Recent from talks
Nothing was collected or created yet.
Epilepsy
View on Wikipedia
| Epilepsy | |
|---|---|
| Other names | Seizure disorder Neurological disability |
 | |
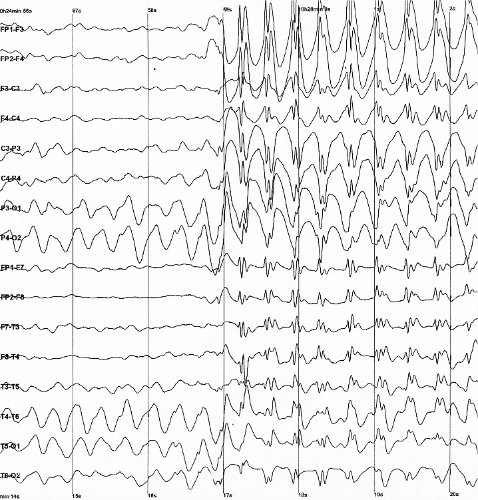
| Generalized 3 Hz spike-and-wave discharges on an electroencephalogram | |
| Specialty | Neurology |
| Symptoms | Periods of loss of consciousness, abnormal shaking, staring, change in vision, mood changes and/or other cognitive disturbances [1] |
| Duration | Long term[1] |
| Causes | Unknown, brain injury, stroke, brain tumors, infections of the brain, birth defects[1][2][3] |
| Diagnostic method | Electroencephalogram, ruling out other possible causes[4] |
| Differential diagnosis | Fainting, alcohol withdrawal, electrolyte problems[4] |
| Treatment | Medication, surgery, neurostimulation, dietary changes[5][6] |
| Prognosis | Controllable in 69%[7] |
| Frequency | 51.7 million/0.68% (2021)[8] |
| Deaths | 140,000 (2021)[9] |
Epilepsy is a group of neurological disorders characterized by a tendency for recurrent, unprovoked seizures.[10] A seizure is a sudden burst of abnormal electrical activity in the brain that can cause a variety of symptoms, ranging from brief lapses of awareness or muscle jerks to prolonged convulsions.[1] These episodes can result in physical injuries, either directly, such as broken bones, or through causing accidents. The diagnosis of epilepsy typically requires at least two unprovoked seizures occurring more than 24 hours apart.[11] In some cases, however, it may be diagnosed after a single unprovoked seizure if clinical evidence suggests a high risk of recurrence.[10] Isolated seizures that occur without recurrence risk or are provoked by identifiable causes are not considered indicative of epilepsy.[12]
The underlying cause is often unknown,[11] but epilepsy can result from brain injury, stroke, infections, tumors, genetic conditions, or developmental abnormalities.[13][2][3] Epilepsy that occurs as a result of other issues may be preventable.[1] Diagnosis involves ruling out other conditions that can resemble seizures, and may include neuroimaging, blood tests, and electroencephalography (EEG).[4]
Most cases of epilepsy — approximately 69% — can be effectively controlled with anti-seizure medications,[7] and inexpensive treatment options are widely available. For those whose seizures do not respond to drugs, other approaches, such as surgery, neurostimulation or dietary changes, may be considered.[5][6] Not all cases of epilepsy are lifelong, and many people improve to the point that treatment is no longer needed.[1]
As of 2024[update], approximately 50 million people worldwide have epilepsy, with nearly 80% of cases occurring in low- and middle-income countries.[1] The burden of epilepsy in low-income countries is more than twice that in high-income countries, likely due to higher exposure to risk factors such as perinatal injury, infections, and traumatic brain injury, combined with limited access to healthcare.[14] In 2021, epilepsy was responsible for an estimated 140,000 deaths, an increase from 125,000 in 1990.[9]
Epilepsy is more common in both children and older adults.[15][16] About 5–10% of people will have an unprovoked seizure by the age of 80.[17] The chance of experiencing a second seizure within two years after the first is around 40%.[18][19]
People with epilepsy may be treated differently in various areas of the world and experience varying degrees of social stigma due to the alarming nature of their symptoms.[11][20] In many countries, people with epilepsy face driving restrictions and must be seizure-free for a set period before regaining eligibility to drive.[21] The word epilepsy is from Ancient Greek ἐπιλαμβάνειν, 'to seize, possess, or afflict'.[22]
Signs and symptoms
[edit]
Epilepsy is characterized by a long-term tendency to experience recurrent, unprovoked seizures.[23] They may vary widely in their presentation depending on the affected brain regions, age of onset, and type of epilepsy.[24]
Seizures
[edit]According to the 2025 classification by the International League Against Epilepsy (ILAE), seizures are grouped into four main classes: focal, generalized, unknown (whether focal or generalized), and unclassified.[25]
Focal seizures
[edit]Focal seizures originate in one area of the brain and may involve localized or distributed networks.[23] For a given seizure type, the site of onset tends to be consistent across episodes. Once initiated, the seizure may remain localized or spread to adjacent areas, and in some cases, may propagate to the opposite hemisphere (contralateral spread).[25]
They are further classified based on the state of consciousness during the episode:[25]
- Focal preserved consciousness seizure: the person remains aware and responsive.
- Focal impaired consciousness seizure: awareness and/or responsiveness are affected.
Experiences known as auras often precede focal seizures.[26] The seizures can include sensory (visual, hearing, or smell), psychic, autonomic, and motor phenomena depending on which part of the brain is involved.[23][27] Muscle jerks may start in a specific muscle group and spread to surrounding muscle groups, a pattern known as a Jacksonian march.[28] Automatisms, or non-consciously generated activities, may occur; these may be simple repetitive movements like smacking the lips or more complex activities such as attempts to pick up something.[28] Some focal seizures can evolve into focal-to-bilateral tonic-clonic seizures, where abnormal brain activity spreads to both hemispheres.[25]
Generalized seizures
[edit]Generalized seizures originate at a specific point within, and quickly spread across both hemispheres through interconnected brain networks. Although the spread is rapid, the onset may appear asymmetric in some cases. These seizures typically impair consciousness from the outset and can take several forms, including:[25]
- Generalized tonic–clonic seizures, often with an initial tonic phase followed by clonic jerking;
- Absence seizures, which may present with eye blinking or automatisms;
- Other generalized seizures, a category that includes tonic, clonic, myoclonic, atonic seizures and epileptic spasms.
Tonic–clonic seizures are among the most recognizable seizure types, typically involving sudden loss of consciousness, stiffening (tonic phase), and rhythmic jerking (clonic phase) of the limbs.[29] This form of seizure — whether focal to bilateral, generalized, or of unknown onset — is given particular emphasis due to their clinical severity; they are associated with the highest risk of injury, medical complications, and sudden unexpected death in epilepsy (SUDEP).[25]
Myoclonic seizures involve sudden, brief muscle jerks, which may affect specific muscle groups or the whole body.[30][31] They can cause falls and injury.[30] Absence seizures are characterized by brief lapses in awareness, sometimes accompanied by subtle movements such as blinking or slight head turning.[2] The person typically recovers immediately afterward without confusion. Atonic seizures involve a sudden loss of muscle tone, often resulting in falls.[26]
Triggers and reflex seizures
[edit]Certain external or internal factors may increase the likelihood of a seizure in individuals with epilepsy. These triggers do not cause epilepsy but can lower the seizure threshold in people who are already susceptible. Common triggers include sleep deprivation, stress, fever, illness, menstruation, alcohol, and certain medications. These do not cause seizures by themselves, but lower the threshold in people who are already susceptible.[32][33][34][35]
A small subset of individuals have reflex epilepsy, in which seizures are reliably provoked by specific stimuli. These reflex seizures account for about 6% of epilepsy cases.[36][37] Common triggers include flashing lights (photosensitive epilepsy), sudden sounds, or specific cognitive tasks such as reading or performing calculations. In some epilepsy syndromes, seizures occur more frequently during sleep or upon awakening.[38][39]
Seizure clusters
[edit]Seizure clusters refer to multiple seizures occurring over a short period of time, with incomplete recovery between events. They are distinct from status epilepticus, though the two may overlap. Definitions vary across studies, but seizure clusters are typically described as two or more seizures within 24 hours or a noticeable increase in seizure frequency over a person's usual baseline. Estimates of their prevalence range widely — from 5% to 50% of people with epilepsy — largely due to differing definitions and populations studied.[40][41] Seizure clusters are more common in individuals with drug-resistant epilepsy, high baseline seizure frequency, or certain epilepsy syndromes.[42] They are associated with increased emergency care utilization, worse quality of life, impaired psychosocial functioning, and possibly elevated risk of mortality.[43]
Postictal state
[edit]After the active portion of a seizure (the ictal state) there is typically a period of recovery during which there is confusion, referred to as the postictal state, before a normal level of consciousness returns,[44] lasting minutes to days.[27] This period is marked by confusion, headache, fatigue, or speech and motor disturbances. Some may experience Todd's paralysis, a transient focal weakness.[45] Postictal psychosis occurs in approximately 2% of individuals with epilepsy, particularly after clusters of generalized tonic–clonic seizures.[46][47]
Psychosocial
[edit]Epilepsy can have substantial effects on psychological and social well-being. People with the condition may experience social isolation, stigma, or functional disability, which can contribute to lower educational attainment and reduced employment opportunities. These challenges often extend to family members, who may also encounter stigma and increased caregiving burden.[48]
Several psychiatric and neurodevelopmental disorders are more common in individuals with epilepsy. These include depression, anxiety, obsessive–compulsive disorder (OCD),[49] and migraine.[50] Attention deficit hyperactivity disorder (ADHD) is particularly prevalent among children with epilepsy, occurring three to five times more often than in the general population. ADHD and epilepsy together can markedly affect behavior, learning, and social development.[51] Epilepsy is also more common in children with autism spectrum disorder.[52]
Approximately, one-in-three people with epilepsy have a lifetime history of a psychiatric disorder.[53] This association is thought to reflect a combination of shared neurobiological mechanisms and the psychosocial impact of living with a chronic neurological condition.[54] Some research also suggests that psychiatric conditions such as depression may precede the onset of epilepsy in certain individuals, particularly those with focal epilepsy. However, the nature of this association remains under investigation and may involve shared pathways, diagnostic overlap, or other confounding factors.[55]
Comorbid depression and anxiety are associated with poorer quality of life,[56] increased healthcare utilization, reduced treatment response (including to surgery), and higher mortality.[57] Some studies suggest that these psychiatric conditions may influence quality of life more than seizure type or frequency.[58] Despite their clinical importance, depression and anxiety often go underdiagnosed and undertreated in people with epilepsy.[59]
Causes
[edit]Epilepsy can result from a wide range of genetic and acquired factors, and in many cases, both play a role.[60][61] Acquired causes include serious traumatic brain injury, stroke, brain tumors, and central nervous system infections.[60] Despite advances in diagnostic tools, no clear cause is identified in approximately 50% of cases.[1] The distribution of causes often varies with age. Epilepsies associated with genetic, congenital, or developmental conditions are more common in children, while epilepsy related to stroke or tumors is more frequently seen in older adults.[48]
Seizures may also occur as a direct response to acute health conditions such as stroke, head trauma, metabolic disturbances, or toxic exposures.[62] These are known as acute symptomatic seizures and are distinct from epilepsy, which involves a recurrent tendency to have unprovoked seizures over time.[63]
The International League Against Epilepsy (ILAE) classifies the causes of epilepsy into six broad categories: structural, genetic, infectious, metabolic, immune, and unknown. These categories are not mutually exclusive, and more than one may apply in an individual case.[64]
Structural
[edit]Structural causes of epilepsy refer to abnormalities in the anatomy of the brain that increase the risk of seizures. These may be acquired — such as from a stroke, traumatic brain injury, brain tumor, or central nervous system infection — or developmental and genetic in origin, as seen in conditions like focal cortical dysplasia or certain congenital brain malformations. A major example is mesial temporal sclerosis (MTS), a common cause of temporal lobe epilepsy.[65][64]
Traumatic brain injury is estimated to cause between 6% and 20% of epilepsy cases, depending on severity, mechanism, and study population. Mild brain injury increases the risk about two-fold, while severe brain injury increases the risk seven-fold. In those who have experienced a high-powered gunshot wound to the head, the risk is about 50%.[66] Stroke is a major cause of epilepsy, particularly in older adults.[67] Approximately 6% to 10% of individuals who experience a stroke develop epilepsy, most often within the first few years after the event. The risk is highest following severe strokes that involve cortical regions, especially in cases of intracerebral hemorrhage.[68] Brain tumors are implicated in approximately 4% of epilepsy cases, with seizures occurring in nearly 30% of individuals with intracranial neoplasms.[66]
In clinical practice, a structural cause is typically identified through neuroimaging (such as MRI), which reveals an abnormality that plausibly accounts for the individual's seizure semiology and EEG findings. The lesion must be epileptogenic, meaning that it is capable of generating seizures. Infections like encephalitis or brain abscess may lead to permanent structural damage, increasing the risk of epilepsy even after the infection resolves.[64]
Structural damage can also result from perinatal brain injury, such as hypoxic-ischemic encephalopathy, especially in low- and middle-income countries where access to prenatal and neonatal care may be limited. When seizures are linked to a clearly defined structural lesion, epilepsy surgery may be considered — particularly in individuals whose seizures do not respond to medication.[64]
Genetics
[edit]Genetic causes of epilepsy are those in which a person's genes directly contribute to the development of seizures. This includes cases where a specific mutation has been identified, as well as situations where the family history and clinical features strongly suggest a genetic basis, even if no known mutation is found. In the updated classification by the ILAE, the term genetic replaces the older term idiopathic, to highlight that these epilepsies arise from inherited or spontaneous changes in a person's biology — not from injury or infection.[64]
Genetic factors are believed to contribute to many cases of epilepsy, either directly or by increasing vulnerability to other causes.[69] Some forms are caused by a single gene defect, which account for around 1–2% of cases. However, most are due to a combination of multiple genes and environmental influences.[13] Many of the genes known to play a role in epilepsy affect how brain cells send electrical signals, especially those involved in ion channels, receptors, or signaling proteins.[30]
Genetics is believed to play an important role in epilepsies by a number of mechanisms. Simple and complex modes of inheritance have been identified for some of them. However, extensive screening have failed to identify many single gene variants of large effect.[70] More recent exome and genome sequencing studies have begun to reveal a number of de novo gene mutations that are responsible for some epileptic encephalopathies, including CHD2 and SYNGAP1[71][72][73] and DNM1, GABBR2, FASN and RYR3.[74]
Some genetic disorders, including phakomatoses such as tuberous sclerosis complex and Sturge–Weber syndrome, are strongly associated with epilepsy.[75]
Infectious
[edit]Infectious causes include infections of the central nervous system that directly affect brain tissue and lead to long-term seizure susceptibility.[66] Examples include herpes simplex encephalitis, which carries a high risk of developing epilepsy, and neurocysticercosis, a major preventable cause of epilepsy in endemic regions. Other infections such as cerebral malaria, toxoplasmosis, and toxocariasis.[66]
Immune
[edit]Immune causes include conditions like autoimmune encephalitis, in which the immune system attacks brain tissue, often presenting with seizures. Certain autoimmune epilepsies are associated with specific autoantibodies, including those against the NMDA receptor, LGI1, and CASPR2. These cases often present with rapid-onset, difficult-to-treat seizures.[64]
Celiac disease has also been associated with epilepsy in rare syndromic forms, such as the triad of epilepsy, cerebral calcifications, and celiac disease.[76][77]
Metabolic
[edit]Metabolic causes of epilepsy include metabolic disorders that disrupt the brain's normal function. In rare cases, epilepsy may result from inborn errors of metabolism, such as mitochondrial diseases, urea cycle disorders, or glucose transporter type 1 (GLUT1) deficiency. These often present early in life and may be associated with developmental delays, movement disorders, or other neurological symptoms.[64]
Seizures can also occur in the context of acquired metabolic disturbances, such as hypoglycemia, hyponatremia, or hypocalcemia. These seizures are often considered acute symptomatic seizures, and are not epilepsy.[78]
Some forms of malnutrition, particularly in low- and middle-income countries, have been associated with a higher risk of epilepsy, although it remains unclear whether the relationship is causal or due to other contributing factors.[14]
Unknown
[edit]Unknown causes of epilepsy refer to cases where no clear structural, genetic, infectious, immune, or metabolic origin can be identified despite thorough evaluation. This category acknowledges the limits of current diagnostic techniques and scientific understanding. A substantial proportion of epilepsy cases still fall into this group, particularly in regions with limited access to advanced testing.[64]
Mechanism
[edit]Understanding the mechanism of epilepsy involves two related but distinct questions: how the brain develops a long-term tendency to generate seizures (epileptogenesis), and how individual seizures begin and spread (ictogenesis). While these processes are not yet fully understood, research has identified a number of cellular, molecular, and network-level changes that contribute to each.[79]
Seizures
[edit]In a healthy brain, neurons communicate through electrical signals that are generally desynchronized. This activity is tightly regulated by a balance between excitatory and inhibitory influences. Intracellular factors that influence neuronal excitability include the type, number, and distribution of ion channels, as well as alterations in receptor function and gene expression. Extracellular factors include ionic concentrations in the surrounding environment, synaptic plasticity, and the regulation of neurotransmitter breakdown by glial cells.[80][81]
During a seizure, this balance breaks down, leading to a sudden and excessive synchronization of neuronal firing. A localized group of neurons may begin firing together in an abnormal and repetitive pattern, overwhelming normal inhibitory controls. This abnormal activity can remain confined to a specific region of the brain or propagate to other areas. The process by which this transition occurs is known as ictogenesis. It involves a shift in network dynamics, typically beginning with excessive excitatory activity in a susceptible area of cortex — known as a seizure focus — and failure of inhibitory mechanisms to contain it. At the cellular level, ictogenesis is often marked by a paroxysmal depolarizing shift, a characteristic pattern of sustained neuronal depolarization followed by rapid repetitive firing.[82] As excitatory feedback loops engage and inhibition further declines, the seizure may become self-sustaining and spread to other regions of the brain.[83]
There is evidence that epileptic seizures are usually not a random event. Seizures are often brought on by factors (also known as triggers) such as stress, excessive alcohol use, flickering light, or a lack of sleep, among others. The term seizure threshold is used to indicate the amount of stimulus necessary to bring about a seizure; this threshold is lowered in epilepsy.[84] The seizures can be described on different scales, from the cellular level[85] to the whole brain.[86]
Epilepsy
[edit]While ictogenesis explains how individual seizures arise, it does not account for why the brain develops a persistent tendency to generate them. This longer-term process is known as epileptogenesis — the sequence of biological events that transforms a previously non-epileptic brain into one capable of producing spontaneous seizures. It can occur after a wide range of brain insults, including traumatic brain injury, stroke, central nervous system infections, brain tumors, or prolonged seizures (such as status epilepticus). In most cases, no clear cause is identified. Although not fully understood, it involves a range of biological changes, including neuronal loss, synaptic reorganization, gliosis, neuroinflammation, and disruption of the blood–brain barrier.[79][87]
Together, these changes contribute to the formation of hyperexcitable neural networks, often anchored around a seizure focus. Once established, this pathological network increases the brain's susceptibility to seizures, even in the absence of ongoing injury. Although many of the processes underlying ictogenesis and epileptogenesis have been identified, the exact mechanisms by which the brain transitions into a seizure or becomes epileptic remain unknown.[87]
Diagnosis
[edit]The diagnosis of epilepsy is primarily clinical, based on a thorough evaluation of the person's history, seizure features, and risk of recurrence. Diagnostic tests such as electroencephalograms and neuroimaging can support the diagnosis. Clinicians must also distinguish epileptic seizures from other conditions that can mimic them and determine whether the event was provoked by an acute, reversible cause or if it suggests a long-term tendency for unprovoked seizures.[26][23]
Definition
[edit]According to the International League Against Epilepsy (ILAE), a diagnosis of epilepsy can be made when any one of the following criteria is met:[10]
- At least two unprovoked (or reflex) seizures occurring more than 24 hours apart
- One unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years
- Diagnosis of an epilepsy syndrome
The ILAE also introduced the concept of resolved epilepsy, which applies to individuals who are past the typical age range for an age-dependent syndrome, or who have remained seizure-free for at least 10 years, including the last 5 years without medication.[10]
This 2014 practical definition built upon the broader 2005 conceptual framework, which defined epilepsy as a disorder involving an enduring predisposition to generate epileptic seizures. The updated criteria incorporated recurrence risk and reflected the realities of clinical decision-making. While widely adopted in clinical settings, other definitions—such as the traditional "two unprovoked seizures" rule still used by the World Health Organization — remain appropriate in epidemiology and public health contexts, provided they are clearly stated. The 2014 revision also shifted terminology, referring to epilepsy as a disease rather than a disorder, to reflect its medical seriousness and public health impact.[88][10]
Classification
[edit]
Once epilepsy is diagnosed, the ILAE recommends a three-level framework to guide further classification and management:[64]
- Identify the seizure type, based on clinical features and EEG (e.g., focal aware seizure, generalized absence)
- Determine the epilepsy type, such as focal, generalized, combined, or unknown
- Identify an epilepsy syndrome, if applicable
Not all levels can always be determined; in some cases, only the seizure type is identifiable. The etiology — whether structural, genetic, infectious, metabolic, immune, or unknown — should be considered at each stage of classification, as it often influences treatment and prognosis.[89][64]
The classification of epilepsies has evolved significantly over time.[90] Earlier systems emphasized seizure location and used terms such as "partial" or "cryptogenic," which have been replaced in the modern framework.[91][92] The current system, introduced in 2017, reflects advances in neuroimaging, genetics, and clinical understanding, and allows for a more individualized and dynamic diagnostic approach.[93]
Syndromes
[edit]An epilepsy syndrome is a specific diagnosis based on a combination of features, including seizure types, age of onset, EEG patterns, imaging findings, and associated symptoms or comorbidities. In many cases, a known genetic or structural cause may also support the diagnosis. Recognizing a syndrome can guide treatment decisions, inform prognosis, and provide clarity for individuals and families navigating an epilepsy diagnosis.[94][95]
Some syndromes are self-limited and age-dependent, such as childhood absence epilepsy, juvenile myoclonic epilepsy, and self-limited epilepsy with centrotemporal spikes.[63] These typically respond well to treatment or remit with age. In contrast, more severe syndromes fall under the category of developmental and epileptic encephalopathies (DEEs).[96] These include Lennox–Gastaut syndrome, West syndrome, and Dravet syndrome, which are associated with early onset, drug-resistant seizures, and significant neurodevelopmental impairments.[97]
Some epilepsy syndromes do not yet fit neatly within current etiological categories, particularly when no definitive cause has been identified. In many cases, a genetic cause is presumed based on age of onset, family history, and electroclinical features, even if no mutation has been found. As genetic and neuroimaging technologies continue to evolve, the classification of epilepsy syndromes is expected to become more precise.[89]
Tests
[edit]
The diagnostic evaluation of epilepsy begins with confirming whether the reported event was in fact a seizure. A detailed clinical history remains essential, supported by eyewitness accounts and, when possible, video recordings. The initial assessment aims to distinguish epileptic seizures from common mimics such as syncope, psychogenic non-epileptic seizures, or transient ischemic attacks.[98][99]
Following clinical evaluation, selected tests may be used to rule out acute causes and seizure mimics. A 12-lead electrocardiogram (ECG) is recommended for all individuals presenting with a first seizure, to screen for cardiac arrhythmias and other cardiovascular conditions that may resemble epilepsy. Blood tests may be performed to identify metabolic disturbances such as hypoglycemia, electrolyte imbalances, or renal and hepatic dysfunction, particularly in acute settings.[100]
Once epilepsy is suspected, electroencephalography (EEG) is used to support the diagnosis, classify seizure types, and help identify specific epilepsy syndromes. A routine EEG may include activation techniques such as hyperventilation or photic stimulation. However, a normal EEG does not rule out epilepsy. When initial EEG findings are inconclusive, further studies such as sleep-deprived EEG, ambulatory EEG, or long-term video EEG monitoring may be considered.[100]
Neuroimaging, usually with magnetic resonance imaging (MRI), is recommended to detect structural causes of epilepsy. If MRI is contraindicated or unavailable, computed tomography (CT) may be considered. Imaging should be interpreted by radiologists with expertise in epilepsy.[100]
Additional tests may be guided by clinical context. Genetic testing may be considered in individuals with early-onset epilepsy, developmental delay, or features of a known genetic epilepsy syndrome. Testing for neuronal antibodies may be appropriate in suspected cases of autoimmune encephalitis, particularly when seizures are new-onset, rapidly progressive, or resistant to standard treatment. Metabolic testing may be pursued in infants or children with unexplained epilepsy, especially when developmental regression or multisystem involvement is present.[100]
Serum prolactin may occasionally be measured after a suspected seizure, particularly to help distinguish epileptic seizures from non-epileptic events. While it can be elevated following certain seizure types, the test lacks sufficient sensitivity and specificity and is not recommended for routine use.[101]
Differential diagnosis
[edit]A number of conditions can resemble epileptic seizures, leading to potential misdiagnosis. Accurate diagnosis is essential, as inappropriate treatment may delay effective care or cause harm. Common mimics include fainting (syncope), psychogenic non-epileptic seizures (PNES), transient ischemic attacks, migraine, narcolepsy, and various sleep or movement disorders.[102][103] In children, reflux, breath-holding spells, and parasomnias such as night terrors may also resemble seizures.[103]
Psychogenic non-epileptic seizures (PNES) are a particularly important consideration, especially in individuals with refractory epilepsy. PNES are involuntary episodes that resemble epileptic seizures but are not associated with abnormal electrical discharges. They are classified as functional neurological disorders and are typically associated with psychological distress or trauma. Studies suggest that approximately 20% of individuals referred to epilepsy centers are diagnosed with PNES,[17] and up to 10% of these individuals also have coexisting epilepsy.[104] Differentiating between the two can be difficult and often requires prolonged video EEG monitoring.[104]
Misdiagnosis remains a significant concern in epilepsy. Reported rates vary widely — from 2% to 71% — depending on factors such as clinical setting, patient population, diagnostic criteria, and physician expertise.[105][106]
Prevention
[edit]Although many causes of epilepsy are not preventable, several known risk factors are modifiable. Perinatal care, including prevention of birth trauma, hypoxia, and maternal infections, can lower the risk of epilepsy in infants.[7] Vaccination programs, especially against neurotropic infections such as measles and meningitis, play a key role in preventing epilepsy caused by central nervous system infections. In low- and middle-income countries, neurocysticercosis remains a major preventable cause of epilepsy, which can be reduced through improved sanitation and food safety.[14][20] Eliminating or reducing risk factors for seizures in older adults such as inactivity, smoking, diabetes, high blood pressure, and excessive alcohol consumption have been suggested as strategies to help prevent epilepsy in older adults.[107]
Complications
[edit]Epilepsy can lead to a range of medical, psychological, and social complications, particularly when seizures are frequent or uncontrolled.[11] One of the most serious risks is injury during a seizure, including falls, burns, or accidents while driving, swimming, or operating machinery.[108][109] The risk of drowning is significantly increased in people with epilepsy, especially those with poor seizure control.[110]
People with epilepsy are at greater risk for mental health conditions, including depression, anxiety, and social isolation. These challenges are often compounded by stigma, employment difficulties, and driving restrictions.[111][112] In children, epilepsy — especially when drug-resistant — can interfere with cognitive development and academic performance.[113]
A rare but serious complication is sudden unexpected death in epilepsy (SUDEP), which is most often associated with uncontrolled generalized tonic–clonic seizures, particularly during sleep.[114]
Management
[edit]
The primary goals of epilepsy management are to control seizures, minimize treatment side effects, and optimize quality of life. Management strategies are individualized based on the type of seizures or epilepsy syndrome, the underlying cause when known, the person's age and comorbidities, and their preferences and life circumstances.[100]
Supporting people's self-management of their condition may be useful.[115] In drug-resistant cases different management options may be considered, including special diets, the implantation of a neurostimulator, or neurosurgery.[23]
First aid and acute management of seizures
[edit]During a generalized tonic–clonic seizure, the primary goals are to ensure safety and prevent injury. The following steps should be taken:[116]
- Stay calm and remove any potential hazards from the area. Clear the space of sharp objects, furniture, or anything that might cause injury.
