Recent from talks
Contribute something
Nothing was collected or created yet.
Indium
View on Wikipedia
 | ||||||||||||||||||||||||||
| Indium | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈɪndiəm/ ⓘ | |||||||||||||||||||||||||
| Appearance | silvery lustrous gray | |||||||||||||||||||||||||
| Standard atomic weight Ar°(In) | ||||||||||||||||||||||||||
| Indium in the periodic table | ||||||||||||||||||||||||||
| Atomic number (Z) | 49 | |||||||||||||||||||||||||
| Group | group 13 (boron group) | |||||||||||||||||||||||||
| Period | period 5 | |||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p1 | |||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 3 | |||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||
| Melting point | 429.7485 K (156.5985 °C, 313.8773 °F) | |||||||||||||||||||||||||
| Boiling point | 2345 K (2072 °C, 3762 °F) | |||||||||||||||||||||||||
| Density (at 20° C) | 7.290 g/cm3[3] | |||||||||||||||||||||||||
| when liquid (at m.p.) | 7.02 g/cm3 | |||||||||||||||||||||||||
| Triple point | 429.7445 K, ~1 kPa[4] | |||||||||||||||||||||||||
| Heat of fusion | 3.281 kJ/mol | |||||||||||||||||||||||||
| Heat of vaporization | 231.8 kJ/mol | |||||||||||||||||||||||||
| Molar heat capacity | 26.74 J/(mol·K) | |||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||
| Oxidation states | common: +3 −5,[5] −2,[6] −1,[7] 0,[8] +1,[9] +2[9] | |||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.78 | |||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||
| Atomic radius | empirical: 167 pm | |||||||||||||||||||||||||
| Covalent radius | 142±5 pm | |||||||||||||||||||||||||
| Van der Waals radius | 193 pm | |||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||
| Crystal structure | body-centered tetragonal (tI2) | |||||||||||||||||||||||||
| Lattice constants | a = 325.16 pm c = 494.71 pm (at 20 °C)[3] | |||||||||||||||||||||||||
| Thermal expansion | 32.2×10−6/K (at 20 °C)[a] | |||||||||||||||||||||||||
| Thermal conductivity | 81.8 W/(m⋅K) | |||||||||||||||||||||||||
| Electrical resistivity | 83.7 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[10] | |||||||||||||||||||||||||
| Molar magnetic susceptibility | −64.0×10−6 cm3/mol (298 K)[11] | |||||||||||||||||||||||||
| Young's modulus | 11 GPa | |||||||||||||||||||||||||
| Speed of sound thin rod | 1215 m/s (at 20 °C) | |||||||||||||||||||||||||
| Mohs hardness | 1.2 | |||||||||||||||||||||||||
| Brinell hardness | 8.8–10.0 MPa | |||||||||||||||||||||||||
| CAS Number | 7440-74-6 | |||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||
| Naming | for the indigo blue line in its spectrum | |||||||||||||||||||||||||
| Discovery | Ferdinand Reich and Hieronymous Theodor Richter (1863) | |||||||||||||||||||||||||
| First isolation | Hieronymous Theodor Richter (1864) | |||||||||||||||||||||||||
| Isotopes of indium | ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
Indium is a chemical element; it has symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are largely intermediate between the two. It was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods and named for the indigo blue line in its spectrum.[13]
Indium is used primarily in the production of flat-panel displays as indium tin oxide (ITO), a transparent and conductive coating applied to glass.[14] It is also used in the semiconductor industry, in low-melting-point metal alloys such as solders and soft-metal high-vacuum seals.[15] It is used in the manufacture of blue and white LED circuits, mainly to produce Indium gallium nitride p-type semiconductor substrates.[16] It is produced exclusively as a by-product during the processing of the ores of other metals, chiefly from sphalerite and other zinc sulfide ores.[17]
Indium has no biological role and its compounds are toxic when inhaled or injected into the bloodstream, although they are poorly absorbed following ingestion.[18][19]
Etymology
[edit]The name comes from the Latin word indicum meaning violet or indigo.[20] The word indicum means "Indian", as the naturally based dye indigo was originally exported to Europe from India.
Properties
[edit]Physical
[edit]
Indium is a shiny silvery-white, highly ductile post-transition metal with a bright luster.[21] It is so soft (Mohs hardness 1.2) that it can be cut with a knife and leaves a visible line like a pencil when rubbed on paper.[22] It is a member of group 13 on the periodic table and its properties are mostly intermediate between its vertical neighbors gallium and thallium. As with tin, a high-pitched cry is heard when indium is bent – a crackling sound due to crystal twinning.[21] Like gallium, indium is able to wet glass. Like both, indium has a low melting point, 156.60 °C (313.88 °F); higher than its lighter homologue, gallium, but lower than its heavier homologue, thallium, and lower than tin.[23] The boiling point is 2072 °C (3762 °F), higher than that of thallium, but lower than gallium, conversely to the general trend of melting points, but similarly to the trends down the other post-transition metal groups because of the weakness of the metallic bonding with few electrons delocalized.[24]
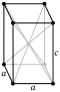
The density of indium, 7.31 g/cm3, is also greater than gallium, but lower than thallium. Below the critical temperature, 3.41 K, indium becomes a superconductor. Indium crystallizes in the body-centered tetragonal crystal system in the space group I4/mmm (lattice parameters: a = 325 pm, c = 495 pm):[23] this is a slightly distorted face-centered cubic structure, where each indium atom has four neighbours at 324 pm distance and eight neighbours slightly further (336 pm).[25] Indium has greater solubility in liquid mercury than any other metal (more than 50 mass percent of indium at 0 °C).[26] Indium displays a ductile viscoplastic response, found to be size-independent in tension and compression. However it does have a size effect in bending and indentation, associated to a length-scale of order 50–100 μm,[27] significantly large when compared with other metals.
