Recent from talks
Nothing was collected or created yet.
Senescence
View on Wikipedia
Senescence (/ˌsɪˈnɛsəns/) or biological aging is the gradual deterioration of functional characteristics in living organisms. Whole organism senescence involves an increase in death rates or a decrease in fecundity with increasing age, at least in the later part of an organism's life cycle.[1][2] However, the effects of senescence can be delayed. The 1934 discovery that calorie restriction can extend lifespans by 50% in rats, the existence of species having negligible senescence, and the existence of potentially immortal organisms such as members of the genus Hydra have motivated research into delaying senescence and thus age-related diseases. Rare human mutations can cause accelerated aging diseases.
Environmental factors may affect aging – for example, overexposure to ultraviolet radiation accelerates skin aging. Different parts of the body may age at different rates and distinctly, including the brain, the cardiovascular system, and muscle. Similarly, functions may distinctly decline with aging, including movement control and memory. Two organisms of the same species can also age at different rates, making biological aging and chronological aging distinct concepts.
Definition and characteristics
[edit]Organismal senescence is the aging of whole organisms. Actuarial senescence can be defined as an increase in mortality or a decrease in fecundity with age. The Gompertz–Makeham law of mortality says that the age-dependent component of the mortality rate increases exponentially with age.
Aging is characterized by the declining ability to respond to stress, increased homeostatic imbalance, and increased risk of aging-associated diseases, including cancer and heart disease. Aging has been defined as "a progressive deterioration of physiological function, an intrinsic age-related process of loss of viability and increase in vulnerability."[3]
In 2013, a group of scientists defined nine hallmarks of aging that are common between organisms with emphasis on mammals:
- genomic instability,
- telomere attrition,
- epigenetic alterations,
- loss of proteostasis,
- deregulated nutrient sensing,
- mitochondrial dysfunction,
- cellular senescence,
- stem cell exhaustion,
- altered intercellular communication[4]
In a decadal update, three hallmarks have been added, totaling 12 proposed hallmarks:
The environment induces damage at various levels, e.g., damage to DNA, and damage to tissues and cells by oxygen radicals (widely known as free radicals), and some of this damage is not repaired and thus accumulates with time.[6] Cloning from somatic cells rather than germ cells may begin life with a higher initial load of damage. Dolly the sheep died young from a contagious lung disease, but data on an entire population of cloned individuals would be necessary to measure mortality rates and quantify aging.[citation needed]
The evolutionary theorist George Williams wrote, "It is remarkable that after a seemingly miraculous feat of morphogenesis, a complex metazoan should be unable to perform the much simpler task of merely maintaining what is already formed."[7]
Variation among species
[edit]Different speeds with which mortality increases with age correspond to different maximum life span among species. For example, a mouse is elderly at 3 years, a human is elderly at 80 years,[8] and ginkgo trees show little effect of age even at 667 years.[9]
Almost all organisms senesce, including bacteria which have asymmetries between "mother" and "daughter" cells upon cell division, with the mother cell experiencing aging, while the daughter is rejuvenated.[10][11] There is negligible senescence in some groups, such as the genus Hydra.[12] Planarian flatworms have "apparently limitless telomere regenerative capacity fueled by a population of highly proliferative adult stem cells."[13] These planarians are not biologically immortal, but rather their death rate slowly increases with age. Organisms that are thought to be biologically immortal would, in one instance, be Turritopsis dohrnii, also known as the "immortal jellyfish", due to its ability to revert to its youth when it undergoes stress during adulthood.[14] The reproductive system is observed to remain intact, and even the gonads of Turritopsis dohrnii exist.[15]
Some species exhibit "negative senescence", in which reproduction capability increases or is stable, and mortality falls with age, resulting from the advantages of increased body size during aging.[16]
Theories of aging
[edit]This section needs expansion. You can help by adding to it. (March 2023) |
More than 300 different theories have been posited to explain the nature (mechanisms) and causes (reasons for natural emergence or factors) of aging.[17][additional citation(s) needed] Good theories would both explain past observations and predict the results of future experiments. Some of the theories may complement each other, overlap, contradict, or may not preclude various other theories.[citation needed]
Theories of aging fall into two broad categories: evolutionary theories of aging and mechanistic theories of aging. Evolutionary theories of aging primarily explain why aging happens,[18] but do not concern themselves with the molecular mechanism(s) that drive the process. All evolutionary theories of aging rest on the basic mechanisms that the force of natural selection declines with age.[19][20] Mechanistic theories of aging can be divided into theories that propose aging is programmed, and damage accumulation theories, i.e. those that propose aging to be caused by specific molecular changes occurring over time.
Evolutionary aging theories
[edit]Antagonistic pleiotropy
[edit]One theory was proposed by George C. Williams[7] and involves antagonistic pleiotropy. A single gene may affect multiple traits. Some traits that increase fitness early in life may also have negative effects later in life. But, because many more individuals are alive at young ages than at old ages, even small positive effects early can be strongly selected for, and large negative effects later may be very weakly selected against. Williams suggested the following example: Perhaps a gene codes for calcium deposition in bones, which promotes juvenile survival and will therefore be favored by natural selection; however, this same gene promotes calcium deposition in the arteries, causing negative atherosclerotic effects in old age. Thus, harmful biological changes in old age may result from selection for pleiotropic genes that are beneficial early in life but harmful later on. In this case, selection pressure is relatively high when Fisher's reproductive value is high and relatively low when Fisher's reproductive value is low.
Cancer versus cellular senescence tradeoff theory of aging
[edit]Senescent cells within a multicellular organism can be purged by competition between cells, but this increases the risk of cancer. This leads to an inescapable dilemma between two possibilities—the accumulation of physiologically useless senescent cells and cancer, both of which lead to increasing rates of mortality with age.[2]
Disposable soma
[edit]The disposable soma theory of aging was proposed by Thomas Kirkwood in 1977.[1][21] The theory suggests that aging occurs due to a strategy in which an individual only invests in maintenance of the soma for as long as it has a realistic chance of survival.[22] A species that uses resources more efficiently will live longer, and therefore be able to pass on genetic information to the next generation. The demands of reproduction are high, so less effort is invested in the repair and maintenance of somatic cells, compared to germline cells, to focus on reproduction and species survival.[23]
Programmed aging theories
[edit]Programmed theories of aging posit that aging is adaptive, normally invoking selection for evolvability or group selection.
