Recent from talks
Nothing was collected or created yet.
Lead(II,IV) oxide
View on WikipediaThis article needs additional citations for verification. (December 2023) |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Lead tetroxide [1] | |
| Other names
Minium, red lead, triplumbic tetroxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.851 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1479 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Pb3O4 | |
| Molar mass | 685.6 g·mol−1 |
| Appearance | Vivid orange crystals |
| Density | 8.3 g/cm3 |
| Melting point | 500 °C (decomposition) |
| Vapor pressure | 1.3 kPa (at 0 °C) |
| Structure | |
| Tetragonal, tP28 | |
| P42/mbc, No. 135 | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H272, H302, H332, H360, H373, H410 | |
| P201, P220, P273, P308+P313, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
| Lead(II) oxide Lead(IV) oxide | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lead(II,IV) oxide, also called red lead or minium, is the inorganic compound with the formula Pb3O4. A bright red or orange solid, it is used as pigment, in the manufacture of batteries, and rustproof primer paints. It is an example of a mixed valence compound, being composed of both Pb(II) and Pb(IV) in the ratio of two to one.[2]
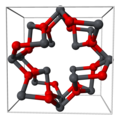
Structure
[edit]Lead(II,IV) oxide is lead(II) orthoplumbate(IV) [Pb2+]2[PbO4−4].[3] It has a tetragonal crystal structure at room temperature, which then transforms to an orthorhombic (Pearson symbol oP28, Space group Pbam, No. 55) form at temperature 170 K (−103 °C). This phase transition only changes the symmetry of the crystal and slightly modifies the interatomic distances and angles.[4]
-
Part of tetragonal red lead's crystal structure
Preparation
[edit]Lead(II,IV) oxide is prepared by calcination of lead(II) oxide (PbO; also called litharge) in air at about 450–480 °C:[5]
- 6 PbO + O2 → 2 Pb3O4
The resulting material is contaminated with PbO. If a pure compound is desired, PbO can be removed by a potassium hydroxide solution:
- PbO + KOH + H2O → K[Pb(OH)3]
Another method of preparation relies on annealing of lead(II) carbonate (cerussite) in air:
- 6 PbCO3 + O2 → 2 Pb3O4 + 6 CO2
Yet another method is oxidative annealing of white lead:
- 3 Pb2CO3(OH)2 + O2 → 2 Pb3O4 + 3 CO2 + 3 H2O
In solution, lead(II,IV) oxide can be prepared by reaction of potassium plumbate with lead(II) acetate, yielding yellow insoluble lead(II,IV) oxide monohydrate Pb3O4·H2O, which can be turned into the anhydrous form by gentle heating:
- K2PbO3 + 2 Pb(OCOCH3)2 + H2O → Pb3O4 + 2 KOCOCH3 + 2 CH3COOH
Natural minium is uncommon, forming only in extreme oxidizing conditions of lead ore bodies. The best known natural specimens come from Broken Hill, New South Wales, Australia, where they formed as the result of a mine fire.[6]
Reactions
[edit]Red lead is virtually insoluble in water and in ethanol. However, it is soluble in hydrochloric acid present in the stomach, and is therefore toxic when ingested. It also dissolves in glacial acetic acid and a diluted mixture of nitric acid and hydrogen peroxide.
When heated to 500 °C, it decomposes to lead(II) oxide and oxygen. At 580 °C, the reaction is complete.
- 2 Pb3O4 → 6 PbO + O2
Nitric acid dissolves the lead(II) oxide component, leaving behind the insoluble lead(IV) oxide:
- Pb3O4 + 4 HNO3 → PbO2 + 2 Pb(NO3)2 + 2 H2O
With iron oxides and with elemental iron, lead(II,IV) oxide forms insoluble iron(II) and iron(III) plumbates, which is the basis of the anticorrosive properties of lead-based paints applied to iron objects.
Use
[edit]Red lead has been used as a pigment for primer paints for iron objects. Due to its toxicity, its use is being limited. It finds limited use in some amateur pyrotechnics as a delay charge and was used in the past in the manufacture of dragon's egg pyrotechnic stars.
Red lead is used as a curing agent in some polychloroprene rubber compounds. It is used in place of magnesium oxide to provide better water resistance properties.
Red lead was used for engineer's scraping, before being supplanted by engineer's blue. Although red lead still offers more accurate markings since it doesn't flow as readily as engineer's blue under pressure.
It is also used as an adulterating agent in turmeric powder.