Isotopes
[edit]Indium has 39 known isotopes, ranging in mass number from 97 to 135. Only two isotopes occur naturally as primordial nuclides: indium-113, the only stable isotope, and indium-115, which has a half-life of 4.41×1014 years, four orders of magnitude greater than the age of the Universe and nearly 30,000 times greater than half-life of thorium-232.[28] The half-life of 115In is very long because the beta decay to 115Sn is spin-forbidden.[29] Indium-115 makes up 95.7% of all indium. Indium is one of three known elements (the others being tellurium and rhenium) of which the stable isotope is less abundant in nature than the long-lived primordial radioisotopes.[30]
The stablest artificial isotope is indium-111, with a half-life of approximately 2.8 days. All other isotopes have half-lives shorter than 5 hours. Indium also has 47 meta states, among which indium-114m1 (half-life about 49.51 days) is the most stable, more stable than the ground state of any indium isotope other than the primordial. All decay by isomeric transition. The indium isotopes lighter than 113In predominantly decay through electron capture or positron emission to form cadmium isotopes, while the indium isotopes heavier than 113In predominantly decay through beta-minus decay to form tin isotopes.[28]
Chemistry
[edit]Indium has 49 electrons, with an electronic configuration of [Kr]4d105s25p1. In compounds, indium most commonly donates the three outermost electrons to become indium(III), In3+. In some cases, the pair of 5s-electrons are not donated, resulting in indium(I), In+. The stabilization of the monovalent state is attributed to the inert pair effect, in which relativistic effects lowers the energy of the 5s-orbital, observed in heavier elements. Thallium (indium's heavier homolog) shows an even stronger effect, manifested by the pervasiveness of thallium(I) vs thallium(III),[31] Gallium (indium's lighter homolog) is only rarely observed in the +1 oxidation state. Thus, although thallium(III) is a moderately strong oxidizing agent, indium(III) is not, and many indium(I) compounds are powerful reducing agents.[32] While the energy required to include the s-electrons in chemical bonding is lowest for indium among the group 13 metals, bond energies decrease down the group so that by indium, the energy released in forming two additional bonds and attaining the +3 state is not always enough to outweigh the energy needed to involve the 5s-electrons.[33] Indium(I) oxide and hydroxide are more basic and indium(III) oxide and hydroxide are more acidic.[33]
A number of standard electrode potentials, depending on the reaction under study,[34] are reported for indium, reflecting the decreased stability of the +3 oxidation state:[25]
In2+ + e− ⇌ In+ E0 = −0.40 V In3+ + e− ⇌ In2+ E0 = −0.49 V In3+ + 2 e− ⇌ In+ E0 = −0.443 V In3+ + 3 e− ⇌ In E0 = −0.3382 V In+ + e− ⇌ In E0 = −0.14 V
Indium metal does not react with water, but it is oxidized by stronger oxidizing agents such as halogens to give indium(III) compounds. It does not form a boride, silicide, or carbide. Indium is rather basic in aqueous solution, showing only slight amphoteric characteristics, and unlike its lighter homologs aluminium and gallium, it is insoluble in aqueous alkaline solutions.[35]
Indium(III) compounds
[edit]
Hydrides and halides
[edit]The hydride InH3 has at best a transitory existence in ethereal solutions at low temperatures. It polymerizes in the absence of bases.[32] Lewis bases stabilize a rich collection of indium hydrides of the formula LInH3 (L = tertiary phosphine and N-Heterocyclic carbenes).[36]
Chlorination, bromination, and iodination of In produce colorless InCl3, InBr3, and yellow InI3. The compounds are Lewis acids, somewhat akin to the better known aluminium trihalides. Again like the related aluminium compound, InF3 is polymeric.[37]
Indium halides dissolves in water to give aquo complexes such as [Ir(H2O)6]3+ and [IrCl2(H2O)4]+. Similar complexes can be prepared from nitrates and acetates. Overall, the pattern is similar to that for aluminium(III).[36]
Chalcogenides and pnictides
[edit]Indium derivatives of chalcogenides (O, S, Se, Te) are well developed. Indium(III) oxide, In2O3, forms when indium metal is burned in air or when the hydroxide or nitrate is heated.[38] The analogous sesqui-chalcogenides with sulfur, selenium, and tellurium are also known.[39]
The chemistry of indium pnictides (N, P, As, Sb) is also well known, motivated by their relevance to semiconductor technology. Direct reaction of indium metal with the pnictogens For applications in microelectronics, the P, As, and Sb derivatives are made by reactions of trimethylindium:
- In(CH3)3 + H3E → InE + 3 CH4 (E = P, As, Sb)
Many of these derivatives are prone to hydrolysis.[40]
Indium(I) compounds
[edit]Indium(I) compounds are not common. The chloride, bromide, and iodide are deeply colored, unlike the parent trihalides from which they are prepared. The fluoride is known only as an unstable gas.[41] Indium(I) oxide black powder is produced when indium(III) oxide decomposes upon heating to 700 °C.[38]
Compounds in other oxidation states
[edit]Less frequently, indium forms compounds in oxidation state +2 and even fractional oxidation states. Usually such materials feature In–In bonding, most notably in the halides In2X4 and [In2X6]2−,[42] and various subchalcogenides such as In4Se3.[43] Several other compounds are known to combine indium(I) and indium(III), such as InI6(InIIICl6)Cl3,[44] InI5(InIIIBr4)2(InIIIBr6),[45] and InIInIIIBr4.[42]
Organoindium compounds
[edit]Organoindium compounds feature In–C bonds. Most are In(III) derivatives, but cyclopentadienylindium(I) is an exception. It was the first known organoindium(I) compound,[46] and is polymeric, consisting of zigzag chains of alternating indium atoms and cyclopentadienyl complexes.[47] Perhaps the best-known organoindium compound is trimethylindium, In(CH3)3, used to prepare certain semiconducting materials.[48][49]
History
[edit]In 1863, German chemists Ferdinand Reich and Hieronymus Theodor Richter were testing ores from the mines around Freiberg, Saxony. They dissolved the minerals pyrite, arsenopyrite, galena and sphalerite in hydrochloric acid and distilled raw zinc chloride. Reich, who was color-blind, employed Richter as an assistant for detecting the colored spectral lines. Knowing that ores from that region sometimes contain thallium, they searched for the green thallium emission spectrum lines. Instead, they found a bright blue line. Because that blue line did not match any known element, they hypothesized a new element was present in the minerals. They named the element indium, from the indigo color seen in its spectrum, after the Latin indicum, meaning 'of India'.[50][51][52][53]
Richter went on to isolate the metal in 1864.[54] An ingot of 0.5 kg (1.1 lb) was presented at the World Fair 1867.[55] Reich and Richter later fell out when the latter claimed to be the sole discoverer.[53]
Occurrence
[edit]
Indium is created by the long-lasting (up to thousands of years) s-process (slow neutron capture) in low-to-medium-mass stars (range in mass between 0.6 and 10 solar masses). When a silver-109 atom captures a neutron, it transmutes into silver-110, which then undergoes beta decay to become cadmium-110. Capturing further neutrons, it becomes cadmium-115, which decays to indium-115 by another beta decay. This explains why the radioactive isotope is more abundant than the stable one.[56] The stable indium isotope, indium-113, is one of the p-nuclei, the origin of which is not fully understood; although indium-113 is known to be made directly in the s- and r-processes (rapid neutron capture), and also as the daughter of very long-lived cadmium-113, which has a half-life of about eight quadrillion years, this cannot account for all indium-113.[57][58]
Indium is the 68th most abundant element in Earth's crust at approximately 50 ppb. This is similar to the crustal abundance of silver, bismuth and mercury. It very rarely forms its own minerals, or occurs in elemental form. Fewer than 10 indium minerals such as roquesite (CuInS2) are known, and none occur at sufficient concentrations for economic extraction.[59] Instead, indium is usually a trace constituent of more common ore minerals, such as sphalerite and chalcopyrite.[60][61] From these, it can be extracted as a by-product during smelting.[17] While the enrichment of indium in these deposits is high relative to its crustal abundance, it is insufficient, at current prices, to support extraction of indium as the main product.[59]
