosseous membrane
- Le Fort fracture of ankle – a vertical fracture of the antero-medial part of the distal fibula with avulsion of the anterior tibiofibular ligament[16]
- Bosworth fracture – a fracture with an associated fixed posterior dislocation of the distal fibular fragment that becomes trapped behind the posterior tibial tubercle; the injury is caused by severe external rotation of the ankle [18]
- Combined tibia and fibula fracture
- Trimalleolar fracture – involving the lateral malleolus, medial malleolus, and the distal posterior aspect of the tibia
- Bimalleolar fracture – involving the lateral malleolus and the medial malleolus
- Pott's fracture
- Tibia fracture
- Foot fracture
- Lisfranc fracture – in which one or all of the metatarsals are displaced from the tarsus[19]
- Jones fracture – a fracture of the proximal end of the fifth metatarsal
- March fracture – a fracture of the distal third of one of the metatarsals occurring because of recurrent stress
- Cuneiform fracture – a fracture of one of the three cuneiform bones typically due to direct blow, axial load, or avulsion [20]
- Calcaneal fracture – a fracture of the calcaneus (heel bone)
- Broken toe – a fracture of the pedal phalanges
OTA/AO classification
[edit]The Orthopaedic Trauma Association Committee for Coding and Classification published its classification system [21] in 1996, adopting a similar system to the 1987 AO Foundation system.[22] In 2007, they extended their system,[23] unifying the two systems regarding wrist, hand, foot, and ankle fractures.
Classifications named after people
[edit]Several classifications are named after the person (eponymous) who developed it.
- "Denis classification" for spinal fractures [24]
- "Frykman classification" for forearm fractures (fractures of radius and ulna)
- "Gustilo open fracture classification" [25]
- "Letournel and Judet Classification" for Acetabular fractures [26]
- "Neer classification" for humerus fractures [27][28]
- Seinsheimer classification, Evans-Jensen classification, Pipkin classification, and Garden classification for hip fractures[29]
Prevention
[edit]Both high- and low-force trauma can cause bone fracture injuries.[30][31] Preventive efforts to reduce motor vehicle crashes, the most common cause of high-force trauma, include reducing distractions while driving.[32] Common distractions are driving under the influence and texting or calling while driving, both of which lead to an approximate 6-fold increase in crashes.[32] Wearing a seatbelt can also reduce the likelihood of injury in a collision.[32] 30 km/h or 20 mph speed limits (as opposed to the more common intracity 50 km/h / 30 mph) also drastically reduce the risk of accident, serious injury and even death in crashes between motor vehicles and humans. Vision Zero aims to reduce traffic deaths to zero through better traffic design and other measures and to drastically reduce traffic injuries, which would prevent many bone fractures.
A common cause of low-force trauma is an at-home fall.[30][31] When considering preventative efforts, the National Institute of Health (NIH) examines ways to reduce the likelihood of falling, the force of the fall, and bone fragility.[33] To prevent at-home falls they suggest keeping cords out of high-traffic areas where someone could trip, installing handrails and keeping stairways well-lit, and installing an assistive bar near the bathtub in the washroom for support.[33] To reduce the impact of a fall the NIH recommends to try falling straight down on your buttocks or onto your hands.[33]
Some sports have a relatively high risk of bone fractures as a common sports injury. Preventive measures depend to some extent on the specific sport, but learning proper technique, wearing protective gear and having a realistic estimation of one's own capabilities and limitations can all help reduce the risk of bone fracture. In contact sports, rules have been put in place to protect athlete health, such as the prohibition of unnecessary roughness in American football.
Taking calcium and vitamin D supplements can help strengthen your bones.[33] Vitamin D supplements combined with additional calcium marginally reduces the risk of hip fractures and other types of fracture in older adults; however, vitamin D supplementation alone did not reduce the risk of fractures.[34] Taking vibration therapy can also help strengthening bones and reducing the risk of a fracture.[35][36]
Patterns
[edit]| Photo | Type | Description | Causes | Effects |
|---|---|---|---|---|
 |
Linear fracture | Parallel to the bone's long axis | ||
 |
Transverse fracture | At a right angle to the bone's long axis | May occur when the bone is bent,[37]and snaps in the middle. | |
 |
Oblique fracture | Diagonal to a bone's long axis (more than 30°) | ||
 |
Spiral fracture or torsion fracture | At least one part of the bone has been twisted (image shows an arm-wrestler) | Torsion on the bone[37] | May rotate, and must be reduced to heal properly |
 |
Compression fracture/wedge fracture | Usually occurs in the vertebrae, for example, when the front portion of a vertebra in the spine collapses due to osteoporosis (a medical condition which causes bones to become brittle and susceptible to fracture, with or without trauma) | ||
| Impacted fracture | Bone fragments are driven into each other | |||
 |
Avulsion fracture | A fragment of bone is separated from the main mass (image shows a Busch fracture) | ||
 |
Comminuted fracture | The bone is shattered | often from crushing injuries[37] |
Treatment
[edit]


Treatment of bone fractures are broadly classified as surgical or conservative, the latter basically referring to any non-surgical procedure, such as pain management, immobilization or other non-surgical stabilization. A similar classification is open versus closed treatment, in which open treatment refers to any treatment in which the fracture site is opened surgically, regardless of whether the fracture is an open or closed fracture.[38]
Pain management
[edit]In arm fractures in children, ibuprofen is as effective as a combination of paracetamol and codeine.[39] In the EMS setting it might be applicable to administer 1mg/kg of iv ketamine to achieve a dissociated state.
Immobilization
[edit]Since bone healing is a natural process that will occur most often, fracture treatment aims to ensure the best possible function of the injured part after healing. Bone fractures typically are treated by restoring the fractured pieces of bone to their natural positions (if necessary), and maintaining those positions while the bone heals. Often, aligning the bone, called reduction, in a good position and verifying the improved alignment with an X-ray is all that is needed. This process is extremely painful without anaesthesia, about as painful as breaking the bone itself. To this end, a fractured limb usually is immobilized with a plaster or fibreglass cast or splint that holds the bones in position and immobilizes the joints above and below the fracture.
When the initial post-fracture oedema or swelling goes down, the fracture may be placed in a removable brace or orthosis. If being treated with surgery, surgical nails, screws, plates, and wires are used to hold the fractured bone together more directly. Alternatively, fractured bones may be treated by the Ilizarov method, which is a form of an external fixator.
Occasionally, smaller bones, such as phalanges of the toes and fingers, may be treated without the cast, by buddy wrapping them, which serves a similar function to making a cast. A device called a Suzuki frame may be used in cases of deep, complex intra-articular digit fractures.[40] By allowing only limited movement, immobilization helps preserve anatomical alignment while enabling callus formation, toward the target of achieving union.
Splinting results in the same outcome as casting in children who have a distal radius fracture with little shifting.[41]
Surgery
[edit]Surgical methods of treating fractures have their own risks and benefits, but usually, surgery is performed only if conservative treatment has failed, is very likely to fail, or is likely to result in a poor functional outcome.[42] With some fractures such as hip fractures (usually caused by osteoporosis), surgery is offered routinely because non-operative treatment results in prolonged immobilisation, which commonly results in complications including chest infections, pressure sores, deconditioning, deep vein thrombosis (DVT), and pulmonary embolism, which are more dangerous than surgery.[43] When a joint surface is damaged by a fracture, surgery is also commonly recommended to make an accurate anatomical reduction and restore the smoothness of the joint.
Infection is especially dangerous in bones, due to the recrudescent nature of bone infections. Bone tissue is predominantly extracellular matrix, rather than living cells, and the few blood vessels needed to support this low metabolism are only able to bring a limited number of immune cells to an injury to fight infection. For this reason, open fractures and osteotomies call for very careful antiseptic procedures and prophylactic use of antibiotics.
Occasionally, bone grafting is used to treat a fracture.[44]
Sometimes bones are reinforced with metal.[45] These implants must be designed and installed with care. Stress shielding occurs when plates or screws carry too large of a portion of the bone's load, causing atrophy. This problem is reduced, but not eliminated, by the use of low-modulus materials, including titanium and its alloys. The heat generated by the friction of installing hardware can accumulate easily and damage bone tissue, reducing the strength of the connections. If dissimilar metals are installed in contact with one another (i.e., a titanium plate with cobalt-chromium alloy or stainless steel screws), galvanic corrosion will result. The metal ions produced can damage the bone locally and may cause systemic effects as well.
Bone stimulation
[edit]Bone stimulation with either electromagnetic or ultrasound waves may be suggested as an alternative to surgery to reduce the healing time for non-union fractures.[46][47] The proposed mechanism of action is by stimulating osteoblasts and other proteins that form bones using these modalities. The evidence supporting the use of ultrasound and shockwave therapy for improving unions is very weak[46] and it is likely that these approaches do not make a clinically significant difference for a delayed union or non-union.[48]
Physical therapy
[edit]Physical therapy exercises (either home-based or physiotherapist-led) to improve functional mobility and strength, gait training for hip fractures, and other physical exercises are also often suggested to help recover physical capacities after a fracture has healed.[49][50]
Children
[edit]In children, whose bones are still developing, there are risks of either a growth plate injury or a greenstick fracture.