Physiological effects
[edit]When inhaled, lead(II,IV) oxide irritates the lungs. In case of high dose, the victim experiences a metallic taste, chest pain, and abdominal pain. When ingested, it is dissolved in the gastric acid and absorbed, leading to lead poisoning. High concentrations can be absorbed through skin as well, and it is important to follow safety precautions when working with lead-based paint.
Long-term contact with lead(II,IV) oxide may lead to accumulation of lead compounds in organisms, with development of symptoms of acute lead poisoning. Chronic poisoning displays as agitation, irritability, vision disorders, hypertension, and a grayish facial hue.
Lead(II,IV) oxide was shown to be carcinogenic for laboratory animals. Its carcinogenicity for humans was not proven.

History
[edit]This compound's Latin name minium originates from the Minius, a river in northwest Iberia where it was first mined.
Lead(II,IV) oxide was used as a red pigment in ancient Rome, where it was prepared by calcination of white lead. In the ancient and medieval periods it was used as a pigment in the production of illuminated manuscripts, and gave its name to the minium or miniature, a style of picture painted with the colour.
Made into a paint with linseed oil, red lead was used as a durable paint to protect exterior ironwork. In 1504 the portcullis at Stirling Castle in Scotland was painted with red lead, as were cannons including Mons Meg.[7]
As a finely divided powder, it was also sprinkled on dielectric surfaces to study Lichtenberg figures.
In traditional Chinese medicine, red lead is used to treat ringworms and ulcerations, though the practice is limited due to its toxicity. Also, azarcón, a Mexican folk remedy for gastrointestinal disorders, contains up to 95% lead(II,IV) oxide.[8]
It was also used before the 18th century as medicine.[9]
See also
[edit]References
[edit]- ^ "VOLUNTARY RISK ASSESSMENT REPORT ON LEAD AND SOME INORGANIC LEAD COMPOUNDS". Retrieved 2012-12-25.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 920. ISBN 0-12-352651-5.
- ^ Gavarri, J; Weigel, Dominique; Hewat, A. W. (1978). "Oxydes de plomb. IV. Évolution structurale de l'oxyde Pb3O4 entre 240 et 5 K et mécanisme de la transition" [Lead oxides. IV. Structural evolution of the oxide Pb3O4 between 240 and 5 K and mechanism of transition]. Journal of Solid State Chemistry. 23 (3–4): 327. Bibcode:1978JSSCh..23..327G. doi:10.1016/0022-4596(78)90081-6.
- ^ Carr, Dodd S. "Lead Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_249. ISBN 978-3-527-30673-2.
- ^ Minium
- ^ James Balfour Paul, Accounts of the Treasurer of Scotland, vol. 2 (Edinburgh, 1900), p. 277.
- ^ Bose, A.; Vashistha, K; O'Loughlin, B. J. (1983). "Azarcón por empacho – another cause of lead toxicity". Pediatrics. 72: 108–118. doi:10.1542/peds.72.1.106. S2CID 37730169.
- ^ "The London Lancet: A Journal of British and Foreign Medicine, Physiology, Surgery, Chemistry, Criticism, Literature and News". 1853.
External links
[edit]Lead(II,IV) oxide
View on GrokipediaLead(II,IV) oxide is an inorganic compound with the chemical formula Pb₃O₄, consisting of lead in mixed +2 and +4 oxidation states and commonly known as red lead or minium.[1][2] It appears as a dense, bright red to orange crystalline powder with a density of approximately 9.1 g/cm³, insoluble in water but soluble in acids, and occurs naturally as the rare mineral minium.[1][3] The compound can be viewed as a composite of lead(II) oxide (PbO) and lead(IV) oxide (PbO₂), specifically 2 PbO · PbO₂, which influences its redox properties and reactivity.[1] Historically employed as an opaque pigment in paints, ceramics, and glass for its vibrant color and durability, it has been largely phased out in consumer products due to lead's toxicity, though it persists in specialized industrial uses such as the paste in lead-acid battery plates and rust-inhibiting primers.[3][4] Exposure to Pb₃O₄ poses significant health risks, including reproductive toxicity, organ damage from chronic inhalation or ingestion, and environmental persistence leading to bioaccumulation, classifying it as a hazardous substance requiring strict handling protocols.[5][6]
Chemical Identity and Properties
Nomenclature and Formula
Lead(II,IV) oxide has the chemical formula Pb₃O₄, corresponding to a molecular weight of 685.60 g/mol.[7] The systematic IUPAC name is lead(II,IV) oxide, which denotes the mixed oxidation states of lead (+2 and +4), while the preferred IUPAC name is lead tetroxide.[8] Common synonyms include red lead, minium, and orange lead, historically used to describe its bright red pigment form.[9] The formula Pb₃O₄ can be expressed as 2PbO·PbO₂, representing a combination of lead(II) oxide (PbO) and lead(IV) oxide (PbO₂), with two Pb²⁺ cations and one Pb⁴⁺ cation per unit.[10] This structural interpretation aligns with its composition as a mixed-valence compound rather than a simple stoichiometric oxide.[11]Crystal Structure