- If the person is standing, gently guide them to the ground to avoid a fall.
- Position the person on their side and into the recovery position, which helps keep the airway clear and reduces the risk of choking. If possible, place something soft (e.g., a jacket or cushion) under their head to prevent injury.
- Do not restrain their movements or attempt to hold them down. Do not put anything in their mouth, as this may cause harm.[44][116]
If the seizure lasts longer than 5 minutes or if multiple seizures occur without full recovery in between, it is important to call for emergency medical assistance immediately, as it is considered a medical emergency known as status epilepticus.[117]
Convulsive status epilepticus requires immediate medical attention to prevent serious complications. In a community setting (such as at home or in the ambulance), first-line treatment includes the administration of benzodiazepines. If the person has an individualized emergency management plan — which may have been developed with healthcare providers and outlines personalized treatment steps (such as the use of buccal midazolam or rectal diazepam) — this plan should be followed immediately.[100] In hospital, intravenous lorazepam is preferred.[100]
If seizures continue after the first dose of benzodiazepine, emergency medical services should be contacted, and further doses can be given. For ongoing seizures, levetiracetam, phenytoin, or sodium valproate may be used as second-line treatments, with levetiracetam preferred for its quicker action and fewer side effects.[100]
Most institutions have a preferred pathway or protocol to be used in a seizure emergency like status epilepticus. These protocols have been found to be effective in reducing time to delivery of treatment.[100]
Medications
[edit]
The primary treatment for epilepsy involves the use of antiseizure medications (ASMs), which aim to control seizures while minimizing side effects. Treatment plans should be individualized, taking into account the seizure type, epilepsy syndrome, patient age, sex, comorbidities, lifestyle factors, and the potential for drug interactions.[100]
First-line treatment for most individuals with epilepsy is monotherapy with a single ASM. For many people with epilepsy, seizure control is achieved with a single medication, but some may require combination therapy if seizures are not well-controlled with monotherapy.[100]
There are a number of medications available including phenytoin, carbamazepine and valproate. Evidence suggests that these drugs are similarly effective for both focal and generalized seizures, although their side-effect profiles vary.[118][119] Controlled release carbamazepine appears to work as well as immediate release carbamazepine, and may have fewer side effects.[120] In the UK, carbamazepine or lamotrigine are recommended as first-line treatments for focal seizures, with levetiracetam and valproate used as second-line treatments due to concerns about cost and side effects. Valproate is the first-line choice for generalized seizures, while lamotrigine is used as second-line. For absence seizures, ethosuximide or valproate are recommended, with valproate also being effective for myoclonic and tonic–clonic seizures.[100][121]
Controlled-release formulations of carbamazepine may be preferred in some cases, as they appear to be equally effective as immediate-release carbamazepine but may have fewer side effects. Once a person's seizures are well-controlled on a specific treatment, it is generally not necessary to routinely check medication blood levels, unless there are concerns about side effects or toxicity.[100]
In low- and middle-income countries (LMICs), the management of epilepsy is often hindered by limited access to medications, diagnostic tools, and specialized care.[14] While phenytoin and carbamazepine are commonly used as first-line treatments due to their availability and low cost, newer drugs like levetiracetam and lamotrigine may not be accessible. Additionally, surgical options and advanced therapies, such as vagus nerve stimulation or resective surgery, are typically inaccessible due to high costs and lack of infrastructure.
The least expensive anticonvulsant is phenobarbital at around US$5 a year.[14] The World Health Organization gives it a first-line recommendation in LMICs and it is commonly used in these countries.[122][123] Access, however, may be difficult as some countries label it as a controlled drug.[14]
Adverse effects from medications are reported in 10% to 90% of people, depending on how and from whom the data is collected.[124] Most adverse effects are dose-related and mild.[124] Some examples include mood changes, sleepiness, or an unsteadiness in gait.[124] Certain medications have side effects that are not related to dose such as rashes, liver toxicity, or suppression of the bone marrow.[124] Up to a quarter of people stop treatment due to adverse effects.[124] Some medications are associated with birth defects when used in pregnancy.[125] Many of the common used medications, such as valproate, phenytoin, carbamazepine, phenobarbital, and gabapentin have been reported to cause increased risk of birth defects,[126] especially when used during the first trimester.[127] Despite this, treatment is often continued once effective, because the risk of untreated epilepsy is believed to be greater than the risk of the medications.[127] Among the antiepileptic medications, levetiracetam and lamotrigine seem to carry the lowest risk of causing birth defects.[126]
Slowly stopping medications may be reasonable in some people who do not have a seizure for two to four years; however, around a third of people have a recurrence, most often during the first six months.[125][128] Stopping is possible in about 70% of children and 60% of adults.[20] Measuring medication levels is not generally needed in those whose seizures are well controlled.[129]
Surgery
[edit]Epilepsy surgery is an important treatment option for individuals with drug-resistant epilepsy,[15][130] typically defined as the failure of at least two appropriately chosen and tolerated antiseizure medications.[131] Surgery is most effective in cases of focal epilepsy, where seizures originate from a specific area of the brain that can be safely removed.[132][133]
Although epilepsy surgery has demonstrated strong evidence of efficacy — especially in drug-resistant focal epilepsy — it remains underutilized worldwide and is often reserved for individuals whose condition has reached an advanced or chronic stage.[130] Early consideration and referral for surgical evaluation can improve long-term outcomes and quality of life. This evaluation, conducted in specialized epilepsy centers, includes seizure classification, long-term video EEG monitoring, high-resolution MRI with epilepsy-specific protocols, neuropsychological assessment, and sometimes functional imaging or invasive monitoring. Early referral improves the likelihood of successful outcomes and avoids prolonged periods of unnecessary disability.[134]
The primary goal of epilepsy surgery is to achieve seizure freedom,[135] but even when that is not possible, palliative procedures that significantly reduce seizure frequency can lead to meaningful improvements in quality of life and development — particularly in children. Studies suggest that 60-70% of individuals with drug-resistant focal epilepsy experience a substantial reduction in seizures following surgery.[136]
Common procedures include anterior temporal lobe resection, which often involves removal of the hippocampus in cases of mesial temporal lobe epilepsy, as well as lesionectomy for tumors or cortical dysplasia, and lobectomy for larger seizure foci.[136] In cases where resection is not possible, procedures such as corpus callosotomy may help reduce the severity and spread of seizures. In addition to traditional resective techniques, minimally invasive approaches such as MRI-guided laser interstitial thermal therapy (LITT) have gained traction as safer alternatives in select cases, particularly where reducing cognitive impact and recovery time is a priority.[137] In many cases, antiseizure medications can be tapered following successful surgery, though long-term monitoring remains essential.[133][136] Surgical treatment is not limited to adults. A 2023 systematic review found that early surgery in children under 3 years with drug-resistant epilepsy can result in meaningful seizure reduction or freedom when other treatments have failed.[138]
Although epilepsy surgery has demonstrated efficacy, it is still rarely used around the world, and is typically reserved for cases where the condition has reached an advanced stage.[130]
Neuromodulation
[edit]Neurotherapy or Neuromodulation therapies, including vagus nerve stimulation (VNS), deep brain stimulation (DBS), Neuromodulation through Radiotherapy (e.g. Leksell Gamma Knife) and responsive neurostimulation (RNS), are treatment options for individuals with drug-resistant epilepsy who are not candidates for resective surgery, or for whom previous surgery has not resulted in seizure freedom.[139][140][141] These neurotherapies aim to reduce seizure frequency and severity by delivering controlled electrical stimulation to targeted neural circuits.
Diet
[edit]Dietary therapy, particularly the ketogenic diet (high-fat, low-carbohydrate, adequate-protein), is a non-pharmacological treatment option used primarily in children with drug-resistant epilepsy. Evidence suggests that children on a classical ketogenic diet may be up to three times more likely to achieve seizure freedom and up to six times more likely to experience a ≥50% reduction in seizure frequency compared to those receiving standard care. Modified versions of the diet, such as the modified Atkins diet, are better tolerated but may be less effective.[6][142] In adults, the ketogenic diet has shown limited evidence of achieving seizure freedom, though it may increase the likelihood of seizure reduction. However, further research is necessary.[6]
It is typically supervised by a multidisciplinary team, including neurologists and dietitians, due to its restrictive nature and potential side effects, such as vomiting, constipation and diarrhoea. Regular monitoring of nutritional status, blood parameters, and growth is recommended.[6] It is unclear why this diet works.[143] A gluten-free diet has been proposed in rare cases of epilepsy associated with celiac disease and occipital calcifications, though evidence is limited and based on small case series.[76]
Adjunctive and complementary therapies
[edit]There is moderate-quality evidence supporting the use of psychological interventions — such as cognitive behavioral therapy (CBT), relaxation techniques, and self-management training — alongside standard treatment.[144] These approaches may improve quality of life, emotional wellbeing, and treatment adherence; however, evidences targeting seizure control are uncertain.[145] Avoidance therapy consists of minimizing or eliminating triggers. For example, those who are sensitive to light may have success with using a small television, avoiding video games, or wearing dark glasses.[146] Biofeedback, particularly EEG-based operant conditioning, has shown preliminary benefit in some people with drug-resistant epilepsy.[147] However, these methods are considered adjunctive and are not recommended as standalone treatments.
Cannabidiol (CBD) has shown benefit as an add-on therapy in certain severe childhood epilepsies. A purified form of CBD was approved by the U.S. FDA in 2018 and by the European Medicines Agency (EMA) in 2020 for the treatment of Dravet syndrome, Lennox–Gastaut syndrome, and tuberous sclerosis complex.[148][149][150]
Regular physical activity is generally considered safe and may have beneficial effects on seizure frequency, mood, and overall wellbeing.[151] While evidence remains limited, some studies suggest that moderate exercise can reduce seizure burden in certain individuals.[152] Seizure response dogs have been trained to assist individuals during or after seizures by providing physical support or alerting others.[153][154] Although anecdotal reports claim that some dogs can anticipate seizures, there is no conclusive scientific evidence supporting the consistent ability of dogs to predict seizures before they occur.[155]
Various forms of alternative medicine, including acupuncture,[156] routine vitamins,[157] and yoga,[158] have no reliable evidence to support their use in epilepsy. Melatonin, as of 2016[update], is insufficiently supported by evidence.[159] The trials were of poor methodological quality and it was not possible to draw any definitive conclusions.[159]
Contraception and pregnancy
[edit]Women of child-bearing age, including those with epilepsy, are at risk of unintended pregnancies if they are not using an effective form of contraception.[160] Women with epilepsy may experience a temporary increase in seizure frequency when they begin hormonal contraception.[160]
Some anti-seizure medications interact with enzymes in the liver and cause the drugs in hormonal contraception to be broken down more quickly. These enzyme inducing drugs make hormonal contraception less effective, and this is particularly hazardous if the anti-seizure medication is associated with birth defects.[161] Potent enzyme-inducing anti-seizure medications include carbamazepine, eslicarbazepine acetate, oxcarbazepine, phenobarbital, phenytoin, primidone, and rufinamide. The drugs perampanel and topiramate can be enzyme-inducing at higher doses.[162] Conversely, hormonal contraception can lower the amount of the anti-seizure medication lamotrigine circulating in the body, making it less effective.[160] The failure rate of oral contraceptives, when used correctly, is 1%, but this increases to between 3–6% in women with epilepsy.[161] Overall, intrauterine devices (IUDs) are preferred for women with epilepsy who are not intending to become pregnant.[160]
Women with epilepsy, especially if they have other medical conditions, may have a slightly lower, but still high, chance of becoming pregnant.[160] Women with infertility have about the same chance of success with in vitro fertilisation or other forms of assisted reproductive technology as women without epilepsy.[160] There may be a higher risk of pregnancy loss.[160]
Once pregnant, there are two main concerns related to pregnancy. The first concern is about the risk of seizures during pregnancy, and the second concern is that the anti-seizure medications may result in birth defects.[126] Most women with epilepsy must continue treatment with anti-seizure drugs, and the treatment goal is to balance the need to prevent seizures with the need to prevent drug-induced birth defects.[160][163]
Pregnancy does not seem to change seizure frequency very much.[160] When seizures happen, however, they can cause some pregnancy complications, such as pre-term births or the babies being smaller than usual when they are born.[160]
All pregnancies have a risk of birth defects, e.g., due to smoking during pregnancy.[160] In addition to this typical level of risk, some anti-seizure drugs significantly increase the risk of birth defects and intrauterine growth restriction, as well as developmental, neurocognitive, and behavioral disorders.[163] Most women with epilepsy receive safe and effective treatment and have typical, healthy children.[163] The highest risks are associated with specific anti-seizure drugs, such as valproic acid and carbamazepine, and with higher doses.[126][160] Folic acid supplementation, such as through prenatal vitamins, reduced the risk.[160] Planning pregnancies in advance gives women with epilepsy an opportunity to switch to a lower-risk treatment program and reduced drug doses.[160]
Although anti-seizure drugs can be found in breast milk, women with epilepsy can breastfeed their babies, and the benefits usually outweigh the risks.[160]
Prognosis
[edit]Epilepsy is generally considered a chronic neurological condition, but its long-term course can vary widely depending on factors such as seizure type, underlying cause, and response to treatment. Although epilepsy is not typically "cured," in many cases it may be considered resolved. According to the ILAE, epilepsy is considered to be resolved in individuals who have been seizure-free for at least 10 years, with no antiseizure medications for the last 5 of those years.[10]
Approximately 60–70% of individuals with epilepsy achieve good seizure control with appropriate antiseizure medications, and many can maintain long-term remission.[7] However, outcomes vary significantly by epilepsy type and etiology. Early treatment response is one of the strongest predictors of long-term outcome, with poor early control correlating with lower chances of remission. Several factors — such as structural brain abnormalities, comorbid developmental disorders, or a high frequency of seizures at onset — have been associated with worse outcomes, although findings are not always consistent.[164]
Epilepsy disproportionately affects low- and middle-income countries, where nearly 80% of the global epilepsy population resides.[165] In these countries, to 75% of individuals with epilepsy do not receive the treatment they need.[11] Untreated epilepsy is associated with elevated risk of injury, psychiatric comorbidities, and early death, including sudden unexpected death in epilepsy (SUDEP).
Cognition
[edit]Cognitive impairment frequently accompanies epilepsy, although it is difficult to determine to what extant it is caused by the epilepsy itself.[166][167] This is because observed cognitive decline could be a result of the cause of the epilepsy (e.g. epilepsy caused by mesial temporal sclerosis), or be secondary to the epilepsy (e.g. brain damage from falling due to a seizure, or impairment from pharmacological or surgical treatment of the epilepsy).[166][167]
In the majority of people who achieve seizure control, there is no associated progressive cognitive decline.[166] However, severe intractable epilepsy does cause negative cognitive effects.[166] Due to its variability, it is unclear whether any given case of epilepsy will lead to cognitive decline, but a few points are noted:
- Epilepsy is associated with increased risk for Alzheimer's disease (and vice-versa).[166][167]
- Longer seizures cause more damage than shorter seizures.[166]
- Progressive thinning of the cerebral cortex occurs with recurring seizures.[166]
Mortality
[edit]People with epilepsy may have a higher risk of premature death compared to those without the condition.[168] This risk is estimated to be between 1.6 and 4.1 times greater than that of the general population.[169] The greatest increase in mortality from epilepsy is among the elderly.[169] Those with epilepsy due to an unknown cause have a relatively low increase in risk.[169]
Mortality is often related to the underlying cause of the seizures, status epilepticus, suicide, trauma, and sudden unexpected death in epilepsy (SUDEP).[168] Death from status epilepticus is primarily due to an underlying problem rather than missing doses of medications.[168] The risk of suicide is between two and six times higher in those with epilepsy;[170][171] the cause of this is unclear.[170] SUDEP appears to be partly related to the frequency of generalized tonic-clonic seizures[172] and accounts for about 15% of epilepsy-related deaths;[173] it is unclear how to decrease its risk.[172] Risk factors for SUDEP include nocturnal generalized tonic-clonic seizures, seizures, sleeping alone and medically intractable epilepsy.[174]
In the United Kingdom, it is estimated that 40–60% of deaths are possibly preventable.[48] In the developing world, many deaths are due to untreated epilepsy leading to falls or status epilepticus.[14]
Epidemiology
[edit]Epilepsy is one of the most common serious neurological disorders, affecting approximately 50 million people globally as of 2021,[8][175] with the majority living in low- and middle-income countries.[11][176] The point prevalence of active epilepsy is generally reported between 5 and 7 per 1,000 people, while lifetime prevalence is slightly higher, typically between 6 and 9 per 1,000.[177] Both prevalence and incidence are higher in low-income regions. The annual incidence of epilepsy — the rate of new diagnoses each year — is estimated at 50 to 70 new cases per 100,000 people globally, based on population studies.[177] Rates are significantly higher in low- and middle-income countries, and, within high-income countries, higher incidence has also been observed among lower socioeconomic groups and some ethnic minorities.
Epilepsy can develop at any age, but its incidence is highest in early infancy and in older adults, following a bimodal distribution. In high-income countries, the incidence peaks during the first year of life, declines during adulthood, and rises again in people over age 85. The increase in older adults is associated with age-related conditions such as stroke, brain tumors, and neurodegenerative diseases. In low- and middle-income countries, incidence more often peaks in older children and young adults, which may reflect the effects of trauma, infections, and underdiagnosis in the elderly. Epilepsy is slightly more common in males than females, a difference that may be influenced by risk factor exposure and underreporting in women in some regions due to sociocultural factors.[178]
Beyond prevalence and incidence, epilepsy imposes a significant global burden in terms of disability, stigma, and premature mortality. The disorder is responsible for an estimated 13 million disability-adjusted life years (DALYs) worldwide each year, with the majority of this burden falling on individuals in low-resource settings where access to diagnosis and treatment remains limited.[175]
History
[edit]
The oldest medical records show that epilepsy has been affecting people at least since the beginning of recorded history.[179] Throughout ancient history, the condition was thought to be of a spiritual cause.[179] The world's oldest description of an epileptic seizure comes from a text in Akkadian (a language used in ancient Mesopotamia) and was written around 2000 BC.[22] The person described in the text was diagnosed as being under the influence of a moon god, and underwent an exorcism.[22] Epileptic seizures are listed in the Code of Hammurabi (c. 1790 BC) as reason for which a purchased slave may be returned for a refund,[22] and the Edwin Smith Papyrus (c. 1700 BC) describes cases of individuals with epileptic convulsions.[22]
The oldest known detailed record of the condition itself is in the Sakikku, a Babylonian cuneiform medical text from 1067–1046 BC.[179] This text gives signs and symptoms, details treatment and likely outcomes,[22] and describes many features of the different seizure types.[179] As the Babylonians had no biomedical understanding of the nature of epilepsy, they attributed the seizures to possession by evil spirits and called for treating the condition through spiritual means.[179] Around 900 BC, Punarvasu Atreya described epilepsy as loss of consciousness;[180] this definition was carried forward into the Ayurvedic text of Charaka Samhita (c. 400 BC).[181]
The ancient Greeks had contradictory views of the condition. They thought of epilepsy as a form of spiritual possession, but also associated the condition with genius and the divine. One of the names they gave to it was the sacred disease (Ancient Greek: ἠ ἱερὰ νόσος).[22][182] Epilepsy appears in Greek mythology: it is associated with the Moon goddesses Selene and Artemis, who afflicted those who upset them. The Greeks thought that important figures such as Julius Caesar and Hercules had the condition.[22] The notable exception to this divine and spiritual view was that of the school of Hippocrates. In the fifth century BC, Hippocrates rejected the idea that the condition was caused by spirits. In his landmark work On the Sacred Disease, he proposed that epilepsy was not divine in origin and instead was a medically treatable problem originating in the brain.[22][179] He accused those of attributing a sacred cause to the condition of spreading ignorance through a belief in superstitious magic.[22] Hippocrates proposed that heredity was important as a cause, described worse outcomes if the condition presents at an early age, and made note of the physical characteristics as well as the social shame associated with it.[22] Instead of referring to it as the sacred disease, he used the term great disease, giving rise to the modern term grand mal, used for tonic–clonic seizures.[22] Despite his work detailing the physical origins of the condition, his view was not accepted at the time.[179] Evil spirits continued to be blamed until at least the 17th century.[179]
In Ancient Rome people did not eat or drink with the same pottery as that used by someone who was affected.[183] People of the time would spit on their chest believing that this would keep the problem from affecting them.[183] According to Apuleius and other ancient physicians, to detect epilepsy, it was common to light a piece of gagates, whose smoke would trigger the seizure.[184] Occasionally a spinning potter's wheel was used, perhaps a reference to photosensitive epilepsy.[185]
In most cultures, persons with epilepsy have been stigmatized, shunned, or even imprisoned. As late as in the second half of the 20th century, in Tanzania and other parts of Africa epilepsy was associated with possession by evil spirits, witchcraft, or poisoning and was believed by many to be contagious.[186] In the Salpêtrière, the birthplace of modern neurology, Jean-Martin Charcot found people with epilepsy side by side with the mentally ill, those with chronic syphilis, and the criminally insane.[187] In Ancient Rome, epilepsy was known as the morbus comitialis or 'disease of the assembly hall' and was seen as a curse from the gods. In northern Italy, epilepsy was traditionally known as Saint Valentine's malady.[188] In at least the 1840s in the United States of America, epilepsy was known as the falling sickness or the falling fits, and was considered a form of medical insanity.[189] Around the same time period, epilepsy was known in France as the haut-mal lit. 'high evil', mal-de terre lit. 'earthen sickness', mal de Saint Jean lit. 'Saint John's sickness', mal des enfans lit. 'child sickness', and mal-caduc lit. 'falling sickness'.[189] People of epilepsy in France were also known as tombeurs lit. 'people who fall', due to the seizures and loss of consciousness in an epileptic episode.[189]
In the mid-19th century, the first effective anti-seizure medication, bromide, was introduced.[124] The first modern treatment, phenobarbital, was developed in 1912, with phenytoin coming into use in 1938.[190]
Society and culture
[edit]Epilepsy has significant social and cultural implications that vary across regions and contexts. People with epilepsy may experience social stigma, legal restrictions, economic disadvantage, and barriers to education and employment. Public perceptions of the condition are shaped by cultural beliefs, media portrayals, and the level of awareness in a given society. Efforts by advocacy groups and international organizations aim to improve public understanding, reduce stigma, and promote access to care. Social consequences, such as educational exclusion, unemployment, and social isolation, further compound the impact on quality of life. Despite the availability of effective antiseizure medications and cost-effective treatment strategies, a large treatment gap persists in many countries, underscoring the need for strengthened health systems and public health interventions.