The reproductive-cell cycle theory suggests that aging is regulated by changes in hormonal signaling over the lifespan.[24]
Damage accumulation theories
[edit]The free radical theory of aging
[edit]One of the most prominent theories of aging was first proposed by Harman in 1956.[25] It posits that free radicals produced by dissolved oxygen, radiation, cellular respiration, and other sources cause damage to the molecular machines in the cell and gradually wear them down. This is also known as oxidative stress.
There is substantial evidence to back up this theory. Old animals have larger amounts of oxidized proteins, DNA, and lipids than their younger counterparts.[26][27]
Chemical damage
[edit]
One of the earliest aging theories was the Rate of Living Hypothesis described by Raymond Pearl in 1928[28] (based on earlier work by Max Rubner), which states that fast basal metabolic rate corresponds to short maximum life span.
While there may be some validity to the idea that for various types of specific damage detailed below that are by-products of metabolism, all other things being equal, a fast metabolism may reduce lifespan, in general this theory does not adequately explain the differences in lifespan either within, or between, species. Calorically restricted animals process as much, or more, calories per gram of body mass, as their ad libitum fed counterparts, yet exhibit substantially longer lifespans.[citation needed] Similarly, metabolic rate is a poor predictor of lifespan for birds, bats and other species that, it is presumed, have reduced mortality from predation, and therefore have evolved long lifespans even in the presence of very high metabolic rates.[29] In a 2007 analysis it was shown that, when modern statistical methods for correcting for the effects of body size and phylogeny are employed, metabolic rate does not correlate with longevity in mammals or birds.[30]
Concerning specific types of chemical damage caused by metabolism, it is suggested that damage to long-lived biopolymers, such as structural proteins or DNA, caused by ubiquitous chemical agents in the body such as oxygen and sugars, are in part responsible for aging. The damage can include breakage of biopolymer chains, cross-linking of biopolymers, or chemical attachment of unnatural substituents (haptens) to biopolymers.[citation needed] Under normal aerobic conditions, approximately 4% of the oxygen metabolized by mitochondria is converted to superoxide ion, which can subsequently be converted to hydrogen peroxide, hydroxyl radical and eventually other reactive species including other peroxides and singlet oxygen, which can, in turn, generate free radicals capable of damaging structural proteins and DNA.[6] Certain metal ions found in the body, such as copper and iron, may participate in the process. (In Wilson's disease, a hereditary defect that causes the body to retain copper, some of the symptoms resemble accelerated senescence.) These processes termed oxidative stress are linked to the potential benefits of dietary polyphenol antioxidants, for example in coffee,[31] and tea.[32] However their typically positive effects on lifespans when consumption is moderate[33][34][35] have also been explained by effects on autophagy,[36] glucose metabolism[37] and AMPK.[38]
Sugars such as glucose and fructose can react with certain amino acids such as lysine and arginine and certain DNA bases such as guanine to produce sugar adducts, in a process called glycation. These adducts can further rearrange to form reactive species, which can then cross-link the structural proteins or DNA to similar biopolymers or other biomolecules such as non-structural proteins. People with diabetes, who have elevated blood sugar, develop senescence-associated disorders much earlier than the general population, but can delay such disorders by rigorous control of their blood sugar levels. There is evidence that sugar damage is linked to oxidant damage in a process termed glycoxidation.
Free radicals can damage proteins, lipids or DNA. Glycation mainly damages proteins. Damaged proteins and lipids accumulate in lysosomes as lipofuscin. Chemical damage to structural proteins can lead to loss of function; for example, damage to collagen of blood vessel walls can lead to vessel-wall stiffness and, thus, hypertension, and vessel wall thickening and reactive tissue formation (atherosclerosis); similar processes in the kidney can lead to kidney failure. Damage to enzymes reduces cellular functionality. Lipid peroxidation of the inner mitochondrial membrane reduces the electric potential and the ability to generate energy. It is probably no accident that nearly all of the so-called "accelerated aging diseases" are due to defective DNA repair enzymes.[39][40]
It is believed that the impact of alcohol on aging can be partly explained by alcohol's activation of the HPA axis, which stimulates glucocorticoid secretion, long-term exposure to which produces symptoms of aging.[41]
DNA damage
[edit]DNA damage was proposed in a 2021 review to be the underlying cause of aging because of the mechanistic link of DNA damage to nearly every aspect of the aging phenotype.[42] Slower rate of accumulation of DNA damage as measured by the DNA damage marker gamma H2AX in leukocytes was found to correlate with longer lifespans in comparisons of dolphins, goats, reindeer, American flamingos and griffon vultures.[43] DNA damage-induced epigenetic alterations, such as DNA methylation and many histone modifications, appear to be of particular importance to the aging process.[42] Evidence for the theory that DNA damage is the fundamental cause of aging was first reviewed in 1981.[44]
Mutation accumulation
[edit]Natural selection can support lethal and harmful alleles, if their effects are felt after reproduction. The geneticist J. B. S. Haldane wondered why the dominant mutation that causes Huntington's disease remained in the population, and why natural selection had not eliminated it. The onset of this neurological disease is (on average) at age 45 and is invariably fatal within 10–20 years. Haldane assumed that, in human prehistory, few survived until age 45. Since few were alive at older ages and their contribution to the next generation was therefore small relative to the large cohorts of younger age groups, the force of selection against such late-acting deleterious mutations was correspondingly small. Therefore, a genetic load of late-acting deleterious mutations could be substantial at mutation–selection balance. This concept came to be known as the selection shadow.[45]
Peter Medawar formalised this observation in his mutation accumulation theory of aging.[46][47] "The force of natural selection weakens with increasing age—even in a theoretically immortal population, provided only that it is exposed to real hazards of mortality. If a genetic disaster... happens late enough in individual life, its consequences may be completely unimportant". Age-independent hazards such as predation, disease, and accidents, called 'extrinsic mortality', mean that even a population with negligible senescence will have fewer individuals alive in older age groups.