- A greenstick fracture occurs due to mechanical failure on the tension side. That is, since the bone is not so brittle as it would be in an adult, it does not completely fracture, but rather exhibits bowing without complete disruption of the bone's cortex in the surface opposite the applied force.
- Growth plate injuries, as in Salter-Harris fractures, require careful treatment and accurate reduction to make sure that the bone continues to grow normally.
- Plastic deformation of the bone, in which the bone permanently bends, but does not break, is also possible in children. These injuries may require an osteotomy (bone cut) to realign the bone if it is fixed and cannot be realigned by closed methods.
- Certain fractures mainly occur in children, including fracture of the clavicle and supracondylar fracture of the humerus.[citation needed]
See also
[edit]- Stress fracture
- Distraction osteogenesis
- Rickets
- Catagmatic
- H. Winnett Orr, U.S. Army surgeon who developed Orthopedic plaster casts
References
[edit]- ^ Katherine, Abel (2013). Official CPC Certification Study Guide. American Medical Association. p. 108.
- ^ "Open Fractures - OrthoInfo - AAOS". www.orthoinfo.org. Retrieved 15 November 2024.
- ^ Witmer, Daniel K.; Marshall, Silas T.; Browner, Bruce D. (2016). "Emergency Care of Musculoskeletal Injuries". In Townsend, Courtney M.; Beauchamp, R. Daniel; Evers, B. Mark; Mattox, Kenneth L. (eds.). Sabiston Textbook of Surgery (20th ed.). Elsevier. pp. 462–504. ISBN 978-0-323-40163-0. Archived from the original on 17 January 2023. Retrieved 4 December 2016.
- ^ MedicineNet – Fracture Archived 2008-12-21 at the Wayback Machine Medical Author: Benjamin C. Wedro, MD, FAAEM.
- ^ Danielle Campagne (September 2022). "Overview of Fractures". mdmanuals.com. Archived from the original on 12 October 2022. Retrieved 12 October 2022.
- ^ "Compartment Syndrome". The Lecturio Medical Concept Library. Archived from the original on 25 June 2021. Retrieved 25 June 2021.
- ^ Silva, Joana Cavaco (11 July 2018). "Bone fracture repair: Procedures, risks, and healing time". Medical News Today. William Morrison, M.D. (medical reviewer). Archived from the original on 23 March 2022. Retrieved 21 April 2022.
- ^ Sloan, A.; Hussain, I.; Maqsood, M.; Eremin, O.; El-Sheemy, M. (2010). "The effects of smoking on fracture healing". The Surgeon. 8 (2): 111–6. doi:10.1016/j.surge.2009.10.014. PMID 20303894.
- ^ Pountos, Ippokratis; Georgouli, Theodora; Calori, Giorgio M.; Giannoudis, Peter V. (2012). "Do Nonsteroidal Anti-Inflammatory Drugs Affect Bone Healing? A Critical Analysis". The Scientific World Journal. 2012: 1–14. doi:10.1100/2012/606404. PMC 3259713. PMID 22272177.
- ^ Kanis, J. A.; Johnell, O.; Oden, A.; Johansson, H.; De Laet, C.; Eisman, J. A.; Fujiwara, S.; Kroger, H.; McCloskey, E. V.; Mellstrom, D.; Melton, L. J.; Pols, H.; Reeve, J.; Silman, A.; Tenenhouse, A. (2004). "Smoking and fracture risk: A meta-analysis". Osteoporosis International. 16 (2): 155–62. doi:10.1007/s00198-004-1640-3. PMID 15175845. S2CID 19890259.
- ^ "Simple fracture | pathology". Encyclopedia Britannica. Archived from the original on 19 May 2021. Retrieved 19 May 2021.
- ^ Metsemakers, WJ; Onsea, J; Neutjens, E; Steffens, E; Schuermans, A; McNally, M; Nijs, S (December 2017). "Prevention of fracture-related infection: a multidisciplinary care package". International Orthopaedics. 41 (12): 2457–2469. doi:10.1007/s00264-017-3607-y. PMID 28831576. S2CID 12894601.
- ^ Roberto Schubert. "Fractures of the extremities (general rules and nomenclature)". Radiopaedia. Archived from the original on 13 May 2021. Retrieved 21 February 2018.
- ^ Essex Lopresti fracture Archived 2009-10-01 at the Wayback Machine at Wheeless' Textbook of Orthopaedics online
- ^ "Bennett's fracture-subluxation". GPnotebook.
- ^ a b Hunter, Tim B.; Peltier, Leonard F.; Lund, Pamela J. (2000). "Radiologic History Exhibit". RadioGraphics. 20 (3): 819–36. doi:10.1148/radiographics.20.3.g00ma20819. PMID 10835130.
- ^ Mellick, Larry B.; Milker, Laura; Egsieker, Erik (1999). "Childhood accidental spiral tibial (CAST) fractures". Pediatric Emergency Care. 15 (5): 307–9. doi:10.1097/00006565-199910000-00001. PMID 10532655.
- ^ Perry, C. R.; Rice, S; Rao, A; Burdge, R (1983). "Posterior fracture-dislocation of the distal part of the fibula. Mechanism and staging of injury". The Journal of Bone and Joint Surgery. American Volume. 65 (8): 1149–57. doi:10.2106/00004623-198365080-00016. PMID 6630259.[permanent dead link]
- ^ "Lisfranc's fracture". Mosby's Medical Dictionary. 9th edition. Elsevier. 2009. Archived from the original on 4 April 2019 – via The Free Dictionary.
- ^ Mabry, LM; Patti, TN; Ross, MD; Bleakley, CM; Gisselman, AS (July 2021). "Isolated Medial Cuneiform Fractures: A Systematic Search and Qualitative Analysis of Case Studies" (PDF). J Am Podiatr Med Assoc. 111 (4): 1–9. doi:10.7547/20-047. PMID 34478529. S2CID 225705519. Archived from the original (PDF) on 2 March 2022.
- ^ "Fracture and dislocation compendium. Orthopaedic Trauma Association Committee for Coding and Classification". Journal of Orthopaedic Trauma. 10 (Suppl 1): v–ix, 1–154. 1996. PMID 8814583.
- ^ Müller ME, Nazarian S, Koch P (1987). Classification AO des fractures. Tome I. Les os longs. Berlin: Springer-Verlag.[page needed]
- ^ Marsh, J. L.; Slongo, T. F.; Agel, J; Broderick, J. S.; Creevey, W; Decoster, T. A.; Prokuski, L; Sirkin, M. S.; Ziran, B; Henley, B; Audigé, L (2007). "Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee". Journal of Orthopaedic Trauma. 21 (10 Suppl): S1–133. doi:10.1097/00005131-200711101-00001. PMID 18277234. S2CID 24535478.
- ^ "Denis classification of spinal fractures". GPnotebook.
- ^ Rüedi, etc. all; Thomas P. Rüedi; Richard E. Buckley; Christopher G. Moran (2007). AO principles of fracture management, Volume 1. Thieme. p. 96. ISBN 978-3-13-117442-0. Archived from the original on 26 July 2024. Retrieved 9 November 2020.
- ^ "Fractures of the Acetabulum". wheelessonline.com. Archived from the original on 26 September 2009.
- ^ Mourad, L (1997). "Neer classification of fractures of the proximal humerus". Orthopedic Nursing. 16 (2): 76. PMID 9155417.
- ^ Proximal Humerus Fractures at eMedicine
- ^ "Hip Fractures". The Lecturio Medical Concept Library. Archived from the original on 24 July 2021. Retrieved 24 July 2021.
- ^ a b "Open Fractures - OrthoInfo - AAOS". Archived from the original on 4 May 2021. Retrieved 3 December 2018.
- ^ a b Court-Brown, Charles M.; Bugler, Kate E.; Clement, Nicholas D.; Duckworth, Andrew D.; McQueen, Margaret M. (June 2012). "The epidemiology of open fractures in adults. A 15-year review". Injury. 43 (6): 891–897. doi:10.1016/j.injury.2011.12.007. ISSN 1879-0267. PMID 22204774.
- ^ a b c Sidwell, Richard; Matar, Maher M.; Sakran, Joseph V. (1 October 2017). "Trauma Education and Prevention". Surgical Clinics of North America. 97 (5): 1185–1197. doi:10.1016/j.suc.2017.06.010. ISSN 0039-6109. PMID 28958365.
- ^ a b c d "Preventing Falls and Related Fractures | NIH Osteoporosis and Related Bone Diseases National Resource Center". www.bones.nih.gov. Archived from the original on 14 May 2021. Retrieved 3 December 2018.