Stigma
[edit]Social stigma is commonly experienced by people with epilepsy worldwide, and it can have economic, social, and cultural consequences.[11][191] Misconceptions about the condition — including beliefs that it is contagious, a form of madness, or caused by supernatural forces — persist in many communities. In parts of Africa, including Tanzania and Uganda, epilepsy is sometimes associated with spirit possession, witchcraft, or poisoning, and is incorrectly believed to be contagious.[186][192] Similar stigmatizing beliefs have been reported in other regions, such as India and China, where epilepsy may be cited as grounds for denying marriage.[20] In the United Kingdom, epilepsy was legally considered valid grounds for annulling a marriage until 1971.[63]
Stigma can also affect how people respond to a diagnosis. Some individuals with epilepsy may deny having had seizures, fearing discrimination.[63] A 2024 cross-sectional study found that 64.8% of relatives of people with epilepsy reported experiencing moderate levels of stigma, which was associated with more negative attitudes toward the condition. Greater stigma was observed among relatives of patients with more frequent seizures or poor medication adherence.[193]
Negative perceptions of epilepsy can also affect educational opportunities and academic outcomes.[194] Children with epilepsy are at increased risk of underachievement in school due to a combination of neurological factors, medication side effects, and the effects of social exclusion.[195]
In adulthood, stigma can result in reduced employment opportunities and workplace discrimination. Adults with epilepsy are more likely to be unemployed or underemployed than the general population, a disparity often driven by employer concerns about safety, productivity, or liability.[194] Disclosure of an epilepsy diagnosis in job applications or interviews may lead to discrimination, although nondisclosure can limit access to workplace accommodations.[196]
Economic impact
[edit]Epilepsy is associated with a substantial economic burden at both the individual and societal levels. Direct costs include expenses related to diagnosis, treatment, and long-term management, such as antiseizure medications and hospitalizations. Indirect costs may arise from lost productivity, unemployment, and premature death. In many countries, especially those with limited health infrastructure, individuals with epilepsy and their families often bear the majority of healthcare expenses out of pocket. A 2021 modeling study estimated the total global cost of epilepsy at approximately $119.27 billion annually, based on per capita cost projections applied to an estimated 52.51 million people living with epilepsy worldwide, while accounting for the treatment gap.[197] The treatment gap — referring to the proportion of people with epilepsy who do not receive appropriate care — remains high in low- and middle-income countries, exacerbating the economic burden through avoidable seizures, injuries, and emergency care. Seizures result in direct economic costs of about one billion dollars in the United States.[17] Epilepsy resulted in economic costs in Europe of around 15.5 billion euros in 2004.[48] In India, epilepsy is estimated to result in costs of US$1.7 billion or 0.5% of the GDP.[20] It is the cause of about 1% of emergency department visits (2% for emergency departments for children) in the United States.[198]
Driving and legal restrictions
[edit]Those with epilepsy are at about twice the risk of being involved in a motor vehicular collision and thus in many areas of the world are not allowed to drive or able to drive only if certain conditions are met.[21] Diagnostic delay has been suggested to be a cause of some potentially avoidable motor vehicle collisions since at least one study showed that most motor vehicle accidents occurred in those with undiagnosed non-motor seizures as opposed to those with motor seizures at epilepsy onset.[199] In some places physicians are required by law to report if a person has had a seizure to the licensing body while in others the requirement is only that they encourage the person in question to report it themselves.[21] Countries that require physician reporting include Sweden, Austria, Denmark and Spain.[21] Countries that require the individual to report include the UK and New Zealand, and physicians may report if they believe the individual has not already.[21] In Canada, the United States and Australia the requirements around reporting vary by province or state.[21] If seizures are well controlled most feel allowing driving is reasonable.[200] The amount of time a person must be free from seizures before they can drive varies by country.[200] Many countries require one to three years without seizures.[200] In the United States the time needed without a seizure is determined by each state and is between three months and one year.[200]
Those with epilepsy or seizures are typically denied a pilot license.[201]
- In Canada if an individual has had no more than one seizure, they may be considered after five years for a limited license if all other testing is normal.[202] Those with febrile seizures and drug related seizures may also be considered.[202]
- In the United States, the Federal Aviation Administration does not allow those with epilepsy to get a commercial pilot license.[203] Rarely, exceptions can be made for persons who have had an isolated seizure or febrile seizures and have remained free of seizures into adulthood without medication.[204]
- In the United Kingdom, a full National Private Pilot Licence requires the same standards as a professional driver's license.[205] This requires a period of ten years without seizures while off medications.[206] Those who do not meet this requirement may acquire a restricted license if free from seizures for five years.[205]
Advocacy and support organizations
[edit]There are organizations that provide support for people and families affected by epilepsy. In 1997 the International Bureau for Epilepsy (IBE), the International League Against Epilepsy (ILAE) and the World Health Organization launched the Global Campaign Against Epilepsy (GCAE) to bring epilepsy 'out of the shadows' by raising awareness of, and improving treatment and services for epilepsy.[1][207] In the United States, the Epilepsy Foundation is a national organization that works to increase the acceptance of those with the disorder, their ability to function in society and to promote research for a cure.[208] The Epilepsy Foundation, some hospitals, and some individuals also run support groups in the United States.[209] In Australia, the Epilepsy Foundation provides support, delivers education and training and funds research for people living with epilepsy.
International Epilepsy Day (World Epilepsy Day) began in 2015 and occurs on the second Monday in February.[210][211]
Purple Day, a different world-wide epilepsy awareness day for epilepsy, was initiated by a nine-year-old Canadian named Cassidy Megan in 2008, and is every year on 26 March.[212]
Research directions
[edit]Epilepsy research aims to uncover the causes of seizures, improve diagnosis, and develop more effective treatments. It spans genetics, neuroscience, pharmacology, and biomedical engineering, with the shared goal of reducing the burden of disease. Researchers also study how epilepsy develops (epileptogenesis), seeking ways to prevent it entirely.
Animal models
[edit]Animal models are widely used in epilepsy research, providing insight into seizure mechanisms, disease progression, and treatment effects. Rodents are most commonly used, with models based on chemical induction (e.g. kainic acid, pilocarpine), electrical stimulation (e.g. kindling), genetic mutations, and others.[213] Other species, including zebrafish, dogs, and non-human primates, are also employed to capture features not easily replicated in rodents, such as complex behaviors or chronic seizure patterns. These models help researchers study epileptogenesis, test antiseizure drugs, and explore surgical or neuromodulatory interventions. While no model captures the full complexity of human epilepsy, they remain essential for translational research.[214]
Genetics and molecular research
[edit]Advances in genetics have transformed the understanding of epilepsy, particularly in early-onset and treatment-resistant forms. Mutations in genes affecting ion channels, synaptic transmission, and mTOR signaling pathways have been linked to a growing number of epilepsy syndromes, including Dravet syndrome (SCN1A), PCDH19-related epilepsy, and familial focal epilepsies. High-throughput sequencing has enabled the discovery of de novo mutations in severe developmental and epileptic encephalopathies. In parallel, research into polygenic risk and epigenetic mechanisms is expanding the view of common epilepsies as complex traits. Molecular studies also support the development of targeted therapies, such as precision treatments for specific genetic subtypes.[215] Variations within the sodium channel SCN3A, and Na+/K+,ATPase (ATP1A3), has been implicated some of earliest onset epilepsies with cortical malformations.[216][217]
Epileptogenesis and biomarkers
[edit]Understanding how epilepsy develops (epileptogenesis) is a major focus of current research. This includes identifying biomarkers that predict who is at risk of developing epilepsy. EEG patterns, neuroimaging features, and molecular signals in blood or cerebrospinal fluid are being investigated as early indicators. The goal is to detect epilepsy before chronic seizures begin and to develop interventions that prevent or halt this process. While no validated biomarker is yet in clinical use, this area holds promise for future disease-modifying therapies.[218]
Antiseizure drug development
[edit]The development of new antiseizure medications remains a priority, especially for people with drug-resistant epilepsy. Current research focuses on compounds with novel mechanisms of action, better safety profiles, and disease-modifying potential. High-throughput screening, including zebrafish and organoid models, accelerates early-stage discovery, while pharmacogenomic studies aim to personalize drug selection. Cannabinoids and neurosteroids are also under investigation for specific syndromes and seizure types.[219][220]
Seizure prediction
[edit]The unpredictability of seizures is a major concern for many people with epilepsy, and seizure prediction has been a longstanding focus of research. Early efforts were limited by small datasets and inconsistent results; however, advances in computational modeling, long-term EEG recording, and machine learning have led to renewed interest in the field. Public EEG databases and algorithm competitions have helped standardize evaluation and fostered the development of more accurate methods. In one clinical trial, prospective seizure prediction using intracranial EEG was achieved in a small group of participants. Current approaches often integrate network models of brain activity, multimodal data sources, and closed-loop systems capable of both detecting and responding to pre-ictal changes. These developments have laid the groundwork for future large-scale clinical trials and the potential integration of seizure forecasting into clinical practice.[221]
Mechanistic modeling and alternative pathways
[edit]Mathematical and computational models are increasingly used to simulate the neural dynamics underlying seizures. Reductionist models such as the Epileptor use ordinary differential equations to replicate interictal and ictal discharges observed in experimental data.[222] More detailed versions, including the Epileptor-2, incorporate physiological variables such as ion concentrations and synaptic resource availability.[223] These models suggest that fluctuations in extracellular potassium and intracellular sodium levels may play a key role in the emergence and termination of seizures.[224]
Potential future therapies
[edit]Several novel therapeutic strategies are under investigation for epilepsy. Gene therapy is being studied in some types of epilepsy.[225] Medications that alter immune function, such as intravenous immunoglobulins, may reduce the frequency of seizures when including in normal care as an add-on therapy; however, further research is required to determine whether these medications are very well tolerated in children and in adults with epilepsy.[226] Noninvasive stereotactic radiosurgery is, as of 2012[update], being compared to standard surgery for certain types of epilepsy.[227]
Other animals
[edit]Epilepsy has also been documented in several animal species, particularly dogs and cats.[228] Veterinary treatments often use similar antiepileptic drugs, such as phenobarbital or levetiracetam. In horses, diagnosis can be challenging, especially in focal seizures,[229] and such conditions as juvenile idiopathic epilepsy have been reported in foals.[230]
See also
[edit]References
[edit]- ^ a b c d e f g h i "Epilepsy Fact sheet". WHO. February 2024. Archived from the original on 11 March 2016. Retrieved 28 September 2024.
- ^ a b c Hammer GD, McPhee SJ, eds. (2010). "7". Pathophysiology of disease: an introduction to clinical medicine (6th ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-162167-0.
- ^ a b Goldberg EM, Coulter DA (May 2013). "Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction". Nature Reviews. Neuroscience. 14 (5): 337–349. doi:10.1038/nrn3482. PMC 3982383. PMID 23595016.
- ^ a b c Longo DL (2012). "369 Seizures and Epilepsy". Harrison's principles of internal medicine (18th ed.). McGraw-Hill. p. 3258. ISBN 978-0-07-174887-2.
- ^ a b Bergey GK (June 2013). "Neurostimulation in the treatment of epilepsy". Experimental Neurology. 244: 87–95. doi:10.1016/j.expneurol.2013.04.004. PMID 23583414.
- ^ a b c d e Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN (June 2020). "Ketogenic diets for drug-resistant epilepsy". The Cochrane Database of Systematic Reviews. 2020 (6) CD001903. doi:10.1002/14651858.CD001903.pub5. PMC 7387249. PMID 32588435.
- ^ a b c d Eadie MJ (December 2012). "Shortcomings in the current treatment of epilepsy". Expert Review of Neurotherapeutics. 12 (12): 1419–1427. doi:10.1586/ern.12.129. PMID 23237349.
- ^ a b "GBD 2021 Cause and Risk Summary: EPILEPSY". Institute for Health Metrics and Evaluation (IHME). Seattle, USA: University of Washington. 2021. Archived (PDF) from the original on 19 July 2024. Retrieved 19 July 2024.
- ^ a b Sinmetz JD, Seeher KM, Schiess N, Nichols E, Cao B, Servili C, et al. (1 April 2024). "Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021". The Lancet Neurology. 23 (4). Elsevier: 344–381. doi:10.1016/S1474-4422(24)00038-3. hdl:1959.4/102176. PMC 10949203. PMID 38493795.
- ^ a b c d e f Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. (April 2014). "ILAE official report: a practical clinical definition of epilepsy". Epilepsia. 55 (4): 475–482. doi:10.1111/epi.12550. PMID 24730690.
- ^ a b c d e f g "Epilepsy". World Health Organization. Retrieved 1 April 2023.
- ^ Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. (April 2005). "Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE)". Epilepsia. 46 (4): 470–472. doi:10.1111/j.0013-9580.2005.66104.x. PMID 15816939.
- ^ a b Pandolfo M (November 2011). "Genetics of epilepsy". Seminars in Neurology. 31 (5): 506–518. doi:10.1055/s-0031-1299789. PMID 22266888.
- ^ a b c d e f g Newton CR, Garcia HH (September 2012). "Epilepsy in poor regions of the world". Lancet. 380 (9848): 1193–1201. doi:10.1016/S0140-6736(12)61381-6. PMID 23021288.
- ^ a b Brodie MJ, Elder AT, Kwan P (November 2009). "Epilepsy in later life". The Lancet. Neurology. 8 (11): 1019–1030. doi:10.1016/S1474-4422(09)70240-6. PMID 19800848.
- ^ Holmes TR, Browne GL (2008). Handbook of epilepsy (4th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 7. ISBN 978-0-7817-7397-3.
- ^ a b c Wilden JA, Cohen-Gadol AA (August 2012). "Evaluation of first nonfebrile seizures". American Family Physician. 86 (4): 334–340. PMID 22963022.
- ^ Neligan A, Adan G, Nevitt SJ, Pullen A, Sander JW, Bonnett L, et al. (Cochrane Epilepsy Group) (January 2023). "Prognosis of adults and children following a first unprovoked seizure". The Cochrane Database of Systematic Reviews. 1 (1) CD013847. doi:10.1002/14651858.CD013847.pub2. PMC 9869434. PMID 36688481.
- ^ Epilepsy: what are the chances of having a second seizure? (Report). 16 August 2023. doi:10.3310/nihrevidence_59456.
- ^ a b c d e "Epilepsy". Fact Sheets. World Health Organization. October 2012. Retrieved 24 January 2013.
- ^ a b c d e f L Devlin A, Odell M, L Charlton J, Koppel S (December 2012). "Epilepsy and driving: current status of research". Epilepsy Research. 102 (3): 135–152. doi:10.1016/j.eplepsyres.2012.08.003. PMID 22981339.
- ^ a b c d e f g h i j k l Magiorkinis E, Sidiropoulou K, Diamantis A (January 2010). "Hallmarks in the history of epilepsy: epilepsy in antiquity". Epilepsy & Behavior. 17 (1): 103–108. doi:10.1016/j.yebeh.2009.10.023. PMID 19963440.
- ^ a b c d e Milligan TA (2021). "Epilepsy: A Clinical Overview". The American Journal of Medicine. 134 (7): 840–847. doi:10.1016/j.amjmed.2021.01.038. PMID 33775643. Retrieved 19 September 2025.
- ^ Haneef Z, Matsumoto JH (2024). "Seizures and Epilepsy Syndromes". Epilepsy Fundamentals. Cham: Springer Nature Switzerland. p. 9–36. doi:10.1007/978-3-031-77741-7_2. ISBN 978-3-031-77740-0. Retrieved 20 September 2025.
- ^ a b c d e f Beniczky S, Trinka E, Wirrell E, Abdulla F, Al Baradie R, Alonso Vanegas M, et al. (23 April 2025). "Updated classification of epileptic seizures: Position paper of the International League Against Epilepsy". Epilepsia. 66 (6): 1804–1823. doi:10.1111/epi.18338. ISSN 0013-9580. PMC 12169392. PMID 40264351.
- ^ a b c Asadi-Pooya AA, Brigo F, Lattanzi S, Blumcke I (2023). "Adult epilepsy". The Lancet. 402 (10399): 412–424. doi:10.1016/S0140-6736(23)01048-6. PMID 37459868. Retrieved 19 September 2025.
- ^ a b Haneef Z, Hegazy M, Gavvala JR (2024). "Semiology". Epilepsy Fundamentals. Cham: Springer Nature Switzerland. pp. 193–207. doi:10.1007/978-3-031-77741-7_15. ISBN 978-3-031-77740-0. Retrieved 20 September 2025.
- ^ a b Bradley WG (2012). "67". Bradley's neurology in clinical practice (6th ed.). Philadelphia, PA: Elsevier/Saunders. ISBN 978-1-4377-0434-1.
- ^ Engel J (2008). Epilepsy: a comprehensive textbook (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 2797. ISBN 978-0-7817-5777-5. Archived from the original on 20 May 2016.
- ^ a b c Simon DA, Greenberg MJ, Aminoff RP (2012). "12". Clinical neurology (8th ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-175905-2.
- ^ Stephenson JB (1990). Fits and faints. London: Mac Keith Press. ISBN 0-632-02811-4. OCLC 25711319.
- ^ Hu M, Zhang C, Xiao X, Guo J, Sun H (2020). "Effect of intensive self-management education on seizure frequency and quality of life in epilepsy patients with prodromes or precipitating factors". Seizure. 78: 38–42. doi:10.1016/j.seizure.2020.03.003. PMID 32155576. Retrieved 21 September 2025.
- ^ Woo KN, Kim K, Ko DS, Kim HW, Kim YH (2022). "Alcohol consumption on unprovoked seizure and epilepsy: An updated meta-analysis". Drug and Alcohol Dependence. 232 109305. doi:10.1016/j.drugalcdep.2022.109305. PMID 35042100. Retrieved 21 September 2025.
- ^ Samsonsen C, Mestvedthagen G, Uglem M, Brodtkorb E (2023). "Disentangling the cascade of seizure precipitants: A prospective observational study". Epilepsy & Behavior. 145 109339. doi:10.1016/j.yebeh.2023.109339. PMID 37413785. Retrieved 21 September 2025.
- ^ Maguire MJ, Nevitt SJ (16 September 2021). "Treatments for seizures in catamenial (menstrual-related) epilepsy". Cochrane Database of Systematic Reviews. 2021 (9) CD013225. doi:10.1002/14651858.CD013225.pub3. PMC 8444032. PMID 34528245.
- ^ Xue LY, Ritaccio AL (March 2006). "Reflex seizures and reflex epilepsy". American Journal of Electroneurodiagnostic Technology. 46 (1): 39–48. doi:10.1080/1086508X.2006.11079556. PMID 16605171.
- ^ Steven C. Schachter, ed. (2008). Behavioral aspects of epilepsy: principles and practice ([Online-Ausg.]. ed.). New York: Demos. p. 125. ISBN 978-1-933864-04-4.
- ^ Malow BA (November 2005). "Sleep and epilepsy". Neurologic Clinics. 23 (4): 1127–1147. doi:10.1016/j.ncl.2005.07.002. PMID 16243619.
- ^ Tinuper P, Provini F, Bisulli F, Vignatelli L, Plazzi G, Vetrugno R, et al. (August 2007). "Movement disorders in sleep: guidelines for differentiating epileptic from non-epileptic motor phenomena arising from sleep". Sleep Medicine Reviews. 11 (4): 255–267. doi:10.1016/j.smrv.2007.01.001. hdl:11380/1205981. PMID 17379548.
- ^ Jafarpour S, Hirsch LJ, Gaínza-Lein M, Kellinghaus C, Detyniecki K (May 2019). "Seizure cluster: Definition, prevalence, consequences, and management". Seizure. 68: 9–15. doi:10.1016/j.seizure.2018.05.013. PMID 29871784.
- ^ Faught E (September 2022). "Economic aspects of treating seizure clusters". Epilepsia. 63 (Suppl 1): S45 – S54. doi:10.1111/epi.17340. PMID 35999172.
- ^ Haut SR, Shinnar S, Moshé SL (January 2005). "Seizure clustering: risks and outcomes". Epilepsia. 46 (1): 146–149. doi:10.1111/j.0013-9580.2005.29004.x. PMID 15660781.
- ^ Chung S, Szaflarski JP, Choi EJ, Wilson JC, Kharawala S, Kaur G, et al. (November 2021). "A systematic review of seizure clusters: Prevalence, risk factors, burden of disease and treatment patterns". Epilepsy Research. 177 106748. doi:10.1016/j.eplepsyres.2021.106748. PMID 34521043.
- ^ a b Shearer P. "Seizures and Status Epilepticus: Diagnosis and Management in the Emergency Department". Emergency Medicine Practice. Archived from the original on 30 December 2010.
- ^ Larner AJ (2010). A dictionary of neurological signs (3rd ed.). New York: Springer. p. 348. ISBN 978-1-4419-7095-4.
- ^ Clancy MJ, Clarke MC, Connor DJ, Cannon M, Cotter DR (13 March 2014). "The prevalence of psychosis in epilepsy; a systematic review and meta-analysis". BMC Psychiatry. 14 (1): 75. doi:10.1186/1471-244X-14-75. ISSN 1471-244X. PMC 3995617. PMID 24625201.
- ^ Panayiotopoulos CP (2010). A clinical guide to epileptic syndromes and their treatment based on the ILAE classifications and practice parameter guidelines (Rev. 2nd ed.). London: Springer. p. 445. ISBN 978-1-84628-644-5.