Different estimates exist of the amounts of indium contained within the ores of other metals.[62][63] However, these amounts are not extractable without mining of the host materials (see Production and availability). Thus, the availability of indium is fundamentally determined by the rate at which these ores are extracted, and not their absolute amount. This is an aspect that is often forgotten in the current debate, e.g. by the Graedel group at Yale in their criticality assessments,[64] explaining the paradoxically low depletion times some studies cite.[65][17]
Production and availability
[edit]
Indium is produced exclusively as a by-product during the processing of the ores of other metals. Its main source material are sulfidic zinc ores, where it is mostly hosted by sphalerite.[17] Minor amounts are also extracted from sulfidic copper ores. During the roast-leach-electrowinning process of zinc smelting, indium accumulates in the iron-rich residues. From these, it can be extracted in different ways. It may also be recovered directly from the process solutions. Further purification is done by electrolysis.[67] The exact process varies with the mode of operation of the smelter.[21][17]
Its by-product status means that indium production is constrained by the amount of sulfidic zinc (and copper) ores extracted each year. Therefore, its availability needs to be discussed in terms of supply potential. The supply potential of a by-product is defined as that amount which is economically extractable from its host materials per year under current market conditions (i.e. technology and price).[68] Reserves and resources are not relevant for by-products, since they cannot be extracted independently from the main-products.[17] Recent estimates put the supply potential of indium at a minimum of 1,300 t/yr from sulfidic zinc ores and 20 t/yr from sulfidic copper ores.[17] These figures are significantly greater than current production (655 t in 2016).[69] Thus, major future increases in the by-product production of indium will be possible without significant increases in production costs or price. The average indium price in 2016 was US$240/kg, down from US$705/kg in 2014.[70]
China is a leading producer of indium (290 tonnes in 2016), followed by South Korea (195 t), Japan (70 t) and Canada (65 t).[69] The Teck Resources refinery in Trail, British Columbia, is a large single-source indium producer, with an output of 32.5 tonnes in 2005, 41.8 tonnes in 2004 and 36.1 tonnes in 2003.
The primary consumption of indium worldwide is LCD production. Demand rose rapidly from the late 1990s to 2010 with the popularity of LCD computer monitors and television sets, which now account for 50% of indium consumption.[71] Increased manufacturing efficiency and recycling (especially in Japan) maintain a balance between demand and supply. According to the UNEP, indium's end-of-life recycling rate is less than 1%.[72]
Applications
[edit]Industrial uses
[edit]
In 1924, indium was found to have a valued property of stabilizing non-ferrous metals, and that became the first significant use for the element.[73] The first large-scale application for indium was coating bearings in high-performance aircraft engines during World War II, to protect against damage and corrosion; this is no longer a major use of the element.[67] New uses were found in fusible alloys, solders, and electronics. In the 1950s, tiny beads of indium were used for the emitters and collectors of PNP alloy-junction transistors. In the middle and late 1980s, the development of indium phosphide semiconductors and indium tin oxide thin films for liquid-crystal displays (LCD) aroused much interest. By 1992, the thin-film application had become the largest end use.[74][75]
Indium(III) oxide and indium tin oxide (ITO) are used as a transparent conductive coating on glass substrates in electroluminescent panels.[76] Indium tin oxide is used as a light filter in low-pressure sodium-vapor lamps. The infrared radiation is reflected back into the lamp, which increases the temperature within the tube and improves the performance of the lamp.[75]
Indium has many semiconductor-related applications. Some indium compounds, such as indium antimonide and indium phosphide,[77] are semiconductors with useful properties: one precursor is usually trimethylindium (TMI), which is also used as the semiconductor dopant in II–VI compound semiconductors.[49] InAs and InSb are used for low-temperature transistors and InP for high-temperature transistors.[67] The compound semiconductors InGaN and InGaP are used in light-emitting diodes (LEDs) and laser diodes.[78] Indium is used in photovoltaics as the semiconductor copper indium gallium selenide (CIGS), also called CIGS solar cells, a type of second-generation thin-film solar cell.[79] Indium is used in PNP bipolar junction transistors with germanium: when soldered at low temperature, indium does not stress the germanium.[67]

Indium wire is used as a vacuum seal and a thermal conductor in cryogenics and ultra-high-vacuum applications, in such manufacturing applications as gaskets that deform to fill gaps.[80] Owing to its great plasticity and adhesion to metals, Indium sheets are sometimes used for cold-soldering in microwave circuits and waveguide joints, where direct soldering is complicated. Indium is an ingredient in the gallium–indium–tin alloy galinstan, which is liquid at room temperature and replaces mercury in some thermometers.[81] Other alloys of indium with bismuth, cadmium, lead, and tin, which have higher but still low melting points (between 50 and 100 °C), are used in fire sprinkler systems and heat regulators.[67]
Indium is one of many substitutes for mercury in alkaline batteries to prevent the zinc from corroding and releasing hydrogen gas.[82] Indium is added to some dental amalgam alloys to decrease the surface tension of the mercury and allow for less mercury and easier amalgamation.[83]
Indium's high neutron-capture cross-section for thermal neutrons makes it suitable for use in control rods for nuclear reactors, typically in an alloy of 80% silver, 15% indium, and 5% cadmium.[84] In nuclear engineering, the (n,n') reactions of 113In and 115In are used to determine magnitudes of neutron fluxes.[85]
In 2009, Professor Mas Subramanian and former graduate student Andrew Smith at Oregon State University discovered that indium can be combined with yttrium and manganese to form an intensely blue, non-toxic, inert, fade-resistant pigment, YInMn blue, the first new inorganic blue pigment discovered in 200 years.[86]
Medical applications
[edit]Radioactive indium-111 (in very small amounts) is used in nuclear medicine tests, as a radiotracer to follow the movement of labeled proteins and white blood cells to diagnose different types of infection.[87][88] Indium compounds are mostly not absorbed upon ingestion and are only moderately absorbed on inhalation; they tend to be stored temporarily in the muscles, skin, and bones before being excreted, and the biological half-life of indium is about two weeks in humans.[89] It is also tagged to growth hormone analogues like octreotide to find growth hormone receptors in neuroendocrine tumors.[90]
Biological role and precautions
[edit]| Hazards | |
|---|---|
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P280, P305+P351+P338[91] | |
| NFPA 704 (fire diamond) | |
Indium has no metabolic role in any organism. According to one overview "no evidence of any health hazard from industrial use of indium."[92]
Notes
[edit]- ^ The thermal expansion is anisotropic: the parameters (at 20 °C) for each crystal axis are αa = 53.2×10−6/K, αc = −9.75×10−6/K, and αaverage = αV/3 = 32.2×10−6/K.[3]
References
[edit]- ^ "Standard Atomic Weights: Indium". CIAAW. 2011.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Mangum, B. W. (1989). "Determination of the Indium Freezing-point and Triple-point Temperatures". Metrologia. 26 (4): 211. Bibcode:1989Metro..26..211M. doi:10.1088/0026-1394/26/4/001.