- ^ Avenell, Alison; Mak, Jenson C. S.; O'Connell, Dianne (14 April 2014). "Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men". The Cochrane Database of Systematic Reviews. 2021 (4) CD000227. doi:10.1002/14651858.CD000227.pub4. ISSN 1469-493X. PMC 7032685. PMID 24729336.
- ^ "Update on vibration therapy for bone health". Harvard Health. 1 October 2011. Retrieved 10 December 2024.
- ^ Lee, Jong Hwa; Kim, Sang Beom; Lee, Kyeong Woo; Lee, Sook Joung; Park, Hyuntae; Kim, Dong Won (18 August 2017). "The effect of a whole-body vibration therapy on the sitting balance of subacute stroke patients: a randomized controlled trial". Topics in Stroke Rehabilitation. 24 (6): 457–462. doi:10.1080/10749357.2017.1305655. ISSN 1074-9357.
- ^ a b c McDaniel, Dalton J.; Rehman, Uzma H. (2 November 2021). "Phalanx Fractures of the Hand". StatPearls. StatPearls Publishing. PMID 32491557. Archived from the original on 29 December 2020. Retrieved 3 January 2022 – via PubMed.
- ^ "Overview of Bone Fractures". The Lecturio Medical Concept Library. Archived from the original on 25 July 2021. Retrieved 25 July 2021.
- ^ Drendel, Amy L.; Gorelick, Marc H.; Weisman, Steven J.; Lyon, Roger; Brousseau, David C.; Kim, Michael K. (2009). "A Randomized Clinical Trial of Ibuprofen Versus Paracetamol with Codeine for Acute Pediatric Arm Fracture Pain". Annals of Emergency Medicine. 54 (4): 553–60. doi:10.1016/j.annemergmed.2009.06.005. PMID 19692147.
- ^ Keramidas EG, Miller G (October 2005). "The Suzuki frame for complex intraarticular fractures of the thumb". Plastic and Reconstructive Surgery. 116 (5): 1326–31. doi:10.1097/01.prs.0000181786.39062.0b. PMID 16217475. S2CID 31890854.
- ^ Boutis, K.; Willan, A.; Babyn, P.; Goeree, R.; Howard, A. (2010). "Cast versus splint in children with minimally angulated fractures of the distal radius: A randomized controlled trial". Canadian Medical Association Journal. 182 (14): 1507–12. doi:10.1503/cmaj.100119. PMC 2950182. PMID 20823169.
- ^ "Fractures". Johns Hopkins Medicine. 28 February 2020. Archived from the original on 25 July 2021. Retrieved 25 July 2021.
- ^ "Hip Fractures". The Lecturio Medical Concept Library. Archived from the original on 24 July 2021. Retrieved 24 July 2021.
- ^ Klokkevold PR, Jovanovic SA (2002). "Advanced Implant Surgery and Bone Grafting Techniques". In Newman MG, Takei HM, Carranza FA (eds.). Carranza's Clinical Periodontology (9th ed.). W.B. Saunders. pp. 907–8. ISBN 978-0-7216-8331-7.
- ^ "Fractures". Johns Hopkins Medicine. 28 February 2020. Archived from the original on 26 July 2024. Retrieved 25 July 2021.
- ^ a b Leighton, R.; Watson, J.T; Giannoudis, P.; Papakostidis, C.; Harrison, A.; Steen, R.G. (May 2017). "Healing of fracture nonunions treated with low-intensity pulsed ultrasound (LIPUS): A systematic review and meta-analysis". Injury. 48 (7): 1339–1347. doi:10.1016/j.injury.2017.05.016. PMID 28532896.
- ^ Victoria, Galkowski; Petrisor, Brad; Drew, Brian; Dick, David (2009). "Bone stimulation for fracture healing: What′s all the fuss?". Indian Journal of Orthopaedics. 43 (2): 117–20. doi:10.4103/0019-5413.50844 (inactive 11 July 2025). ISSN 0019-5413. PMC 2762251. PMID 19838359.
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ Searle, Henry Kc; Lewis, Sharon R.; Coyle, Conor; Welch, Matthew; Griffin, Xavier L. (3 March 2023). "Ultrasound and shockwave therapy for acute fractures in adults". The Cochrane Database of Systematic Reviews. 2023 (3) CD008579. doi:10.1002/14651858.CD008579.pub4. ISSN 1469-493X. PMC 9983300. PMID 36866917.
- ^ Pradhan, Sara; Chiu, Sarah; Burton, Claire; Forsyth, Jacky; Corp, Nadia; Paskins, Zoe; van der Windt, Danielle A.; Babatunde, Opeyemi O. (3 June 2022). "Overall Effects and Moderators of Rehabilitation in Patients With Wrist Fracture: A Systematic Review". Physical Therapy. 102 (6) pzac032. doi:10.1093/ptj/pzac032. ISSN 1538-6724. PMID 35421234.
- ^ Fairhall, Nicola J.; Dyer, Suzanne M.; Mak, Jenson Cs; Diong, Joanna; Kwok, Wing S.; Sherrington, Catherine (7 September 2022). "Interventions for improving mobility after hip fracture surgery in adults". The Cochrane Database of Systematic Reviews. 2022 (9) CD001704. doi:10.1002/14651858.CD001704.pub5. ISSN 1469-493X. PMC 9451000. PMID 36070134.
External links
[edit]- Authoritative information in orthopaedic surgery American Association of Orthopedic Surgeons (AAOS)
- Radiographic Atlas of Fracture
Bone fracture
View on GrokipediaOverview
Definition
A bone fracture is defined as a break or crack in the continuity of bone tissue, resulting in a disruption of the bone's structural integrity. This distinguishes fractures from soft tissue injuries such as sprains, which involve ligament damage, or strains, which affect muscles or tendons, as fractures specifically pertain to the skeletal system.[6] Bone anatomy plays a key role in understanding fractures, with bones composed of cortical (compact) bone, which forms the dense outer layer providing strength and support, and cancellous (trabecular) bone, which is spongy and found primarily in the interior, offering flexibility and shock absorption. The periosteum, a tough fibrous membrane covering the outer surface of bone (except at joints), is crucial in fracture contexts as it supplies blood vessels, nerves, and progenitor cells essential for bone repair and remodeling.[7][8][6] Fractures are broadly classified by extent and exposure: complete fractures involve a full break across the bone, separating it into distinct segments, while incomplete fractures feature partial cracks that do not fully divide the bone, such as greenstick or hairline types often seen in children. Additionally, closed (simple) fractures occur when the bone breaks without piercing the skin, maintaining an intact overlying soft tissue envelope, whereas open (compound) fractures result in the bone protruding through the skin or communicating with an external wound, increasing infection risk.[9][10] The medical recognition of bone fractures dates back to ancient times, with the earliest detailed descriptions appearing in the works of Hippocrates around 400 BCE, who classified various fracture patterns and outlined basic reduction techniques in texts like On Fractures.[11] Fractures commonly affect long bones such as the femur, though they can occur in any skeletal element depending on the circumstances.Epidemiology
Bone fractures impose a substantial global health burden, with an estimated 178 million new cases occurring worldwide in 2019, marking a 33.4% increase in absolute numbers since 1990 despite a decline in age-standardized incidence rates to 2296.2 per 100,000 population.[12] In the United States, approximately 1.5 to 2 million fractures are treated annually, though rates are notably higher in developing countries, where trauma from road traffic accidents and violence is a major contributor to severe fracture cases.[13][14] Demographic patterns reveal distinct variations by age and gender. Incidence peaks in children aged 11-14 years, primarily from falls, with rates around 1,300-1,700 per 100,000 in boys at peak ages.[15] Among adults under 50, males experience higher rates due to high-energy trauma, such as sports injuries or accidents, with male-to-female ratios exceeding 1.6:1 in this group.[16] In contrast, females over 50 face elevated risk from low-energy falls linked to osteoporosis, accounting for about one-third of women and one-fifth of men in this age group sustaining an osteoporotic fracture in their lifetime.[17] The most common fracture sites differ by population. In adults overall, distal radius fractures predominate, comprising about 18% of cases, followed by proximal femur, ankle, proximal humerus, and proximal tibia.[18] Among the elderly, proximal femur (hip) fractures are a major type of fragility fracture in women over 65, accounting for approximately 20% of such cases.[19] Epidemiological trends indicate a rising burden driven by aging populations. Worldwide, hip fracture incidence is projected to increase by 310% in men and 240% in women by 2050 compared to 1990 levels. More recent projections from 2023 indicate that hip fractures will nearly double globally by 2050 compared to 2018.[20][21] Post-2020 data from the COVID-19 pandemic suggest a potential uptick in fragility fractures in some regions, attributed to sedentary lifestyles, reduced physical activity, and vitamin D deficiencies during lockdowns.[22] Morbidity and mortality remain high, especially for hip fractures in the elderly, with one-year post-fracture mortality rates ranging from 20% to 30%.[23][24]Causes and Risk Factors
Mechanisms of Injury
Bone fractures occur when the applied mechanical forces exceed the bone's capacity to withstand them, leading to structural failure. The primary mechanisms involve various types of loading that deform the bone beyond its elastic limit, resulting in crack initiation and propagation. These forces can act singly or in combination, depending on the injury event.[25] The main types of forces responsible for fractures include tensile, compressive, shear, torsional, and bending loads. Tensile forces stretch the bone, pulling it apart along its length and often leading to transverse fractures; cortical bone typically fails in tension at stresses around 130 MPa. Compressive forces crush the bone, shortening it and commonly producing impacted or compression fractures, with failure thresholds near 190 MPa longitudinally. Shear forces cause layers of bone to slide past one another, resulting in oblique or irregular fracture patterns, as bone is weakest under shear loading. Torsional forces twist the bone, generating spiral fractures due to the rotational stress along the bone's axis. Bending loads combine tension on the convex side and compression on the concave side, often seen in three- or four-point bending scenarios, leading to bowing or transverse fractures at the midpoint of the load application.[26][25] Fractures are broadly classified by the energy level of the trauma: high-energy versus low-energy. High-energy trauma involves substantial force, such as motor vehicle accidents or falls from height, which can cause comminuted or displaced fractures due to the rapid, intense loading that overwhelms bone's toughness. In contrast, low-energy trauma, like a simple fall from standing height, typically results in simpler fracture patterns, such as stable transverse breaks, as the force is more gradual and localized.[28] Mechanisms can also be direct or indirect based on force application. Direct mechanisms apply force immediately at the fracture site, such as a blow from an object causing a localized impact fracture. Indirect mechanisms transmit force along the limb from a distant point, like a fall on an outstretched hand propagating axial load to the humerus, resulting in fractures away from the impact area.[29] Biomechanical thresholds for fracture vary by bone type, age, and loading direction, but cortical bone generally yields at stresses of 100-200 MPa under physiological conditions, beyond which plastic deformation and failure occur. For instance, the yield stress in tension is approximately 100-150 MPa, while compression can tolerate up to 150-200 MPa before buckling. These limits highlight bone's anisotropic nature, being strongest longitudinally but vulnerable transversely.[25][30] Specific examples illustrate these mechanisms. Avulsion fractures arise from indirect tensile forces where a sudden muscle contraction pulls a bone fragment at its tendon or ligament attachment, common in the ankle or pelvis during sports. Stress fractures develop from repetitive low-energy loading, such as prolonged running, causing cumulative microdamage that accumulates beyond repair capacity, often in weight-bearing bones like the tibia or metatarsals.[31][32]Predisposing Factors