- ^ a b c d National Clinical Guideline Centre (January 2012). The Epilepsies: The diagnosis and management of the epilepsies in adults and children in primary and secondary care (PDF). National Institute for Health and Clinical Excellence. pp. 21–28. Archived (PDF) from the original on 16 December 2013.
- ^ Kaplan PW (November 2011). "Obsessive-compulsive disorder in chronic epilepsy". Epilepsy & Behavior. 22 (3): 428–432. doi:10.1016/j.yebeh.2011.07.029. PMID 21889913.
- ^ Stefan H (2012). Epilepsy Part I: Basic Principles and Diagnosis E-Book: Handbook of Clinical Neurology (Volume 107 of Handbook of Clinical Neurology ed.). Newnes. p. 471. ISBN 978-0-444-53505-4.
- ^ Reilly CJ (May–June 2011). "Attention deficit hyperactivity disorder (ADHD) in childhood epilepsy". Research in Developmental Disabilities. 32 (3): 883–893. doi:10.1016/j.ridd.2011.01.019. PMID 21310586.
- ^ Levisohn PM (2007). "The autism-epilepsy connection". Epilepsia. 48 (Suppl 9): 33–35. doi:10.1111/j.1528-1167.2007.01399.x. PMID 18047599.
- ^ Lin JJ, Mula M, Hermann BP (September 2012). "Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan". Lancet. 380 (9848): 1180–1192. doi:10.1016/s0140-6736(12)61455-x. PMC 3838617. PMID 23021287.
- ^ Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Mula M, Trimble M, et al. (June 2012). "Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence". Epilepsy & Behavior. 24 (2): 156–168. doi:10.1016/j.yebeh.2012.01.007. PMID 22632406.
- ^ Adelöw C, Andersson T, Ahlbom A, Tomson T (February 2012). "Hospitalization for psychiatric disorders before and after onset of unprovoked seizures/epilepsy". Neurology. 78 (6): 396–401. doi:10.1212/wnl.0b013e318245f461. PMID 22282649.
- ^ Taylor RS, Sander JW, Taylor RJ, Baker GA (December 2011). "Predictors of health-related quality of life and costs in adults with epilepsy: a systematic review". Epilepsia. 52 (12): 2168–2180. doi:10.1111/j.1528-1167.2011.03213.x. PMID 21883177.
- ^ Lacey CJ, Salzberg MR, Roberts H, Trauer T, D'Souza WJ (August 2009). "Psychiatric comorbidity and impact on health service utilization in a community sample of patients with epilepsy". Epilepsia. 50 (8): 1991–1994. doi:10.1111/j.1528-1167.2009.02165.x. PMID 19490049.
- ^ Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K, Devinsky O (January 2004). "Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy". Neurology. 62 (2): 258–261. doi:10.1212/01.wnl.0000103282.62353.85. PMID 14745064.
- ^ Munger Clary HM, Croxton RD, Allan J, Lovato J, Brenes G, Snively BM, et al. (March 2020). "Who is willing to participate in research? A screening model for an anxiety and depression trial in the epilepsy clinic". Epilepsy & Behavior. 104 (Pt A) 106907. doi:10.1016/j.yebeh.2020.106907. PMC 7282472. PMID 32000099.
- ^ a b Berkovic SF, Mulley JC, Scheffer IE, Petrou S (July 2006). "Human epilepsies: interaction of genetic and acquired factors". Trends in Neurosciences. 29 (7): 391–397. doi:10.1016/j.tins.2006.05.009. PMID 16769131.
- ^ Balestrini S, Arzimanoglou A, Blümcke I, Scheffer IE, Wiebe S, Zelano J, et al. (February 2021). "The aetiologies of epilepsy". Epileptic Disorders. 23 (1): 1–16. doi:10.1684/epd.2021.1255. hdl:2158/1262349. PMID 33720020.
- ^ Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, et al. (September 2011). "Standards for epidemiologic studies and surveillance of epilepsy". Epilepsia. 52 (Suppl 7): 2–26. doi:10.1111/j.1528-1167.2011.03121.x. PMID 21899536.
- ^ a b c d Neligan A, Hauser WA, Sander JW (2012). "The epidemiology of the epilepsies". Epilepsy. Handbook of Clinical Neurology. Vol. 107. pp. 113–33. doi:10.1016/B978-0-444-52898-8.00006-9. ISBN 978-0-444-52898-8. PMID 22938966.
- ^ a b c d e f g h i j Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. (2017). "ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology". Epilepsia. 58 (4): 512–521. doi:10.1111/epi.13709. ISSN 0013-9580. PMC 5386840. PMID 28276062.
- ^ Thom M (2014). "Review: Hippocampal sclerosis in epilepsy: a neuropathology review". Neuropathology and Applied Neurobiology. 40 (5): 520–543. doi:10.1111/nan.12150. ISSN 1365-2990. PMC 4265206. PMID 24762203.
- ^ a b c d Bhalla D, Godet B, Druet-Cabanac M, Preux PM (June 2011). "Etiologies of epilepsy: a comprehensive review". Expert Review of Neurotherapeutics. 11 (6): 861–876. doi:10.1586/ern.11.51. PMID 21651333.
- ^ Zelano J, Holtkamp M, Agarwal N, Lattanzi S, Trinka E, Brigo F (June 2020). "How to diagnose and treat post-stroke seizures and epilepsy". Epileptic Disorders. 22 (3): 252–263. doi:10.1684/epd.2020.1159. PMID 32597766.
- ^ Zöllner JP, Schmitt FC, Rosenow F, Kohlhase K, Seiler A, Strzelczyk A, et al. (December 2021). "Seizures and epilepsy in patients with ischaemic stroke". Neurological Research and Practice. 3 (1) 63. doi:10.1186/s42466-021-00161-w. PMC 8647498. PMID 34865660.
- ^ Steinlein OK (31 March 2008). "Genetics and epilepsy". Dialogues in Clinical Neuroscience. 10 (1): 29–38. doi:10.31887/DCNS.2008.10.1/oksteinlein. PMC 3181863. PMID 18472482.
- ^ Heinzen EL, Depondt C, Cavalleri GL, Ruzzo EK, Walley NM, Need AC, et al. (August 2012). "Exome sequencing followed by large-scale genotyping fails to identify single rare variants of large effect in idiopathic generalized epilepsy". American Journal of Human Genetics. 91 (2): 293–302. doi:10.1016/j.ajhg.2012.06.016. PMC 3415540. PMID 22863189.
- ^ Carvill GL, Heavin SB, Yendle SC, McMahon JM, O'Roak BJ, Cook J, et al. (July 2013). "Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1". Nature Genetics. 45 (7): 825–830. doi:10.1038/ng.2646. PMC 3704157. PMID 23708187.
- ^ Chénier S, Yoon G, Argiropoulos B, Lauzon J, Laframboise R, Ahn JW, et al. (2014). "CHD2 haploinsufficiency is associated with developmental delay, intellectual disability, epilepsy and neurobehavioural problems". Journal of Neurodevelopmental Disorders. 6 (1) 9. doi:10.1186/1866-1955-6-9. PMC 4022362. PMID 24834135.
- ^ Suls A, Jaehn JA, Kecskés A, Weber Y, Weckhuysen S, Craiu DC, et al. (November 2013). "De novo loss-of-function mutations in CHD2 cause a fever-sensitive myoclonic epileptic encephalopathy sharing features with Dravet syndrome". American Journal of Human Genetics. 93 (5): 967–975. doi:10.1016/j.ajhg.2013.09.017. PMC 3824114. PMID 24207121.
- ^ EuroEPINOMICS-RES Consortium (October 2014). "De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies". American Journal of Human Genetics. 95 (4): 360–370. doi:10.1016/j.ajhg.2014.08.013. PMC 4185114. PMID 25262651.
- ^ Stafstrom CE, Staedtke V, Comi AM (2017). "Epilepsy Mechanisms in Neurocutaneous Disorders: Tuberous Sclerosis Complex, Neurofibromatosis Type 1, and Sturge-Weber Syndrome". Frontiers in Neurology. 8: 87. doi:10.3389/fneur.2017.00087. PMC 5355446. PMID 28367137.
- ^ a b Jackson JR, Eaton WW, Cascella NG, Fasano A, Kelly DL (March 2012). "Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity". The Psychiatric Quarterly. 83 (1): 91–102. doi:10.1007/s11126-011-9186-y. PMC 3641836. PMID 21877216.
- ^ Grossman G (April 2008). "Neurological complications of coeliac disease: what is the evidence?". Practical Neurology. 8 (2): 77–89. doi:10.1136/jnnp.2007.139717. PMID 18344378.
- ^ Mauritz M, Hirsch LJ, Camfield P, Chin R, Nardone R, Lattanzi S, et al. (2022). "Acute symptomatic seizures: an educational, evidence-based review". Epileptic Disorders. 24 (1): 26–49. doi:10.1684/epd.2021.1376. ISSN 1294-9361. PMID 34789447. Retrieved 19 September 2025.
- ^ a b Pitkänen A, Engel J (2014). "Past and present definitions of epileptogenesis and its biomarkers". Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 11 (2): 231–241. doi:10.1007/s13311-014-0257-2. ISSN 1878-7479. PMC 3996117. PMID 24492975.
- ^ Blumenfeld H (2005). "Cellular and network mechanisms of spike-wave seizures". Epilepsia. 46 (Suppl.9): 21–33. doi:10.1111/j.1528-1167.2005.00311.x. PMID 16302873.
- ^ Bromfield EB (2006). "Basic Mechanisms Underlying Seizures and Epilepsy". An Introduction to Epilepsy. American Epilepsy Society.
- ^ Somjen GG (2004). Ions in the Brain Normal Function, Seizures, and Stroke. New York: Oxford University Press. p. 167. ISBN 978-0-19-803459-9.
- ^ Engel J, Pedley TA, eds. (2008). Epilepsy: a comprehensive textbook (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 483. ISBN 978-0-7817-5777-5.
- ^ Le Van Quyen M, Navarro V, Martinerie J, Baulac M, Varela FJ (2003). "Toward a neurodynamical understanding of ictogenesis". Epilepsia. 44 (Suppl.12): 30–43. doi:10.1111/j.0013-9580.2003.12007.x. PMID 14641559.
- ^ Depannemaecker D, Ivanov A, Lillo D, Spek L, Bernard C, Jirsa V (February 2022). "A unified physiological framework of transitions between seizures, sustained ictal activity and depolarization block at the single neuron level". Journal of Computational Neuroscience. 50 (1): 33–49. doi:10.1007/s10827-022-00811-1. PMC 8818009. PMID 35031915.
- ^ Depannemaecker D, Destexhe A, Jirsa V, Bernard C (August 2021). "Modeling seizures: From single neurons to networks". Seizure. 90: 4–8. doi:10.1016/j.seizure.2021.06.015. PMID 34219016.
- ^ a b Noebels JL, Avoli M (29 June 2012). Jasper's Basic Mechanisms of the Epilepsies. Oxford University Press. pp. 466, 470. ISBN 978-0-19-974654-5. Retrieved 16 October 2014.
- ^ Panayiotopoulos CP (December 2011). "The new ILAE report on terminology and concepts for organization of epileptic seizures: a clinician's critical view and contribution". Epilepsia. 52 (12): 2155–2160. doi:10.1111/j.1528-1167.2011.03288.x. PMID 22004554.
- ^ a b Shorvon SD (June 2011). "The etiologic classification of epilepsy". Epilepsia. 52 (6): 1052–1057. doi:10.1111/j.1528-1167.2011.03041.x. PMID 21449936.
- ^ "Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy". Epilepsia. 30 (4): 389–399. 1989. doi:10.1111/j.1528-1157.1989.tb05316.x. PMID 2502382.
- ^ Engel J (August 2006). "ILAE classification of epilepsy syndromes". Epilepsy Research. 70 (Suppl 1): S5-10. doi:10.1016/j.eplepsyres.2005.11.014. PMID 16822650.
- ^ Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. (April 2010). "Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009". Epilepsia. 51 (4): 676–685. doi:10.1111/j.1528-1167.2010.02522.x. PMID 20196795.
- ^ Beniczky S (2025). "Updated classification of epileptic seizures: Position paper of the International League Against Epilepsy". Epilepsia. 66 (6): 1804–1823. doi:10.1111/epi.18338. PMC 12169392. PMID 40264351.
- ^ "Epilepsy syndromes". International league against epilepsy. Archived from the original on 6 October 2014. Retrieved 6 October 2014.
- ^ Wirrell EC, Nabbout R, Scheffer IE, Alsaadi T, Bogacz A, French JA, et al. (2022). "Methodology for classification and definition of epilepsy syndromes with list of syndromes: Report of the ILAE Task Force on Nosology and Definitions". Epilepsia. 63 (6): 1333–1348. doi:10.1111/epi.17237. ISSN 1528-1167. PMID 35503715.
- ^ Raga S, Specchio N, Rheims S, Wilmshurst JM (1 February 2021). "Developmental and epileptic encephalopathies: recognition and approaches to care". Epileptic Disorders: International Epilepsy Journal with Videotape. 23 (1): 40–52. doi:10.1684/epd.2021.1244. ISSN 1950-6945. PMID 33632673.
- ^ Nordli DR (October 2012). "Epileptic encephalopathies in infants and children". Journal of Clinical Neurophysiology. 29 (5): 420–424. doi:10.1097/WNP.0b013e31826bd961. PMID 23027099.
- ^ Ropper AH, Samuels MA, Klein JP, Prasad S (6 May 2023). "Chapter 15: Epilepsy and Other Seizure Disorders". Adams and Victor's Principles of Neurology, Twelfth Edition. McGraw Hill Professional. ISBN 978-1-264-26453-7.
- ^ Ricci L, Boscarino M, Assenza G, Tombini M, Lanzone J, Di Lazzaro V, et al. (2021). "Clinical utility of home videos for diagnosing epileptic seizures: a systematic review and practical recommendations for optimal and safe recording" (PDF). Neurological Sciences. 42 (4): 1301–1309. doi:10.1007/s10072-021-05040-5. ISSN 1590-1874. PMC 7815499. PMID 33471259. Retrieved 19 September 2025.
- ^ a b c d e f g h i j k l m Epilepsies in children, young people and adults. National Institute for Health and Care Excellence: Guidelines. London: National Institute for Health and Care Excellence (NICE). 2022. ISBN 978-1-4731-4513-9. PMID 35700280.
- ^ Wang YQ, Wen Y, Wang MM, Zhang YW, Fang ZX (2021). "Prolactin levels as a criterion to differentiate between psychogenic non-epileptic seizures and epileptic seizures: A systematic review". Epilepsy Research. 169 106508. doi:10.1016/j.eplepsyres.2020.106508. Retrieved 19 September 2025.
- ^ Brodtkorb E (2013). "Common imitators of epilepsy". Acta Neurologica Scandinavica. Supplementum. 127 (196): 5–10. doi:10.1111/ane.12043. PMID 23190285.
- ^ a b Marx JA, ed. (2010). Rosen's emergency medicine: concepts and clinical practice (7th ed.). Philadelphia: Mosby/Elsevier. p. 2228. ISBN 978-0-323-05472-0.
- ^ a b Jerome E (2013). Seizures and epilepsy (2nd ed.). New York: Oxford University Press. p. 462. ISBN 978-0-19-532854-7.
- ^ Oto M( (1 January 2017). "The misdiagnosis of epilepsy: Appraising risks and managing uncertainty". Seizure. 25th Anniversary Issue. 44: 143–146. doi:10.1016/j.seizure.2016.11.029. ISSN 1059-1311. PMID 28017581.
- ^ Xu Y, Nguyen D, Mohamed A, Carcel C, Li Q, Kutlubaev MA, et al. (1 October 2016). "Frequency of a false positive diagnosis of epilepsy: A systematic review of observational studies". Seizure - European Journal of Epilepsy. 41: 167–174. doi:10.1016/j.seizure.2016.08.005. ISSN 1059-1311. PMID 27592470.
- ^ Sen A, Jette N, Husain M, Sander JW (2020). "Epilepsy in older people". The Lancet. 395 (10225): 735–748. doi:10.1016/S0140-6736(19)33064-8. Retrieved 8 October 2025.
- ^ Haynes J, Patel K, Baca CM (2024). "Social Considerations in Epilepsy". Epilepsy Fundamentals. Cham: Springer Nature Switzerland. p. 171–181. doi:10.1007/978-3-031-77741-7_13. ISBN 978-3-031-77740-0. Retrieved 8 October 2025.
- ^ Glauser T, Becker DA, Long L, Detyniecki K, Penovich P, Sirven J, et al. (2024). "Short-Term Impact of Seizures and Mitigation Opportunities" (PDF). Current Neurology and Neuroscience Reports. 24 (8): 303–314. doi:10.1007/s11910-024-01350-1. ISSN 1528-4042. PMC 11258047. PMID 38940995. Retrieved 8 October 2025.
- ^ Duignan KM, Luu H, Delgado JH, London S, Ratzan RM (2024). "Drowning incidents precipitated by unusual causes (DIPUCs): A narrative review of their diagnoses, evaluation and management". Resuscitation Plus. 20 100770. doi:10.1016/j.resplu.2024.100770. PMC 11415818. PMID 39309751.
- ^ Modiano Y, Sullivan-Baca E (2024). "Neuropsychiatric Comorbidities". Epilepsy Fundamentals. Cham: Springer Nature Switzerland. p. 157–169. doi:10.1007/978-3-031-77741-7_12. ISBN 978-3-031-77740-0. Retrieved 8 October 2025.
- ^ Lu E, Pyatka N, Burant CJ, Sajatovic M (2021). "Systematic Literature Review of Psychiatric Comorbidities in Adults with Epilepsy". Journal of Clinical Neurology. 17 (2): 176. doi:10.3988/jcn.2021.17.2.176. ISSN 1738-6586. PMC 8053555. PMID 33835737.
- ^ Emerton BC, Morgan AK (2022). "Neuropsychological Comorbidities in Pediatric Epilepsy". Handbook of Pediatric Epilepsy. Cham: Springer International Publishing. p. 199–211. doi:10.1007/978-3-319-08290-5_9. ISBN 978-3-319-08289-9. Retrieved 8 October 2025.
- ^ Luo Y (2022). "Prognosis of Childhood Epilepsy". Handbook of Pediatric Epilepsy. Cham: Springer International Publishing. p. 213–226. doi:10.1007/978-3-319-08290-5_10. ISBN 978-3-319-08289-9. Retrieved 8 October 2025.
- ^ Helmers SL, Kobau R, Sajatovic M, Jobst BC, Privitera M, Devinsky O, et al. (March 2017). "Self-management in epilepsy: Why and how you should incorporate self-management in your practice". Epilepsy & Behavior. 68: 220–224. doi:10.1016/j.yebeh.2016.11.015. PMC 5381244. PMID 28202408.
- ^ a b Michael GE, O'Connor RE (February 2011). "The diagnosis and management of seizures and status epilepticus in the prehospital setting". Emergency Medicine Clinics of North America. 29 (1): 29–39. doi:10.1016/j.emc.2010.08.003. PMID 21109100.
- ^ Wheless JW, Willmore J, Brumback RA (2009). Advanced therapy in epilepsy. Shelton, Conn.: People's Medical Pub. House. p. 144. ISBN 978-1-60795-004-2.
- ^ Nevitt SJ, Marson AG, Tudur Smith C (July 2019). "Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review". The Cochrane Database of Systematic Reviews. 2019 (7) CD001911. doi:10.1002/14651858.CD001911.pub4. PMC 6637502. PMID 31318037.
- ^ Nevitt SJ, Marson AG, Weston J, Tudur Smith C (August 2018). "Sodium valproate versus phenytoin monotherapy for epilepsy: an individual participant data review". The Cochrane Database of Systematic Reviews. 2018 (8) CD001769. doi:10.1002/14651858.CD001769.pub4. PMC 6513104. PMID 30091458.
- ^ Powell G, Saunders M, Rigby A, Marson AG (9 December 2016). "Immediate-release versus controlled-release carbamazepine in the treatment of epilepsy". Cochrane Database of Systematic Reviews. 2017 (4) CD007124. doi:10.1002/14651858.CD007124.pub5. PMC 6463840. PMID 27933615.
- ^ Nevitt SJ, Sudell M, Cividini S, Marson AG, Tudur Smith C (April 2022). "Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data". The Cochrane Database of Systematic Reviews. 2022 (4) CD011412. doi:10.1002/14651858.CD011412.pub4. PMC 8974892. PMID 35363878.
- ^ Ilangaratne NB, Mannakkara NN, Bell GS, Sander JW (December 2012). "Phenobarbital: missing in action". Bulletin of the World Health Organization. 90 (12): 871–871A. doi:10.2471/BLT.12.113183. PMC 3524964. PMID 23284189.
- ^ Shorvon S, Perucca E, Engel Jr J, eds. (2009). The treatment of epilepsy (3rd ed.). Chichester, UK: Wiley-Blackwell. p. 587. ISBN 978-1-4443-1667-4. Archived from the original on 21 May 2016.
- ^ a b c d e f Perucca P, Gilliam FG (September 2012). "Adverse effects of antiepileptic drugs". The Lancet. Neurology. 11 (9): 792–802. doi:10.1016/S1474-4422(12)70153-9. PMID 22832500.
- ^ a b National Clinical Guideline Centre (January 2012). The Epilepsies: The diagnosis and management of the epilepsies in adults and children in primary and secondary care (PDF). National Institute for Health and Clinical Excellence. pp. 57–83. Archived (PDF) from the original on 16 December 2013.
- ^ a b c d Bromley R, Adab N, Bluett-Duncan M, Clayton-Smith J, Christensen J, Edwards K, et al. (August 2023). "Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child". The Cochrane Database of Systematic Reviews. 2023 (8) CD010224. doi:10.1002/14651858.CD010224.pub3. PMC 10463554. PMID 37647086.
- ^ a b Kamyar M, Varner M (June 2013). "Epilepsy in pregnancy". Clinical Obstetrics and Gynecology. 56 (2): 330–341. doi:10.1097/GRF.0b013e31828f2436. PMID 23563876.
- ^ Lawrence S. Neinstein, ed. (2008). Adolescent health care: a practical guide (5th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 335. ISBN 978-0-7817-9256-1.
- ^ "American Epilepsy Society Choosing Wisely". www.choosingwisely.org. 14 August 2018. Retrieved 30 August 2018.
- ^ a b c Engel J (2018). "The current place of epilepsy surgery". Current Opinion in Neurology. 31 (2): 192–197. doi:10.1097/WCO.0000000000000528. ISSN 1473-6551. PMC 6009838. PMID 29278548.
- ^ Kwan P, Brodie MJ (3 February 2000). "Early identification of refractory epilepsy". The New England Journal of Medicine. 342 (5): 314–319. doi:10.1056/NEJM200002033420503. ISSN 0028-4793. PMID 10660394.
- ^ Benoit PW, Yagiela A, Fort NF (February 1980). "Pharmacologic correlation between local anesthetic-induced myotoxicity and disturbances of intracellular calcium distribution". Toxicology and Applied Pharmacology. 52 (2): 187–198. Bibcode:1980ToxAP..52..187B. doi:10.1016/0041-008x(80)90105-2. PMID 7361318.
- ^ a b Krucoff MO, Chan AY, Harward SC, Rahimpour S, Rolston JD, Muh C, et al. (December 2017). "Rates and predictors of success and failure in repeat epilepsy surgery: A meta-analysis and systematic review". Epilepsia. 58 (12): 2133–2142. doi:10.1111/epi.13920. PMC 5716856. PMID 28994113.
- ^ Rosenow F, Bast T, Czech T, Feucht M, Hans VH, Helmstaedter C, et al. (2016). "Revised version of quality guidelines for presurgical epilepsy evaluation and surgical epilepsy therapy issued by the Austrian, German, and Swiss working group on presurgical epilepsy diagnosis and operative epilepsy treatment". Epilepsia. 57 (8): 1215–1220. doi:10.1111/epi.13449. ISSN 1528-1167. PMID 27354263.
- ^ Birbeck GL, Hays RD, Cui X, Vickrey BG (May 2002). "Seizure reduction and quality of life improvements in people with epilepsy". Epilepsia. 43 (5): 535–538. doi:10.1046/j.1528-1157.2002.32201.x. PMID 12027916.