Other damage
[edit]A study concluded that retroviruses in the human genomes can become awakened from dormant states and contribute to aging which can be blocked by neutralizing antibodies, alleviating "cellular senescence and tissue degeneration and, to some extent, organismal aging".[48]
Stem cell theories of aging
[edit]The stem cell theory of aging postulates that the aging process is the result of the inability of various types of stem cells to continue to replenish the tissues of an organism with functional differentiated cells capable of maintaining that tissue's (or organ's) original function. Damage and error accumulation in genetic material is always a problem for systems regardless of the age. The number of stem cells in young people is very much higher than older people and thus creates a better and more efficient replacement mechanism in the young contrary to the old. In other words, aging is not a matter of the increase in damage, but a matter of failure to replace it due to a decreased number of stem cells. Stem cells decrease in number and tend to lose the ability to differentiate into progenies or lymphoid lineages and myeloid lineages.
Maintaining the dynamic balance of stem cell pools requires several conditions. Balancing proliferation and quiescence along with homing (See niche) and self-renewal of hematopoietic stem cells are favoring elements of stem cell pool maintenance while differentiation, mobilization and senescence are detrimental elements. These detrimental effects will eventually cause apoptosis.
There are also several challenges when it comes to therapeutic use of stem cells and their ability to replenish organs and tissues. First, different cells may have different lifespans even though they originate from the same stem cells (See T-cells and erythrocytes), meaning that aging can occur differently in cells that have longer lifespans as opposed to the ones with shorter lifespans. Also, continual effort to replace the somatic cells may cause exhaustion of stem cells.[49]- Hematopoietic stem cell aging
- Hematopoietic stem cell diversity aging
- Hematopoietic mosaic loss of chromosome Y
Biomarkers of aging
[edit]If different individuals age at different rates, then fecundity, mortality, and functional capacity might be better predicted by biomarkers than by chronological age.[58][59] However, graying of hair,[60] face aging, skin wrinkles, and other common changes seen with aging are not better indicators of future functionality than chronological age. Biogerontologists have continued efforts to find and validate biomarkers of aging, but success thus far has been limited.
Levels of CD4 and CD8 memory T cells and naive T cells have been used to give good predictions of the expected lifespan of middle-aged mice.[61]
Aging clocks
[edit]There is increasing interest in epigenetic clocks as biomarkers of aging, based on their ability to predict human chronological age. Many epigenetic aging clocks are based on DNA methylation [62][63], but alternative epigenetic clocks are also starting to emerge, e.g. based on nucleosome positioning derived from cell-free DNA.[64] Basic blood biochemistry and cell counts can also be used to accurately predict the chronological age.[65] It is also possible to predict the human chronological age using transcriptomic aging clocks.[66]
There is research and development of further biomarkers, detection systems, and software systems to measure the biological age of different tissues or systems or overall. For example, a deep learning (DL) software using anatomic magnetic resonance images estimated brain age with relatively high accuracy, including detecting early signs of Alzheimer's disease and varying neuroanatomical patterns of neurological aging,[67] and a DL tool was reported as to calculate a person's inflammatory age based on patterns of systemic age-related inflammation.[68]
Aging clocks have been used to evaluate the impacts of interventions on humans, including combination therapies.[69][additional citation(s) needed] Employing aging clocks to identify and evaluate longevity interventions represents a fundamental goal in aging biology research. However, achieving this goal requires overcoming numerous challenges and implementing additional validation steps.[70][71]
Genetic determinants of aging
[edit]Several genetic components of aging have been identified using model organisms, ranging from the simple budding yeast Saccharomyces cerevisiae to worms such as Caenorhabditis elegans and fruit flies (Drosophila melanogaster). Study of these organisms has revealed the presence of at least two conserved aging pathways.
Gene expression is imperfectly controlled, and random fluctuations in the expression levels of many genes may contribute to the aging process, as suggested by a study of such genes in yeast.[72] Individual cells, which are genetically identical, nonetheless can have substantially different responses to outside stimuli, and markedly different lifespans, indicating the epigenetic factors play an important role in gene expression and aging as well as genetic factors. There is research into epigenetics of aging.
The ability to repair DNA double-strand breaks declines with aging in mice[73] and humans.[74]
A set of rare hereditary (genetics) disorders, each called progeria, has been known for some time. Sufferers exhibit symptoms resembling accelerated aging, including wrinkled skin. The cause of Hutchinson–Gilford progeria syndrome was reported in the journal Nature in May 2003.[75] This report suggests that DNA damage, not oxidative stress, is the cause of this form of accelerated aging.
A study indicates that aging may shift activity toward short genes or shorter transcript length and that this can be countered by interventions.[76]
Healthspans and aging in society
[edit]

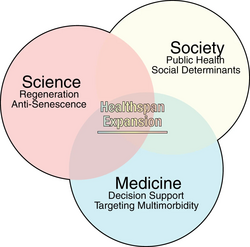
Healthspan can broadly be defined as the period of one's life that one is healthy, such as being free of significant diseases[78] or declines of capacities (e.g., senses such as hearing, muscle, endurance and cognition).
Biological aging or the LHG comes with a great cost burden to society, including potentially rising health care costs (also depending on types and costs of treatments).[77][81] This, along with global quality of life or wellbeing, highlight the importance of extending healthspans.[77]
Although interventions which extend lifespan may also extend healthspan, this is not always the case, suggesting that "lifespan can no longer be the sole parameter of interest," in related research.[82] When increases in life expectancy outpaced improvements in healthspan,[77] public awareness of these 'healthspan lags' began rising around 2017.[78] Scientists have noted that "Chronic diseases of aging are increasing and are inflicting untold costs on human quality of life".[81]
Interventions
[edit]Life extension is the concept of extending the human lifespan, either modestly through improvements in medicine or dramatically by increasing the maximum lifespan beyond its generally-settled biological limit of around 125 years.[83] Several researchers in the area, along with "life extensionists", "immortalists", or "longevists" (those who wish to achieve longer lives themselves), postulate that future breakthroughs in tissue rejuvenation, stem cells, regenerative medicine, molecular repair, gene therapy, pharmaceuticals, and organ replacement (such as with artificial organs or xenotransplantations) will eventually enable humans to have indefinite lifespans through complete rejuvenation to a healthy youthful condition (agerasia[84]). The ethical ramifications, if life extension becomes a possibility, are debated by bioethicists.