Lead(II,IV) oxide, Pb₃O₄, crystallizes in the tetragonal space group P4₂/mbc (No. 135) at room temperature.[12][13] The lattice parameters are a = b = 8.811 Å and c = 6.563 Å.[14] This structure features a three-dimensional network with two inequivalent lead sites: one corresponding to Pb(IV) in octahedral coordination and two Pb(II) sites exhibiting pyramidal coordination due to the stereochemically active 6s² lone pair.[12][15] The arrangement can be conceptualized as a composite of PbO-like layers and PbO₂ units, reflecting its mixed-valence composition equivalent to 2 PbO · PbO₂.[16] Pb(IV) atoms occupy positions akin to those in the rutile structure of PbO₂, surrounded by six oxygen atoms, while Pb(II) atoms form distorted square pyramidal geometries with four oxygen neighbors, the lone pair occupying the apical position.[14] Oxygen atoms bridge the lead polyhedra, forming a framework that stabilizes the mixed oxidation states.[17] At temperatures below 170 K, the structure undergoes a phase transition to an orthorhombic form (space group Pbam, No. 55), involving distortions that lower the symmetry while preserving the overall connectivity.[18][19] This low-temperature phase exhibits slightly altered bond lengths and angles, as determined by X-ray diffraction studies.[20]Physical Properties
Lead(II,IV) oxide manifests as a bright red crystalline solid or amorphous powder, typically odorless.[21][22] Its density is 9.1 g/cm³.[23][22] The compound decomposes at around 500 °C under standard conditions, though it melts at 830 °C when subjected to high pressure in an oxygen atmosphere.[24][22] It exhibits negligible solubility in water, approximately 67.3 mg/L at 20 °C, rendering it effectively insoluble for most practical purposes.[25] However, it dissolves readily in acids such as hydrochloric acid and in alkalis.[26][27]Synthesis and Preparation
Industrial Methods
Lead(II,IV) oxide is primarily produced industrially by the calcination of lead(II) oxide (litharge, PbO) in an oxidizing atmosphere at controlled temperatures of 450–500 °C, yielding the reaction 6 PbO + O₂ → 2 Pb₃O₄.[28] This process occurs in refractory-lined batch ovens or reverberatory furnaces, where leady oxide feedstock is heated electrically under oxygen-enriched conditions to achieve conversion rates of 20–98% Pb₃O₄, with residual PbO in tetragonal or orthorhombic forms.[28][29] Agitators ensure uniform mixing, and automated oxygen addition maintains optimal partial pressure for oxidation without exceeding decomposition thresholds above 500 °C.[28] Alternative configurations include rotary tube furnaces, where molten lead is initially oxidized to litharge via an oxidizing flame at the lower end of an inclined refractory tube, followed by further conversion to red lead under sustained airflow and moderate heating between 327.5 °C (lead melting point) and 888 °C (PbO melting point).[30] These methods prioritize particulate emission control, with baghouses or scrubbers capturing approximately 90% lead-bearing dust at efficiencies of 99% or 70–95%, respectively, reflecting environmental regulations on lead pigment production.[29] Litharge feedstock is often derived upstream from Barton-pot or ball-mill oxidation of metallic lead, integrating red lead synthesis into broader lead oxide manufacturing.[29] Production capacities vary by furnace model; for instance, batch systems can output 1,000–16,000 kg/day of red lead depending on purity targets (23–98% Pb₃O₄).[28] Fluidized-bed variants accelerate oxidation by suspending litharge in high-velocity air or oxygen streams, reducing reaction times while minimizing agglomeration, though they remain less common than static or rotary setups.[31] Overall, these processes emphasize precise temperature and oxygen control to balance yield, particle size (typically 1–10 μm for pigment applications), and avoidance of free lead contamination.[28]Laboratory Methods
Lead(II,IV) oxide is prepared in the laboratory primarily through the controlled oxidation of lead(II) oxide (PbO, or litharge) by heating it in air at temperatures between 450 °C and 500 °C for several hours, yielding the characteristic red-orange product via the reaction 6 PbO + O₂ → 2 Pb₃O₄.[32][33] This thermal process exploits the partial oxidation of Pb²⁺ to Pb⁴⁺ in the lattice, forming the mixed-valence compound, and requires an oxygen-rich atmosphere to prevent incomplete conversion or formation of higher oxides like PbO₂.[34] Yields approach quantitative under optimized conditions, such as gradual heating to avoid sintering, with the product often ground to a fine powder post-cooling for uniformity.[35] An alternative laboratory route involves calcining basic lead carbonate (PbCO₃·Pb(OH)₂, known as lead white) in air at approximately 450 °C, which decomposes to PbO intermediate before further oxidation to Pb₃O₄; this method mirrors historical pigment production and is suitable for smaller scales due to the availability of the precursor.