Bone density issues significantly predispose individuals to fractures by reducing skeletal strength and increasing fragility. Osteoporosis, characterized by low bone mineral density (BMD) and microarchitectural deterioration, affects approximately 30-50% of postmenopausal women, with prevalence estimates reaching up to 37.5% for osteoporosis and 44.7% for osteopenia in this group.[33] Osteopenia, a milder form of bone loss, serves as a precursor and elevates fracture risk, with 10-year cumulative fragility fracture incidence at 37.5% in affected postmenopausal women compared to 31.1% in those with normal BMD.[34] Lifestyle factors further compromise bone integrity through modifiable behaviors that impair BMD accrual or maintenance. Sedentary behavior contributes to muscle weakness and reduced bone loading, thereby heightening fracture susceptibility by negatively impacting BMD.[35] Poor nutrition, particularly deficiencies in calcium and vitamin D, weakens bone structure; low vitamin D levels are linked to decreased BMD and increased fracture risk, while inadequate calcium intake exacerbates this vulnerability.[36] Smoking accelerates bone loss and increases fracture risk, with heavy smokers facing up to 70% higher risk.[37] Excessive alcohol consumption accelerates bone loss and increases fracture risk, independent of other factors.[37] Certain medical conditions and treatments inherently elevate fracture propensity by disrupting bone metabolism. Rheumatoid arthritis (RA) doubles the risk of hip and vertebral fractures due to chronic inflammation and associated corticosteroid therapy.[38] Hyperparathyroidism promotes excessive bone resorption through elevated parathyroid hormone levels, leading to secondary osteoporosis and heightened fragility fracture rates.[39] Long-term corticosteroid use, common in inflammatory conditions, induces rapid bone loss, with up to 50% of users developing fractures, particularly vertebral and hip, within months of initiation.[40] Genetic influences play a foundational role in fracture susceptibility by modulating bone mass and quality. A family history of fractures doubles the risk among descendants, independent of BMD, as genetic factors account for 60-85% of BMD variability.[41] In rare cases, mutations in collagen type I alpha 1 genes, as seen in osteogenesis imperfecta, severely impair bone matrix formation, resulting in extreme fragility and recurrent fractures from minimal trauma.[42] Recent data indicate that SARS-CoV-2 infection induces persistent inflammation and immobility, leading to bone loss and an approximately 22% increased fragility fracture risk (HR 1.22) in affected populations, particularly older adults with preexisting vulnerabilities, as of 2024.[43]Clinical Presentation
Signs and Symptoms
Bone fractures typically present with sudden, severe pain that is localized to the injury site and intensifies with any movement or pressure on the affected area.[9] Swelling and bruising often develop rapidly, within hours of the injury, due to the formation of a hematoma from bleeding at the fracture site.[6] Deformity may be evident, such as angulation or shortening of the limb, along with loss of normal function, including inability to bear weight or move the area effectively.[44] Crepitus, a grating sensation or sound produced by bone fragments rubbing together, can occur when attempting to move the injured part.[45] In open fractures, where the bone pierces the skin, systemic signs include visible bleeding and exposed bone, which heighten the risk of infection if not addressed promptly.[46] Variations in presentation depend on the fracture location; for instance, a Colles' fracture of the distal radius often results in a characteristic "dinner fork" deformity, where the wrist appears bent backward with dorsal angulation.[47] If left untreated, these initial signs can progress to more severe issues, underscoring the need for timely medical evaluation.[9]Immediate Complications
Immediate complications of bone fractures encompass acute adverse events that can arise shortly after injury, potentially threatening limb viability and systemic stability if not promptly addressed. Neurovascular compromise represents a critical immediate risk, particularly in fractures of the extremities where swelling or direct trauma can impair blood flow and nerve function. Compartment syndrome, a hallmark of this compromise, occurs when intracompartmental pressure exceeds 30 mmHg, leading to ischemia of muscles and nerves within the fascial envelope.[48] This condition is most common following tibial or forearm fractures, with pressures approaching or surpassing diastolic blood pressure causing irreversible tissue damage within hours.[49] Arterial injuries further exacerbate neurovascular threats; for instance, popliteal artery disruption is a well-documented sequela of proximal tibial fractures, often resulting from traction or direct laceration during high-energy trauma, with rates approaching 5-10% in such cases.[50] These vascular insults can manifest as absent distal pulses or cool extremities, necessitating urgent vascular imaging and intervention to prevent amputation.[51] Hemorrhage constitutes another profound immediate complication, especially in fractures involving vascular-rich regions like the pelvis, where disruption of venous plexuses or arteries can lead to significant blood loss. In unstable pelvic fractures, hemorrhage volumes can reach 1-2 liters, contributing to hemorrhagic shock in up to 10-20% of severe cases, with venous bleeding accounting for approximately 85-90% of the total.[52] This rapid exsanguination arises from the retroperitoneal space's capacity to accommodate large volumes, often exceeding 1.5 liters before tamponade effects, and is a leading cause of early mortality in polytrauma patients.[53] In open fractures, where bone protrudes through the skin, infection emerges as an immediate concern due to bacterial contamination from the external environment. The Gustilo-Anderson classification stratifies this risk: type I fractures (clean wounds <1 cm) carry a low infection rate of 0-2%, while type II (wounds 1-10 cm without extensive damage) range from 2-5%; type III fractures, characterized by extensive soft-tissue injury, contamination, or vascular compromise, exhibit risks escalating to 10-50%, particularly in IIIB subtypes with periosteal stripping.[54] Factors such as wound contamination level and delay in debridement amplify this peril, with gram-negative and anaerobic organisms predominant in high-grade cases.[55] Fat embolism syndrome (FES) poses a systemic immediate complication primarily after fractures of long bones, such as the femur or tibia, where marrow fat globules enter the circulation. Symptoms typically onset within 24-72 hours post-injury, including petechial rash on the conjunctiva or axillae, acute respiratory distress with hypoxemia, and neurological alterations like confusion or seizures, fulfilling Gurd's criteria in 1-5% of at-risk patients.[56] This triad arises from pulmonary vascular occlusion and cerebral embolization, with early recognition vital to mitigate multi-organ involvement.[57] Recent studies as of 2025 have heightened awareness of thromboembolic events as an underrecognized immediate sequela following lower limb fractures, with deep vein thrombosis (DVT) incidence approximating 10-20% in immobilized patients, driven by venous stasis and endothelial injury.[58] Lower extremity fractures, particularly of the femur or tibia, elevate pulmonary embolism risk within the first week, underscoring the need for early thromboprophylaxis in high-risk cohorts.[59]Pathophysiology
Fracture Healing Mechanisms