- ^ a b c Duncan JS (April 2007). "Epilepsy surgery". Clinical Medicine. 7 (2): 137–142. doi:10.7861/clinmedicine.7-2-137. PMC 4951827. PMID 17491501.
- ^ Sharma M, Ball T, Alhourani A, Ugiliweneza B, Wang D, Boakye M, et al. (1 April 2020). "Inverse national trends of laser interstitial thermal therapy and open surgical procedures for refractory epilepsy: a Nationwide Inpatient Sample–based propensity score matching analysis". Neurosurgical Focus. 48 (4): E11. doi:10.3171/2020.1.FOCUS19935. ISSN 1092-0684. PMID 32234991.
- ^ Tsou AY, Kessler SK, Wu M, Abend NS, Massey SL, Treadwell JR (3 January 2023). "Surgical Treatments for Epilepsies in Children Aged 1–36 Months: A Systematic Review". Neurology. 100 (1): e1 – e15. doi:10.1212/WNL.0000000000201012. PMC 9827129. PMID 36270898.
- ^ Panebianco M, Rigby A, Marson AG (July 2022). "Vagus nerve stimulation for focal seizures". The Cochrane Database of Systematic Reviews. 2022 (7) CD002896. doi:10.1002/14651858.CD002896.pub3. PMC 9281624. PMID 35833911.
- ^ Edwards CA, Kouzani A, Lee KH, Ross EK (September 2017). "Neurostimulation Devices for the Treatment of Neurologic Disorders". Mayo Clinic Proceedings. 92 (9): 1427–1444. doi:10.1016/j.mayocp.2017.05.005. PMID 28870357.
- ^ Englot DJ, Chang EF, Auguste KI (2011). "Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response". Journal of Neurosurgery. 115 (6): 1248–1255. doi:10.3171/2011.7.JNS11977. ISSN 1933-0693. PMID 21838505.
- ^ Treadwell JR, Wu M, Tsou AY (2022). Management of Infantile Epilepsies: A Systematic Review (Report). doi:10.23970/AHRQEPCCER252. PMID 36383706.
- ^ Maria BL, ed. (2009). Current management in child neurology (4th ed.). Hamilton, Ont.: BC Decker. p. 180. ISBN 978-1-60795-000-4. Archived from the original on 24 June 2016.
- ^ Michaelis R, Tang V, Wagner JL, Modi AC, LaFrance WC, Goldstein LH, et al. (October 2017). "Psychological treatments for people with epilepsy". The Cochrane Database of Systematic Reviews. 10 (10) CD012081. doi:10.1002/14651858.CD012081.pub2. PMC 6485515. PMID 29078005.
- ^ Li D, Song Y, Zhang S, Qiu J, Zhang R, Wu J, et al. (1 January 2023). "Cognitive behavior therapy for depression in people with epilepsy: A systematic review and meta-analysis". Epilepsy & Behavior. 138 109056. doi:10.1016/j.yebeh.2022.109056. ISSN 1525-5050. PMID 36571868.
- ^ Verrotti A, Tocco AM, Salladini C, Latini G, Chiarelli F (November 2005). "Human photosensitivity: from pathophysiology to treatment". European Journal of Neurology. 12 (11): 828–841. doi:10.1111/j.1468-1331.2005.01085.x. PMID 16241971.
- ^ Tan G, Thornby J, Hammond DC, Strehl U, Canady B, Arnemann K, et al. (July 2009). "Meta-analysis of EEG biofeedback in treating epilepsy". Clinical EEG and Neuroscience. 40 (3): 173–179. doi:10.1177/155005940904000310. PMID 19715180.
- ^ Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, et al. (July 2018). "Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence". Journal of Neurology, Neurosurgery, and Psychiatry. 89 (7): 741–753. doi:10.1136/jnnp-2017-317168. hdl:1959.4/unsworks_50076. PMID 29511052.
- ^ Cannabis derivative may reduce seizures in some severe drug-resistant epilepsies, but adverse events increase (Report). 26 June 2018. doi:10.3310/signal-000606.
- ^ "Press Announcements - FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy". www.fda.gov. 25 June 2018. Retrieved 4 October 2018.[dead link]
- ^ Arida RM, Scorza FA, Scorza CA, Cavalheiro EA (March 2009). "Is physical activity beneficial for recovery in temporal lobe epilepsy? Evidences from animal studies". Neuroscience and Biobehavioral Reviews. 33 (3): 422–431. doi:10.1016/j.neubiorev.2008.11.002. PMID 19059282.
- ^ Arida RM, Cavalheiro EA, da Silva AC, Scorza FA (2008). "Physical activity and epilepsy: proven and predicted benefits". Sports Medicine. 38 (7): 607–615. doi:10.2165/00007256-200838070-00006. PMID 18557661.
- ^ Di Vito L, Naldi I, Mostacci B, Licchetta L, Bisulli F, Tinuper P (June 2010). "A seizure response dog: video recording of reacting behaviour during repetitive prolonged seizures". Epileptic Disorders. 12 (2): 142–145. doi:10.1684/epd.2010.0313. PMID 20472528.
- ^ Kirton A, Winter A, Wirrell E, Snead OC (October 2008). "Seizure response dogs: evaluation of a formal training program". Epilepsy & Behavior. 13 (3): 499–504. doi:10.1016/j.yebeh.2008.05.011. PMID 18595778.
- ^ Doherty MJ, Haltiner AM (January 2007). "Wag the dog: skepticism on seizure alert canines". Neurology. 68 (4): 309. doi:10.1212/01.wnl.0000252369.82956.a3. PMID 17242343.
- ^ Cheuk DK, Wong V (May 2014). "Acupuncture for epilepsy". The Cochrane Database of Systematic Reviews. 2014 (5) CD005062. doi:10.1002/14651858.CD005062.pub4. PMC 10105317. PMID 24801225.
- ^ Ranganathan LN, Ramaratnam S (April 2005). "Vitamins for epilepsy". The Cochrane Database of Systematic Reviews (2) CD004304. doi:10.1002/14651858.CD004304.pub2. PMID 15846704.
- ^ Panebianco M, Sridharan K, Ramaratnam S (October 2017). "Yoga for epilepsy". The Cochrane Database of Systematic Reviews. 2017 (10) CD001524. doi:10.1002/14651858.CD001524.pub3. PMC 6485327. PMID 28982217.
- ^ a b Brigo F, Igwe SC, Del Felice A (August 2016). "Melatonin as add-on treatment for epilepsy". The Cochrane Database of Systematic Reviews. 2016 (8) CD006967. doi:10.1002/14651858.CD006967.pub4. PMC 7386917. PMID 27513702.
- ^ a b c d e f g h i j k l m n o King A, Gerard EE (April 2022). "Contraception, fecundity, and pregnancy in women with epilepsy: an update on recent literature". Current Opinion in Neurology. 35 (2): 161–168. doi:10.1097/WCO.0000000000001039. PMC 9230745. PMID 35191408.
- ^ a b Reimers A, Brodtkorb E, Sabers A (May 2015). "Interactions between hormonal contraception and antiepileptic drugs: Clinical and mechanistic considerations". Seizure. 28: 66–70. doi:10.1016/j.seizure.2015.03.006. PMID 25843765.
- ^ "Enzyme-inducing antiepileptic drugs". NICE. May 2023. Retrieved 2 November 2023.
- ^ a b c Tomson T, Battino D, Bromley R, Kochen S, Meador K, Pennell P, et al. (December 2019). "Management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy". Epileptic Disorders. 21 (6): 497–517. doi:10.1684/epd.2019.1105. hdl:11336/119061. PMID 31782407.
- ^ Mohanraj R, Brodie MJ (1 June 2013). "Early predictors of outcome in newly diagnosed epilepsy". Seizure. 22 (5): 333–344. doi:10.1016/j.seizure.2013.02.002. ISSN 1059-1311. PMID 23583115.
- ^ Vergonjeanne M, Auditeau E, Erazo D, Luna J, Gelle T, Gbessemehlan A, et al. (21 September 2021). "Epidemiology of Epilepsy in Low- and Middle-Income Countries: Experience of a Standardized Questionnaire over the Past Two Decades". Neuroepidemiology. 55 (5): 369–380. doi:10.1159/000517065. ISSN 0251-5350. PMID 34315167.
- ^ a b c d e f g Klein P, Carrazana E, Glauser T, Herman BP, Penovich P, Rabinowicz AL, et al. (16 April 2025). "Do Seizures Damage the Brain?-Cumulative Effects of Seizures and Epilepsy: A 2025 Perspective". Epilepsy Currents 15357597251331927. doi:10.1177/15357597251331927. ISSN 1535-7597. PMC 12003328. PMID 40256117.
- ^ a b c Hoxhaj P, Habiya SK, Sayabugari R, Balaji R, Xavier R, Ahmad A, et al. (June 2023). "Investigating the Impact of Epilepsy on Cognitive Function: A Narrative Review". Cureus. 15 (6) e41223. doi:10.7759/cureus.41223. ISSN 2168-8184. PMC 10387362. PMID 37525802.
- ^ a b c Hitiris N, Mohanraj R, Norrie J, Brodie MJ (May 2007). "Mortality in epilepsy". Epilepsy & Behavior. 10 (3): 363–376. doi:10.1016/j.yebeh.2007.01.005. PMID 17337248.
- ^ a b c Shorvon S, Perucca E, Engel J, eds. (2009). The treatment of epilepsy (3rd ed.). Chichester, UK: Wiley-Blackwell. p. 28. ISBN 978-1-4443-1667-4. Archived from the original on 10 June 2016.
- ^ a b Bagary M (April 2011). "Epilepsy, antiepileptic drugs and suicidality". Current Opinion in Neurology. 24 (2): 177–182. doi:10.1097/WCO.0b013e328344533e. PMID 21293270.
- ^ Mula M, Sander JW (August 2013). "Suicide risk in people with epilepsy taking antiepileptic drugs". Bipolar Disorders. 15 (5): 622–627. doi:10.1111/bdi.12091. PMID 23755740.
- ^ a b Ryvlin P, Nashef L, Tomson T (May 2013). "Prevention of sudden unexpected death in epilepsy: a realistic goal?". Epilepsia. 54 (Suppl 2): 23–28. doi:10.1111/epi.12180. PMID 23646967.
- ^ Kwan P (2012). Fast facts: epilepsy (5th ed.). Abingdon, Oxford, UK: Health Press. p. 10. ISBN 978-1-908541-12-3.
- ^ Kløvgaard M, Sabers A, Ryvlin P (November 2022). "Update on Sudden Unexpected Death in Epilepsy". Neurologic Clinics. 40 (4): 741–754. doi:10.1016/j.ncl.2022.06.001. PMID 36270688.
- ^ a b Feigin VL, Vos T, Nair BS, Hay SI, Abate YH, Magied AH, et al. (1 March 2025). "Global, regional, and national burden of epilepsy, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021". The Lancet Public Health. 10 (3): e203 – e227. doi:10.1016/S2468-2667(24)00302-5. hdl:10067/1585180151162165141. ISSN 2468-2667. PMC 11876103. PMID 40015291.
- ^ Espinosa-Jovel C, Toledano R, Aledo-Serrano Á, García-Morales I, Gil-Nagel A (March 2018). "Epidemiological profile of epilepsy in low income populations". Seizure. 56: 67–72. doi:10.1016/j.seizure.2018.02.002. PMID 29453113.
- ^ a b Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. (2017). "Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies". Neurology. 88 (3): 296–303. doi:10.1212/WNL.0000000000003509. ISSN 0028-3878. PMC 5272794. PMID 27986877.
- ^ Beghi E (2019). "The Epidemiology of Epilepsy". Neuroepidemiology. 54 (2): 185–191. doi:10.1159/000503831. PMID 31852003.
- ^ a b c d e f g h Saraceno B, Avanzini G, Lee P, eds. (2005). Atlas: Epilepsy Care in the World. World Health Organization. ISBN 978-92-4-156303-1. Retrieved 21 October 2023.
- ^ Eadie MJ, Bladin PF (2001). A Disease Once Sacred: A History of the Medical Understanding of Epilepsy. John Libbey Eurotext. ISBN 978-0-86196-607-3.
- ^ "Epilepsy: An historical overview". World Health Organization. February 2001. Archived from the original on 30 October 2013. Retrieved 27 December 2013.
- ^ "Epilepsy: historical overview". World Health Organization. Archived from the original on 20 January 2011. Retrieved 20 March 2011.
- ^ a b Temkin O (1 March 1994). The Falling Sickness: A History of Epilepsy from the Greeks to the Beginnings of Modern Neurology. JHU Press. p. Section 1. ISBN 978-1-4214-0053-2.
- ^ Stol M (1993). Epilepsy in Babylonia. BRILL. p. 143. ISBN 978-90-72371-63-8.
- ^ Harding GF, Jeavons PM (1994). Photosensitive Epilepsy. Cambridge University Press. p. 2. ISBN 978-1-898683-02-5.
- ^ a b Jilek-Aall L (March 1999). "Morbus sacer in Africa: some religious aspects of epilepsy in traditional cultures". Epilepsia. 40 (3): 382–386. doi:10.1111/j.1528-1157.1999.tb00723.x. PMID 10080524.
- ^ "Epilepsy and its Management: A Review". ResearchGate. January 2012. Retrieved 22 February 2022.
- ^ Illes J (2011). Encyclopedia of Mystics, Saints & Sages. HarperCollins. p. 1238. ISBN 978-0-06-209854-2. Archived from the original on 11 January 2014.
Saint Valentine is invoked for healing as well as love. He protects against fainting and is requested to heal epilepsy and other seizure disorders. In northern Italy, epilepsy was once traditionally known as Saint Valentine's Malady.
- ^ a b c Lewis E (17 February 2012). Report of The Trial and Conviction of John Haggerty, for The Murder of Melchoir Fordney, Late of The City of Lancaster, Pennsylvania. Gale, Making of Modern Law. p. 62. ISBN 978-1-275-31136-7.
- ^ Caravati EM (2004). Medical toxicology (3rd ed.). Philadelphia [u.a.]: Lippincott Williams & Wilkins. p. 789. ISBN 978-0-7817-2845-4.
- ^ de Boer HM (December 2010). "Epilepsy stigma: moving from a global problem to global solutions". Seizure. 19 (10): 630–636. doi:10.1016/j.seizure.2010.10.017. PMID 21075013.
- ^ Mayor R, Gunn S, Reuber M, Simpson J (2022). "Experiences of stigma in people with epilepsy: A meta-synthesis of qualitative evidence". Seizure. 94: 142–160. doi:10.1016/j.seizure.2021.11.021.
- ^ Erkal E, Kiyak E, Uren Y, Milanlioglu A (October 2024). "Determination of stigma and attitude in relatives of patients with epilepsy". Seizure: European Journal of Epilepsy. 121: 64–69. doi:10.1016/j.seizure.2024.07.022. PMID 39089140.
- ^ a b Jacoby A, Austin JK (2007). "Social stigma for adults and children with epilepsy". Epilepsia. 48 (s9): 6–9. doi:10.1111/j.1528-1167.2007.01391.x. ISSN 1528-1167. PMID 18047591.
- ^ Camfield CS, Camfield PR (2007). "Long-term social outcomes for children with epilepsy". Epilepsia. 48 (s9): 3–5. doi:10.1111/j.1528-1167.2007.01390.x. ISSN 0013-9580. PMID 18047590.
- ^ Shi Y, Liu S, Wang J, Li C, Zhang J (2021). "Stigma experienced by patients with epilepsy: A systematic review and meta-synthesis of qualitative studies". Epilepsy & Behavior. 118 107926. doi:10.1016/j.yebeh.2021.107926. Retrieved 10 October 2025.
- ^ Begley C, Wagner RG, Abraham A, Beghi E, Newton C, Kwon CS, et al. (2022). "The global cost of epilepsy: A systematic review and extrapolation". Epilepsia. 63 (4): 892–903. doi:10.1111/epi.17165. ISSN 0013-9580. PMID 35195894.
- ^ Martindale JL, Goldstein JN, Pallin DJ (February 2011). "Emergency department seizure epidemiology". Emergency Medicine Clinics of North America. 29 (1): 15–27. doi:10.1016/j.emc.2010.08.002. PMID 21109099.
- ^ Pellinen J, Tafuro E, Yang A, Price D, Friedman D, Holmes M, et al. (December 2020). "Focal nonmotor versus motor seizures: The impact on diagnostic delay in focal epilepsy". Epilepsia. 61 (12): 2643–2652. doi:10.1111/epi.16707. PMID 33078409.
- ^ a b c d Engel J, Pedley TA, eds. (2008). Epilepsy: a comprehensive textbook (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 2279. ISBN 978-0-7817-5777-5.
- ^ Bor R (2012). Aviation Mental Health: Psychological Implications for Air Transportation. Ashgate Publishing. p. 148. ISBN 978-1-4094-8491-2.
- ^ a b "Seizure Disorders". Transport Canada. Government of Canada. Archived from the original on 30 December 2013. Retrieved 29 December 2013.
- ^ Wilner AN (2008). Epilepsy 199 answers: a doctor responds to his patients' questions (3rd ed.). New York: Demos Health. p. 52. ISBN 978-1-934559-96-3. Archived from the original on 17 May 2016.
- ^ "Guide for Aviation Medical Examiners". Federal Aviation Administration. Archived from the original on 17 October 2013. Retrieved 29 December 2013.
- ^ a b "National PPL (NPPL) Medical Requirements". Civil Aviation Authority. Archived from the original on 16 October 2013. Retrieved 29 December 2013.
- ^ Drivers Medical Group (2013). "For Medical Practitioners: At a glance Guide to the current Medical Standards of Fitness to Drive" (PDF). p. 8. Archived (PDF) from the original on 30 December 2013. Retrieved 29 December 2013.
- ^ "Global Campaign Against Epilepsy // International League Against Epilepsy". www.ilae.org. ILAE. Retrieved 28 September 2025.
- ^ "Epilepsy Foundation of America – EFA". Healthfinder.gov. US Department of Health and Human Services. 28 April 2011. Archived from the original on 16 July 2014. Retrieved 28 July 2014.
- ^ Engel J, Pedley TA, eds. (2008). Epilepsy: a comprehensive textbook (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 2245. ISBN 978-0-7817-5777-5.
- ^ Aleem MA (February 2015). "World epilepsy day". Epilepsia. 56 (2): 168. doi:10.1111/epi.12814. PMID 25404065.
- ^ Perucca E (February 2015). "Commentary: why an international epilepsy day?". Epilepsia. 56 (2): 170–171. doi:10.1111/epi.12813. PMID 25403985.
- ^ Carr F (26 March 2018). "People Are Wearing Purple Today for Epilepsy Awareness Day. Here's What That Is". Time. Retrieved 18 April 2018.
- ^ Guillemain, I., Kahane, P. & Depaulis, A. Animal models to study aetiopathology of epilepsy: what are the features to model? Epileptic Disord 14, 217–225 (2012).
- ^ Grone BP, Baraban SC (2015). "Animal models in epilepsy research: legacies and new directions". Nature Neuroscience. 18 (3): 339–343. doi:10.1038/nn.3934. ISSN 1097-6256. PMID 25710835.
- ^ Perucca P, Perucca E (1 May 2019). "Identifying mutations in epilepsy genes: Impact on treatment selection". Epilepsy Research. 152: 18–30. doi:10.1016/j.eplepsyres.2019.03.001. ISSN 0920-1211. PMID 30870728.
- ^ Smith RS, Kenny CJ, Ganesh V, Jang A, Borges-Monroy R, Partlow JN, et al. (5 September 2018). "Sodium Channel SCN3A (NaV1.3) Regulation of Human Cerebral Cortical Folding and Oral Motor Development". Neuron. 99 (5): 905–913.e7. doi:10.1016/j.neuron.2018.07.052. PMC 6226006. PMID 30146301.
- ^ Smith RS, Florio M, Akula SK, Neil JE, Wang Y, Hill RS, et al. (22 June 2021). "Early role for a Na + ,K + -ATPase ( ATP1A3 ) in brain development". Proceedings of the National Academy of Sciences. 118 (25) e2023333118. doi:10.1073/pnas.2023333118. PMC 8237684. PMID 34161264.
- ^ Pitkänen A, Engel J (1 April 2014). "Past and Present Definitions of Epileptogenesis and Its Biomarkers". Neurotherapeutics. 11 (2): 231–241. doi:10.1007/s13311-014-0257-2. ISSN 1878-7479. PMC 3996117. PMID 24492975.
- ^ Löscher W, Klein P (2021). "The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond". CNS Drugs. 35 (9): 935–963. doi:10.1007/s40263-021-00827-8. ISSN 1179-1934. PMC 8408078. PMID 34145528.
- ^ Rho JM, White HS (2018). "Brief history of anti-seizure drug development". Epilepsia Open. 3 (Suppl Suppl 2): 114–119. doi:10.1002/epi4.12268. ISSN 2470-9239. PMC 6293064. PMID 30564769.
- ^ Kuhlmann L, Lehnertz K, Richardson MP, Schelter B, Zaveri HP (2018). "Seizure prediction — ready for a new era". Nature Reviews Neurology. 14 (10): 618–630. doi:10.1038/s41582-018-0055-2. hdl:2164/11941. ISSN 1759-4766. PMID 30131521.
- ^ Jirsa VK, Stacey WC, Quilichini PP, Ivanov AI, Bernard C (August 2014). "On the nature of seizure dynamics". Brain. 137 (8): 2210–2230. doi:10.1093/brain/awu133. ISSN 1460-2156. PMC 4107736. PMID 24919973.
- ^ Chizhov AV, Zefirov AV, Amakhin DV, Smirnova EY, Zaitsev AV (31 May 2018). "Minimal model of interictal and ictal discharges "Epileptor-2"". PLOS Computational Biology. 14 (5) e1006186. Bibcode:2018PLSCB..14E6186C. doi:10.1371/journal.pcbi.1006186. ISSN 1553-7358. PMC 6005638. PMID 29851959.
- ^ "Epileptor-2 model".
- ^ Walker MC, Schorge S, Kullmann DM, Wykes RC, Heeroma JH, Mantoan L (September 2013). "Gene therapy in status epilepticus". Epilepsia. 54 (Suppl 6): 43–45. doi:10.1111/epi.12275. PMID 24001071.
- ^ Panebianco M, Walker L, Marson AG, et al. (Cochrane Epilepsy Group) (October 2023). "Immunomodulatory interventions for focal epilepsy". The Cochrane Database of Systematic Reviews. 2023 (10) CD009945. doi:10.1002/14651858.CD009945.pub3. PMC 10577807. PMID 37842826.
- ^ Quigg M, Rolston J, Barbaro NM (January 2012). "Radiosurgery for epilepsy: clinical experience and potential antiepileptic mechanisms". Epilepsia. 53 (1): 7–15. doi:10.1111/j.1528-1167.2011.03339.x. PMC 3519388. PMID 22191545.
- ^ Thomas WB (January 2010). "Idiopathic epilepsy in dogs and cats". The Veterinary Clinics of North America. Small Animal Practice. 40 (1): 161–179. doi:10.1016/j.cvsm.2009.09.004. PMID 19942062.
- ^ van der Ree M, Wijnberg I (2012). "A review on epilepsy in the horse and the potential of Ambulatory EEG as a diagnostic tool". The Veterinary Quarterly. 32 (3–4): 159–167. doi:10.1080/01652176.2012.744496. PMID 23163553.
- ^ Ciosek J, Kimes A, Vinardell T, Miller DC, Antczak DF, Brooks S (23 August 2023). "Juvenile idiopathic epilepsy in Arabian horses is not a single-gene disorder". Journal of Heredity. 114 (5): 488–491. doi:10.1093/jhered/esad029. PMID 37145017.
Further reading
[edit]- Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. (April 2017). "ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology". Epilepsia. 58 (4): 512–521. doi:10.1111/epi.13709. PMC 5386840. PMID 28276062.