[32][35] Direct oxidation of metallic lead is possible but less common in labs, requiring initial melting and surface oxidation at higher temperatures (around 600 °C) followed by grinding and re-oxidation, as 6 Pb + 3 O₂ → 6 PbO, then proceeding as above; this stepwise approach minimizes impurities but demands careful temperature control to avoid lead vaporization.[33] Electrochemical synthesis offers a modern variant for thin films or controlled deposits, involving anodic oxidation of lead electrodes in alkaline electrolytes to form PbO layers that are then annealed in air to yield Pb₃O₄, though this is more specialized and yields lower bulk quantities compared to thermal methods.[36] Purity in all methods is verified by X-ray diffraction confirming the tetragonal structure, with contamination risks from over-oxidation to PbO₂ mitigated by precise thermogravimetric monitoring.[34]Chemical Reactivity
Key Reactions
Lead(II,IV) oxide, formulated as Pb₃O₄ and equivalent to 2PbO·PbO₂, exhibits reactivity characteristic of both Pb(II) oxide (basic, amphoteric tendencies) and Pb(IV) oxide (oxidizing agent). With concentrated hydrochloric acid, it undergoes a redox reaction where the Pb(IV) component oxidizes chloride ions to chlorine gas, while Pb(IV) is reduced to Pb(II), yielding lead(II) chloride: Pb₃O₄ + 8HCl → 3PbCl₂ + Cl₂ + 4H₂O. This differs from its behavior with nitric acid, where the oxidizing nature of HNO₃ prevents chloride-like oxidation; instead, the Pb(II) oxide component dissolves to form lead(II) nitrate, leaving PbO₂ intact: Pb₃O₄ + 4HNO₃ → 2Pb(NO₃)₂ + PbO₂ + 2H₂O. In reductive environments, Pb₃O₄ serves as an oxygen source. Heating with carbon reduces it to metallic lead and carbon dioxide: Pb₃O₄ + 2C → 3Pb + 2CO₂.[37] Similarly, strong reducing agents like hydrogen gas at elevated temperatures yield lead metal and water. Pb₃O₄ also participates in pigment degradation reactions, such as with atmospheric hydrogen sulfide, forming black lead(II) sulfide: 2Pb₃O₄ + 6H₂S → 3PbS + 3PbO + 3S + 6H₂O, contributing to darkening in exposed coatings.[38] The compound's mixed-valence structure enables disproportionation-like processes in certain media. In aqueous acidic conditions (pH < 2), hydronium ions preferentially attack Pb(II) sites, leading to structural rearrangement and partial dissolution into soluble lead species, though full solubility requires stronger acids.[39] These reactions underscore Pb₃O₄'s utility in historical applications like glassmaking fluxes, where it acts as both a base and oxidant, but also highlight its environmental persistence due to incomplete reduction in mild conditions.Stability and Decomposition
Lead(II,IV) oxide is thermally stable under ambient conditions, remaining intact in dry air at room temperature without significant decomposition. Safety assessments classify it as stable when stored in tightly sealed containers, protected from extreme temperatures, direct sunlight, and incompatible materials such as strong reducing agents or combustible organics, which could promote hazardous reactions.[24] Upon heating, Pb₃O₄ decomposes exothermically to lead(II) oxide and dioxygen gas via the reaction 2 Pb₃O₄(s) → 6 PbO(s) + O₂(g), with onset temperatures typically ranging from 500 °C to 540 °C depending on purity, particle size, and atmospheric conditions.[40][41][42] Structural defects, such as those introduced by mechanical milling, lower the decomposition threshold by stabilizing intermediate phases like orthorhombic PbO, facilitating the Pb₃O₄ to PbO conversion at reduced temperatures.[43] The process is reversible under controlled high-pressure oxygen environments at elevated temperatures, but irreversible decomposition predominates in standard heating scenarios, yielding litharge (tetragonal PbO) as the primary residue.[44]Applications and Uses
Pigments and Protective Coatings
Lead(II,IV) oxide, commonly known as red lead or minium, functions as an inhibitive pigment in paints, imparting a bright red-orange hue while providing corrosion protection to ferrous metals.[1] Its effectiveness stems from the formation of insoluble lead compounds, such as carbonates and hydroxides, that passivate steel surfaces upon exposure to atmospheric CO2 and moisture.[45] Historically, red lead was a primary component in anti-corrosive primers applied to iron and steel structures, including bridges, ships, and concrete reinforcements, due to its durability and rust-preventive qualities.[46][1] The pigment's use dates back to antiquity, with evidence of application in Egyptian, Roman, and Asian artifacts for decorative and protective purposes.[3] In the 19th and early 20th centuries, red lead primers became standard for industrial coatings, often mixed with linseed oil for enhanced adhesion and longevity on exposed metalwork.