The sale of purported anti-aging products such as supplements and hormone replacement is a lucrative global industry. For example, the industry that promotes the use of hormones as a treatment for consumers to slow or reverse the aging process in the US market generated about $50 billion of revenue a year in 2009.[85] The use of such hormone products has not been proven to be effective or safe.[85][86][87][88] Similarly, a variety of apps make claims to assist in extending the life of their users, or predicting their lifespans.[89][90][91]See also
[edit]- Anti-aging movement
- Antimuscarinics
- Dementia
- DNA repair
- Geriatrics
- Gerontology
- Homeostatic capacity
- Immortality
- Index of topics related to life extension
- Mitohormesis
- Old age
- Phenoptosis
- Plant senescence
- Programmed cell death
- Strategies for engineered negligible senescence (SENS)
- Sub-lethal damage
- Transgenerational design
- Timeline of senescence research
References
[edit]- ^ a b Kirkwood TB (1977). "Evolution of ageing". Nature. 270 (5635): 301–4. Bibcode:1977Natur.270..301K. doi:10.1038/270301a0. PMID 593350. S2CID 492012.
- ^ a b Nelson P, Masel J (December 2017). "Intercellular competition and the inevitability of multicellular aging". Proceedings of the National Academy of Sciences of the United States of America. 114 (49): 12982–7. Bibcode:2017PNAS..11412982N. doi:10.1073/pnas.1618854114. PMC 5724245. PMID 29087299.
- ^ "Aging and Gerontology Glossary". Archived from the original on 19 October 2019. Retrieved 26 February 2011.
- ^ López-Otín C, Blasco MA, Partridge L, et al. (June 2013). "The hallmarks of aging". Cell. 153 (6): 1194–217. doi:10.1016/j.cell.2013.05.039. PMC 3836174. PMID 23746838.
- ^ López-Otín C, Blasco MA, Partridge L, et al. (19 January 2023). "Hallmarks of aging: An expanding universe". Cell. 186 (2): 243–278. doi:10.1016/j.cell.2022.11.001. PMID 36599349. S2CID 255394876.
- ^ a b Holmes GE, Bernstein C, Bernstein H (September 1992). "Oxidative and other DNA damages as the basis of aging: a review". Mutat. Res. 275 (3–6): 305–15. doi:10.1016/0921-8734(92)90034-m. PMID 1383772.
- ^ a b Williams GC (1957). "Pleiotropy, natural selection, and the evolution of senescence". Evolution. 11 (4): 398–411. doi:10.2307/2406060. JSTOR 2406060.
- ^ Austad SN (February 2009). "Comparative biology of aging". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 64 (2): 199–201. doi:10.1093/gerona/gln060. PMC 2655036. PMID 19223603.
- ^ Wang L, Cui J, Jin B, et al. (January 2020). "Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees". Proceedings of the National Academy of Sciences of the United States of America. 117 (4): 2201–10. Bibcode:2020PNAS..117.2201W. doi:10.1073/pnas.1916548117. PMC 6995005. PMID 31932448.
- ^ Ackermann M, Stearns SC, Jenal U (June 2003). "Senescence in a bacterium with asymmetric division". Science. 300 (5627): 1920. doi:10.1126/science.1083532. PMID 12817142. S2CID 34770745.
- ^ Stewart EJ, Madden R, Paul G, et al. (February 2005). "Aging and death in an organism that reproduces by morphologically symmetric division". PLOS Biology. 3 (2) e45. doi:10.1371/journal.pbio.0030045. PMC 546039. PMID 15685293.
- ^ Dańko MJ, Kozłowski J, Schaible R (October 2015). "Unraveling the non-senescence phenomenon in Hydra". Journal of Theoretical Biology. 382: 137–49. Bibcode:2015JThBi.382..137D. doi:10.1016/j.jtbi.2015.06.043. PMID 26163368.
- ^ Tan TC, Rahman R, Jaber-Hijazi F, et al. (March 2012). "Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms". Proceedings of the National Academy of Sciences of the United States of America. 109 (11): 4209–14. Bibcode:2012PNAS..109.4209T. doi:10.1073/pnas.1118885109. PMC 3306686. PMID 22371573.
- ^ Lisenkova AA, Grigorenko AP, Tyazhelova TV, et al. (February 2017). "Complete mitochondrial genome and evolutionary analysis of Turritopsis dohrnii, the "immortal" jellyfish with a reversible life-cycle". Molecular Phylogenetics and Evolution. 107: 232–8. Bibcode:2017MolPE.107..232L. doi:10.1016/j.ympev.2016.11.007. PMID 27845203.
- ^ Piraino S, Boero F, Aeschbach B, et al. (June 1996). "Reversing the Life Cycle: Medusae Transforming into Polyps and Cell Transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa)". The Biological Bulletin. 190 (3): 302–312. doi:10.2307/1543022. JSTOR 1543022. PMID 29227703.
- ^ Vaupel JW, Baudisch A, Dölling M, et al. (June 2004). "The case for negative senescence". Theoretical Population Biology. 65 (4): 339–51. Bibcode:2004TPBio..65..339W. doi:10.1016/j.tpb.2003.12.003. PMID 15136009.
- ^ Viña J, Borrás C, Miquel J (2007). "Theories of ageing". IUBMB Life. 59 (4–5): 249–54. doi:10.1080/15216540601178067. PMID 17505961.
- ^ Kirkwood TB, Austad SN (2000). "Why do we age?". Nature. 408 (6809): 233–8. Bibcode:2000Natur.408..233K. doi:10.1038/35041682. PMID 11089980. S2CID 2579770.
- ^ Medawar PB (1952). An unsolved problem of biology. Published for the College by H.K. Lewis. OCLC 869293719.
- ^ Rose MR (1991). Evolutionary biology of aging. Oxford University Press. ISBN 1-4237-6520-6. OCLC 228167629.
- ^ Kirkwood T (2006). Time of Our Lives: the Science of Human Aging. Oxford University Press. ISBN 978-0-19-802939-7. OCLC 437175125.
- ^ Hammers M, Richardson DS, Burke T, et al. (September 2013). "The impact of reproductive investment and early-life environmental conditions on senescence: support for the disposable soma hypothesis". Journal of Evolutionary Biology. 26 (9): 1999–2007. doi:10.1111/jeb.12204. hdl:11370/9cc6749c-f67d-40ab-a253-a06650c32102. PMID 23961923. S2CID 46466320.