- ^ Guloy, A. M.; Corbett, J. D. (1996). "Synthesis, Structure, and Bonding of Two Lanthanum Indium Germanides with Novel Structures and Properties". Inorganic Chemistry. 35 (9): 2616–22. doi:10.1021/ic951378e. PMID 11666477.
- ^ In(−2) has been observed in Na2In, see [1], p. 69.
- ^ In(−1) has been observed in NaIn; see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2008). Lehrbuch der Anorganischen Chemie (in German) (102 ed.). Walter de Gruyter. p. 1185. ISBN 978-3-11-020684-5.
- ^ Unstable In(0) carbonyls and clusters have been detected, see [2], p. 6.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3) 030001. doi:10.1088/1674-1137/abddae.
- ^ "Indium | In (Element) - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2025-09-12.
- ^ "Indium Tin Oxide Targets for Mobile Phone and Tablet Display Screens". www.samaterials.com. Retrieved 2025-09-12.
- ^ US4153317A, Ljung, Bo H. G. & Koper, James G., "Indium seal for gas laser", issued 1979-05-08
- ^ "Why It Was Almost Impossible to Make the Blue LED". YouTube. 2024-03-06. Retrieved 2025-08-15.
- ^ a b c d e f g Frenzel, Max; Mikolajczak, Claire; Reuter, Markus A.; Gutzmer, Jens (June 2017). "Quantifying the relative availability of high-tech by-product metals – The cases of gallium, germanium and indium". Resources Policy. 52: 327–335. Bibcode:2017RePol..52..327F. doi:10.1016/j.resourpol.2017.04.008.
- ^ "Indium and compounds". app.croneri.co.uk. Retrieved 2025-09-12.
- ^ Bomhard, Ernst. (2018). The toxicology of indium oxide. Environmental Toxicology and Pharmacology. 58. 10.1016/j.etap.2018.02.003.
- ^ Royal Society of Chemistry, https://www.rsc.org/ Archived 2021-04-20 at the Wayback Machine
- ^ a b c Alfantazi, A. M.; Moskalyk, R. R. (2003). "Processing of indium: a review". Minerals Engineering. 16 (8): 687–694. Bibcode:2003MiEng..16..687A. doi:10.1016/S0892-6875(03)00168-7.
- ^ Binder, Harry H. (1999). Lexicon der chemischen Elemente (in German). S. Hirzel Verlag. ISBN 978-3-7776-0736-8.
- ^ a b Dean, John A. (523). Lange's handbook of chemistry (Fifteenth ed.). McGraw-Hill, Inc. ISBN 978-0-07-016190-0.
{{cite book}}: ISBN / Date incompatibility (help) - ^ Greenwood and Earnshaw, p. 222
- ^ a b Greenwood and Earnshaw, p. 252
- ^ Okamoto, H. (2012). "Hg-In phase diagram". Journal of Phase Equilibria and Diffusion. 33 (2): 159–160. doi:10.1007/s11669-012-9993-3. S2CID 93043767.
- ^ Iliev, S. P.; Chen, X.; Pathan, M. V.; Tagarielli, V. L. (2017-01-23). "Measurements of the mechanical response of Indium and of its size dependence in bending and indentation". Materials Science and Engineering: A. 683: 244–251. doi:10.1016/j.msea.2016.12.017. hdl:10044/1/43082.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ Dvornický, R.; Šimkovic, F. (13–16 June 2011). "Second unique forbidden β decay of 115In and neutrino mass". AIP Conf. Proc. AIP Conference Proceedings. 1417 (33): 33. Bibcode:2011AIPC.1417...33D. doi:10.1063/1.3671032.
- ^ "IUPAC Periodic Table of the Isotopes" (PDF). ciaaw.org. IUPAC. 1 October 2013. Archived (PDF) from the original on 14 February 2019. Retrieved 21 June 2016.
- ^ Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Thallium". Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 892–893. ISBN 978-3-11-007511-3.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ a b Greenwood and Earnshaw, p. 256
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 8.20. ISBN 1-4398-5511-0.
- ^ Greenwood and Earnshaw, p. 255
- ^ a b Zhao, Yanbao; Zhang, Zhijun (2005). "Indium: Inorganic Chemistry". Encyclopedia of Inorganic and Bioinorganic Chemistry. doi:10.1002/9781119951438.eibc0090. ISBN 978-1-119-95143-8.
- ^ Greenwood and Earnshaw, pp. 263–7
- ^ a b Anthony John Downs (1993). Chemistry of aluminium, gallium, indium, and thallium. Springer. ISBN 978-0-7514-0103-5.
- ^ Greenwood and Earnshaw, p. 286
- ^ Greenwood and Earnshaw, p. 288
- ^ Greenwood and Earnshaw, pp. 270–1
- ^ a b Sinclair, Ian; Worrall, Ian J. (1982). "Neutral complexes of the indium dihalides". Canadian Journal of Chemistry. 60 (6): 695–698. doi:10.1139/v82-102.
- ^ Greenwood and Earnshaw, p. 287
- ^ Beck, Horst Philipp; Wilhelm, Doris (1991). "In7Cl9—A New"Old" Compound in the System In-Cl". Angewandte Chemie International Edition in English. 30 (7): 824–825. doi:10.1002/anie.199108241.
- ^ Dronskowski, Richard (1995). "Synthesis, Structure, and Decay of In4Br7". Angewandte Chemie International Edition in English. 34 (10): 1126–1128. doi:10.1002/anie.199511261.
- ^ Fischer, E. O.; Hofmann, H. P. (1957). "Metall-cyclopentadienyle des Indiums". Angewandte Chemie (in German). 69 (20): 639–640. Bibcode:1957AngCh..69..639F. doi:10.1002/ange.19570692008.
- ^ Beachley O. T.; Pazik J. C.; Glassman T. E.; Churchill M. R.; Fettinger J.C.; Blom R. (1988). "Synthesis, characterization and structural studies of In(C5H4Me) by x-ray diffraction and electron diffraction techniques and a reinvestigation of the crystalline state of In(C5H5) by x-ray diffraction studies". Organometallics. 7 (5): 1051–1059. doi:10.1021/om00095a007.
- ^ Shenai, Deo V.; Timmons, Michael L.; Dicarlo, Ronald L.; Lemnah, Gregory K.; Stennick, Robert S. (2003). "Correlation of vapor pressure equation and film properties with trimethylindium purity for the MOVPE grown III–V compounds". Journal of Crystal Growth. 248: 91–98. Bibcode:2003JCrGr.248...91S. doi:10.1016/S0022-0248(02)01854-7.