Fracture healing is a dynamic biological process that restores bone integrity following injury, involving coordinated cellular and molecular events to regenerate tissue structurally and functionally similar to the original bone. This regenerative capacity relies on the activation of resident stem cells, inflammatory responses, and biomechanical influences at the fracture site. The process typically progresses through distinct but overlapping stages, modulated by local factors such as vascularity and mechanical stability.[6] The initial stage, hematoma formation, occurs within days 1-5 after fracture, when disrupted blood vessels lead to bleeding and clot formation at the site, providing a scaffold rich in growth factors and hematopoietic cells that initiates repair. This is followed by the inflammatory phase (weeks 1-3), characterized by the influx of inflammatory cells like macrophages and neutrophils, which clear debris and release cytokines to recruit mesenchymal stem cells (MSCs) for tissue regeneration; during this period, a soft callus of granulation tissue and fibrocartilage begins to bridge the fracture gap. The repair stage (weeks 3-12) involves the formation of a hard callus through endochondral ossification, where the soft callus is mineralized into woven bone, offering mechanical strength. Finally, the remodeling stage spans months to years, during which osteoclasts resorb excess bone and osteoblasts deposit organized lamellar bone, restoring the bone's original architecture.[60] Key cellular players include osteoclasts, which resorb damaged bone matrix and excess callus during remodeling; osteoblasts, derived from MSCs, that synthesize new bone matrix and express RANKL to regulate osteoclast activity; and chondrocytes, which produce the cartilaginous soft callus and later undergo apoptosis to facilitate ossification. Growth factors such as bone morphogenetic protein-2 (BMP-2) play a pivotal role by promoting the differentiation of MSCs into osteoblasts and chondrocytes, enhancing chondrogenesis and osteogenesis within the callus.[6][61] Fracture healing occurs via two primary pathways: primary (direct) healing, which involves intramembranous ossification without callus formation and requires absolute stability with interfragmentary strain below 2%, as seen in rigidly fixed fractures allowing direct osteonal bridging; and secondary (indirect) healing, the more common pathway involving callus formation through endochondral ossification under relative stability with strain between 2% and 10%, typically achieved with non-rigid immobilization like casts or intramedullary nails.[6][60] Timelines vary based on fracture characteristics and patient factors; simple fractures in young adults often unite in 6-8 weeks, while complex or unstable fractures may extend beyond this due to delayed callus formation. Adequate blood supply is essential, peaking at 2 weeks post-fracture to support angiogenesis and nutrient delivery, with impaired vascularity leading to delayed progression through the stages. Immobilization stability is crucial, as appropriate mechanical loading promotes callus development, whereas excessive motion (>10% strain) can disrupt early repair and lead to nonunion. Smoking can impair these mechanisms by reducing vascularity and delaying union, increasing nonunion risk.[60][6][62]Modifying Influences
Several factors can modify the outcomes of fracture healing by influencing vascular supply, cellular activity, and inflammatory responses. Smoking is a prominent external modifier that impairs bone repair. It reduces vascularity at the fracture site, leading to delayed union and increased risk of nonunion or complications. Studies indicate that fractures in smokers take approximately six weeks longer to heal compared to non-smokers, with nicotine specifically inhibiting osteoblast function and angiogenesis essential for callus formation.[63][62][64] Nutritional status also significantly affects healing timelines. Protein deficiency can prolong the inflammatory phase by limiting the availability of amino acids needed for tissue repair and matrix synthesis. Conversely, adequate vitamin C intake supports collagen cross-linking and synthesis, which are critical for early callus development; deficiency has been linked to delayed healing in both preclinical and clinical settings.[65][66] Certain medications alter healing dynamics through their effects on inflammation and bone turnover. Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit prostaglandin synthesis, which may delay callus formation and increase the risk of nonunion, particularly with prolonged use. Bisphosphonates, commonly prescribed for osteoporosis to enhance bone mineral density, generally do not significantly prolong fracture healing time but carry a risk of atypical fractures with long-term use due to suppressed remodeling.[67][68][69] Comorbidities introduce internal modifiers that impair repair processes. In diabetes, hyperglycemia promotes advanced glycation end-products and chronic inflammation, impairing angiogenesis and prolonging healing by 87%, alongside reduced bone formation. Advanced age contributes through diminished mesenchymal stem cell function and proliferative capacity, leading to slower union rates and higher complication risks independent of other factors.[70][71][72] As of 2025, emerging research highlights the role of biologics in modulating healing among patients with inflammatory diseases. Anti-TNF agents, such as adalimumab, reduce systemic inflammation and may preserve or increase bone mineral density, potentially mitigating delays in fracture repair by countering excessive cytokine-driven resorption in conditions like rheumatoid arthritis.[73][74]Diagnosis
History and Physical Examination
The initial clinical assessment for a suspected bone fracture begins with a detailed patient history to guide the diagnosis and management. Key elements include the mechanism of injury, such as high-energy trauma from motor vehicle accidents, low-energy falls in older adults, or twisting forces in sports-related incidents, which helps predict fracture patterns and associated soft tissue or visceral injuries.[75] The onset, location, quality, and severity of pain should be documented, along with any immediate symptoms like inability to bear weight or use the limb, as these indicate acute structural compromise.[76] Inquiry into comorbidities, such as osteoporosis, diabetes, or malignancy, is essential, as they predispose to fractures or complicate healing, while current medications like anticoagulants or bisphosphonates must be noted for their impact on bleeding risk and bone metabolism.[77] Physical examination proceeds systematically to localize the injury and assess for complications, starting with inspection of the affected area for deformity, swelling, ecchymosis, or skin breaks suggestive of an open fracture.[75] Palpation follows, evaluating for focal tenderness, crepitus (grating sensation from bone ends), or abnormal motion, which confirm bony disruption while avoiding excessive manipulation to prevent further damage.[78] A critical component is neurovascular assessment, involving palpation of distal pulses (e.g., radial, dorsalis pedis), evaluation of capillary refill time, skin temperature and color, sensation to light touch, and motor strength, to detect vascular compromise or nerve injury that could lead to ischemia if untreated.[79] This examination must compare the injured limb to the contralateral side for baseline. Red flags during assessment demand urgent escalation, including open wounds exposing bone (indicating an open fracture with infection risk) or signs of acute compartment syndrome, such as pain disproportionate to the injury, worsening with passive stretch, progressive paresthesia or numbness, pallor, or tense compartments.[48] These findings, particularly in high-risk scenarios like tibial shaft fractures, signal potential limb-threatening emergencies requiring immediate surgical consultation.[80] Standardized clinical decision rules enhance efficiency and reduce unnecessary imaging. The Ottawa Ankle Rules, developed to identify malleolar or midfoot fractures after acute injury, involve checking for bony tenderness at specific sites (e.g., medial malleolus, base of fifth metatarsal) and inability to bear weight; their prospective validation showed near 100% sensitivity (pooled 97.6%) for clinically significant fractures, safely decreasing ankle radiographs by 30-35%.[81] Similar rules exist for the foot, emphasizing load-bearing capacity and tenderness to minimize radiation exposure without missing injuries. Limitations in history and physical examination arise in certain populations, where diagnostic accuracy diminishes. In polytrauma patients, the sensitivity of clinical findings for detecting significant fractures or injuries may be reduced due to distracting injuries, altered mental status, or systemic instability masking localized signs.[82] Similarly, obesity impairs palpation reliability and visualization, with studies indicating reduced accuracy of physical examination for certain orthopedic injuries such as shoulder assessments, often necessitating earlier reliance on adjunctive tests.[83]Imaging and Diagnostic Tests
X-rays remain the cornerstone of initial imaging for suspected bone fractures, typically obtained in two orthogonal views (anteroposterior and lateral) to assess alignment, displacement, and fracture characteristics.[84] This modality demonstrates high sensitivity, ranging from 90% to 95% for detecting most fractures in long bones and extremities, though it may miss subtle or nondisplaced fractures.[85] Advanced imaging techniques are employed when X-rays are inconclusive or for specific fracture types. Computed tomography (CT) provides detailed three-dimensional visualization, particularly useful for complex articular fractures, intra-articular involvement, or spinal fractures where precise assessment of fragment position is critical.[86] Magnetic resonance imaging (MRI) excels in identifying stress or occult fractures not visible on plain radiographs, offering superior soft tissue contrast to evaluate associated ligamentous or marrow edema.[87] Ultrasound serves as a portable, radiation-free option for detecting cortical disruptions in superficial bones, such as the forearm or tibia, and is especially valuable in pediatric or resource-limited settings for initial screening.[88] Laboratory tests complement imaging by assessing associated risks and healing potential. A complete blood count (CBC) helps identify infection or anemia that may complicate fractures, while coagulation profiles, including prothrombin time and partial thromboplastin time, are essential prior to surgical intervention to evaluate bleeding risks.[84] Bone turnover markers, such as bone-specific alkaline phosphatase, provide insights into fracture healing by measuring osteoblast activity, with elevated levels indicating active bone formation during the reparative phase.[89] The American College of Radiology (ACR) Appropriateness Criteria guide modality selection based on clinical suspicion and fracture location; for instance, radiography is rated as usually appropriate for initial evaluation of suspected extremity fractures, with MRI or CT recommended for occult or complex cases.[90] Recent advances as of 2025 include AI-assisted fracture detection algorithms applied to X-rays, which enhance diagnostic accuracy by automating identification of subtle fractures; these tools achieve specificities up to 98% when integrated with radiologist review, reducing missed diagnoses in emergency settings.[91]Classification
By Mechanism and Pattern