- ^ Kirkwood TB, Rose MR (April 1991). "Evolution of senescence: late survival sacrificed for reproduction". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 332 (1262): 15–24. Bibcode:1991RSPTB.332...15K. doi:10.1098/rstb.1991.0028. PMID 1677205.
- ^ Atwood CS, Bowen RL (2011). "The reproductive-cell cycle theory of aging: an update". Experimental Gerontology. 46 (2–3): 100–7. doi:10.1016/j.exger.2010.09.007. PMID 20851172. S2CID 20998909.
- ^ Harman D (July 1956). "Aging: a theory based on free radical and radiation chemistry". Journal of Gerontology. 11 (3): 298–300. doi:10.1093/geronj/11.3.298. hdl:2027/mdp.39015086547422. PMID 13332224.
- ^ Stadtman ER (August 1992). "Protein oxidation and aging". Science. 257 (5074): 1220–4. Bibcode:1992Sci...257.1220S. doi:10.1126/science.1355616. PMID 1355616. Archived from the original on 31 July 2021. Retrieved 21 July 2021.
- ^ Sohal RS, Agarwal S, Dubey A, et al. (August 1993). "Protein oxidative damage is associated with life expectancy of houseflies". Proceedings of the National Academy of Sciences of the United States of America. 90 (15): 7255–9. Bibcode:1993PNAS...90.7255S. doi:10.1073/pnas.90.15.7255. PMC 47115. PMID 8346242.
- ^ Pearl R (1928). The Rate of Living, Being an Account of Some Experimental Studies on the Biology of Life Duration. New York: Alfred A. Knopf. LCCN 28000834.[page needed]
- ^ Brunet-Rossinni AK, Austad SN (2004). "Ageing studies on bats: a review". Biogerontology. 5 (4): 211–22. doi:10.1023/B:BGEN.0000038022.65024.d8. PMID 15314271. S2CID 22755811.
- ^ de Magalhães JP, Costa J, Church GM (February 2007). "An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 62 (2): 149–60. CiteSeerX 10.1.1.596.2815. doi:10.1093/gerona/62.2.149. PMC 2288695. PMID 17339640.
- ^ Freedman ND, Park Y, Abnet CC, et al. (May 2012). "Association of coffee drinking with total and cause-specific mortality". The New England Journal of Medicine. 366 (20): 1891–904. doi:10.1056/NEJMoa1112010. PMC 3439152. PMID 22591295.
- ^ Yang Y, Chan SW, Hu M, et al. (2011). "Effects of some common food constituents on cardiovascular disease". ISRN Cardiology. 2011 397136. doi:10.5402/2011/397136. PMC 3262529. PMID 22347642.
- ^ Poole R, Kennedy OJ, Roderick P, et al. (22 November 2017). "Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes". BMJ. 359 j5024. doi:10.1136/bmj.j5024. PMC 5696634. PMID 29167102.
- ^ O'Keefe JH, DiNicolantonio JJ, Lavie CJ (1 May 2018). "Coffee for Cardioprotection and Longevity". Progress in Cardiovascular Diseases. 61 (1): 38–42. doi:10.1016/j.pcad.2018.02.002. PMID 29474816.
- ^ Grosso G, Godos J, Galvano F, et al. (21 August 2017). "Coffee, Caffeine, and Health Outcomes: An Umbrella Review". Annual Review of Nutrition. 37 (1): 131–156. doi:10.1146/annurev-nutr-071816-064941. PMID 28826374.
- ^ Dirks-Naylor AJ (15 December 2015). "The benefits of coffee on skeletal muscle". Life Sciences. 143: 182–6. doi:10.1016/j.lfs.2015.11.005. PMID 26546720.
- ^ Reis CE, Dórea JG, da Costa TH (1 July 2019). "Effects of coffee consumption on glucose metabolism: A systematic review of clinical trials". Journal of Traditional and Complementary Medicine. 9 (3): 184–191. doi:10.1016/j.jtcme.2018.01.001. PMC 6544578. PMID 31193893.
- ^ Loureiro LM, Reis CE, Costa TH (1 May 2018). "Effects of Coffee Components on Muscle Glycogen Recovery: A Systematic Review". International Journal of Sport Nutrition and Exercise Metabolism. 28 (3): 284–293. doi:10.1123/ijsnem.2017-0342. PMID 29345166.
- ^ Bernstein H, Payne CM, Bernstein C, et al. (2008). "Cancer and aging as consequences of un-repaired DNA damage.". In Kimura H, Suzuki A (eds.). New Research on DNA Damage. Nova Science Publishers. pp. 1–47. ISBN 978-1-60456-581-2. Archived from the original on 15 November 2023. Retrieved 4 February 2016.
- ^ Pan MR, Li K, Lin SY, et al. (May 2016). "Connecting the Dots: From DNA Damage and Repair to Aging". International Journal of Molecular Sciences. 17 (5): 685. doi:10.3390/ijms17050685. PMC 4881511. PMID 27164092.
- ^ Spencer RL, Hutchison KE (1999). "Alcohol, aging, and the stress response" (PDF). Alcohol Research & Health. 23 (4): 272–83. PMC 6760387. PMID 10890824. Archived from the original (PDF) on 11 December 2018. Retrieved 8 April 2008.
- ^ a b Schumacher B, Pothof J, Vijg J, et al. (April 2021). "The central role of DNA damage in the ageing process". Nature. 592 (7856): 695–703. Bibcode:2021Natur.592..695S. doi:10.1038/s41586-021-03307-7. PMC 9844150. PMID 33911272.
- ^ Whittemore K, Martínez-Nevado E, Blasco MA (November 2019). "Slower rates of accumulation of DNA damage in leukocytes correlate with longer lifespans across several species of birds and mammals". Aging (Albany NY). 11 (21): 9829–45. doi:10.18632/aging.102430. PMC 6874430. PMID 31730540.
- ^ Gensler HL, Bernstein H (September 1981). "DNA damage as the primary cause of aging". The Quarterly Review of Biology. 56 (3): 279–303. doi:10.1086/412317. PMID 7031747. S2CID 20822805.
- ^ Fabian D, Flatt T (2011). "The Evolution of Aging" (PDF). Nature Education.
- ^ Medawar PB (1946). "Old age and natural death". Modern Quarterly. 1: 30–56.