- ^ a b Shenai, Deodatta V.; Timmons, Michael L.; Dicarlo, Ronald L.; Marsman, Charles J. (2004). "Correlation of film properties and reduced impurity concentrations in sources for III/V-MOVPE using high-purity trimethylindium and tertiarybutylphosphine". Journal of Crystal Growth. 272 (1–4): 603–608. Bibcode:2004JCrGr.272..603S. doi:10.1016/j.jcrysgro.2004.09.006.
- ^ Reich, F.; Richter, T. (1863). "Ueber das Indium". Journal für Praktische Chemie (in German). 90 (1): 172–176. doi:10.1002/prac.18630900122. S2CID 94381243. Archived from the original on 2020-02-02. Retrieved 2019-06-30.
- ^ Venetskii, S. (1971). "Indium". Metallurgist. 15 (2): 148–150. doi:10.1007/BF01088126.
- ^ Greenwood and Earnshaw, p. 244
- ^ a b Weeks, Mary Elvira (1932). "The Discovery of the Elements: XIII. Some Spectroscopic Studies". Journal of Chemical Education. 9 (8): 1413–1434. Bibcode:1932JChEd...9.1413W. doi:10.1021/ed009p1413.[permanent dead link]
- ^ Reich, F.; Richter, T. (1864). "Ueber das Indium". Journal für Praktische Chemie (in German). 92 (1): 480–485. doi:10.1002/prac.18640920180.
- ^ Schwarz-Schampera, Ulrich; Herzig, Peter M. (2002). Indium: Geology, Mineralogy, and Economics. Springer. ISBN 978-3-540-43135-0.
- ^ Boothroyd, A. I. (2006). "Heavy elements in stars". Science. 314 (5806): 1690–1691. doi:10.1126/science.1136842. PMID 17170281. S2CID 116938510.
- ^ Arlandini, C.; Käppeler, F.; Wisshak, K.; Gallino, R.; Lugaro, M.; Busso, M.; Straniero, O. (1999). "Neutron Capture in Low-Mass Asymptotic Giant Branch Stars: Cross Sections and Abundance Signatures". The Astrophysical Journal. 525 (2): 886–900. arXiv:astro-ph/9906266. Bibcode:1999ApJ...525..886A. doi:10.1086/307938. S2CID 10847307.
- ^ Zs; Käppeler, F.; Theis, C.; Belgya, T.; Yates, S. W. (1994). "Nucleosynthesis in the Cd-In-Sn region". The Astrophysical Journal. 426: 357–365. Bibcode:1994ApJ...426..357N. doi:10.1086/174071.
- ^ a b Frenzel, Max (2016). "The distribution of gallium, germanium and indium in conventional and non-conventional resources - Implications for global availability (PDF Download Available)". ResearchGate. doi:10.13140/rg.2.2.20956.18564. Archived from the original on 2018-10-06. Retrieved 2017-06-02.
- ^ Frenzel, Max; Hirsch, Tamino; Gutzmer, Jens (July 2016). "Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type — A meta-analysis". Ore Geology Reviews. 76: 52–78. Bibcode:2016OGRv...76...52F. doi:10.1016/j.oregeorev.2015.12.017.
- ^ Bachmann, Kai; Frenzel, Max; Krause, Joachim; Gutzmer, Jens (June 2017). "Advanced Identification and Quantification of In-Bearing Minerals by Scanning Electron Microscope-Based Image Analysis". Microscopy and Microanalysis. 23 (3): 527–537. Bibcode:2017MiMic..23..527B. doi:10.1017/S1431927617000460. ISSN 1431-9276. PMID 28464970. S2CID 6751828.
- ^ "Mineral Commodities Summary 2007: Indium" (PDF). United States Geological Survey. Archived (PDF) from the original on 2008-05-09. Retrieved 2007-12-26.
- ^ Werner, T. T.; Mudd, G. M.; Jowitt, S. M. (2015-10-02). "Indium: key issues in assessing mineral resources and long-term supply from recycling". Applied Earth Science. 124 (4): 213–226. Bibcode:2015ApEaS.124..213W. doi:10.1179/1743275815Y.0000000007. ISSN 0371-7453. S2CID 128555024.
- ^ Graedel, T. E.; Barr, Rachel; Chandler, Chelsea; Chase, Thomas; Choi, Joanne; Christoffersen, Lee; Friedlander, Elizabeth; Henly, Claire; Jun, Christine (2012-01-17). "Methodology of Metal Criticality Determination". Environmental Science & Technology. 46 (2): 1063–1070. Bibcode:2012EnST...46.1063G. doi:10.1021/es203534z. ISSN 0013-936X. PMID 22191617.
- ^ Harper, E. M.; Kavlak, Goksin; Burmeister, Lara; Eckelman, Matthew J.; Erbis, Serkan; Sebastian Espinoza, Vicente; Nuss, Philip; Graedel, T. E. (2015-08-01). "Criticality of the Geological Zinc, Tin, and Lead Family". Journal of Industrial Ecology. 19 (4): 628–644. Bibcode:2015JInEc..19..628H. doi:10.1111/jiec.12213. ISSN 1530-9290. S2CID 153380535.[permanent dead link]
- ^ U.S. Geological Survey – Historical Statistics for Mineral and Material Commodities in the United States; INDIUM STATISTICS // USGS, April 1, 2014
- ^ a b c d e Greenwood and Earnshaw, p. 247
- ^ Frenzel, Max; Tolosana-Delgado, Raimon; Gutzmer, Jens (December 2015). "Assessing the supply potential of high-tech metals – A general method". Resources Policy. 46, Part 2: 45–58. Bibcode:2015RePol..46...45F. doi:10.1016/j.resourpol.2015.08.002.
- ^ a b Indium - in: USGS Mineral Commodity Summaries (PDF). United States Geological Survey. 2017. Archived (PDF) from the original on 2019-01-11. Retrieved 2017-06-02.
- ^ Kelly, TD; Matos, GR (2015). "Historical Statistics for Mineral and Material Commodities in the United States". Archived from the original on 2017-05-11. Retrieved 2017-06-02.
- ^ "Indium Price Supported by LCD Demand and New Uses for the Metal". Geology.com. Archived from the original (PDF) on 2007-12-21. Retrieved 2007-12-26.
- ^ "USGS Mineral Commodity Summaries 2011" (PDF). USGS and USDI. Archived (PDF) from the original on January 11, 2019. Retrieved August 2, 2011.
- ^ French, Sidney J. (1934). "A story of indium". Journal of Chemical Education. 11 (5): 270. Bibcode:1934JChEd..11..270F. doi:10.1021/ed011p270.
- ^ Tolcin, Amy C. "Mineral Yearbook 2007: Indium" (PDF). United States Geological Survey. Archived (PDF) from the original on 2016-12-31. Retrieved 2009-12-03.