Bone fractures are systematically classified by mechanism and pattern to elucidate the injury dynamics, inform prognosis, and optimize treatment strategies, as these categories reflect the biomechanical forces involved and the resulting structural integrity of the bone.[92] This approach distinguishes fractures based on etiology—whether from acute trauma, underlying disease, or repetitive stress—and morphological features, such as the orientation and completeness of the break, which directly influence stability and healing potential.[9] Imaging modalities like X-rays are essential for accurately identifying these patterns, though clinical history provides initial clues to the mechanism.[93]Mechanism-Based Classification
Fractures arising from traumatic mechanisms occur when acute mechanical forces exceed the strength of normal bone, often subdivided into high-energy injuries from severe impacts like motor vehicle collisions or falls from height, and low-energy injuries from minor forces such as simple falls in the elderly.[9] High-energy traumatic fractures typically involve greater soft tissue damage and comminution, leading to higher complication rates, while low-energy ones are more common in osteoporotic bone but generally heal well with conservative management.[92] Pathologic fractures, in contrast, result from minimal or no trauma applied to bone already compromised by underlying conditions, such as metastatic cancer (e.g., from breast or lung primaries), primary bone tumors, or metabolic disorders like osteoporosis, which alter bone biomechanics and reduce healing potential— with union rates as low as 0% in cases of lung carcinoma metastases.[5] Stress fractures develop from repetitive cyclical loading on normal or weakened bone, causing microdamage accumulation and eventual incomplete or complete breaks; they are classified as fatigue fractures in healthy bone (e.g., from athletic overuse) or insufficiency fractures in abnormal bone (e.g., due to osteoporosis), with high-risk sites like the femoral neck requiring prompt intervention to prevent displacement.[94]Pattern-Based Classification
The morphological pattern of a fracture describes the configuration of the break, which correlates with the applied force and guides reduction techniques. Transverse fractures feature a straight line perpendicular to the bone's long axis, often from direct perpendicular loading, resulting in relatively stable fragments if nondisplaced.[9] Oblique fractures occur at an angle to the bone axis, typically from combined axial and bending forces, making them less stable and prone to shortening.[92] Spiral fractures exhibit a twisting pattern around the bone, indicative of rotational torque, and are common in torsional injuries like sports twists, often requiring surgical stabilization due to instability.[9] Comminuted fractures involve the bone shattering into three or more fragments, usually from high-energy impacts, complicating alignment and increasing infection risk if open.[92] Incomplete patterns predominate in children due to bone flexibility; greenstick fractures partially break one cortex while the other bends, resembling a snapped green twig, and torus (or buckle) fractures cause cortical buckling without full disruption, both often managed nonoperatively.[92] Impacted fractures, where one fragment is driven into the opposing end, are frequent in elderly patients with low-energy falls and may appear stable despite compression.[9]Displacement Characteristics
Displacement refers to malalignment of fracture fragments and is quantified to assess severity and need for intervention. Angulation describes an alteration in the bone's normal axis, where the distal fragment tilts relative to the proximal, potentially causing deformity if uncorrected—measured in degrees and direction (e.g., dorsal or volar).[95] Translation, or side-to-side shift, occurs when fragments move laterally relative to each other, expressed as a percentage of bone width (e.g., 50% overlap or complete override), and exceeding full width is termed "off-ended."[96] Rotation involves twisting of the distal fragment around the bone's long axis relative to the proximal, often subtle on imaging but detectable clinically through joint malalignment, and can impair function if significant, as in forearm fractures.[97]Soft Tissue Involvement in Open Fractures
Open fractures, where bone protrudes through the skin, are further classified by soft tissue injury using the Gustilo-Anderson system, which stratifies risk of infection and guides debridement urgency based on wound characteristics and contamination.[98]| Type | Description |
|---|---|
| I | Wound <1 cm long, clean or minimal contamination, low soft tissue damage.[98] |
| II | Wound 1–10 cm long, moderate soft tissue injury without extensive flaps, avulsions, or periosteal stripping.[98] |
| IIIA | Extensive laceration or high-energy trauma with adequate soft tissue coverage despite flaps or contamination.[98] |
| IIIB | Extensive soft tissue damage with periosteal stripping, bone exposure, and massive contamination, often requiring flap coverage.[98] |
| IIIC | Any open fracture with associated vascular injury requiring repair for limb viability.[98] |
By Location and Soft Tissue Involvement
Bone fractures are categorized by their anatomical location within the body, which directly impacts clinical management, potential complications, and healing outcomes due to variations in blood supply, mechanical stresses, and surrounding structures.[99] This approach highlights site-specific risks, such as neurovascular compromise in the extremities or instability in the axial skeleton. Additionally, the degree of associated soft tissue involvement is assessed separately, particularly for closed fractures, to guide surgical timing and infection risk.[100] Periprosthetic fractures, occurring adjacent to orthopedic implants, represent a distinct subset influenced by implant stability and bone quality.[101] In the upper extremity, clavicle fractures are among the most prevalent, comprising up to 10% of all fractures and being the most common in pediatric patients, with midshaft fractures accounting for approximately 80% of cases due to their central location under mechanical stress during falls.[102] These often result in cosmetic deformity but rarely cause long-term disability if managed conservatively. Proximal humerus fractures, particularly at the surgical neck, frequently occur in older adults from low-energy falls and involve the metaphyseal region just below the humeral head, potentially disrupting rotator cuff attachments and leading to shoulder instability.[103] Distal radius fractures are the most common upper extremity injury in adults over 50, with Colles' fractures featuring dorsal angulation and displacement of the distal fragment, typically from outstretched hand falls, while Smith's fractures exhibit volar displacement and are often linked to direct impacts or flexion forces.[47][104] Lower extremity fractures carry significant morbidity due to weight-bearing demands. Femur fractures are critical, with neck fractures common in the elderly from osteoporosis-related falls, disrupting the femoral head's blood supply and risking avascular necrosis, whereas shaft fractures arise from high-energy trauma and involve the diaphysis, often requiring intramedullary nailing for stabilization.[105][106] Tibia and fibula fractures, particularly bimalleolar ankle fractures, involve both the medial malleolus of the tibia and lateral malleolus of the fibula, compromising ankle mortise stability and frequently necessitating surgical fixation to prevent chronic instability or arthritis.[107] These distal fractures highlight the interplay between bone and ligamentous structures in the lower leg.[108] Axial skeleton fractures affect core stability and visceral protection. Spinal compression fractures primarily involve vertebral body collapse, most often in the thoracolumbar region due to osteoporosis or trauma, leading to height loss and potential kyphosis; they are graded by severity using the Genant system, where grade 1 indicates 20-25% height reduction and grade 3 exceeds 40%.[109] Pelvic fractures, including acetabular fractures, disrupt the hip socket formed by the ilium, ischium, and pubis, often from high-impact events, with implications for pelvic ring integrity and hip joint function; the Judet-Letournel classification delineates elementary patterns like posterior wall fractures from associated ones involving both columns.[110][111] Soft tissue involvement modifies fracture severity, especially in closed injuries, where the Tscherne classification grades damage from 0 to 3 based on trauma energy and tissue compromise. Grade 0 denotes minimal soft tissue injury from indirect forces with a simple fracture pattern, suitable for immediate fixation. Grade 1 involves superficial abrasions or contusions from indirect violence with moderate fracture severity. Grade 2 features deep contaminated abrasions and potential compartment syndrome from direct forces, requiring delayed intervention. Grade 3 encompasses high-energy crush injuries with extensive muscle damage, vascular issues, or nerve injury, often mandating staged reconstruction or amputation consideration.[100] This system underscores soft tissue's prognostic role beyond bony disruption.[112] Periprosthetic fractures occur near joint implants, complicating revision due to altered biomechanics and bone stock. In the hip, the Vancouver classification subtypes them as type A (trochanteric), type B (stem region: B1 with well-fixed prosthesis, B2 with loose stem but intact bone, B3 with loose stem and deficient bone), and type C (distal to prosthesis), guiding choices between fixation and revision arthroplasty.[113] These fractures, increasingly common with aging populations and implant longevity, emphasize preoperative implant assessment for optimal outcomes.[101]Management
Initial Stabilization and Pain Control