[29] By the mid-20th century, formulations typically included 20-40% red lead by weight in solvent-based paints to achieve optimal inhibition without excessive opacity.[47] Due to lead's toxicity, including risks of neurological damage and environmental persistence, regulatory restrictions have curtailed its application since the 1970s. In the United States, the Consumer Product Safety Commission banned lead concentrations exceeding 0.009% by weight in consumer paints in 1978, prompting a shift away from red lead in residential and general-purpose coatings.[48] Industrial use has similarly declined, with zinc phosphate and other non-toxic alternatives supplanting it in most anti-corrosive formulations for compliance with OSHA and EPA standards.[49][50] Nonetheless, red lead persists in select high-performance maintenance coatings for aging infrastructure in jurisdictions with exemptions or where superior passivation justifies controlled exposure risks.[51]Industrial and Manufacturing Uses
Lead(II,IV) oxide is incorporated into lead-acid battery production as an additive for positive electrode plates, where it accelerates formation processes and enhances deep-cycle performance by improving electrochemical properties during manufacturing.[4] In battery assembly, it is typically mixed with other lead compounds to form active pastes applied to grids, contributing to higher initial capacity and conductivity in the cured plates.[1] Global demand for such batteries, including those in automotive and industrial stationary applications, sustains its use despite regulatory pressures on lead content.[52] In glass manufacturing, lead(II,IV) oxide serves as a flux and stabilizer for producing lead crystal and heat-resistant glassware, where it lowers melting temperatures and imparts refractive index enhancements during fritting and forming stages.[1] Typical formulations include 20-30% Pb3O4 by weight in batch compositions for optical and decorative glasses, enabling precise control over viscosity and crystallization.[53] Its role persists in specialized high-temperature glass for laboratory equipment and industrial sight glasses, though substitution with non-lead alternatives is increasing in consumer products.[52] Ceramic production utilizes lead(II,IV) oxide in enamel frits and glazes for electrical insulators and porcelain, providing adhesion to metal substrates and corrosion resistance through vitrification at 800-1000°C.[1] It functions as a maturing agent, reducing firing times by 10-20% in formulations for spark plugs and switchgear components.[54] Additionally, it acts as a vulcanizing agent in rubber compounding for cable insulation and seals, cross-linking polymers under heat to yield durable, flexible materials resistant to oils and weathering.[54] These applications leverage its oxidative properties to ensure product integrity in demanding manufacturing environments.[55]Niche and Historical Applications
Red lead (Pb₃O₄) served as a pigment in ancient cosmetics and artwork due to its vibrant red hue, with evidence of its use dating back to early civilizations for decorative and ceremonial purposes.[56] It was particularly favored in Byzantine and Persian illuminated manuscripts and paintings for its durability and color intensity.[57] Additionally, historical records indicate its application in traditional medicines, plasters, and ointments, though such uses were later recognized for associated health risks from lead exposure.[1] In the industrial era, red lead found extensive historical use as an anticorrosive primer in paints for structural iron, steel, and ship bottoms, providing effective rust inhibition through its chemical reactivity with metal oxides until regulatory restrictions in the late 20th century curtailed its application.[3][1] It was also employed as a flux in porcelain painting and a glaze component for faience ceramics, enhancing adhesion and color development in specialized pottery production.[1] Niche applications persist in select areas despite broader phase-outs, including its historical role in lead-acid battery manufacturing where it was added to pasted and tubular positive plates to accelerate formation and improve deep-cycle performance, a practice common among early producers but largely supplanted by alternatives.[4][58] Limited contemporary uses include catalysts in certain chemical processes and components in specialty electronics and coatings where lead's properties remain advantageous, though declining due to toxicity concerns.[59][60] Red lead continues to color specific glass formulations and ceramic glazes in artisanal or heritage contexts valuing traditional aesthetics.[3]Toxicology and Health Effects
Mechanisms of Lead Toxicity