- ^ a b Downs, Anthony John (1993). Chemistry of Aluminium, Gallium, Indium, and Thallium. Springer. pp. 89 and 106. ISBN 978-0-7514-0103-5.
- ^ "The Electroluminescent Light Sabre". Nanotechnology News Archive. Azonano. June 2, 2005. Archived from the original on October 12, 2007. Retrieved 2007-08-29.[dead link]
- ^ Bachmann, K. J. (1981). "Properties, Preparation, and Device Applications of Indium Phosphide". Annual Review of Materials Science. 11: 441–484. Bibcode:1981AnRMS..11..441B. doi:10.1146/annurev.ms.11.080181.002301.
- ^ Schubert, E. Fred (2003). Light-Emitting Diodes. Cambridge University Press. p. 16. ISBN 978-0-521-53351-5.
- ^ Powalla, M.; Dimmler, B. (2000). "Scaling up issues of CIGS solar cells". Thin Solid Films. 361–362 (1–2): 540–546. Bibcode:2000TSF...361..540P. doi:10.1016/S0040-6090(99)00849-4.
- ^ Weissler, G. L., ed. (1990). Vacuum physics and technology. San Diego: Acad. Press. p. 296. ISBN 978-0-12-475914-5.
- ^ Surmann, P; Zeyat, H (Nov 2005). "Voltammetric analysis using a self-renewable non-mercury electrode". Analytical and Bioanalytical Chemistry. 383 (6): 1009–13. doi:10.1007/s00216-005-0069-7. PMID 16228199. S2CID 22732411.
- ^ Geological Survey (U.S.) (2010). Minerals Yearbook, 2008, V. 1, Metals and Minerals. Government Printing Office. pp. 35–2. ISBN 978-1-4113-3015-3.
- ^ Powell L. V.; Johnson G. H.; Bales D. J. (1989). "Effect of Admixed Indium on Mercury Vapor Release from Dental Amalgam". Journal of Dental Research. 68 (8): 1231–3. CiteSeerX 10.1.1.576.2654. doi:10.1177/00220345890680080301. PMID 2632609. S2CID 28342583.
- ^ Scoullos, Michael J. (2001-12-31). "Other types of cadmium alloys". Mercury, cadmium, lead: handbook for sustainable heavy metals policy and regulation. Springer. p. 222. ISBN 978-1-4020-0224-3.
- ^ Berger, Harold; National Bureau Of Standards, United States; Committee E-7 On Nondestructive Testing, American Society for Testing and Materials (1976). "Image Detectors for Other Neutron Energies". Practical applications of neutron radiography and gaging: a symposium. pp. 50–51.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ^ Kupferschmidt, Kai (2019-05-02). "In search of blue". Science. 364 (6439). American Association for the Advancement of Science (AAAS): 424–429. Bibcode:2019Sci...364..424K. doi:10.1126/science.364.6439.424. ISSN 0036-8075. PMID 31048474. S2CID 143434096.
- ^ "IN-111 FACT SHEET" (PDF). Nordion(Canada), Inc. Archived from the original (PDF) on 3 December 2011. Retrieved 23 September 2012.
- ^ Van Nostrand, D.; Abreu, S. H.; Callaghan, J. J.; Atkins, F. B.; Stoops, H. C.; Savory, C. G. (May 1988). "In-111-labeled white blood cell uptake in noninfected closed fracture in humans: prospective study". Radiology. 167 (2): 495–498. doi:10.1148/radiology.167.2.3357961. PMID 3357961.
- ^ Nordberg, Gunnar F.; Fowler, Bruce A.; Nordberg, Monica (7 August 2014). Handbook on the Toxicology of Metals (4th ed.). Academic Press. p. 845. ISBN 978-0-12-397339-9.
- ^ Krenning, E. P.; Bakker, W. H.; Kooij, P. P.; Breeman, W. A.; Oei, H. Y.; De Jong, M.; Reubi, J. C.; Visser, T. J.; Bruns, C.; Kwekkeboom, D. J. (1992). "Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: Metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide". Journal of Nuclear Medicine. 33 (5): 652–658. PMID 1349039.
- ^ "Indium 57083". Archived from the original on 2018-10-02. Retrieved 2018-10-02.
- ^ Felix, Noël (2000). "Indium and Indium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a14_157. ISBN 978-3-527-30385-4.
Sources
[edit]- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
External links
[edit]- Indium Archived 2023-03-13 at the Wayback Machine at The Periodic Table of Videos (University of Nottingham)
- Reducing Agents > Indium low valent Archived 2023-07-09 at the Wayback Machine
- NIOSH Pocket Guide to Chemical Hazards Archived 2015-12-08 at the Wayback Machine (Centers for Disease Control and Prevention)
- usgs.gov (Mineral Commodity Summaries 2025): Indium
Indium
View on GrokipediaCharacteristics
Physical properties
Indium is a silvery-white, lustrous post-transition metal that appears soft and malleable, easily cut with a knife due to its low hardness.[5] The density of indium is 7.31 g/cm³ at 20°C, and unlike water but like most metals, it exhibits contraction upon solidification, resulting in a volume decrease of approximately 2.5%.[9] Indium has a low melting point for a metal at 156.60 °C and a high boiling point of 2072 °C, contributing to its wide liquidus range.[10] In its solid form, indium adopts a body-centered tetragonal crystal structure with space group I4/mmm and lattice parameters a = 3.252 Å and c = 4.946 Å.[11] Key thermal properties include a specific heat capacity of 0.233 J/g·K and a thermal conductivity of 81.8 W/m·K at room temperature.[12][13] Electrically, indium is a good conductor with an electrical conductivity of 11.6 × 10⁶ S/m at 20°C, corresponding to a resistivity of 8.6 × 10⁻⁸ Ω·m.[14] Indium is diamagnetic, exhibiting a negative magnetic susceptibility of -64.0 × 10⁻⁶ cm³/mol at 298 K.[14] In binary alloys, indium commonly forms eutectic systems and solid solutions, with phase diagrams showing peritectic and congruent melting behaviors depending on the alloying element, often resulting in depressed melting temperatures.[15]Chemical properties
Indium (In) is a post-transition metal in group 13 of the periodic table, with atomic number 49 and positioned in period 5.[2] Its electron configuration is [Kr] 4d^{10} 5s^2 5p^1, reflecting the filling of the 5p subshell typical for p-block elements in this group.[2] Indium exhibits an electronegativity of 1.78 on the Pauling scale, indicating moderate electron-attracting ability compared to other group 13 elements.[2] The first ionization energy is 558.3 kJ/mol, while the second is significantly higher at 1820.7 kJ/mol, highlighting the energy required to remove successive electrons from the neutral atom and In⁺ ion, respectively.[2] The predominant oxidation state of indium is +3, consistent with its group valence, though +1 and +2 states are accessible due to the inert pair effect, where the 5s² electron pair becomes increasingly reluctant to participate in bonding down the group.[16] The standard reduction potential for the In³⁺/In couple is -0.342 V, signifying that indium is a moderately strong reducing agent relative to the standard hydrogen electrode.[17] Indium displays moderate reactivity, reacting directly with halogens to form trihalides and with oxygen at elevated temperatures to yield indium(III) oxide, though it remains stable in ambient air owing to a protective oxide layer that forms on the surface.[3] It dissolves readily in non-oxidizing acids such as hydrochloric and sulfuric acid, but resists nitric acid due to surface passivation by the oxide film.[18] In coordination chemistry, indium(III) commonly adopts octahedral geometry in six-coordinate complexes or tetrahedral arrangements in four-coordinate species, influenced by ligand field effects and steric factors.[19] Thermodynamically, elemental indium in its standard state has a formation enthalpy ΔH_f° of 0 kJ/mol and a standard molar entropy S° of 57.65 J/mol·K at 298 K, providing baseline values for assessing reaction spontaneity involving the metal.[20]Isotopes