In the initial management of bone fractures, particularly in polytrauma scenarios, the Advanced Trauma Life Support (ATLS) protocol prioritizes the primary survey addressing airway, breathing, and circulation (ABCs) to stabilize the patient before focusing on fracture-specific interventions. Airway patency must be secured with cervical spine immobilization in blunt trauma cases to prevent secondary neurologic injury, while breathing is assessed to ensure oxygenation (target SpO₂ >93%) and ventilation, addressing issues like tension pneumothorax via needle decompression if present. Circulation involves controlling hemorrhage through direct pressure, tourniquets for extremity bleeding, or pelvic binders for unstable pelvic fractures, with permissive hypotension (systolic blood pressure 80-90 mmHg) until bleeding is controlled to minimize further blood loss.[114] Following ABC stabilization, immobilization is essential to reduce pain, prevent further soft tissue damage, and control bleeding. For upper extremity fractures such as those of the forearm, a sugar-tong splint is applied with the elbow at 90 degrees, forearm neutral, and wrist in slight extension to immobilize the radius, ulna, and wrist while allowing elbow flexion; this technique stabilizes without restricting circulation and is used temporarily until definitive care. In lower extremity injuries like mid-shaft femur fractures, traction splinting is recommended to align the fracture, alleviate pain, and improve alignment during transport, applied by securing an ankle hitch and providing inline traction counteracted by a pubic or ischial strap.[115][116] Pain management employs a multimodal approach to optimize relief while minimizing opioid use. Intravenous morphine, titrated at 0.05-0.1 mg/kg every 5-15 minutes to effect, is a cornerstone for severe pain in opioid-naive adults, often combined with intravenous acetaminophen (1 g every 6 hours) for synergistic analgesia without increasing adverse effects. Regional nerve blocks, such as femoral nerve blocks for femur fractures, provide targeted relief by interrupting sensory pathways, reducing systemic opioid requirements by up to 50% in acute settings. For open fractures, initial care includes urgent irrigation with at least 3 liters of normal saline to reduce bacterial load, followed by intravenous antibiotics like cefazolin 2 g within 1 hour of injury to cover gram-positive organisms and lower infection risk, and urgent surgical debridement within 24 hours to remove necrotic tissue and contaminants.[117][118][119] Ongoing monitoring for compartment syndrome is critical post-immobilization, involving serial clinical examinations every 1-2 hours in high-risk fractures (e.g., tibia or forearm) to detect early signs like disproportionate pain on passive stretch, tense swelling, or paresthesia. These exams guide the need for intracompartmental pressure measurement if clinical suspicion persists, as pressures exceeding 30 mmHg warrant emergent fasciotomy to prevent irreversible muscle necrosis.[48]Definitive Treatment Options
Definitive treatment for bone fractures aims to restore anatomical alignment, promote union, and enable functional recovery through either conservative or surgical approaches, selected based on fracture stability, displacement, and patient factors. Conservative management is preferred for stable, minimally displaced fractures, while surgical intervention is indicated for unstable or significantly displaced cases to prevent complications like malunion.[120][121] Conservative TreatmentsFor stable fractures, such as buckle (torus) fractures in children, immobilization with casting is the standard approach, allowing natural healing without surgical intervention. These compression injuries of the metaphysis are inherently stable and typically unite within 3-4 weeks under a short-arm cast or splint. Functional bracing, which permits controlled motion while providing support, is another option for select upper extremity fractures like distal radius buckle types, reducing stiffness compared to rigid casting.[122][123][124] Surgical Treatments
Open reduction and internal fixation (ORIF) involves surgical realignment of fracture fragments followed by stabilization using plates and screws, indicated for displaced fractures where closed methods fail to achieve alignment. This technique is widely used for periarticular fractures, such as those of the distal radius or proximal humerus, to restore joint congruity and prevent deformity. Intramedullary (IM) nailing is the gold standard for diaphyseal fractures of long bones like the femur and tibia, where a metal rod is inserted into the medullary canal to provide axial stability and load-sharing. For open or infected fractures, external fixation uses pins connected to an external frame to stabilize the bone temporarily or definitively, minimizing soft tissue disruption and infection risk in contaminated wounds.[125][126][127] Site-specific considerations guide treatment selection; for example, in elderly patients with displaced femoral neck fractures, arthroplasty (hemi- or total hip replacement) is often preferred over internal fixation to address comminution and reduce reoperation rates. Similarly, percutaneous pinning or screw fixation is recommended for scaphoid waist fractures to maintain alignment and promote vascularity in this avascular-prone bone.[128][129][130] Advanced Techniques
As of 2025, 3D-printed implants offer personalized solutions for complex fractures, enabling custom-fit titanium plates or scaffolds that match patient anatomy and enhance integration with host bone. Biologic augments, such as recombinant human bone morphogenetic protein (BMP-7 or BMP-2), are used adjunctively for non-unions, stimulating osteogenesis in recalcitrant cases with union rates exceeding 80% when combined with fixation.[131][132][133] Treatment decisions hinge on factors like displacement greater than 2 mm or instability, where conservative approaches risk malunion. Randomized controlled trials (RCTs) demonstrate that surgical fixation reduces malunion rates compared to conservative management, particularly in long bone shaft fractures, with operative groups showing up to 50% lower incidence in select pediatric and adult cohorts.[120][134][135]
Rehabilitation and Follow-Up
Rehabilitation following definitive treatment of a bone fracture focuses on restoring function, preventing stiffness, and ensuring proper healing through structured therapy and ongoing monitoring. The process is tailored to the fracture type, location, and patient factors, with timing aligned to the biological stages of healing, such as the transition from inflammatory to reparative phases.[6] Physical therapy is a cornerstone of recovery, beginning with gentle range-of-motion exercises as early as the first week after immobilization or surgery to maintain joint mobility and reduce the risk of contractures.[136] These exercises progress to strengthening protocols around 6 weeks, once initial callus formation provides stability, incorporating resistance training to rebuild muscle mass and support bone loading.[137] Follow-up care includes serial radiographic imaging, typically X-rays at 2, 6, and 12 weeks post-treatment, to evaluate fracture alignment, callus formation, and progression toward union.[138] This monitoring helps detect deviations early, guiding adjustments to weight-bearing or therapy intensity. Complications are vigilantly assessed during follow-up, with malunion—defined as healing with angular deformity exceeding 10 degrees—potentially leading to functional impairment if not addressed.[139] Non-union, characterized by failure of the fracture to consolidate by 6 months despite adequate treatment, requires intervention to stimulate healing.[140] Return to daily activities and sports follows progressive weight-bearing protocols, starting with partial loading under supervision and advancing as radiographic and clinical evidence confirms stability; most patients achieve full recovery within 3 to 6 months.[141] A multidisciplinary team enhances outcomes, with occupational therapists aiding in regaining independence in activities of daily living through adaptive strategies and functional training.[142] Psychological support is integrated to address chronic pain, anxiety, or adjustment issues, promoting adherence to rehabilitation and overall well-being.[143]Prevention
Bone Health Promotion
Promoting bone health through lifestyle and medical interventions is essential for reducing the risk of fractures associated with low bone mineral density (BMD). Adequate nutrition, regular physical activity, screening, pharmacotherapy, and public health initiatives form the cornerstone of these strategies, targeting intrinsic bone strength in at-risk populations such as postmenopausal women and older adults.[144] Nutritional strategies emphasize sufficient intake of calcium and vitamin D, which are critical for bone mineralization and remodeling. The recommended daily calcium intake for adults is 1,000 mg for those aged 19-50 years, 1,000 mg for men 51-70 years, 1,200 mg for women 51-70 years, and 1,200 mg for adults over 70 years, primarily from dietary sources like dairy products (e.g., milk, yogurt, cheese) and leafy green vegetables (e.g., kale, broccoli).[145] Vitamin D intake should be at least 800 international units (IU) per day for postmenopausal women at increased fracture risk, supporting calcium absorption and bone health; sources include fortified foods and sunlight exposure, with supplementation advised if dietary intake is insufficient.[146] These nutrients work synergistically to maintain BMD, with deficiencies linked to higher osteoporosis rates.[147] Weight-bearing exercises are a key non-pharmacological approach to enhance bone density. Activities such as brisk walking for at least 30 minutes daily, combined with higher-impact exercises, can help maintain or modestly increase BMD when performed regularly, with evidence showing small gains in bone health markers among postmenopausal women.[148] Resistance training, like weightlifting, complements this by further promoting bone formation through mechanical loading, with studies showing sustained gains over 2 years when combined with adequate calcium.[149] Consistency is vital, as benefits accrue gradually and diminish without ongoing practice.[150] Screening with dual-energy X-ray absorptiometry (DEXA) scans identifies individuals at high fracture risk by measuring BMD at the hip and spine. A T-score of -2.5 or lower indicates osteoporosis, warranting intervention, while scores between -1.0 and -2.5 suggest osteopenia.[151] Guidelines recommend DEXA screening for all women aged 65 years and older, and earlier for those with risk factors like family history or glucocorticoid use.[152] Early detection allows for targeted prevention, with screening linked to a 6-17% reduction in fractures based on clinical trials.[153] For those diagnosed with osteoporosis, pharmacotherapy such as bisphosphonates provides robust fracture risk reduction. Alendronate, administered at 70 mg weekly, inhibits osteoclast activity to preserve BMD and has been shown to reduce hip fracture risk by approximately 50% in women with low bone density.[154] This treatment is particularly effective in postmenopausal women, with benefits evident within 1-3 years of initiation.[155] Monitoring for side effects like gastrointestinal issues is recommended, alongside calcium and vitamin D co-supplementation.[156] Public health efforts amplify individual strategies through fortification programs and updated guidelines. Food fortification with calcium, such as in plant-based milks and orange juice, addresses dietary gaps in diverse populations, including vegans.[157] Public health efforts promote diverse calcium sources, including fortified plant-based options like almond milk and tofu, to address dietary needs in various populations. These initiatives aim to improve access to nutrient-rich options to support bone health.[158]Trauma Avoidance Strategies