- Programme for Neurological Diseases and Neuroscience (2005). Atlas, epilepsy care in the world, 2005. Global Campaign against Epilepsy; International League against Epilepsy. Geneva: Department of Mental Health and Substance Abuse, World Health Organization. ISBN 978-92-4-156303-1.
- Wilson JV, Reynolds EH (April 1990). "Texts and documents. Translation and analysis of a cuneiform text forming part of a Babylonian treatise on epilepsy". Medical History. 34 (2): 185–198. doi:10.1017/s0025727300050651. PMC 1036070. PMID 2187129.
External links
[edit]- "Epilepsy Basics: An Overview for Behavioral Health Providers". YouTube. Epilepsy Foundation. 30 May 2019. Archived from the original on 11 December 2021.
- "What To Do If Someone Has A Seizure – First Aid Training – St John Ambulance". YouTube. St John Ambulance. 1 February 2017. Archived from the original on 11 December 2021.
- World Health Organization fact sheet
Epilepsy
View on GrokipediaClinical Manifestations
Seizure Characteristics
Epileptic seizures are transient episodes of signs and/or symptoms attributable to abnormally excessive or synchronous neuronal activity in the brain, distinguishing them from nonepileptic events through their recurrent, unprovoked nature and association with epileptiform electroencephalographic patterns.[13] These events typically onset abruptly, last from seconds to a few minutes—most commonly 30 seconds to 2 minutes—and resolve spontaneously, though durations exceeding 5 minutes constitute status epilepticus requiring urgent intervention.[14] [15] Characteristics include stereotyped manifestations that vary by seizure type, influenced by the brain regions involved, with symptoms ranging from subtle behavioral changes to overt motor convulsions or altered consciousness.[16] The International League Against Epilepsy (ILAE) classifies seizures primarily by onset: focal (originating in networks limited to one hemisphere), generalized (involving both hemispheres from the outset), unknown (insufficient information to determine), or unclassified (atypical features).[17] Focal onset seizures may preserve awareness (focal aware) or impair it (focal impaired awareness), manifesting as motor phenomena like jerking or posturing in one body part, or non-motor features such as sensory auras (e.g., tingling, déjà vu), autonomic changes (e.g., nausea, piloerection), or cognitive disturbances (e.g., forced thinking).[18] [16] If focal seizures evolve to bilateral tonic-clonic activity, they exhibit stiffening (tonic phase) followed by rhythmic jerking (clonic phase), often with loss of postural control, urinary incontinence, and cyanosis.[19] Generalized onset seizures engage bilaterally distributed networks immediately, bypassing focal origins, and include absence seizures characterized by brief staring spells with subtle automatisms like eye blinking or lip smacking, lasting 5-10 seconds without post-event confusion.[20] Myoclonic seizures involve sudden, shock-like jerks of limbs, often upon awakening, while tonic seizures feature abrupt muscle stiffening leading to falls, and atonic seizures cause sudden loss of muscle tone resulting in head drops or collapses.[16] Generalized tonic-clonic seizures, previously termed grand mal, encompass widespread convulsions with potential tongue biting and frothing, reflecting diffuse cortical involvement.[5] Unknown onset seizures, such as those observed during sleep without video-EEG capture of onset, may later be reclassified with additional data, underscoring the diagnostic value of prolonged monitoring.[21] Variability in presentation necessitates individualized assessment, as semiological features like versive head turning or gelastic laughter can localize seizure foci precisely in presurgical evaluations.[22]Postictal Period
The postictal period refers to the transitional phase immediately following the cessation of a seizure, during which the brain recovers toward baseline function, often manifesting as transient neurological deficits.[23] This state typically begins when clinical seizure activity ends and persists until the individual regains normal alertness and motor control, with symptoms including confusion, drowsiness, headache, amnesia for the event, and sometimes sensory or language impairments.[24] In generalized tonic-clonic seizures, the postictal phase is often more pronounced, featuring profound somnolence and disorientation due to widespread cortical involvement, whereas focal seizures may produce localized deficits such as unilateral weakness.[25][26] Duration of the postictal state varies widely, averaging 5 to 30 minutes but extending to hours or even days in severe cases, influenced by factors like seizure length, patient age, and baseline neurological status.[27][28] A study of postictal scalp EEG suppression after focal seizures reported a mean duration of 275 seconds (ranging from 7 seconds to over 40 minutes), with longer seizures correlating to slower EEG recovery and more persistent abnormalities compared to pre-seizure baselines.[23][29] Approximately 60% of postictal episodes resolve within one hour, while 10% last longer, and symptoms such as fatigue can persist up to 24 hours on average.[30][31] A notable postictal phenomenon is Todd's paralysis (or paresis), characterized by temporary focal weakness or hemiplegia affecting one side of the body or a limb, typically emerging in the recovery phase after focal motor seizures.[32] This deficit, first described by Robert Bentley Todd in 1849, usually lasts from 30 minutes to 36 hours (mean around 15 hours) and resolves without permanent damage, distinguishing it from stroke via EEG evidence of epileptiform activity or clinical history.[33][34] It occurs in up to 13% of seizure patients and may follow either the first or recurrent events, with pathophysiology linked to transient neuronal exhaustion in the epileptogenic zone rather than structural injury.[35][36] Underlying mechanisms involve cerebral hypoperfusion, neurotransmitter imbalances (e.g., GABAergic inhibition overpowering excitation), and metabolic disruptions akin to hypoxic-ischemic states, leading to slowed neural firing and impaired synaptic function.[37] Postictal EEG often shows polymorphic delta activity or suppression, reflecting widespread cortical depression, while functional imaging reveals reduced blood flow in affected regions that normalizes over time.[23] These processes underscore the postictal state's role as a protective recovery mechanism, though prolonged impairments can mimic ongoing pathology and necessitate differentiation from non-epileptic events via clinical correlation and diagnostics.[38]Psychosocial Consequences
People with epilepsy often experience significant psychosocial challenges, including perceived stigma, which affects up to 80% of patients and correlates with lower socioeconomic status and reduced quality of life.[39] Stigma manifests as discrimination in social interactions, employment, and relationships, leading to isolation and diminished self-efficacy.[40] Empirical studies indicate that perceived stigma prevalence is approximately 35%, strongly linked to depressive symptoms and poorer social support.[41] Comorbid mental health disorders are prevalent, with depression affecting 23-34% of patients and anxiety impacting 31-56%, rates substantially higher than in the general population.[41][40] These conditions arise from factors such as unpredictable seizures, medication side effects, and societal misconceptions about epilepsy as a mental weakness, exacerbating overall psychological burden.[42] Suicide risk is elevated 2-5 times compared to individuals without epilepsy, accounting for about 11.5% of deaths in chronic epilepsy cases versus 1.6% in the general population.[43][44] Employment challenges compound these issues, with epilepsy associated with higher unemployment rates, job layoffs, and perceptions of unfitness for work, independent of seizure control.[45] Quality of life metrics, such as those from the QOLIE-31 survey, reveal low scores in domains like seizure worry (mean 46.05) and overall quality of life (mean 44.21), influenced by ongoing seizures, polypharmacy, and low household income.[46] About one-third of patients exhibit poor quality of life, primarily due to adverse effects from antiseizure medications and stigma-related barriers.[47] Social consequences extend to family dynamics and education, where unpredictable seizures hinder participation and foster dependency, though resilience factors like strong social support can mitigate depressive and anxious symptoms.[48] Interventions targeting stigma reduction and mental health screening are critical, as untreated psychosocial burdens independently predict suicidality and treatment non-adherence.[49][50]Etiology
Genetic Contributions
Epilepsy exhibits a substantial genetic component, with heritability estimates derived from twin studies ranging from 25% to 70%, indicating that genetic factors significantly influence susceptibility across various syndromes.[51] Monozygotic twins demonstrate higher concordance rates for epilepsy than dizygotic twins, with 83% of affected monozygotic pairs sharing the same major epilepsy syndrome compared to 65% in dizygotic pairs, underscoring the role of shared genetics over environmental influences alone.[52] These findings affirm that while epilepsy often follows complex inheritance patterns involving multiple genes and environmental interactions, inherited variants account for a large proportion of risk, particularly in idiopathic generalized epilepsies where common genetic variants explain 39.6% to 90% of the genetic liability.[53] Monogenic forms of epilepsy, characterized by mutations in single genes, predominate in developmental and epileptic encephalopathies, often involving ion channel genes that disrupt neuronal excitability. For instance, mutations in SCN1A, encoding a sodium channel subunit, cause over 80% of Dravet syndrome cases, a severe infantile-onset epilepsy with refractory seizures and cognitive impairment, and also contribute to milder phenotypes like genetic epilepsy with febrile seizures plus (GEFS+).[54] Similarly, variants in KCNQ2 and KCNQ3, which encode potassium channels, underlie benign familial neonatal seizures and sometimes progress to more severe epileptic encephalopathies.[55] Other notable genes include SCN2A for early infantile developmental epileptic encephalopathy and CDKL5 for CDKL5 deficiency disorder, which disproportionately affects females due to X-linked inheritance patterns.[56] Over 900 epilepsy-associated genes have been identified, categorized by their roles in ion transport, synaptic function, and neuronal development, with ion channelopathies representing the most common class in non-structural epilepsies.[57][58] In contrast, common epilepsies like genetic generalized epilepsy (GGE) arise from polygenic risk, where genome-wide association studies (GWAS) have pinpointed 26 loci, implicating 29 causal genes such as SV2A and NRXN1 that influence neuronal signaling pathways.[53] Familial aggregation studies further reveal enrichment of common risk variants in multiplex families, supporting a threshold model where cumulative genetic burden lowers seizure threshold in the presence of triggers.[59] Epigenetic modifications, including DNA methylation and histone alterations, may modulate these genetic risks, potentially explaining variable expressivity, though empirical data linking specific epigenetic changes to epilepsy onset remain preliminary.[51] Genetic testing, such as targeted panels or exome sequencing, confirms diagnoses in up to 40% of suspected genetic cases, guiding precision therapies like sodium channel blockers tailored to SCN1A-related disorders.[60] Despite advances, the "missing heritability" persists, with rare variants and gene-environment interactions accounting for unresolved variance in population-level risk.[61]Structural Abnormalities
Structural abnormalities encompass congenital malformations of cortical development, acquired lesions such as hippocampal sclerosis, neoplasms, and vascular anomalies that disrupt normal brain architecture and neuronal excitability, thereby contributing to epileptogenesis. These lesions often manifest as focal epilepsies, with seizures originating from the irritative focus created by the abnormality. Magnetic resonance imaging (MRI) detects many such structures, guiding diagnosis and surgical planning in refractory cases.[62][63] Malformations of cortical development, particularly focal cortical dysplasia (FCD), involve localized disruptions in neuronal migration, proliferation, or organization during embryogenesis. FCD is characterized by abnormal cortical lamination, giant neurons, or balloon cells, depending on subtype (type I-III per Taylor classification). It accounts for up to 25-40% of pediatric epilepsy surgery cases and is a leading cause of intractable focal seizures in children.[64][65] Surgical resection of FCD lesions achieves seizure freedom in 50-70% of cases, underscoring the direct causal role of the malformation.[66] Hippocampal sclerosis (HS), also termed mesial temporal sclerosis, features neuronal loss and gliosis primarily in the CA1 and CA3 regions of the hippocampus, often bilateral but more epileptogenic when unilateral. It predominates in mesial temporal lobe epilepsy, comprising 50-75% of temporal lobectomy specimens in surgical series. Population prevalence estimates for HS-associated epilepsy range from 19.4 per 100,000 adults, with annual incidence around 2.3 per 100,000. Early febrile seizures or prolonged status epilepticus may precipitate HS via excitotoxic mechanisms, though causality remains debated as imaging often reveals sclerosis post-onset.[67][68][69] Neoplastic causes include low-grade gliomas, gangliogliomas, and dysembryoplastic neuroepithelial tumors (DNETs), which irritate surrounding cortex through mass effect, peritumoral edema, or neurotransmitter dysregulation. Seizures herald 30-80% of supratentorial tumors, particularly in temporal or frontal lobes, and may precede radiographic detection by years in slow-growing lesions. Resection yields seizure control in 60-90% of cases for benign tumors, contrasting poorer outcomes in high-grade malignancies where epileptogenicity stems from rapid invasion.[70][71] Vascular malformations, such as cavernous malformations (cavernomas) and arteriovenous malformations (AVMs), provoke epilepsy via chronic hemorrhage, ischemia, or gliosis in adjacent parenchyma. Cavernomas associate with seizures in 30-70% of supratentorial cases, often supratentorial and presenting with focal seizures. AVMs yield epilepsy in 20-50% of patients, linked to hemodynamic steal or perilesional scarring. Microsurgical or radiosurgical intervention reduces seizure recurrence, with lesionectomy achieving 60-80% freedom rates in select cohorts.[72][73]Infectious and Immune Factors
Infections of the central nervous system (CNS) constitute a significant etiology of acquired epilepsy, primarily through mechanisms involving acute inflammation, neuronal injury, and chronic structural changes such as hippocampal sclerosis or cortical scarring that lower seizure thresholds.[74] Bacterial, viral, parasitic, and fungal pathogens can initiate these processes, with the risk of epileptogenesis persisting months to years post-infection due to persistent inflammation or gliosis.[75] In developed countries, post-infectious epilepsy affects approximately 7-8% of adult survivors of CNS infections, while in resource-limited settings, infectious etiologies account for up to 30-50% of new-onset epilepsy cases, driven by endemic pathogens.[76] [77] Parasitic infections, particularly neurocysticercosis caused by the larval stage of Taenia solium, represent the most common identifiable infectious cause of epilepsy globally, especially in Latin America, sub-Saharan Africa, and Asia where prevalence exceeds 10% in endemic porcine farming communities.[78] Cysts in the brain parenchyma provoke perilesional edema and calcification upon degeneration, fostering epileptogenic foci; surgical or antiparasitic treatment reduces seizure recurrence by 50-70% in symptomatic cases.[79] Other parasites like Toxoplasma gondii and cerebral malaria (Plasmodium falciparum) contribute via vascular occlusion or granuloma formation, with malaria-associated epilepsy reported in 5-10% of severe pediatric cases in endemic regions.[80] [81] Viral encephalitides, including herpes simplex virus type 1 (HSV-1), Japanese encephalitis virus, and emerging pathogens like SARS-CoV-2, induce epilepsy through direct cytopathic effects and secondary excitotoxicity.[82] HSV-1 encephalitis carries a 20-50% risk of refractory temporal lobe epilepsy in survivors, often linked to mesial temporal sclerosis identifiable on MRI.[74] Bacterial meningitides, such as those from Streptococcus pneumoniae or Neisseria meningitidis, yield epilepsy in 5-10% of pediatric survivors, exacerbated by ventriculitis or abscess formation.[81] Tuberculous meningitis, prevalent in high-burden areas, associates with chronic epilepsy in up to 25% of cases due to basal exudates and infarcts.[75] Immune-mediated factors in epilepsy encompass autoimmune encephalitides where autoantibodies target neuronal surface antigens, disrupting synaptic transmission and provoking inflammation independent of prior infection in many instances.[83] Anti-NMDA receptor encephalitis, often affecting young females and linked to ovarian teratomas in 50% of cases, manifests with refractory seizures in 70-80% of patients, responsive to immunotherapy like rituximab or cyclophosphamide.[84] Other antibodies, such as anti-LGI1 or anti-CASPR2, predominate in limbic encephalitis with faciobrachial dystonic seizures, yielding chronic epilepsy if untreated but remission rates exceeding 70% with early steroids and IVIG.[85] Mechanisms include complement activation and T-cell infiltration, with molecular mimicry from infections like HSV proposed but not universally required; Rasmussen's encephalitis exemplifies a unihemispheric autoimmune process with progressive atrophy.[86] [87] Diagnosis relies on serum/CSF antibody panels, as EEG often shows extreme delta brush patterns, and delays beyond 4 weeks correlate with poorer seizure control.[88]Metabolic and Environmental Influences
Metabolic disorders contribute to epilepsy through disruptions in energy production, neurotransmitter synthesis, or accumulation of toxic metabolites, often manifesting as early-onset refractory seizures. Inborn errors of metabolism (IEMs) account for approximately 1-7% of neonatal seizures and are a core feature in certain inherited metabolic diseases, where epilepsy arises directly from the underlying biochemical defect rather than secondary effects.[89][90] Examples include glucose transporter 1 (GLUT1) deficiency syndrome, caused by variants in the SLC2A1 gene that impair glucose transport across the blood-brain barrier, leading to hypoglycorrhachia and pharmacoresistant epilepsy typically presenting in infancy with absence or myoclonic seizures.[91] Urea cycle disorders, such as ornithine transcarbamylase deficiency, result in hyperammonemia, which triggers cerebral edema and seizures through excitotoxic mechanisms, with epilepsy persisting in up to 50% of survivors despite treatment.[92] Mitochondrial disorders, affecting oxidative phosphorylation, and pyridoxine-dependent epilepsy from ALDH7A1 variants, which disrupt vitamin B6 metabolism and GABA synthesis, further exemplify how metabolic pathway failures drive epileptogenesis via neuronal hyperexcitability and energy failure.[93][94] Acute metabolic derangements, such as hyponatremia, hypoglycemia, or hypocalcemia, can provoke seizures but rarely lead to chronic epilepsy unless recurrent or associated with an underlying IEM; for instance, nonketotic hyperglycinemia from glycine cleavage system defects causes intractable neonatal epilepsy due to glycine-mediated NMDA receptor overstimulation.[94] Early identification via newborn screening or targeted metabolic testing is critical, as some forms, like biotinidase deficiency or cerebral folate transporter deficiency, respond to cofactor supplementation (e.g., biotin or folinic acid), potentially halting epileptogenesis.[95] However, many IEM-related epilepsies remain refractory, underscoring the need for causal intervention over symptomatic anticonvulsant therapy alone.[96] Environmental exposures to neurotoxins represent modifiable risk factors for epilepsy, particularly through oxidative stress, excitotoxicity, or disruption of neuronal signaling pathways. Chronic occupational exposure to pesticides has been associated with elevated epilepsy risk, with a 2023 study reporting odds ratios up to 2.5 for generalized and focal seizures in exposed agricultural workers, likely due to organophosphate-induced acetylcholinesterase inhibition and subsequent glutamate dysregulation.[97] Organic solvents, such as toluene or benzene derivatives, correlate with new-onset epilepsy in case series, where solvent-induced GABAergic inhibition and kindling-like effects precipitate recurrent seizures following prolonged inhalation or dermal contact.[98] Air pollution, including fine particulate matter (PM2.5) and nitrogen dioxide (NO2), exacerbates epileptogenesis; a 2025 analysis found PM2.5 exposure increases epilepsy incidence by promoting neuroinflammation and ferroptosis via Nrf2 pathway impairment, with relative risks rising 1.1-1.3 per 10 μg/m³ increment in urban cohorts.[99] Methylmercury, from environmental contamination like fish consumption, acts as a gene-environment interactor, potentiating seizures in susceptible individuals through cerebellar and hippocampal damage.[100] These influences highlight preventable etiology, though causality requires longitudinal evidence beyond acute toxicity, as most exposures provoke isolated seizures rather than idiopathic epilepsy syndromes.[101]Cases of Unknown Origin
Cases of unknown origin, also known as idiopathic or cryptogenic epilepsy, encompass instances where recurrent seizures occur without identifiable structural, genetic, metabolic, infectious, or immune abnormalities following comprehensive evaluation, including neuroimaging, EEG, and laboratory tests.[102] These cases are distinguished by the absence of evident brain lesions or systemic disorders that could precipitate epileptiform activity, often aligning with age-specific generalized seizure syndromes such as childhood absence epilepsy or juvenile myoclonic epilepsy.[103] In such epilepsies, seizures typically manifest as generalized tonic-clonic, absence, or myoclonic types, with patients exhibiting normal interictal neurological function and no progressive cognitive decline.[102] Globally, the etiology remains unknown in approximately 50% of epilepsy cases, affecting an estimated 24.2 million individuals with idiopathic epilepsy as of recent burden assessments.[5] [6] This proportion varies by age and region; in children, up to 65-70% of cases may lack a discernible cause, while in adults, structural factors like stroke reduce the unknown category to around one-third.[104] [105] High-income countries report slightly lower rates of unknown etiology due to advanced diagnostics, yet the figure persists at 40-50% even with genetic testing, underscoring gaps in causal identification.[106] Although labeled "unknown," many such cases implicate subtle genetic susceptibilities, evidenced by familial clustering and polygenic risk factors, though specific mutations are not always pinpointed without whole-genome sequencing.[102] EEG patterns in these epilepsies often show generalized spike-and-wave discharges without focal abnormalities, supporting a primary cortical hyperexcitability origin rather than secondary propagation from lesions.[107] Ongoing research highlights the need for deeper genomic and environmental interaction studies, as current classifications like those from the International League Against Epilepsy (ILAE) increasingly reassign some "unknown" cases to genetic categories upon molecular discovery, yet a substantial remainder defies etiological assignment.[108] This diagnostic uncertainty complicates prognosis, with unknown-origin epilepsies generally responding well to antiepileptic drugs but carrying risks of pharmacoresistance in 20-30% of instances.[109]Pathophysiology
Seizure Generation Mechanisms
Seizure generation, or ictogenesis, involves the abrupt transition from interictal to ictal states through hypersynchronous neuronal firing, primarily driven by an imbalance between excitatory and inhibitory neurotransmission.[110] At the cellular level, this arises from ion channel dysfunctions, such as mutations or dysregulation in voltage-gated sodium channels (e.g., NaV1.1), which prolong action potentials via persistent sodium currents, reducing the threshold for depolarization.[111] Potassium channel impairments, including reduced Kir4.1 expression in astrocytes, fail to buffer extracellular K⁺ effectively, leading to elevated [K⁺]ₒ levels (8–16 mM) that depolarize neurons and promote hyperexcitability, as demonstrated in hippocampal slice models.[110][8] Synaptic mechanisms contribute via excessive glutamate release activating NMDA and AMPA receptors, causing calcium influx and excitotoxicity, while GABAergic inhibition paradoxically facilitates synchronization at high frequencies due to chloride accumulation and depolarizing shifts.[8][110] Homeostatic synaptic plasticity, such as AMPA receptor upregulation following insults like traumatic brain injury, further amplifies excitatory drive, with computational models showing bistable network states where small perturbations trigger seizure-like activity.[110] In focal epilepsies, interictal spikes recruit adjacent neurons through ephaptic interactions and endogenous electric fields, independent of chemical synapses, escalating to ictal onset.[110] Network-level dynamics involve preictal changes, including progressive desynchronization followed by hypersynchrony in thalamocortical circuits for generalized seizures or localized cortical networks for focal ones.[111] Empirical data from rodent models and human intracranial EEG reveal that short-term synaptic plasticity over seconds—either facilitation or depression—can initiate ictal bursts, with Na⁺/K⁺ ATPase activation eventually terminating seizures via postictal hyperpolarization.[110] These processes differ from epileptogenesis, which establishes chronic susceptibility, but share causal roots in ionic homeostasis disruption and synaptic reorganization.[8]Processes of Epileptogenesis