- ^ Medawar 1952[page needed]
- ^ Liu X, Liu Z, Wu Z, et al. (19 January 2023). "Resurrection of endogenous retroviruses during aging reinforces senescence". Cell. 186 (2): 287–304.e26. doi:10.1016/j.cell.2022.12.017. PMID 36610399. S2CID 232060038.
- Expert explanation of the study: "Aging and Retroviruses". Science. Archived from the original on 17 February 2023. Retrieved 17 February 2023.
- ^ Smith J., A., Daniel R. "Stem Cells and Aging: A Chicken-Or-Egg Issue?". Aging and Disease. 2012 Jun, Vol. 3, Number 3; 260–268.
- ^ Mahla RS (2016). "Stem cells application in regenerative medicine and disease threpeutics". International Journal of Cell Biology. 2016 (7): 19. doi:10.1155/2016/6940283. PMC 4969512. PMID 27516776.
- ^ Rossi DJ, Bryder D, Seita J, et al. (2007). "Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age". Nature. 447 (7145): 725–9. Bibcode:2007Natur.447..725R. doi:10.1038/nature05862. PMID 17554309. S2CID 4416445.
- ^ a b Beerman I, Seita J, Inlay MA, et al. (2014). "Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle". Cell Stem Cell. 15 (1): 37–50. doi:10.1016/j.stem.2014.04.016. PMC 4082747. PMID 24813857.
- ^ a b Nijnik A, Woodbine L, Marchetti C, et al. (2007). "DNA repair is limiting for haematopoietic stem cells during ageing". Nature. 447 (7145): 686–90. Bibcode:2007Natur.447..686N. doi:10.1038/nature05875. PMID 17554302. S2CID 4332976.
- ^ "Research may reveal why people can suddenly become frail in their 70s". The Guardian. 1 June 2022. Retrieved 18 July 2022.
- ^ Mitchell E, Spencer Chapman M, Williams N, et al. (June 2022). "Clonal dynamics of haematopoiesis across the human lifespan". Nature. 606 (7913): 343–350. Bibcode:2022Natur.606..343M. doi:10.1038/s41586-022-04786-y. ISSN 1476-4687. PMC 9177428. PMID 35650442.
- ^ Kolata G (14 July 2022). "As Y Chromosomes Vanish With Age, Heart Risks May Grow". The New York Times. Retrieved 21 August 2022.
- ^ Sano S, Horitani K, Ogawa H, et al. (15 July 2022). "Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality". Science. 377 (6603): 292–297. Bibcode:2022Sci...377..292S. doi:10.1126/science.abn3100. ISSN 0036-8075. PMC 9437978. PMID 35857592.
- ^ Gasmi A, Chirumbolo S, Peana M, et al. (17 September 2020). "Biomarkers of Senescence during Aging as Possible Warnings to Use Preventive Measures". Current Medicinal Chemistry. 28 (8): 1471–88. doi:10.2174/0929867327999200917150652. PMID 32942969. S2CID 221789280.
- ^ Baker GT, Sprott RL (1988). "Biomarkers of aging". Experimental Gerontology. 23 (4–5): 223–39. doi:10.1016/0531-5565(88)90025-3. PMID 3058488. S2CID 31039588. Archived from the original on 24 October 2021. Retrieved 12 July 2019.
- ^ Van Neste D, Tobin DJ (2004). "Hair cycle and hair pigmentation: dynamic interactions and changes associated with aging". Micron. 35 (3): 193–200. doi:10.1016/j.micron.2003.11.006. PMID 15036274.
- ^ Miller RA (April 2001). "Biomarkers of aging: prediction of longevity by using age-sensitive T-cell subset determinations in a middle-aged, genetically heterogeneous mouse population". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 56 (4): B180-6. doi:10.1093/gerona/56.4.b180. PMC 7537444. PMID 11283189.
- ^ Naue J (October 2023). "Getting the chronological age out of DNA: using insights of age-dependent DNA methylation for forensic DNA applications". Genes & Genomics. 45 (10): 1239–1261. doi:10.1007/s13258-023-01392-8. PMC 10504122. PMID 37253906.
- ^ Horvath S, Raj K (2018). "DNA methylation-based biomarkers and the epigenetic clock theory of ageing". Nat Rev Genet. 19 (6): 371–384. doi:10.1038/s41576-018-0004-3. PMID 29643443.
- ^ Shtumpf M, Jeong S, Bikova M, et al. (2024). "Aging clock based on nucleosome reorganisation derived from cell-free DNA". Aging Cell. 23 (5) e14100. doi:10.1111/acel.14100. PMC 11113261. PMID 38337183.
- ^ Putin E, Mamoshina P, Aliper A, et al. (May 2016). "Deep biomarkers of human aging: Application of deep neural networks to biomarker development". Aging. 8 (5): 1021–33. doi:10.18632/aging.100968. PMC 4931851. PMID 27191382.
- ^ Peters MJ, Joehanes R, Pilling LC, et al. (October 2015). "The transcriptional landscape of age in human peripheral blood". Nature Communications. 6 8570. Bibcode:2015NatCo...6.8570.. doi:10.1038/ncomms9570. PMC 4639797. PMID 26490707.
- ^ Yin C, Imms P, Cheng M, et al. (10 January 2023). "Anatomically interpretable deep learning of brain age captures domain-specific cognitive impairment". Proceedings of the National Academy of Sciences. 120 (2) e2214634120. Bibcode:2023PNAS..12014634Y. doi:10.1073/pnas.2214634120. PMC 9926270. PMID 36595679.
- University press release: "How old is your brain, really? AI-powered analysis accurately reflects risk of cognitive decline and Alzheimer's disease". University of Southern California via medicalxpress.com. Archived from the original on 17 February 2023. Retrieved 17 February 2023.
- News article about the study: "KI kann wahres Alter des Hirns bestimmen". Deutschlandfunk Nova (in German). Archived from the original on 17 February 2023. Retrieved 17 February 2023.
- ^ Sayed N, Huang Y, Nguyen K, et al. (July 2021). "An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging". Nature Aging. 1 (7): 598–615. doi:10.1038/s43587-021-00082-y. PMC 8654267. PMID 34888528.
- News article about the study: "Tool that calculates immune system age could predict frailty and disease". New Atlas. 13 July 2021. Archived from the original on 26 July 2021. Retrieved 26 July 2021.