Indium has approximately 40 known isotopes, with mass numbers ranging from 97 to 135, including both ground states and metastable isomers. Only two isotopes occur naturally and are considered stable: indium-113 (¹¹³In) and indium-115 (¹¹⁵In), with natural abundances of 4.29(5)% and 95.71(5)%, respectively. These abundances result in a standard atomic weight for indium of 114.818(1) u.[21][5] Both stable isotopes exhibit nuclear spins of 9/2⁺, reflecting their odd-neutron configuration in the nuclear shell model. Although ¹¹⁵In is classified as stable, it undergoes extremely slow beta decay to tin-115 with a half-life of 4.41 × 10¹⁴ years. Among the radioactive isotopes, examples include ¹¹¹In, which decays primarily by electron capture to cadmium-111 with a half-life of 2.80 days, and ¹¹⁴In, which undergoes beta decay to tin-114 with a half-life of 71.9 seconds for the ground state. The long-lived metastable isomer ¹¹⁴ᵐIn has a half-life of 49.51 days and decays via isomeric transition (95.7%) or electron capture (4.3%) to the ground state of ¹¹⁴In.[22][23][24] Radioactive isotopes of indium are produced artificially, often through neutron activation. For instance, ¹¹⁴ᵐIn is generated via the reaction ¹¹³In(n,γ)¹¹⁴ᵐIn in nuclear reactors, leveraging the 4.29% natural abundance of ¹¹³In as a target. These radioisotopes find applications in nuclear physics, such as neutron flux monitoring and as tracers in material science studies, due to their well-characterized decay properties.[25]History
Etymology
The name "indium" originates from the Latin word indicum, meaning "indigo," referring to the prominent indigo-blue spectral lines observed in its emission spectrum during its identification in 1863.[6] This naming convention was common in 19th-century spectroscopy, where newly discovered elements were often designated based on distinctive colors in their spectral signatures, as seen with elements like rubidium (from Latin rubidus, meaning "red") and caesium (from Latin caesius, meaning "sky blue").[26] The element was discovered by German chemists Ferdinand Reich and Hieronymus Theodor Richter while analyzing zinc ores, and they proposed the name to reflect these characteristic lines, distinguishing it from unrelated geographic associations like India.[27] The chemical symbol "In" directly derives from "indium," following the standard practice established by the discoverers in their 1863 publication and later formalized in international nomenclature.[28] The term indicum itself traces to the ancient indigo dye derived from plants, symbolizing the deep blue hue that became emblematic of the element's spectroscopic identity.[29]Discovery and development
Indium was discovered in 1863 by German chemists Ferdinand Reich and Hieronymus Theodor Richter at the Freiberg Mining Academy while spectroscopically examining samples of zinc blende ore from the Himmelfürst mine.[6] Reich, who was color-blind, relied on Richter to interpret the spectrum, where they observed prominent indigo-blue emission lines indicating a previously unknown element.[30] This finding occurred amid a surge in spectrochemical discoveries during the mid-19th century, building on techniques pioneered by Robert Bunsen and Gustav Kirchhoff, and following closely the 1861 identification of thallium by William Crookes.[6] Richter subsequently isolated metallic indium in 1864 through electrolytic reduction, producing a small quantity of the soft, silvery-white metal from an aqueous solution of indium chloride.[27] By 1867, they had refined the process to prepare a larger sample, presenting a 0.5 kg ingot at the Paris World Fair, which demonstrated the element's malleability and luster.[31] Early characterization efforts confirmed indium as a distinct element in group 13 of the periodic table, with Richter initially estimating its atomic weight at 75.6 based on assuming a divalent chloride formula (InCl₂); this was corrected in the 1870s to approximately 113.4 after recognizing its trivalent nature (InCl₃), through analyses by chemists using gravimetric methods on indium halides.[27] Commercial production of indium began in 1934 with the founding of the Indium Corporation of America. In the 1930s, it began to be incorporated into low-melting alloys, marking its transition from laboratory curiosity to industrial material.[32] Postwar advancements in the mid-20th century recognized indium's potential in electronic applications, particularly in semiconductor research.[33]Occurrence and production
Natural occurrence
Indium occurs at low levels in the cosmos, with an estimated abundance of 0.3 parts per billion by weight, comparable to that of silver at 0.6 ppb.[34][35] In Earth's crust, indium is present at an average concentration of 0.25 parts per million, ranking it as the 49th most abundant element.[36] It is predominantly dispersed as a trace element in zinc ores, especially sphalerite ((Zn,Fe)S), where it substitutes for zinc via isomorphous replacement and can reach concentrations up to 1 wt%.[37][38] Although indium rarely forms independent deposits, it is found in primary minerals such as roquesite (CuInS₂) and dzhalindite (In(OH)₃), with pure indium occurrences being exceptionally scarce.[39] It is chiefly associated with sulfide minerals in polymetallic ores of zinc, lead, and copper, serving as a byproduct in these systems.[40] Exploration efforts have recently uncovered new potential sources. In Canada, the Magno project in British Columbia has shown anomalous indium in historical zinc-lead-silver deposits, indicating untapped reserves.[41] In Australia, 2024 discoveries at the Orient project in Queensland by Iltani Resources have delineated what may be the country's largest silver-indium deposit, with high-grade mineralization that could expand known reserves.[42] Extraterrestrially, indium has been identified in meteorites and lunar samples, with concentrations ranging from 3 to 60 parts per billion in the latter.[43]Extraction and refining
Indium is primarily obtained as a byproduct of zinc smelting, accounting for approximately 90% of global production.[44] During zinc ore processing, indium concentrates in residues such as slags, dusts, and fumes generated from roasting and smelting sphalerite ores.[7] The standard industrial process begins with leaching these zinc residues using sulfuric acid, which dissolves indium along with other metals into solution.[45] Indium is then selectively precipitated from the leachate as indium hydroxide, In(OH)₃, by adjusting the pH with a base such as sodium hydroxide. This precipitate is redissolved in sulfuric acid to form indium sulfate, In₂(SO₄)₃, solution.[46] The purified indium sulfate is subjected to electrolysis in an acidic electrolyte, typically using aluminum cathodes, to deposit high-purity indium metal with yields exceeding 99.99%.[46] Alternative methods for indium recovery include solvent extraction using di(2-ethylhexyl)phosphoric acid (D2EHPA) as the extractant in an organic phase, which selectively separates indium from impurities in the leach solution, followed by stripping and precipitation.