Epileptogenesis refers to the dynamic, multifactorial process by which a previously normal brain develops the capacity for spontaneous recurrent seizures, often following an initial precipitating insult such as trauma, infection, or status epilepticus, though it can occur without identifiable triggers in genetic forms.[112] This transformation involves progressive molecular, cellular, and network-level alterations that lower the seizure threshold and promote hyperexcitability, culminating in chronic epilepsy.[8] The process typically unfolds over a latent period—a seizure-free interval lasting days to years—during which maladaptive changes accumulate without overt clinical manifestations, challenging the notion of a discrete "silent" phase as subclinical events may contribute to progression.[112] At the molecular level, epileptogenesis entails transcriptomic and epigenetic reprogramming, including dysregulation of microRNAs such as miR-134 and miR-106b-5p, which modulate immune responses and neuronal excitability.[8] Hyperactivation of pathways like mTOR disrupts autophagy, synapse formation, and neuronal survival, as seen in models with TSC1/TSC2 mutations.[8] Ion channel genes, including SCN1A (voltage-gated sodium) and KCNQ2/KCNQ3 (potassium), exhibit mutations or altered expression, shifting membrane potentials toward depolarization and reducing refractory periods.[8] Neuroinflammation amplifies these effects through cytokines like IL-1β and TNF-α, released by activated microglia and astrocytes, which upregulate NMDA receptor subunits (e.g., GluN2B) and downregulate GABA receptors, fostering a pro-excitatory milieu.[8] [113] Cellular changes during epileptogenesis include selective neuronal loss, particularly in hippocampal regions like CA1 and CA3, coupled with aberrant neurogenesis in the dentate gyrus, where newborn neurons migrate ectopically to hyperexcitable zones such as the hilus or molecular layer.[112] Axonal sprouting, exemplified by mossy fiber collaterals from granule cells synapsing onto neighboring granule cells, creates recurrent excitatory loops that bypass inhibitory interneurons.[112] Dendritic remodeling and gliosis further contribute, with reactive astrocytes impairing potassium buffering and glutamate uptake, while blood-brain barrier leakage permits inflammatory cell infiltration.[112] Loss of GABAergic inhibition, via reduced KCC2 expression or NKCC1 upregulation, shifts chloride gradients to depolarizing levels in immature or injured neurons.[8] Network-level reorganization manifests as imbalanced excitation-inhibition dynamics and rewired circuits, with diminished interneuronal control allowing synchronized population bursts.[112] In animal models of status epilepticus, these changes progressively increase seizure frequency and severity over weeks, reflecting a vicious cycle where early seizures reinforce hyperexcitability through oxidative stress and mitochondrial dysfunction.[112] Human studies corroborate this, showing upregulated chemokines like CCL2 in intractable epilepsy tissues, linking inflammation to sustained circuit pathology.[8] Despite advances in models, the precise causality remains debated, as interventions targeting single mechanisms (e.g., mTOR inhibitors like rapamycin) show promise in preclinical latent-phase blockade but limited translation to humans.[113]Diagnosis
Definitional Criteria
Epilepsy is operationally defined by the International League Against Epilepsy (ILAE) as a brain disorder characterized by at least two unprovoked (or reflex) seizures occurring more than 24 hours apart, reflecting an enduring predisposition to generate epileptic seizures and associated cognitive, psychological, and social consequences.[114] This criterion distinguishes epilepsy from isolated or provoked seizures, where immediate causes such as acute metabolic disturbances, toxins, or structural insults are identifiable and transient.[115] Unprovoked seizures lack such identifiable proximate triggers, implying an underlying chronic brain dysfunction.[116] An alternative diagnostic pathway applies when only one unprovoked seizure occurs but with a confirmed probability of recurrence comparable to the general risk after two unprovoked events—at least 60% over the subsequent 10 years—or when an epilepsy syndrome is diagnosed based on characteristic clinical, electroencephalographic, and genetic features.[114][117] Syndrome-based diagnosis is particularly relevant in pediatric cases, such as infantile spasms or Lennox-Gastaut syndrome, where seizure patterns, age of onset, and EEG abnormalities align with established epileptic entities independent of seizure count.[118] Reflex seizures, triggered by specific stimuli like flashing lights or reading, are incorporated if unprovoked by acute factors and recurrent.[119] The definition excludes conditions resolved by time or intervention, such as childhood absence epilepsy after adolescence or post-surgical remission, to focus on active disease states requiring management.[114] Diagnosis hinges on clinical history corroborated by electroencephalography (EEG) to confirm epileptiform activity, though normal interictal EEG does not preclude epilepsy if history meets criteria.[115] This operational framework, updated in 2014, prioritizes practical clinical utility over purely conceptual descriptions, enabling earlier intervention while minimizing overdiagnosis from nonepileptic events like syncope or psychogenic seizures.[116]Classification Systems
The International League Against Epilepsy (ILAE) provides the predominant frameworks for classifying epileptic seizures and epilepsies, emphasizing observable features, onset location, and clinical utility to support diagnosis, treatment, and research.[120] These systems evolved from earlier iterations, such as the 1981 classifications, to incorporate advances in neuroimaging, genetics, and electrophysiology, prioritizing biological classifiers that influence management over purely descriptive terms.[13] The operational classification of seizure types, revised in 2017 and updated in 2025, categorizes seizures into four main classes: focal (originating in networks limited to one cerebral hemisphere), generalized (involving bilateral networks from onset), unknown (insufficient evidence to determine focal or generalized), and unclassified (lacking sufficient features for any class).[121] [13] The 2025 update refines the 2017 framework by reducing named seizure types from 63 to 21, removing "onset" from class nomenclature, replacing "awareness" with "consciousness" (defined by both awareness and responsiveness), and substituting "observable" (e.g., visible movements or behaviors) for "motor" and "non-observable" (e.g., subjective sensations) for "non-motor" manifestations.[121] [122] Seizures are further described chronologically by semiology (signs and symptoms) using ILAE glossary terms, with additions like epileptic negative myoclonus (brief interruption of ongoing muscle activity) as a distinct type; neonatal seizures are excluded and addressed separately.[121] Examples include focal seizures with impaired consciousness and observable clonic manifestations, generalized absence seizures (brief non-observable lapses), and unknown-onset tonic-clonic seizures.[13] The 2017 ILAE classification of epilepsies builds on seizure type identification to delineate three hierarchical levels: seizure types (per the seizure classification), epilepsy types (focal, generalized, combined generalized and focal, or unknown), and epilepsy syndromes (specific clusters with defined age of onset, seizure types, EEG patterns, and etiology).[123] Focal epilepsy involves seizures from one hemisphere, often linked to structural lesions; generalized epilepsy features bilateral onset without focal features; combined types exhibit both; unknown applies when data are inadequate.[123] Etiology—spanning genetic, structural, metabolic, immune, infectious, or unknown—is evaluated at each level to inform prognosis and therapy, such as targeting ion channel mutations in genetic generalized epilepsies.[123] Syndrome recognition, the highest level, applies to entities like Lennox-Gastaut syndrome (multiple seizure types, cognitive impairment, specific EEG) only when criteria are met, avoiding overgeneralization.[123] These frameworks integrate comorbidities and precision approaches, though ongoing refinements address gaps in unknown etiologies and atypical presentations.[123]Syndrome Recognition
An epilepsy syndrome is defined as a characteristic cluster of electroclinical features, encompassing specific seizure types, age at onset, etiology, comorbidities, and neuroimaging or genetic findings, which collectively predict treatment response and prognosis.[124][118] Recognition requires integration of clinical history, including seizure semiology and developmental status, with ancillary data such as interictal and ictal electroencephalography (EEG) patterns, which often exhibit syndrome-specific signatures like generalized 3 Hz spike-and-wave discharges.[125][126] The International League Against Epilepsy (ILAE) positions syndrome identification as the highest tier in its diagnostic framework, following seizure type and epilepsy type classification, to enable targeted management.[123] Diagnosis typically begins with a thorough patient and family history to establish seizure frequency, triggers, and associated neurological or cognitive impairments, supplemented by prolonged video-EEG monitoring to correlate behavioral events with electrophysiological abnormalities.[127][128] Structural imaging via MRI identifies lesions in focal syndromes, while genetic testing confirms etiologies in developmental syndromes, such as SCN1A mutations in Dravet syndrome.[129] Syndrome recognition is prognostically critical: benign syndromes like childhood absence epilepsy (onset ages 4-10 years, characterized by brief staring spells and 3 Hz generalized spike-wave EEG, with >70% remission by adolescence) contrast with refractory ones like Lennox-Gastaut syndrome (onset 1-8 years, multiple seizure types including tonic and atonic, slow spike-wave EEG <2.5 Hz, poor seizure control in 80-90% of cases).[130][124] Common syndromes illustrate recognition patterns: Juvenile myoclonic epilepsy (onset adolescence, myoclonic jerks on awakening, photosensitivity, 4-6 Hz polyspike-wave EEG, lifetime persistence but >90% seizure freedom with valproate) relies on EEG confirmation of generalized epileptiform discharges.[130][131] Infantile spasms (West syndrome, onset 3-12 months, flexor/extensor spasms in clusters, hypsarrhythmia on EEG, etiology-specific prognosis with 50% developmental delay if untreated) demand early EEG to differentiate from mimics and guide ACTH or vigabatrin therapy.[129][132] Failure to recognize syndromes promptly can delay etiology-directed interventions, as in genetic generalized epilepsies where misclassification as focal delays broad-spectrum antiseizure medications.[133] ILAE classifications, updated through 2022 for neonatal/infant onset, underscore age-stratified criteria to refine diagnostic accuracy across the lifespan.[134][135]Diagnostic Testing
Electroencephalography (EEG) serves as the cornerstone electrophysiological test in epilepsy diagnosis, detecting abnormal brain electrical activity indicative of epileptiform discharges.[136] Routine scalp EEG exhibits low sensitivity of approximately 17% for identifying interictal epileptiform discharges in adults following a first unprovoked seizure, though specificity reaches 94.7%.[137] Despite this, EEG remains essential for classifying seizure types and supporting clinical suspicion, as up to 50% of epilepsy patients may have normal interictal recordings.[136] Prolonged or ambulatory EEG improves yield, with sensitivity for detecting abnormalities rising to 72% compared to 11% for initial routine sessions.[138] Video-EEG monitoring combines EEG with synchronized video to capture ictal events, enabling differentiation between epileptic seizures and nonepileptic events, which is critical given that psychogenic nonepileptic seizures mimic epilepsy in up to 20-30% of refractory cases referred for evaluation.[139] Sleep deprivation or activation procedures, such as hyperventilation and photic stimulation, enhance diagnostic utility during EEG by provoking discharges in susceptible individuals.[136] Intracranial EEG, involving invasive electrodes, is reserved for presurgical localization in drug-resistant epilepsy, offering higher spatial resolution for focal onset identification.[140] Neuroimaging, particularly magnetic resonance imaging (MRI), is recommended for all newly diagnosed patients except those with confirmed idiopathic generalized epilepsy to identify structural lesions such as hippocampal sclerosis, tumors, or malformations contributing to seizures.[141] MRI surpasses computed tomography (CT) in sensitivity for epileptogenic foci, detecting subtle cortical dysplasia or mesial temporal sclerosis missed by CT, which is preferred only in acute settings for rapid exclusion of hemorrhage or mass effect due to its speed and availability.[142] [143] Advanced techniques like functional MRI (fMRI) coupled with EEG or positron emission tomography (PET) aid in noninvasive localization for surgical candidates but are not routine for initial diagnosis.[140] Laboratory evaluations, including complete blood count, electrolytes, glucose, and toxicology screens, rule out acute symptomatic causes like hyponatremia or drug intoxication precipitating seizures.[139] Genetic testing is indicated for suspected syndromes, such as Dravet or Lennox-Gastaut, where mutations in SCN1A or other genes confirm etiology in 10-20% of pediatric cases.[144] Lumbar puncture may be performed if infection or inflammation is suspected, particularly in new-onset seizures with fever or altered mental status.[145] In summary, no single test confirms epilepsy definitively; integration of EEG findings, neuroimaging, and laboratory results with detailed clinical history optimizes accuracy, as isolated normal tests do not exclude the diagnosis.[146] Guidelines emphasize early EEG within 24 hours post-seizure and MRI for comprehensive evaluation, reducing misdiagnosis rates that can exceed 30% without multimodal assessment.[145] [147]Differential Considerations
Accurate diagnosis of epilepsy requires distinguishing true epileptic seizures, characterized by transient hypersynchronous neuronal discharges, from other paroxysmal events that mimic them clinically. Misdiagnosis is common, with up to 20-30% of patients referred to epilepsy centers ultimately found to have nonepileptic conditions, leading to unnecessary antiepileptic drug exposure and delayed appropriate treatment.[148] [149] Key differentials include syncope, psychogenic nonepileptic seizures (PNES), migraines, transient ischemic attacks (TIAs), metabolic derangements, movement disorders, and sleep-related phenomena, differentiated primarily through detailed history, eyewitness accounts, and confirmatory testing like video-electroencephalography (EEG).[4] Syncope, the second most common epilepsy mimic after PNES, involves transient cerebral hypoperfusion leading to loss of consciousness, often with brief myoclonic jerks in convulsive forms that resemble tonic-clonic seizures. Precipitants include vasovagal triggers, orthostatic changes, or cardiac arrhythmias, with characteristic prodromal symptoms such as nausea, pallor, diaphoresis, and visual blurring preceding collapse; recovery is rapid without postictal confusion, unlike epilepsy. Distinction relies on absence of epileptiform EEG changes during events and cardiovascular evaluation, such as tilt-table testing or Holter monitoring.[4] [150] Psychogenic nonepileptic seizures (PNES), the leading misdiagnosis in refractory epilepsy referrals, manifest as episodes of involuntary movements, unresponsiveness, or convulsions driven by psychological factors rather than cerebral electrical abnormalities. Semiology often includes asynchronous thrashing, pelvic thrusting, side-to-side head shaking, and preserved awareness or eye-opening, contrasting with the stereotyped progression and postictal state in epileptic seizures; events may be prolonged, occur in crowds, or cease with distraction. Video-EEG captures normal background rhythms without ictal epileptiform activity, confirming PNES, which affects 2-33% of epilepsy clinic patients depending on setting.[148] [151] [149] Migraines, particularly those with auras, can imitate focal aware or focal impaired awareness seizures through transient visual scintillations, sensory paresthesias, or aphasia, but symptoms build gradually over 5-20 minutes, last 20-60 minutes, and frequently progress to headache with nausea—features atypical for seizures. Basilar-type or hemiplegic migraines may cause confusion or hemiparesis mimicking complex partial seizures, yet EEG remains normal, and response to migraine prophylactics supports the diagnosis over antiepileptics.[150] [4] Transient ischemic attacks (TIAs) produce focal neurological deficits like hemiparesis or speech arrest that may resemble focal seizures, but TIAs feature negative symptoms (e.g., weakness without clonic activity) with abrupt onset and resolution within 24 hours, often linked to vascular risk factors; neuroimaging reveals ischemia without epileptogenic lesions, and EEG lacks seizure correlates.[150] [152] Metabolic disturbances, such as hypoglycemia (blood glucose <50 mg/dL) or electrolyte imbalances like hyponatremia, provoke altered mentation, tremors, or focal deficits mimicking seizures, particularly in diabetics or those on diuretics; urgent laboratory testing reveals reversible biochemical abnormalities, with EEG showing diffuse slowing rather than focal epileptiform discharges.[150] [4] Movement disorders including paroxysmal kinesigenic dyskinesia or tics present with episodic dystonia, chorea, or tremors without loss of consciousness, differing from seizures by trigger association (e.g., sudden movement) and retained awareness; normal interictal EEG and response to specific therapies like carbamazepine aid differentiation.[150] Sleep disorders such as narcolepsy with cataplexy or parasomnias (e.g., night terrors) cause sudden atonia or confusional arousals mimicking absence or myoclonic seizures, but polysomnography demonstrates REM intrusions or non-rapid eye movement disruptions without epileptiform activity, with episodes confined to sleep-wake transitions.[150] [4] Prolonged video-EEG monitoring, capturing stereotypical versus variable event patterns, remains the gold standard for resolving ambiguities, reducing misdiagnosis rates from 30% to under 10% in specialized centers.[148] [151]Prevention
Primary Prevention Measures
Preventing traumatic brain injury (TBI) represents the most effective primary prevention strategy for epilepsy, as TBI is a leading cause of acquired epilepsy, contributing to up to 20% of cases in high-income countries.[5] Measures include mandatory seatbelt and child restraint use in vehicles, helmet wearing during cycling, motorcycling, and contact sports, and environmental modifications to reduce falls, particularly among children and the elderly, such as installing handrails and non-slip flooring.[153] These interventions have demonstrated reductions in TBI incidence; for instance, helmet laws correlate with a 13-29% decrease in motorcycle-related head injuries.[154] Immunization programs targeting pathogens that cause central nervous system infections are critical, as such infections like bacterial meningitis and viral encephalitis precede approximately 10-20% of epilepsy cases globally.[5] Vaccines against Haemophilus influenzae type b, pneumococcus, meningococcus, measles, mumps, and rubella have lowered post-infectious epilepsy rates; measles vaccination alone has prevented millions of encephalitis cases since its introduction in 1963.[153] In endemic regions, deworming and sanitation improvements to curb neurocysticercosis from Taenia solium—responsible for up to 30% of epilepsy in parts of Latin America, Africa, and Asia—have shown efficacy, with mass treatment campaigns reducing prevalence by 50% in pilot areas.[154] Perinatal care interventions address birth-related insults, which cause 10-15% of epilepsy, particularly in low-resource settings. Folic acid supplementation (400-800 mcg daily) preconception and during early pregnancy reduces neural tube defects by 50-70%, some of which progress to epilepsy syndromes like infantile spasms.[153] Optimizing maternal health to avoid complications such as prolonged labor or hypoxia, through skilled birth attendance and emergency obstetric care, further mitigates risks; programs in developing countries have halved perinatal asphyxia-related epilepsy.[5] Preventing cerebrovascular events like stroke, which underlie 10-15% of new-onset epilepsy in adults over 50, involves controlling modifiable risk factors. Lifestyle measures such as smoking cessation, blood pressure management below 130/80 mmHg, diabetes control (HbA1c <7%), and statin use in high-risk individuals reduce stroke incidence by 20-30%, thereby averting post-stroke epilepsy.[155] Public health efforts emphasizing these, including community screening, yield long-term benefits, as evidenced by declines in stroke-related epilepsy following antihypertensive campaigns.[154] For genetic epilepsies, comprising 40-50% of cases with known familial patterns, primary prevention is limited to preconception counseling and prenatal testing in high-risk pedigrees, such as those with Dravet syndrome linked to SCN1A mutations, though ethical and technical barriers persist.[155] Overall, integrated public health approaches combining these strategies could prevent up to 25% of epilepsy cases worldwide, per modeling from the World Health Organization.[5]Secondary Prophylaxis
Secondary prophylaxis in epilepsy refers to interventions aimed at preventing seizure recurrence following an initial unprovoked seizure, prior to a formal diagnosis of epilepsy, which requires at least two such events separated by more than 24 hours. The rationale centers on mitigating the risk of early relapse, which, without treatment, stands at approximately 40-50% within two years of the first seizure, with the highest probability occurring in the initial 6-12 months.[156] [157] Factors elevating recurrence risk include abnormal electroencephalogram (EEG) findings, structural brain lesions identifiable on neuroimaging, a history of remote symptomatic seizures, or seizures arising from sleep, with hazard ratios for recurrence ranging from 1.4 to 3.0 depending on the predictor.[157] [158] Initiation of antiepileptic drugs (AEDs) immediately after a first unprovoked seizure reduces the two-year recurrence risk by about 35% compared to deferred treatment, achieving absolute risk reductions of 15-20% in adults, though this does not alter the long-term probability of developing epilepsy (defined as recurrent unprovoked seizures).[159] [157] Evidence from randomized controlled trials, such as the First Seizure Trial Group study involving 1,078 adults, supports this short-term benefit, with treated patients experiencing seizure-free rates of 75% at two years versus 58% in untreated groups; however, quality-of-life measures and cognitive side effects of AEDs, including sedation and mood alterations, must be weighed, as these can offset gains in seizure control.[160] The American Academy of Neurology (AAN) recommends counseling patients on these trade-offs, favoring immediate AEDs in high-risk cases (e.g., abnormal EEG or epileptiform discharges) but deferring in low-risk scenarios where recurrence odds are below 25% at two years.[157] In pediatric populations, guidelines generally advise against routine AED initiation after a single seizure, as recurrence rates mirror adults but remit spontaneously in up to 70% of cases without intervention, and early treatment does not prevent epilepsy development.[161] [162] Decisions should incorporate patient-specific elements, such as seizure semiology (e.g., focal vs. generalized), comorbidities, and lifestyle impacts; for instance, immediate treatment may be prioritized for those in high-stakes occupations like driving or operating machinery, where a second seizure could pose immediate safety risks.[159] Once a second seizure occurs, transitioning to definitive epilepsy management with sustained AED therapy becomes standard, as cumulative recurrence risk exceeds 60-90%.[156] Non-pharmacological secondary prophylaxis lacks robust evidence in this context, though lifestyle modifications—such as avoiding sleep deprivation, alcohol excess, and triggers like flashing lights—are universally advised as adjuncts, supported by observational data linking these factors to lowered seizure thresholds in susceptible individuals.[159] Ongoing monitoring via follow-up EEG or neuroimaging refines risk stratification, but prophylactic AEDs beyond seven days are not endorsed in provoked seizures (e.g., post-traumatic or metabolic), where addressing the underlying cause suffices.[163] Overall, secondary prophylaxis emphasizes individualized assessment over blanket application, prioritizing empirical risk data to balance seizure prevention against treatment burdens.Management
Acute Intervention Protocols
For witnessed tonic-clonic seizures without immediate life-threatening features, initial interventions prioritize safety and monitoring rather than active pharmacological suppression. Caregivers should remain calm, stay with the individual, time the seizure duration, clear the area of hazards, cushion the head, and avoid restraining movements or inserting objects into the mouth, as these actions risk injury without altering seizure progression.[164][165] Once convulsions cease, position the person in the recovery position on their side to maintain airway patency and monitor breathing and responsiveness.[164] Emergency medical services should be activated if the seizure exceeds 5 minutes, repeats without recovery, involves respiratory compromise, or occurs in someone without known epilepsy.[164][165] Status epilepticus, defined as continuous seizure activity lasting at least 5 minutes or recurrent seizures without recovery between episodes, requires urgent escalation to abort neuronal injury from prolonged excitotoxicity.[166] Protocols begin with stabilization of airway, breathing, and circulation (ABCs), including oxygen administration and intravenous access, within the first 0-5 minutes.[166] First-line treatment involves benzodiazepines: intravenous lorazepam at 0.1 mg/kg (maximum 4 mg per dose, repeatable once after 5 minutes) achieves seizure cessation in approximately 60-80% of cases due to enhancement of GABA-mediated inhibition.[166][167] Alternatives include intramuscular midazolam 10 mg (adults) or intranasal/buccal midazolam if IV access is delayed, with comparable efficacy in prehospital settings.[166] If seizures persist after two benzodiazepine doses (typically by 10-20 minutes), second-line therapies target sustained antiseizure effects: fosphenytoin 20 mg PE/kg IV (maximum 150 mg/min rate) or alternatives like levetiracetam 60 mg/kg IV (maximum 4500 mg) or valproate 40 mg/kg IV, selected based on patient factors such as age, comorbidities, and etiology.[166][167] These agents reduce recurrence risk by 40-60% in this phase, though evidence from randomized trials shows no single drug superior.[166] For refractory cases (>30-60 minutes), transfer to intensive care with continuous EEG monitoring; induce general anesthesia using propofol (1-2 mg/kg bolus then 2-10 mg/kg/h infusion) or midazolam (0.2 mg/kg bolus then 0.4 mg/kg/h), titrated to burst suppression on EEG to halt subclinical activity.[167] Underlying causes, such as metabolic derangements or infections, must be addressed concurrently, as untreated precipitants like hyponatremia contribute to 20-30% of cases.[168]Pharmacological Treatments