- ^ Fitzgerald KN, Hodges R, Hanes D, et al. (2021). "Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial". Aging. 13 (7): 9419–32. doi:10.18632/aging.202913. PMC 8064200. PMID 33844651. Archived from the original on 2 June 2021. Retrieved 28 June 2021.
- ^ Moqri M, Herzog C, Poganik JR, et al. (August 2023). "Biomarkers of aging for the identification and evaluation of longevity interventions". Cell. 186 (18): 3758–75. doi:10.1016/j.cell.2023.08.003. PMC 11088934. PMID 37657418.
- ^ Moqri M, Herzog C, Poganik JR, et al. (February 2024). "Validation of biomarkers of aging". Nature Medicine. 30 (2): 360–372. doi:10.1038/s41591-023-02784-9. ISSN 1546-170X. PMC 11090477. PMID 38355974.
- ^ Ryley J, Pereira-Smith OM (2006). "Microfluidics device for single cell gene expression analysis in Saccharomyces cerevisiae". Yeast. 23 (14–15): 1065–73. doi:10.1002/yea.1412. PMID 17083143. S2CID 31356425.
- ^ Vaidya A, Mao Z, Tian X, et al. (July 2014). "Knock-in reporter mice demonstrate that DNA repair by non-homologous end joining declines with age". PLOS Genet. 10 (7) e1004511. doi:10.1371/journal.pgen.1004511. PMC 4102425. PMID 25033455.
- ^ Li Z, Zhang W, Chen Y, et al. (November 2016). "Impaired DNA double-strand break repair contributes to the age-associated rise of genomic instability in humans". Cell Death Differ. 23 (11): 1765–77. doi:10.1038/cdd.2016.65. PMC 5071568. PMID 27391797.
- ^ Mounkes LC, Kozlov S, Hernandez L, et al. (May 2003). "A progeroid syndrome in mice is caused by defects in A-type lamins". Nature. 423 (6937): 298–301. Bibcode:2003Natur.423..298M. doi:10.1038/nature01631. PMID 12748643. S2CID 4360055. Archived from the original on 30 May 2022. Retrieved 21 July 2021 – via Zenodo.
- ^ Stoeger T, Grant RA, McQuattie-Pimentel AC, et al. (December 2022). "Aging is associated with a systemic length-associated transcriptome imbalance". Nature Aging. 2 (12): 1191–1206. doi:10.1038/s43587-022-00317-6. PMC 10154227. PMID 37118543.
- University press release: "Aging is driven by unbalanced genes, finds AI analysis of multiple species". Northwestern University. 9 December 2022. Archived from the original on 2 February 2023. Retrieved 18 January 2023 – via phys.org.
- News article about the study: Kwon D (6 January 2023). "Aging Is Linked to More Activity in Short Genes Than in Long Genes". Scientific American. Archived from the original on 17 January 2023. Retrieved 18 January 2023.
- ^ a b c d e f Garmany A, Yamada S, Terzic A (23 September 2021). "Longevity leap: mind the healthspan gap". npj Regenerative Medicine. 6 (1): 57. doi:10.1038/s41536-021-00169-5. PMC 8460831. PMID 34556664.
- Non-profit hospital press release: Buckles S (7 October 2021). "A regenerative reset for aging » Center for Regenerative Biotherapeutics". Mayo Clinic. Archived from the original on 1 March 2023. Retrieved 1 March 2023.
- ^ a b Peterson T (30 May 2017). "Healthspan is more important than lifespan, so why don't more people know about it?". Institute for Public Health. Washington University in St. Louis. Harvey A. Friedman Center for Aging. Archived from the original on 1 March 2023. Retrieved 1 March 2023.
- ^ Garmany A, Yamada S, Terzic A (September 2021). "Longevity leap: mind the healthspan gap". npj Regenerative Medicine. 6 (1): 57. doi:10.1038/s41536-021-00169-5. PMC 8460831. PMID 34556664.
- ^ Farrelly C (November 2022). "Aging, Equality and the Human Healthspan". HEC Forum. 36 (2): 187–205. doi:10.1007/s10730-022-09499-3. PMC 9644010. PMID 36348214.
- ^ a b Hansen M, Kennedy BK (1 August 2016). "Does Longer Lifespan Mean Longer Healthspan?". Trends in Cell Biology. 26 (8): 565–8. doi:10.1016/j.tcb.2016.05.002. PMC 4969078. PMID 27238421.
- ^ Bansal A, Zhu LJ, Yen K, et al. (20 January 2015). "Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants". Proceedings of the National Academy of Sciences. 112 (3): E277-86. Bibcode:2015PNAS..112E.277B. doi:10.1073/pnas.1412192112. PMC 4311797. PMID 25561524.
- ^ Turner BS (2009). Can We Live Forever? A Sociological and Moral Inquiry. Anthem Press. p. 3.
- ^ "agerasia". Oxford English Dictionary (Online ed.). Oxford University Press. (Subscription or participating institution membership required.)
- ^ a b Japsen B (15 June 2009). "AMA report questions science behind using hormones as anti-aging treatment". The Chicago Tribune. Retrieved 17 July 2009.
- ^ Holliday R (April 2009). "The extreme arrogance of anti-aging medicine". Biogerontology. 10 (2): 223–228. doi:10.1007/s10522-008-9170-6. PMID 18726707. S2CID 764136.
- ^ Olshansky SJ, Hayflick L, Carnes BA (August 2002). "Position statement on human aging". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 57 (8): B292 – B297. CiteSeerX 10.1.1.541.3004. doi:10.1093/gerona/57.8.B292. PMID 12145354.
- ^ Warner H, Anderson J, Austad S, et al. (November 2005). "Science fact and the SENS agenda. What can we reasonably expect from ageing research?". EMBO Reports. 6 (11): 1006–1008. doi:10.1038/sj.embor.7400555. PMC 1371037. PMID 16264422.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Ellefson L (12 February 2025). "This 'Death Clock' App Made Me Rethink My Life". Lifehacker. Retrieved 27 June 2025.
- ^ Downs M (20 May 2025). "The Death Clock App Uses AI to Predict When You'll Die". SUCCESS. Retrieved 27 June 2025.
- ^ Petrow S (9 June 2025). "How many years do I have left? An app gave me some helpful insights". Retrieved 27 June 2025.