Fall prevention strategies are essential for reducing the incidence of fractures, particularly among older adults who are at higher risk due to diminished balance and mobility. Implementing home modifications such as installing grab bars in bathrooms and along stairways provides crucial support during transitions, significantly lowering the risk of slips and subsequent falls that often result in hip or wrist fractures. Studies indicate that tailored home hazard modifications, including grab bars and improved lighting, can reduce the rate of falls by approximately 21% in community-dwelling older adults.[159] Complementing these environmental changes, balance training programs—such as tai chi or structured exercises focusing on postural stability—enhance proprioception and muscle strength, thereby decreasing fall rates by about 23% among elderly participants according to high-certainty evidence from multiple trials.[160] In sports and recreational activities, protective equipment plays a pivotal role in mitigating fracture risks from high-impact collisions or falls. Helmets, pads, and braces are standard in activities like skiing, cycling, and contact sports to absorb forces that could otherwise cause skull, clavicle, or long-bone fractures. For instance, wrist guards in snowboarding substantially decrease the likelihood of distal radius fractures by up to 77%, as evidenced by systematic reviews of injury data.[161] Similarly, knee braces for individuals with prior ligament issues in skiing help stabilize the joint, reducing the overall incidence of lower extremity injuries, including tibial fractures, during dynamic maneuvers.[162] Occupational settings, especially construction, demand rigorous adherence to ergonomics and personal protective equipment (PPE) to avert fractures from falls, struck-by incidents, or overexertion. Ergonomic practices, such as proper lifting techniques and workstation adjustments, minimize awkward postures that contribute to slips or structural failures leading to limb fractures. The use of PPE like hard hats, safety harnesses, and steel-toed boots in construction helps reduce the risk of injuries, including fractures, by providing protection against common hazards.[163] Road safety measures significantly curb fractures from motor vehicle accidents, which remain a leading cause of traumatic bone injuries. Seatbelts, when used correctly, lower the risk of severe injuries—including chest, pelvic, and extremity fractures—by approximately 50% by preventing ejection or impact with vehicle interiors. Airbags further enhance this protection; frontal airbags combined with seatbelts reduce the risk of serious injury by up to 50% in compatible crash scenarios, particularly for upper body fractures. Enforcing speed limits complements these, as lower velocities correlate with decreased fracture severity in collisions.[164][165] Community-level programs promote broader trauma avoidance through urban planning and public education initiatives. In 2025, the World Health Organization's UN Global Road Safety Week emphasizes infrastructure improvements like pedestrian-friendly crosswalks, bike lanes, and traffic calming measures to minimize collisions that result in lower limb fractures among vulnerable road users. These efforts, integrated into global strategies, aim to foster safer environments and reduce pedestrian trauma by prioritizing walkability and enforcement of safety norms.[166]Special Populations
Pediatric Fractures
Pediatric fractures represent 10-25% of all childhood injuries, with an overall incidence of approximately 20 per 1,000 children annually.[167] These injuries are particularly common in active children, where the porous and flexible nature of immature bone leads to distinct patterns such as torus or buckle fractures, which occur due to the bone's plasticity under compressive forces, often in the distal radius.[168] Falls from low heights account for a significant portion of these cases, with upper extremity fractures predominating.[169] Children exhibit notable advantages in bone healing compared to adults, primarily due to active growth plates and robust periosteal blood supply, enabling faster union times of 3-7 weeks in younger patients depending on age and fracture location.[170] Additionally, the remodeling potential in pediatric bones can reach up to 100% in young children, particularly for diaphyseal fractures, allowing angular deformities to correct over time through physeal growth and Wolff's law.[170] Unique fracture types in children include plastic deformation, a bowing injury without a complete break, most often seen in the forearm bones due to their lower mineral content and ability to absorb energy elastically.[171] Growth plate injuries are classified using the Salter-Harris system, which describes five types: Type I (transverse through the physis), Type II (metaphyseal extension, most common at ~75%), Type III (epiphyseal intra-articular), Type IV (transverse through all layers), and Type V (crush injury), each carrying varying risks of growth disturbance.[172] Management of pediatric fractures emphasizes nonoperative approaches to capitalize on healing potential, with closed reduction and casting preferred over surgical intervention to minimize risks like infection or growth plate damage.[173] Remanipulation under anesthesia may be required if initial alignment is lost, but hardware such as pins or plates is avoided when possible, especially near growth plates, to prevent premature physeal closure or arrest.[173] Long-term complications include a risk of avascular necrosis in the femoral head following certain fractures, such as displaced femoral neck injuries, which can mimic or lead to conditions like Legg-Calvé-Perthes disease through disrupted blood supply and subsequent bone collapse.[174]Geriatric Fractures
Geriatric fractures, often classified as fragility fractures, predominantly affect individuals aged 65 years and older due to age-related bone loss from osteoporosis and increased susceptibility to low-energy trauma. Approximately 87% to 96% of all hip fractures occur in patients aged 65 or older, with women comprising the majority of cases owing to postmenopausal osteoporosis. The lifetime risk of sustaining a hip fracture from age 50 is estimated at approximately 1 in 6 for women and 1 in 17 for men.[175] These risks escalate sharply with advancing age; for instance, women over 85 face a particularly high burden, with incidence rates reaching up to 3,032 per 100,000 person-years. These fractures impose a substantial socioeconomic strain, with over 250,000 hip fractures annually in the United States alone among those 65 and older, frequently leading to hospitalization and long-term care needs. Common fragility fractures in this population include hip fractures, vertebral compression fractures, and distal radius fractures, typically resulting from minimal trauma such as falls from standing height. Vertebral compression fractures, for example, often present insidiously with back pain and height loss, affecting up to 20% of postmenopausal women and contributing to kyphosis and reduced mobility. Osteoporosis underlies most cases, exacerbated by comorbidities like diabetes, cardiovascular disease, and chronic kidney disease, which impair bone quality and increase fall risk. Management must account for frailty, with modified approaches such as comprehensive geriatric assessments to address these factors. Challenges in treating geriatric fractures include delayed presentation due to atypical symptoms or cognitive impairment, which can complicate timely diagnosis and increase complication risks. Polypharmacy, prevalent in over 50% of older adults, interacts adversely with analgesics and anticoagulants, heightening bleeding or sedation risks during perioperative care. Bone healing is also prolonged, often taking twice as long as in younger adults—up to 16 weeks or more for union—due to reduced osteogenic potential, vascularity, and stem cell activity influenced by chronic inflammation ("inflamm-aging"). These issues necessitate tailored strategies, such as cement-augmented fixation for osteoporotic bone to enhance screw stability and reduce failure rates in proximal humerus or femoral fractures, as supported by studies showing improved mechanical outcomes in high-risk patients. Early mobilization within 24-48 hours post-surgery is a cornerstone of management to mitigate complications like delirium, which affects up to 50% of hip fracture patients and prolongs hospital stays. Protocols emphasizing multidisciplinary orthogeriatric care promote ambulation to preserve function and prevent secondary issues such as pneumonia or deep vein thrombosis. Outcomes remain guarded, with 1-year mortality rates ranging from 20% to 30% following hip fractures, driven by frailty, comorbidities, and postoperative complications. Recent advancements as of 2025 emphasize frailty indices, such as the modified Frailty Index (mFI-5) or the HIP-G Index, for prognostication, enabling risk stratification and personalized interventions to improve survival and quality of life. Prevention through bone health promotion, including calcium/vitamin D supplementation and antiresorptive therapies, remains essential to reduce incidence in this vulnerable group.References
- https://wikimsk.org/wiki/Bone_Biomechanics
- https://www.[academia.edu](/page/Academia.edu)/1792569/Biomechanics_of_bone_trauma