Lead toxicity arises primarily from the ionic form Pb²⁺, which has high affinity for sulfhydryl groups on proteins and enzymes, leading to widespread disruption of cellular functions after absorption and distribution in the body.[61] Pb²⁺ mimics essential divalent cations like Ca²⁺ and interferes with signaling pathways, while also promoting oxidative damage and enzymatic inhibition.[62] These effects occur at low exposure levels, with no safe threshold established, as even blood lead concentrations below 5 µg/dL correlate with adverse outcomes.[61] A central mechanism involves inhibition of heme biosynthesis, critical for hemoglobin production and oxygen transport. Pb²⁺ potently inhibits δ-aminolevulinic acid dehydratase (ALAD), an enzyme catalyzing the second step in the pathway, with >80–90% inhibition at blood lead levels of 20–55 µg/dL, resulting in accumulation of δ-aminolevulinic acid (ALA).[61] Ferrochelatase, the final enzyme inserting iron into protoporphyrin IX, is also inhibited, elevating zinc protoporphyrin (ZPP) and free erythrocyte protoporphyrin levels as biomarkers.[61] This disruption causes microcytic hypochromic anemia through reduced heme availability, increased erythrocyte fragility, and hemolysis, compounded by ALA's pro-oxidant properties that generate reactive oxygen species (ROS).[63] ALA accumulation further exacerbates neurotoxicity by overstimulating gamma-aminobutyric acid (GABA) receptors and inhibiting heme-dependent enzymes.[62] Oxidative stress represents another key pathway, where Pb²⁺ directly generates ROS such as superoxide and hydrogen peroxide via auto-oxidation and Fenton-like reactions, while depleting glutathione (GSH) reserves by up to 90% under stress conditions.[61] This leads to lipid peroxidation, protein carbonylation, and DNA damage, with inhibition of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR).[61] In neural tissues, ROS-induced mitochondrial dysfunction reduces membrane potential, releases cytochrome c, and activates apoptosis cascades involving caspases and altered Bcl-2/Bax ratios.[62] In the central nervous system, Pb²⁺ crosses the blood-brain barrier via calcium channels (e.g., DMT1, L-type VGCCs) and competes with Ca²⁺ at binding sites on calmodulin, protein kinase C (PKC), and N-methyl-D-aspartate (NMDA) receptors, dysregulating intracellular calcium homeostasis and impairing synaptic plasticity.[62] This inhibits neurotransmitter release (e.g., glutamate, dopamine) and downregulates NMDA receptor subunits like NR2A, disrupting long-term potentiation essential for learning and memory.[62] Pb²⁺ also alters gene expression by mimicking Zn²⁺ to affect transcription factors (e.g., Sp1, Egr-1) and activating NF-κB pathways, upregulating pro-inflammatory cytokines (IL-1β, TNF-α) and downregulating neurotrophins like BDNF.[62] Epigenetic changes, including DNA hypermethylation of genes like ALAD and p16, contribute to persistent neurodevelopmental deficits.[62] Renal toxicity stems from Pb²⁺ accumulation in proximal tubules, inducing oxidative damage, apoptosis, and fibrosis through ROS-mediated pathways and inhibition of Na⁺/K⁺-ATPase.[63] Reproductive effects involve disruption of steroidogenesis and spermatogenesis via enzymatic inhibition and hormonal imbalance, with reduced testosterone and luteinizing hormone (LH) levels observed in exposed males.[63] Bone serves as the primary storage depot (85–95% of body burden in adults), mobilizing Pb²⁺ during resorption and prolonging exposure.[61] Overall, these mechanisms exhibit dose-dependence, with chronic low-level exposure amplifying risks through bioaccumulation.[61]Physiological Impacts on Humans
Exposure to lead(II,IV) oxide primarily occurs through inhalation of respirable dust in occupational settings or ingestion via contaminated food or traditional remedies, leading to systemic absorption of lead ions after dissolution in gastric acid or lung fluids.[64] Gastrointestinal absorption efficiency varies but can reach 40-50% in children and 10-15% in adults for lead compounds, with lead(II,IV) oxide exhibiting relative bioavailability of 0.25-0.75 compared to soluble salts.[64] Once absorbed, lead distributes rapidly to blood (primarily erythrocytes), soft tissues, and bone, where it has a half-life of decades, mimicking calcium and disrupting enzymatic functions.[64] Hematological effects manifest as inhibition of delta-aminolevulinic acid dehydratase (ALAD) and ferrochelatase, elevating erythrocyte protoporphyrin (EP) at blood lead levels (BLLs) ≥25-30 μg/dL and causing microcytic anemia with basophilic stippling at higher exposures.[64] Neurological impacts include peripheral neuropathy, characterized by wrist drop and paresthesia in adults at BLLs >40 μg/dL, and central nervous system effects such as encephalopathy with seizures and coma at BLLs >70 μg/dL; chronic low-level exposure correlates with IQ decrements of 2-4 points per 10 μg/dL increase in children.