External links
[edit]Senescence
View on GrokipediaDefinition and Observable Features
Core Definition
Senescence, in biological terms, denotes the progressive, time-dependent decline in an organism's physiological functions that compromises survival and reproductive capacity, manifesting as increased age-specific mortality risk and decreased fecundity after reproductive maturity.[7] This deterioration arises from accumulated molecular and cellular damage, including genomic instability, telomere shortening, and proteostatic imbalances, which erode tissue homeostasis and organ performance over chronological time.[2] Unlike extrinsic mortality factors such as predation or infection, senescence is an intrinsic process, empirically quantified by actuarial data showing exponential rises in death probability with age in species exhibiting it, such as humans where mortality doubles roughly every 8 years post-maturity.[8] At the cellular level, senescence involves a stable arrest of the cell cycle in response to stressors like DNA damage or oncogenic signals, preventing proliferation while inducing a senescence-associated secretory phenotype (SASP) that promotes inflammation and tissue remodeling.[9] This response, evolutionarily conserved across eukaryotes, serves as a tumor-suppressive mechanism but contributes to organismal aging when senescent cells accumulate, resisting apoptosis and disrupting neighboring healthy cells via paracrine signaling.00640-8) Empirical hallmarks include enlarged cell morphology, lysosomal hyperactivity (e.g., beta-galactosidase elevation), and epigenetic chromatin changes, verifiable through biomarkers in model organisms like fibroblasts exposed to replicative stress, where division ceases after approximately 50-60 doublings (Hayflick limit).[10] Organismal senescence integrates these cellular events, yielding observable declines in metrics such as muscle strength (sarcopenia) and cognitive acuity, with human data from longitudinal studies confirming a 1-2% annual functional loss post-30 years.[11]Key Characteristics Across Organisms
Senescence manifests as a time-dependent deterioration of physiological functions essential for survival and reproduction, a pattern observed in most multicellular organisms despite variations in expression and rate.[8] This decline typically involves reduced regenerative capacity, heightened vulnerability to environmental stressors, and progressive accumulation of molecular and cellular damage, such as genomic instability and protein misfolding, which disrupt homeostasis at tissue and organismal levels.[2] In animals, these features culminate in functional impairments like diminished sensory acuity, musculoskeletal weakening, and impaired immune responses, correlating with exponential increases in mortality risk.[12][13] Across taxa, a common characteristic is the age-related elevation in mortality and decline in fecundity, though not universally following identical trajectories; for instance, many species exhibit actuarial senescence where post-reproductive survival drops sharply after peak fertility.[14] In plants, senescence often appears modularly, with organ-level changes like leaf chlorosis and reduced photosynthesis preceding whole-plant deterioration, yet some species achieve indefinite growth through meristem renewal, mitigating organismal-level aging.[15] Unicellular organisms, such as yeast, display replicative senescence wherein daughter cells inherit fewer divisions due to asymmetric partitioning of damaged components, mirroring damage accumulation seen in multicellular lineages.[16] Cellular senescence, a conserved response involving irreversible proliferative arrest, contributes to organismal aging by accumulating dysfunctional cells that secrete pro-inflammatory factors, a phenotype documented from invertebrates to vertebrates and linked to tissue remodeling failures.[9][11] However, negligible senescence—characterized by stable mortality rates and sustained function into extreme age—occurs in select species like hydra and certain bivalves, highlighting that while damage accrual is widespread, evolutionary and physiological buffers can suppress overt decline.[16] Comparative analyses reveal conserved signaling pathways, such as those involving nutrient sensing and stress resistance, underpinning these traits, though species-specific adaptations modulate their impact.[17]Variation Across Species
Lifespan and Senescence Patterns
Lifespans across species exhibit extreme variation, from less than one day in mayflies (Ephemeroptera) to over 500 years in the ocean quahog clam (Arctica islandica), reflecting diverse senescence trajectories shaped by evolutionary pressures.[18][19] In most multicellular organisms, particularly mammals and birds, senescence manifests as actuarial aging, where post-reproductive mortality rates increase exponentially with age per the Gompertz-Makeham law, alongside phenotypic declines in fertility, tissue repair, and homeostasis.[20] This pattern arises from accumulating molecular damage and reduced regenerative capacity, leading to frailty and higher extrinsic mortality risks in older individuals.[21] A subset of species demonstrates negligible senescence, defined by stable adult mortality rates, sustained fecundity, and minimal physiological deterioration with age, as documented in the AnAge database of longevity records.[18] Approximately 75% of 52 turtle and tortoise (Testudines) species analyzed in captivity show slow or negligible senescence, with no significant rise in mortality or reproductive decline, contradicting assumptions of universal aging inevitability.[22] Negative senescence, where mortality decreases or functions improve with age, occurs rarely, as in certain cnidarians like Hydra vulgaris, which maintain telomere length and regenerative stem cell pools indefinitely through continuous cell turnover.[20][23] Long-lived exemplars of negligible senescence include the naked mole rat (Heterocephalus glaber), with a maximum lifespan of 32 years, low cancer incidence, and preserved proteostasis; the Greenland shark (Somniosus microcephalus), reaching 400+ years with slow metabolic rates; and the bigmouth buffalo (Ictiobus cyprinellus), living nearly a century without declines in multiple physiological systems such as metabolism or immunity.[19][24] These patterns correlate with enhanced DNA repair, antioxidant defenses, and low extrinsic mortality in protected niches, rather than programmed decay.[20] Empirical data from wild and captive populations underscore that negligible senescence is not confined to invertebrates but appears in select vertebrates, informing comparative gerontology.[25]| Species | Maximum Lifespan (years) | Senescence Pattern | Key Traits Supporting Pattern |
|---|---|---|---|
| House mouse (Mus musculus) | ~4 | Actuarial | Exponential mortality rise; rapid fertility decline post-maturity.[18] |
| Naked mole rat (Heterocephalus glaber) | ~32 | Negligible | Stable mortality; cancer resistance; sustained social reproduction.[19] |
| Ocean quahog (Arctica islandica) | >500 | Negligible | Minimal oxidative damage; low metabolic rate.[19] |
| Galápagos tortoise (Chelonoidis nigra) | ~177 | Negligible/slow | No age-related mortality increase in captivity.[22] |
| Hydra (Hydra vulgaris) | Indefinite (lab) | Negative/negligible | Continuous stem cell renewal; no telomere shortening.[23] |