[47] Another approach is cementation, where zinc dust is added to the acidic solution to reduce and precipitate indium metal selectively.[46] Further refining of the electrolytic indium to ultrahigh purity (up to 99.9999%) for semiconductor applications employs zone melting, where a narrow molten zone is passed along the indium ingot to segregate impurities to one end, or vacuum distillation, which exploits indium's volatility to separate it from non-volatile impurities.[48] Companies involved in refining high-purity indium for semiconductors include Indium Corporation (USA), Vital Materials, and others such as 5N Plus and Umicore. However, the primary production and much of the refining capacity remain heavily tied to China.[49][50][8] Typical recovery rates from zinc residues range from 80% to 95%, depending on the residue type and process efficiency.[45] Recent advances since 2020 include optimized hydrometallurgical techniques, such as oxidative pressure leaching and enhanced solvent extraction systems, enabling efficient recovery from low-grade ores and wastes with indium concentrations below 100 ppm.[51]Global supply and market trends
Global indium production reached 1,020 metric tons in 2023, with an estimated 1,080 metric tons in 2024 driven by increased recovery from zinc processing and recycling efforts.[8] China dominates as the top producer, accounting for about 70% of global output in 2024, followed by South Korea (approximately 20%) and Japan (6%) through refinery operations tied to electronics manufacturing, with much of the refining capacity, particularly for high-purity indium used in semiconductors, heavily tied to China.[8][52] Recycling supplies a significant portion of indium availability, primarily from indium tin oxide (ITO) scrap generated in display and semiconductor production, with major recovery activities in Japan and South Korea.[8] Quantitative estimates of world reserves are not available, as indium is mainly recovered as a byproduct from zinc ores where it occurs at concentrations from less than 1 to 100 ppm.[8] Recent discoveries have bolstered supply potential, including high-grade deposits in Australia's Orient project, identified as the country's largest silver-indium resource, and new exploration sites in Canada such as the Magno project.[53][54] As of November 2025, indium prices were approximately $350-370 per kilogram, following an increase from the 2024 average of $340 per kilogram, influenced by China's export controls on indium and related products implemented on February 4, 2025, to safeguard national security and resources.[55][56] These controls exacerbate supply chain risks stemming from heavy dependence on China, which supplies over 70% of global indium and 25% of U.S. imports, prompting initiatives to enhance recycling from ITO scrap and diversify sourcing.[57] Looking ahead, production is forecasted to grow, fueled by rising demand in electronics and optoelectronics, though potential shortages loom due to the technology sector's expansion outpacing supply diversification.[58]Compounds
Indium(III) compounds
Indium(III) compounds represent the most stable and prevalent class of indium derivatives, featuring the metal in its +3 oxidation state, which dominates due to indium's group 13 position and electronic configuration. These compounds exhibit diverse structures, ranging from simple binary salts to coordination complexes, and display properties such as amphoterism, solubility variations, and utility in materials synthesis. Synthesis often involves direct reaction of indium metal with the corresponding acid or oxidizing agent, followed by precipitation or evaporation, while their reactivity includes hydrolysis and coordination with ligands forming octahedral geometries typical for d^{10} In(III) centers. Indium(III) oxide, In₂O₃, is a key compound prepared by calcination of indium(III) hydroxide or carbonate at high temperatures around 800–1000 °C, yielding a yellow to white powder with a cubic bixbyite structure. It is amphoteric, dissolving in acids to form In³⁺ salts and in strong bases to produce indiumate ions like [In(OH)₄]⁻, which underscores its intermediate electronegativity. Widely used as a precursor for other indium compounds and in transparent conductive films, In₂O₃ has a direct band gap of approximately 3.6 eV, making it a wide-bandgap semiconductor suitable for optoelectronic applications.[59] Indium(III) halides, InX₃ where X = Cl, Br, or I, are hygroscopic solids synthesized by direct combination of indium with the halogen or via metathesis reactions. In the solid state, they adopt layered structures with octahedral InX₆ units, but in the gas phase, they form dimeric In₂X₆ species with bridging halides, while monomeric InX₃ units exhibit trigonal planar geometry around indium. These halides are prone to hydrolysis, reacting with water to form indium oxychlorides (e.g., InOCl) or basic salts, a property exploited in analytical separations but requiring anhydrous conditions for handling.[60] Indium(III) sulfate, In₂(SO₄)₃, is obtained by dissolving indium in sulfuric acid and crystallizing the product, resulting in a colorless, highly water-soluble salt (solubility ~539 g/L at 20 °C) with a monoclinic crystal structure. Its exceptional solubility and stability in aqueous solutions make it valuable as a hardening agent in gold electroplating baths and as a source for indium salts in synthesis. The compound remains stable under typical processing conditions but decomposes upon strong heating to indium oxide and sulfur oxides.[61] Coordination compounds of In(III) often feature six-coordinate octahedral geometries due to the ion's preference for high coordination numbers. A representative example is tris(acetylacetonato)indium(III), [In(acac)₃], where three bidentate acetylacetonate ligands chelate the indium center, forming a propeller-like structure with In–O bond lengths around 2.1 Å, confirmed by X-ray crystallography and NMR studies. These complexes are typically prepared by reacting InX₃ with the ligand in the presence of base and serve as volatile precursors for chemical vapor deposition of indium-containing films.[62] A prominent reaction of In(III) compounds is the hydrolysis of the In³⁺ ion in aqueous solution, leading to precipitation of indium(III) hydroxide, In(OH)₃, as a white gelatinous solid:This process is governed by a very low solubility product constant, at 25 °C, indicating extremely low solubility (~10^{-11} M) and enabling quantitative precipitation for purification. The hydroxide can be dehydrated to In₂O₃ and is amphoteric, redissolving in excess base.[63] In analytical chemistry, indium is detected through precipitation methods, such as forming In(OH)₃ or indium sulfide (In₂S₃) for gravimetric analysis, or via spectroscopic techniques like atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS), which offer detection limits down to ppb levels in complex matrices such as alloys or environmental samples. Spectrophotometric methods using chromogenic agents, like 1-(2-pyridylmethylideneamine)-3-(salicylideneamine)thiourea, provide sensitive colorimetric detection at 450 nm for trace indium in nickel alloys.[64]