[64] Renal toxicity involves proximal tubule damage, forming intranuclear inclusion bodies and leading to aminoaciduria, glycosuria, and chronic nephropathy, with gouty arthritis from urate retention at prolonged BLLs >40 μg/dL.[64] Cardiovascular consequences include hypertension, with odds ratios increasing 1.3-1.9 for BLLs 5-20 μg/dL, and potential endothelial dysfunction contributing to atherosclerosis.[64] Reproductive effects encompass reduced sperm count and motility in males at BLLs >40 μg/dL, and in females, transplacental transfer resulting in fetal BLLs approximating maternal levels, elevating risks of low birth weight and preterm delivery.[64] Documented cases, such as ingestion of Pb3O4-containing folk remedies like azarcón or hongdan, have produced BLLs exceeding 100 μg/dL, yielding acute symptoms including abdominal colic, anemia, and elevated urinary coproporphyrins in affected children and adults.[65][66] Inorganic lead compounds, including oxides, are classified as probably carcinogenic to humans (Group 2A) by IARC, primarily via inhalation-induced lung cancer, though non-genotoxic mechanisms predominate in physiological toxicity.[67]Exposure Assessment and Mitigation
Exposure to lead(II,IV) oxide primarily occurs through inhalation of fine dust particles generated during manufacturing, handling, abrasion, or disposal processes, with secondary routes including incidental ingestion via contaminated hands or surfaces and limited dermal absorption.[1] In occupational settings such as pigment production or anti-corrosion coating application, airborne concentrations can exceed safe limits without controls, leading to systemic lead absorption.[68] Biological monitoring via blood lead levels (BLL) serves as a key metric for assessing cumulative exposure, with OSHA mandating surveillance for workers exposed at or above the action level of 30 μg/m³ air lead equivalent.[68] Air monitoring uses personal sampling pumps to measure respirable lead particulates, calibrated to the OSHA permissible exposure limit (PEL) of 50 μg/m³ as an 8-hour time-weighted average (TWA) for lead compounds including Pb₃O₄.[69] The NIOSH recommended exposure limit (REL) aligns at 0.050 mg/m³ TWA, emphasizing maintenance below levels that elevate BLLs above 40 μg/dL, where medical removal from exposure is required under OSHA standards.[70] Mitigation strategies follow the hierarchy of controls, prioritizing engineering measures such as local exhaust ventilation systems to capture dust at the source and enclosed processes to minimize airborne release.[68] Administrative controls include limiting exposure duration through job rotation, prohibiting eating, drinking, or smoking in work areas, and enforcing handwashing with lead-removal soaps before breaks.[71] Personal protective equipment (PPE) is implemented as a supplementary measure, featuring NIOSH-approved respirators (e.g., half-face with P100 filters for concentrations up to 10 times the PEL), impermeable gloves, and disposable coveralls laundered or discarded to prevent take-home contamination.[68] Employers must provide annual training on hazards, conduct exposure assessments every six months for high-risk operations, and ensure housekeeping via HEPA vacuuming rather than dry sweeping to avoid dust resuspension.[68] For decontamination, dedicated change rooms with showers separate contaminated work attire from street clothes, reducing secondary exposure risks documented in lead industry studies.[6] Substitution with less toxic alternatives, where feasible, represents the most effective long-term mitigation, though Pb₃O₄'s unique properties limit this in certain applications.Environmental and Regulatory Aspects
Ecological Persistence and Impact
Lead(II,IV) oxide (Pb₃O₄), known as red lead, demonstrates high environmental persistence owing to its low aqueous solubility (approximately 0.0001 g/100 mL at 20°C) and resistance to biodegradation, allowing it to remain stable in soils, sediments, and water bodies for extended periods, often indefinitely without natural degradation processes.[72] Anthropogenic releases, such as from weathered paints or industrial effluents, contribute to its deposition primarily as particulate matter, which settles into soils and aquatic sediments where it accumulates due to limited mobility under neutral pH conditions.[73] In acidic environments (pH < 6), solubility increases, facilitating gradual dissolution and release of Pb²⁺ ions, enhancing bioavailability.[74] In terrestrial ecosystems, Pb₃O₄ persists in topsoil layers, inhibiting microbial activity and reducing soil fertility by disrupting enzyme functions and nutrient cycling, with studies showing decreased decomposition rates of organic matter at concentrations exceeding 100 mg/kg soil.[75] Plant uptake occurs primarily through roots, leading to bioaccumulation in foliage and reduced growth, chlorophyll content, and photosynthesis efficiency, particularly in species like lettuce and radish exposed to lead-contaminated soils.