Antiseizure medications (ASMs), formerly known as antiepileptic drugs, constitute the cornerstone of pharmacological management for epilepsy, targeting the suppression of abnormal neuronal excitability to achieve seizure freedom or significant reduction in frequency.[169] Selection of an ASM is guided by seizure type, epilepsy syndrome, patient age, comorbidities, and potential adverse effects, with monotherapy preferred initially for newly diagnosed cases. Approximately 47% of patients become seizure-free on the first ASM, rising to 62% with a second agent, though around 30% develop drug-resistant epilepsy requiring alternative strategies.[170] The International League Against Epilepsy (ILAE) recommends antiseizure over antiepileptic terminology to reflect that these agents primarily control seizures without addressing underlying epileptogenic processes.[171] ASMs are classified by predominant mechanisms of action, including modulation of voltage-gated ion channels, enhancement of inhibitory neurotransmission, or reduction of excitatory signaling. Sodium channel blockers, such as carbamazepine and lamotrigine, stabilize neuronal membranes by prolonging the inactive state of voltage-gated sodium channels, proving effective for focal seizures with response rates of 60-70% in monotherapy trials.[172][170] GABAergic agents like valproate and benzodiazepines augment gamma-aminobutyric acid-mediated inhibition; valproate, a broad-spectrum option, achieves seizure control in 50-60% of generalized epilepsy cases but carries risks of hepatotoxicity and teratogenicity, limiting use in women of childbearing potential.[170] Ethosuximide, targeting T-type calcium channels, remains first-line for absence seizures, with 70% efficacy in controlled studies compared to 50% for broader agents like valproate.[170] Newer ASMs, including levetiracetam (SV2A modulator) and lacosamide (sodium channel and CRMP-2 binder), offer improved tolerability and fewer drug interactions, suitable for focal and generalized epilepsies. Levetiracetam controls seizures in 40-60% of refractory cases as add-on therapy, with behavioral side effects like irritability in 10-15% of users.[173] Perampanel, an AMPA receptor antagonist, reduces focal seizure frequency by 20-30% in adjunctive use but is associated with dizziness and psychiatric effects.[170] Polytherapy for drug-resistant epilepsy involves rational combinations leveraging complementary mechanisms, though it increases risks of adverse events and interactions; for instance, enzyme-inducing ASMs like phenytoin can reduce efficacy of oral contraceptives by accelerating metabolism.[174]| ASM Class/Example | Primary Mechanism | Key Indications | Common Adverse Effects | Efficacy Notes |
|---|---|---|---|---|
| Sodium Channel Blockers (e.g., Carbamazepine, Lamotrigine) | Prolong sodium channel inactivation | Focal seizures | Dizziness, rash (lamotrigine: 5-10% Stevens-Johnson risk) | 60-70% response in focal epilepsy monotherapy[172][170] |
| GABA Enhancers (e.g., Valproate, Vigabatrin) | Increase GABA levels or receptor affinity | Generalized tonic-clonic, myoclonic | Weight gain, tremor, hepatotoxicity (valproate) | Broad-spectrum; 50-60% control in idiopathic generalized epilepsy[170] |
| Calcium Channel Blockers (e.g., Ethosuximide) | Block T-type calcium currents | Absence seizures | Gastrointestinal upset, sedation | Superior to valproate for absence (70% vs. 50%)[170] |
| SV2A Modulators (e.g., Levetiracetam) | Bind synaptic vesicle protein 2A | Focal, generalized | Irritability, somnolence | 40-60% add-on efficacy in refractory cases[173] |
Surgical Options
Surgical interventions are considered for patients with drug-resistant epilepsy, defined as failure to achieve seizure control after adequate trials of at least two appropriately chosen antiepileptic drugs, affecting approximately 30% to 40% of individuals with epilepsy.[178] These procedures aim to either resect or ablate the epileptogenic zone, disconnect seizure propagation pathways, or modulate neural activity through implanted devices, with candidacy determined by presurgical evaluation including video-EEG monitoring, MRI, and sometimes invasive recordings to localize the seizure focus.[179] Outcomes vary by procedure type, epilepsy etiology, and patient factors, but successful surgery can yield seizure freedom rates of 50% to 80% in select cases, alongside reductions in antiepileptic drug requirements and improved quality of life.[180] Resective surgery involves excision of the seizure-onset zone, most commonly temporal lobectomy for mesial temporal sclerosis, achieving seizure freedom in about 60% to 70% of patients at long-term follow-up.[181] Frontal or parietal resections yield lower rates, around 40% to 50%, due to broader networks involved.[182] Risks include visual field deficits, language impairments, or cognitive changes, occurring in 5% to 10% of cases, though mortality is under 1%.[179] Ablative techniques, such as laser interstitial thermal therapy (LITT), use MRI-guided laser probes to thermally destroy deep or eloquent-area foci via small burr holes, offering a minimally invasive alternative to open resection with seizure freedom in 50% to 65% of mesial temporal lobe epilepsy patients.[183] LITT reduces operative time and hospital stay compared to craniotomy but may require repeat procedures in 10% to 20% of cases for incomplete ablation.[184] Disconnective procedures like corpus callosotomy sever interhemispheric connections to halt bilateral synchrony in Lennox-Gastaut syndrome or atonic drop attacks, reducing generalized tonic-clonic or atonic seizures by 70% to 90% without eliminating focal origins.[185] Anterior two-thirds callosotomy minimizes disconnection syndrome risks like alien hand, while full section is reserved for refractory cases.[186] Neuromodulation devices provide palliative options for multifocal or non-resectable epilepsy. Vagus nerve stimulation (VNS) implants electrodes on the vagus nerve to deliver intermittent pulses, yielding 50% to 65% seizure reduction in responders after 6 to 12 months, with efficacy increasing over time.[187] Responsive neurostimulation (RNS) detects electrocorticographic abnormalities and delivers targeted cortical stimulation, reducing disabling seizures by 50% to 75% over years in focal epilepsy.[188] Deep brain stimulation (DBS) to the anterior thalamic nucleus, FDA-approved in 2018, achieves median 50% seizure frequency decrease at 5 years, particularly for tonic-clonic events.[189] These devices carry infection risks (2% to 5%) and require battery replacements but avoid tissue resection.[190] Patient selection emphasizes comprehensive evaluation to balance potential benefits against procedural morbidity.[191]Dietary Interventions
The ketogenic diet, a high-fat, low-carbohydrate regimen that induces ketosis by mimicking fasting states, has been employed as a treatment for epilepsy since the 1920s and is particularly indicated for drug-resistant cases after failure of two or more antiepileptic drugs.[192] Clinical evidence from multicenter studies demonstrates that approximately 51% of patients achieve greater than 50% seizure reduction, with 32% experiencing over 90% reduction, across various seizure types and patient ages.[193] A meta-analysis reports a 53% combined efficacy rate for seizure reduction and 13% for seizure freedom in drug-resistant epilepsy.[194] The diet's multimodal mechanisms, including altered neuronal excitability and enhanced GABAergic inhibition, contribute to its antiseizure effects, though long-term adherence remains challenging due to gastrointestinal side effects like constipation and potential risks such as kidney stones or dyslipidemia, necessitating medical supervision.[195][196] Variants of the ketogenic diet offer less restrictive alternatives, improving tolerability while maintaining efficacy. The classic ketogenic diet enforces a strict 3:1 or 4:1 ratio of fat to combined protein and carbohydrates by weight, typically limiting carbohydrates to 10-20 grams daily.[197] The modified Atkins diet (MAD), allowing 15-20 grams of net carbohydrates per day with emphasis on high-fat intake but without precise weighing, achieves at least 50% seizure reduction in 70% of pediatric patients in small cohorts and is preferred for adolescents and adults due to its palatability and reduced monitoring burden.[198][199] The low glycemic index treatment (LGIT), restricting intake to carbohydrates with a glycemic index below 50 and totaling 40-60 grams daily, stabilizes blood glucose fluctuations and yields seizure reductions comparable to MAD with fewer adverse events, as evidenced in comparative studies of pediatric epilepsy.[200][201] Guidelines from epilepsy specialists recommend initiating these therapies under multidisciplinary oversight, including neurologists and dietitians, with baseline assessments of nutritional status, lipids, and bone health, followed by periodic monitoring to mitigate complications like growth delays in children or weight loss in adults.[202] Efficacy persists in select cohorts beyond one year, with seizure freedom rates of 10-15% in refractory cases, though outcomes vary by epilepsy syndrome, such as superior responses in myoclonic-astatic epilepsy.[203] Discontinuation is considered after two years of seizure freedom, with relapse risks assessed individually.[197] Emerging data suggest microbiome modulation may enhance ketogenic diet benefits, but causal links require further validation.[204] In contrast to the ketogenic diet and its variants, which have established clinical evidence for treating drug-resistant epilepsy, no dietary supplements are conclusively proven by high-quality scientific evidence to effectively treat epilepsy or reduce seizures as a standard therapy. Systematic reviews, including Cochrane reviews, have found insufficient or no reliable evidence for common supplements such as omega-3 fatty acids (polyunsaturated fatty acids), vitamin E, folic acid, thiamine, or vitamin D in improving seizure control.[205][206] Vitamin D may be recommended to prevent bone loss associated with antiepileptic drugs, but not for seizure reduction. Herbal supplements lack evidence of effectiveness and may worsen seizures or interact with medications. The ketogenic diet has evidence for some drug-resistant cases but is not a supplement. Consult a healthcare professional before using any supplements.[207]Adjunctive Approaches
Vagus nerve stimulation (VNS) serves as an FDA-approved neuromodulation therapy for drug-resistant epilepsy, involving surgical implantation of a pulse generator in the chest that delivers intermittent electrical impulses to the left vagus nerve via an electrode, thereby modulating thalamocortical networks to suppress seizure activity.[208] Approved in 1997 for patients aged 12 and older with refractory partial-onset seizures, VNS has demonstrated seizure frequency reductions of approximately 50% in responsive patients, with long-term data from randomized controlled trials indicating sustained benefits over multiple years without curing the underlying condition.[209] [210] Adverse effects include hoarseness, cough, and infection risk at implantation, occurring in up to 20-30% of cases initially but often diminishing with time.[208] Responsive neurostimulation (RNS) represents a closed-loop device system implanted intracranially to detect electrocorticographic seizure patterns in real-time and deliver targeted electrical stimulation to interrupt abnormal activity, primarily for adults with focal epilepsy refractory to medications and ineligible for resective surgery.[211] FDA-cleared in 2013, pivotal trials reported a median 37.9% reduction in seizure frequency at one year and 75% at nine years in open-label extensions, with seizure freedom achieved in subsets of patients through adaptive programming.[212] [213] Risks encompass surgical complications like hemorrhage (1-2%) and device malfunction, alongside potential cognitive effects monitored via integrated electrocorticography recording.[210] Deep brain stimulation (DBS) of the anterior nucleus of the thalamus provides another adjunctive option, where bilateral electrodes deliver continuous high-frequency pulses to disrupt epileptogenic circuits, suitable for multifocal or generalized refractory epilepsy.[208] FDA-approved in 2018 based on the SANTE trial, which showed a 56% median seizure reduction at seven years in randomized and long-term follow-up cohorts, though efficacy varies by epilepsy type and electrode placement precision.[210] Common side effects include stimulation-induced paresthesia, ataxia, and mood alterations, with infection rates below 5% in experienced centers.[208] These therapies, while supported by level I evidence from double-blind trials, function palliatively alongside continued antiseizure medications rather than as standalone cures.[210]Reproductive and Familial Aspects
Contraceptive Interactions
Certain antiepileptic drugs (AEDs), particularly enzyme-inducing agents such as carbamazepine, phenytoin, phenobarbital, primidone, oxcarbazepine, eslicarbazepine acetate, and topiramate (at doses exceeding 200 mg/day), accelerate the hepatic metabolism of hormonal contraceptives via induction of cytochrome P450 3A4 enzymes, thereby reducing serum concentrations of ethinylestradiol and progestins by 40-50% or more.[214]00076-X/fulltext) This pharmacokinetic interaction diminishes the efficacy of combined oral contraceptives (OCs), progestin-only pills, implants, and depot medroxyprogesterone acetate (DMPA) injections, elevating the risk of ovulation and unintended pregnancy.[215][216] In contrast, non-enzyme-inducing AEDs like valproate, gabapentin, lamotrigine (at standard doses), levetiracetam, and lacosamide do not significantly impair hormonal contraceptive effectiveness.[214][217] Bidirectional interactions occur with lamotrigine, where estrogen-containing contraceptives can halve lamotrigine plasma levels through enhanced glucuronidation, potentially precipitating breakthrough seizures in women stabilized on this AED.00076-X/fulltext)[218] For women on enzyme-inducing AEDs, guidelines recommend non-hormonal methods such as copper intrauterine devices (IUDs), which remain unaffected, or barrier methods; if hormonal options are preferred, higher-dose OCs (≥50 μg ethinylestradiol) with shortened pill-free intervals or continuous regimens may partially mitigate reduced efficacy, though failure rates can still exceed 3-6% annually without additional measures.[217][219] Progestin-only methods like etonogestrel implants or DMPA show variable attenuation with inducers, often warranting avoidance or dual protection.[214][220] Emergency contraception, including levonorgestrel or ulipristal acetate pills and copper IUDs, can be used without restriction in women with epilepsy, as AEDs do not substantially alter their pharmacokinetics.[217] Counseling should emphasize preconception planning, as unintended pregnancies in this population carry risks of fetal malformations from teratogenic AEDs like valproate.[221] Clinicians must verify specific AED profiles, as newer agents like cenobamate or brivaracetam may exhibit partial induction, and monitor for clinical outcomes rather than relying solely on theoretical predictions.00076-X/fulltext)[215]Pregnancy Management
Women with epilepsy face elevated risks during pregnancy, including potential increases in seizure frequency and teratogenic effects from antiepileptic drugs (AEDs), which necessitate preconception planning and multidisciplinary care to balance maternal seizure control against fetal harm.[222] Uncontrolled seizures, particularly tonic-clonic types, pose dangers such as maternal injury, hypoxia, and fetal distress, underscoring the need to maintain effective AED therapy rather than discontinuation.[223] Approximately 15-30% of women experience worsened seizure control during pregnancy, often attributable to physiological changes like altered AED pharmacokinetics, sleep disruption, or hormonal fluctuations, while most maintain stable frequencies.[224] [225] Preconception counseling should prioritize switching from high-risk AEDs like valproate, which carries the highest teratogenic potential—including up to 10% risk of major congenital malformations (MCMs) such as neural tube defects, cardiac anomalies, and cleft palate—toward lower-risk options like lamotrigine, levetiracetam, or oxcarbazepine, associated with MCM rates closer to 2-3%.[226] [227] Polytherapy further elevates risks, so monotherapy is preferred when feasible.[228] Folic acid supplementation at minimum 0.4 mg daily, and up to 4-5 mg for those on AEDs, is recommended preconceptionally and throughout pregnancy to mitigate neural tube defect risks, though evidence for higher doses preventing other AED-related malformations remains inconclusive.[223] [229] During pregnancy, therapeutic drug monitoring is essential due to increased AED clearance—e.g., lamotrigine levels may drop by 50-100%—requiring dose adjustments to sustain efficacy without excessive dosing.[230] Fetal ultrasonography and anomaly scans are advised, particularly in the second trimester, to detect MCMs, with overall malformation rates in epilepsy pregnancies ranging 4-8% versus 2-3% in the general population.[222] Seizure precipitants like fatigue and nonadherence should be addressed through lifestyle measures, including consistent sleep and avoidance of triggers.[225] Labor and delivery typically favor vaginal routes unless obstetric indications dictate otherwise; epidural anesthesia is safe but may lower seizure threshold slightly, and operative interventions like vacuum extraction should be minimized to reduce maternal stress.[231] Postpartum, seizure risk surges due to sleep deprivation and rapid AED clearance reversal, warranting close monitoring and prompt dose optimization.[230] Breastfeeding is generally compatible with most AEDs, as infant exposure remains low (e.g., <10% of maternal dose for lamotrigine or levetiracetam), with no demonstrated adverse neurodevelopmental effects and potential benefits for maternal-infant bonding; however, infants of mothers on phenobarbital or benzodiazepines require observation for sedation.[232] [233]Prognosis
Long-Term Outcomes
Approximately 60-68% of individuals with newly diagnosed epilepsy achieve long-term remission, defined as seizure freedom for at least one to five years, depending on the study cohort and duration of follow-up.[234][235] In a cohort of patients followed for 20 years post-onset, two-thirds entered terminal remission, with half achieving this without antiepileptic medications.[236] Remission rates are higher for idiopathic epilepsy compared to structural or symptomatic causes, with early response to initial antiepileptic drugs serving as a strong predictor of sustained seizure control.[237] In childhood-onset epilepsy, outcomes are favorable for many, with 64% of survivors seizure-free for at least five years by adulthood, including 47% off medications; however, psychosocial challenges such as reduced educational attainment and employment persist even among those in remission.[238] Adult-onset cases show similar patterns, with about two-thirds entering five-year terminal remission long-term, though chronic epilepsy affects roughly one-third, often featuring relapsing-remitting seizure patterns rather than unremitting activity.[239][235] Prognosis varies by syndrome; for instance, benign childhood epilepsies like rolandic epilepsy yield near-complete remission by adolescence, whereas temporal lobe epilepsy in children may resolve in over half but carries risks of persistence into adulthood.[240] Relapse risk remains elevated post-remission, with 40% of patients experiencing seizure recurrence five years after entering remission, and 25% developing drug-resistant epilepsy thereafter.[241] Defining sufficient remission duration for low relapse probability improves with longer seizure-free periods: from two to five years markedly reduces risk, stabilizing further beyond five years.[242] Surgical interventions in refractory cases enhance long-term remission, with patterns of initial post-surgical seizure control predicting sustained outcomes in up to 73% without medication adjustments.[243] Long-term quality of life is compromised by comorbidities, even in seizure-controlled patients, including cognitive deficits (e.g., memory difficulties in 55.8% of adults with active epilepsy), chronic pain (40.2%), obesity (38.6%), and psychiatric conditions like depression and anxiety, which exacerbate unemployment, social isolation, and reduced independence.[244][245] Multimorbidity amplifies these effects, correlating with poorer health-related quality of life, higher suicide risk, and premature mortality independent of seizures.[246][247] In children transitioning to adulthood, neurodevelopmental and psychiatric comorbidities contribute to enduring behavioral and social impairments.[248]Mortality Risks
Individuals with epilepsy experience a standardized mortality ratio (SMR) approximately 1.6 to 9.3 times higher than the general population, depending on epilepsy type, duration, and control, with higher ratios observed in those with remote symptomatic etiologies or frequent seizures.[249] [250] Premature mortality arises from both direct epilepsy-related mechanisms, such as seizure-induced physiological disruptions, and indirect factors including comorbidities, accidents, and treatment side effects.[251] Sudden unexpected death in epilepsy (SUDEP) constitutes a primary direct risk, defined as sudden, witnessed or unwitnessed, nontraumatic, and nondrowning death in epilepsy patients without a toxicological or anatomic explanation after thorough postmortem examination.[252] The incidence of SUDEP is estimated at 0.22 per 1,000 patient-years in children with epilepsy, rising to 1.2 per 1,000 patient-years in adults, with rates escalating to 3-9 per 1,000 in those with refractory, frequent generalized tonic-clonic seizures.[253] [254] Key risk factors include uncontrolled seizures, polytherapy with antiepileptic drugs, intellectual disability, and young adult male sex, while seizure freedom reduces risk near general population levels.[253] Mechanistically, SUDEP often links to postictal respiratory arrest, central apnea, or cardiorespiratory instability during or after a generalized seizure, supported by witnessed cases and animal models demonstrating seizure-induced brainstem dysfunction.[252] Beyond SUDEP, status epilepticus accounts for significant mortality, contributing to 16-23% of epilepsy-related deaths, often via neuronal injury, systemic complications like rhabdomyolysis, or cerebral edema.[249] [255] Seizure-associated accidents, including drowning (up to 25% of non-SUDEP epilepsy deaths in some cohorts) and trauma from falls, elevate risks particularly in unsupervised settings or with nocturnal seizures.[255] Suicide rates are 3-10 times higher, driven by psychosocial stressors, medication side effects, and comorbid psychiatric conditions rather than seizures per se.[256] Indirect causes encompass exacerbated comorbidities, such as cerebrovascular disease (SMR 4.50) and pneumonia, where seizures impair airway protection or mobility.[256] In the United States from 2011-2021, epilepsy was listed in 43,231 deaths, underlying in 39% and contributing in 61%, underscoring its pervasive role.[257]| Cause of Death | Approximate Proportion in Epilepsy Cohorts | Key Notes |
|---|---|---|
| SUDEP | 20-23% of epilepsy-related deaths | Highest in refractory cases; postictal cardiorespiratory failure predominant.[255] [253] |
| Status Epilepticus | 16-23% | Often leads to multiorgan failure; prompt treatment critical.[249] |
| Accidents (e.g., drowning, falls) | 25% | Preventable with supervision and seizure alerts.[255] |
| Suicide | Elevated 3-10x general rate | Linked to depression, not directly to seizures.[256] |
| Cerebrovascular/Neoplasms | 15-19% | Underlying etiologies amplify risk.[256] |
Epidemiology
Global Prevalence
Approximately 51.7 million individuals worldwide were living with epilepsy in 2021, corresponding to an age-standardized prevalence rate of 658 cases per 100,000 population.00302-5/fulltext) This estimate encompasses both idiopathic and secondary forms, derived from the Global Burden of Disease (GBD) study, which aggregates data from epidemiological surveys, registries, and modeling to account for underdiagnosis in resource-limited settings.00302-5/fulltext) Earlier World Health Organization (WHO) assessments similarly report over 50 million affected persons, with point prevalence for active epilepsy—defined as ongoing seizures or recent treatment—averaging 6.38 per 1,000 persons based on meta-analyses of 197 studies spanning multiple continents.[5][258] Prevalence varies substantially by economic development, with nearly 80% of cases concentrated in low- and middle-income countries (LMICs), where rates reach 139 incident cases per 100,000 annually compared to 49 per 100,000 in high-income nations.[5] This disparity stems from higher burdens of etiological factors such as parasitic infections (e.g., neurocysticercosis), perinatal trauma, and stroke in LMICs, rather than diagnostic differences alone, as evidenced by community-based studies adjusting for case ascertainment.[5][258] Globally, annual incidence hovers around 61-68 new cases per 100,000 person-years, with lifetime risk estimates indicating one in 26 individuals may develop epilepsy.[258][259]| Region/Income Group | Prevalence (per 1,000) | Key Notes |
|---|---|---|
| Global | 6.38 (active) | Pooled from 197 studies; higher for lifetime (10.98).[258][260] |
| High-Income | ~5.0 | Lower incidence due to better perinatal care and infection control.[5] |
| Low/Middle-Income | ~12.0 | 80% of global cases; driven by preventable causes.[5] |
Demographic Distributions
Epilepsy exhibits a bimodal age distribution in incidence, with peaks in the first year of life and after age 65, while prevalence tends to increase steadily in adulthood due to cumulative cases.[261] In the United States, approximately 456,000 children aged 0-17 years have active epilepsy, representing about 0.6% prevalence, whereas 2.9 million adults (1% of the adult population) report active epilepsy as of 2021-2022 data.[262] Among older adults, those over 65 account for nearly a quarter of new-onset cases, often linked to cerebrovascular events or neurodegeneration.[263] Incidence rates are marginally higher in males than females globally, with male-to-female ratios around 1.1-1.5 in various studies, potentially attributable to greater exposure to traumatic etiologies.[264] [265] Prevalence shows similar patterns, with males exhibiting higher overall rates in population analyses, though idiopathic forms display minimal sex differences.[266] [6] Racial and ethnic disparities in the US reveal higher prevalence among Black individuals (2.13% lifetime prevalence) compared to Whites (0.77%), with nearly threefold elevated active epilepsy rates in African Americans.[267] [268] Incidence is also elevated in Black populations relative to Whites and Hispanics, alongside increased late-onset epilepsy post-stroke in non-Hispanic Blacks.[269] [270] Some broader studies find no significant race/ethnicity differences after adjustment, but unadjusted data consistently indicate disproportionate burden in minorities.[267] Prevalence correlates inversely with socioeconomic status, with lower-income groups and neighborhoods showing higher rates, potentially due to increased etiological risks like perinatal complications or trauma.[271] [272] In pediatric cohorts, children from higher-income households have 30% lower odds of epilepsy diagnosis.[273] Lower SES also associates with greater healthcare utilization disparities and non-adherence to treatment.[274] [275]| Demographic Factor | Key Observation | Example Rate (US unless noted) |
|---|---|---|
| Age <1 year | High incidence peak | Bimodal distribution start[261] |
| Age >65 years | Highest new-onset incidence | ~25% of cases[263] |
| Male vs. Female | Higher male incidence | Ratio 1.1-1.5 globally[264] |
| Black vs. White | Higher Black prevalence | 2.13% vs. 0.77% lifetime[276] |
| Low SES | Elevated prevalence | Inverse correlation[271] |