[76] This persistence disrupts food webs by transferring lead to herbivores and higher trophic levels, causing biomagnification in some cases.[77] Aquatic ecosystems face significant impacts from Pb₃O₄ particulates settling into sediments, where resuspension or acidification can mobilize lead, exerting toxicity on benthic organisms and phytoplankton at levels as low as 1-10 µg/L dissolved lead.[78] Fish and invertebrates exhibit bioaccumulation in gills, liver, and muscle tissues, triggering oxidative stress, impaired reproduction, and histological damage, with chronic exposure linked to population declines in sensitive species like salmonids.[79] Overall, lead from sources including Pb₃O₄ contributes to biodiversity loss, altered community structures, and ecosystem dysfunction near contamination hotspots.[75][80]
Regulations and Risk Management
In the United States, occupational exposure to Lead(II,IV) oxide is governed by the Occupational Safety and Health Administration (OSHA) under 29 CFR 1910.1025, which establishes a permissible exposure limit (PEL) of 50 µg/m³ of lead as an 8-hour time-weighted average and an action level of 30 µg/m³, triggering requirements for exposure monitoring, medical surveillance, and engineering controls such as local exhaust ventilation to minimize airborne dust during handling or processing.[68] For construction activities involving lead pigments like Pb₃O₄, OSHA's 29 CFR 1926.62 applies similar limits and mandates hygiene practices, including prohibiting removal of lead-contaminated clothing by blowing or shaking and requiring disposal of wash water per local regulations.[81] The Environmental Protection Agency (EPA) designates wastes containing Pb₃O₄ as hazardous under code D008 if lead concentrations exceed 5 mg/L via toxicity characteristic leaching procedure, necessitating treatment, storage, and disposal in permitted facilities rather than landfilling or open burning.[1] Risk management protocols emphasize prevention of inhalation, ingestion, and dermal absorption, with recommendations for personal protective equipment including NIOSH-approved respirators (e.g., half-face with P100 filters for concentrations up to 10 times the PEL), impermeable gloves, and protective clothing, alongside administrative controls like restricted access zones and worker training on emergency procedures such as eye washing and decontamination.[68] Facilities handling red lead pigments must conduct regular air sampling and biological monitoring (e.g., blood lead levels below 40 µg/dL for most workers), with removal from exposure if levels exceed 60 µg/dL.[68] For abatement or renovation involving lead-based coatings, EPA's Renovate, Repair, and Paint (RRP) rule requires certified contractors to contain dust, use wet methods, and dispose of debris in sealed containers to prevent community exposure.[82] In the European Union, Lead(II,IV) oxide falls under REACH Regulation (EC) No 1907/2006, where lead compounds are prioritized for restriction due to reproductive toxicity and environmental persistence, with the European Chemicals Agency (ECHA) proposing authorizations for remaining uses and classifying lead as environmentally hazardous effective January 2024, prohibiting certain discharges into water bodies under the Water Framework Directive. The EU's Carcinogens and Mutagens Directive (2004/37/EC) lowered the binding occupational exposure limit for lead and its inorganic compounds to 0.03 mg/m³ (8-hour TWA) and a biological limit value of 150 µg/g creatinine in urine as of March 2024, mandating substitution where feasible and enhanced risk assessments for downstream users like paint manufacturers.[83] Member states enforce these via national laws, such as Germany's WHG (Water Resources Act) restricting lead emissions from industrial processes involving Pb₃O₄.[84] Internationally, the International Lead Association coordinates compliance for lead oxides under frameworks like REACH, while many countries align with WHO guidelines recommending blood lead levels below 5 µg/dL for children and phase-outs of lead in paints per the Global Alliance to Eliminate Lead Paint, though industrial exemptions persist in regions without full bans, requiring site-specific risk assessments and hazardous waste manifests for transboundary shipments under Basel Convention Annex III.[85] In Australia, the National Industrial Chemicals Notification and Assessment Scheme sets a workplace exposure standard of 0.05 mg/m³ for lead oxides including Pb₃O₄, with prohibitions on consumer products exceeding 90 ppm lead.| Jurisdiction | Key Exposure Limit (as Pb) | Source |
|---|---|---|
| US OSHA (general industry) | PEL: 50 µg/m³ (8h TWA) | [68] |
| EU (updated 2024) | OEL: 0.03 mg/m³ (8h TWA) | [83] |
| Australia | ES: 0.05 mg/m³ (8h TWA) |