Recent from talks
Nothing was collected or created yet.
Cassava
View on Wikipedia
| Cassava | |
|---|---|

| |

| |
| Storage root (waxed) | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Rosids |
| Order: | Malpighiales |
| Family: | Euphorbiaceae |
| Genus: | Manihot |
| Species: | M. esculenta
|
| Binomial name | |
| Manihot esculenta | |
| Synonyms[1] | |
| |
Manihot esculenta, commonly called cassava, manioc, or yuca (among numerous regional names), is a woody shrub of the spurge family, Euphorbiaceae, native to South America, from Brazil, Paraguay and parts of the Andes. Although a perennial plant, cassava is extensively cultivated in tropical and subtropical regions as an annual crop for its edible starchy tuberous root. Cassava is predominantly consumed in boiled form, but substantial quantities are processed to extract cassava starch, called tapioca, which is used for food, animal feed, and industrial purposes. The Brazilian farofa, and the related garri of West Africa, is an edible coarse flour obtained by grating cassava roots, pressing moisture off the obtained grated pulp, and finally drying and roasting it.
Cassava is the third-largest source of carbohydrates in food in the tropics, after rice and maize, making it an important staple; more than 500 million people depend on it. It offers the advantage of being exceptionally drought-tolerant, and able to grow productively on poor soil. The largest producer is Nigeria, while Thailand is the largest exporter of cassava starch.
Cassava is grown in sweet and bitter varieties; both contain toxins, but the bitter varieties have them in much larger amounts. Cassava has to be prepared carefully for consumption, as improperly prepared material can contain sufficient cyanide to cause poisoning. The more toxic varieties of cassava have been used in some places as famine food during times of food insecurity. Farmers may however choose bitter cultivars to minimise crop losses.
Etymology
[edit]The generic name Manihot and the common name "manioc" both derive from the Guarani (Tupi) name mandioca or manioca for the plant.[2][3] The specific name esculenta is Latin for 'edible'.[2] The common name "cassava" is a 16th century word from the French or Portuguese cassave, in turn from Taíno caçabi.[4] The common name "yuca" or "yucca" is most likely also from Taíno, via Spanish yuca or juca.[5]
Description
[edit]The harvested part of a cassava plant is the storage root. This is long and tapered, with an easily detached rough brown rind. The white or yellowish flesh is firm and even in texture. Commercial cultivars can be 5 to 10 centimetres (2 to 4 in) wide at the top, and some 15 to 30 cm (6 to 12 in) long, with a woody vascular bundle running down the middle. The tuberous roots are largely starch, with small amounts of calcium (16 milligrams per 100 grams), phosphorus (27 mg/100 g), and vitamin C (20.6 mg/100 g).[6] Cassava roots contain little protein, whereas the leaves are rich in it,[7] except for being low in methionine, an essential amino acid.[8]
-
Cassava plant
-
Unprocessed tuberous roots
-
Tuberous root in cross-section
-
Leaf
-
Leaf detail
-
Flower buds
-
Seeds
Genome
[edit]The complete and haplotype-resolved African cassava (TME204) genome has been reconstructed and made available using the Hi-C technology.[9] The genome shows abundant novel gene loci with enriched functionality related to chromatin organization, meristem development, and cell responses.[9] Differentially expressed transcripts of different haplotype origins were enriched for different functionality during tissue development. In each tissue, 20–30% of transcripts showed allele-specific expression differences with <2% of direction-shifting. Despite high gene synteny, the HiFi genome assembly revealed extensive chromosome rearrangements and abundant intra-genomic and inter-genomic divergent sequences, with significant structural variations mostly related to long terminal repeat retrotransposons.[9]
Although smallholders are otherwise economically inefficient producers, they are vital to productivity at particular times.[10] Small cassava farmers are no exception.[10] Genetic diversity is vital when productivity has declined due to pests and diseases, and smallholders tend to retain less productive but more diverse gene pools.[10]
The molecular genetics of starchy root development in cassava have been analyzed and compared to other root and tuber crops, including possible (unproven) roles for Flowering Locus T (FT) orthologs.[11]
History
[edit]Wild populations of M. esculenta subspecies flabellifolia, shown to be the progenitor of domesticated cassava, are centered in west-central Brazil, where it was likely first domesticated no more than 10,000 years ago.[12] Forms of the modern domesticated species can also be found growing in the wild in the south of Brazil. By 4600 BC, cassava pollen appears in the Gulf of Mexico lowlands, at the San Andrés archaeological site.[13] The oldest direct evidence of cassava cultivation comes from a 1,400-year-old Maya site, Joya de Cerén, in El Salvador.[14] It became a staple food of the native populations of northern South America, southern Mesoamerica, and the Taino people in the Caribbean islands, who grew it using a high-yielding form of shifting agriculture by the time of European contact in 1492.[15] Cassava was a staple food of pre-Columbian peoples in the Americas and is often portrayed in indigenous art. The Moche people often depicted cassava in their ceramics.[16]
Spaniards in their early occupation of Caribbean islands did not want to eat cassava or maize, which they considered insubstantial, dangerous, and not nutritious. They much preferred foods from Spain, specifically wheat bread, olive oil, red wine, and meat, and considered maize and cassava damaging to Europeans.[17] The cultivation and consumption of cassava were nonetheless continued in both Portuguese and Spanish America. Mass production of cassava bread became the first Cuban industry established by the Spanish.[18] Ships departing to Europe from Cuban ports such as Havana, Santiago, Bayamo, and Baracoa carried goods to Spain, but sailors needed to be provisioned for the voyage. The Spanish also needed to replenish their boats with dried meat, water, fruit, and large amounts of cassava bread.[19] Sailors complained that it caused them digestive problems.[20]
Portuguese traders introduced cassava to Africa from Brazil in the 16th century. Around the same period, it was introduced to Asia through Columbian Exchange by Portuguese and Spanish traders, who planted it in their colonies in Goa, Malacca, Eastern Indonesia, Timor and the Philippines.[21] Cassava has also become an important crop in Asia. While it is a valued food staple in parts of eastern Indonesia, it is primarily cultivated for starch extraction and bio-fuel production in Thailand, Cambodia and Vietnam.[22] Cassava is sometimes described as the "bread of the tropics"[23] but should not be confused with the tropical and equatorial bread tree (Encephalartos), the breadfruit (Artocarpus altilis) or the African breadfruit (Treculia africana). This description definitely holds in Africa and parts of South America; in Asian countries such as Vietnam fresh cassava barely features in human diets.[24] Cassava was introduced to East Africa around 1850 by Arab and European settlers, who promoted its cultivation as a reliable crop to mitigate the effects of drought and famine.[25]
There is a legend that cassava was introduced in 1880–1885 to the South Indian state of Kerala by the King of Travancore, Vishakham Thirunal Maharaja, after a great famine hit the kingdom, as a substitute for rice.[26] However, cassava was cultivated in the state before that time.[27] Cassava is called kappa or maricheeni in Malayalam, and tapioca in Indian English usage.[28]
-
Taíno women preparing cassava bread in 1565: grating tuberous roots into paste, shaping the bread, and cooking it on a fire-heated burén
-
17th-century painting by Albert Eckhout in Dutch Brazil
Cultivation
[edit]Optimal conditions for cassava cultivation are mean annual temperatures between 20 and 29 °C (68 and 84 °F), annual precipitation between 1,000 and 2,500 mm (39 and 98 in), and an annual growth period of no less than 240 days.[29] Cassava is propagated by cutting the stem into sections of approximately 15 cm (5.9 in), these being planted prior to the wet season.[30] Cassava growth is favorable under temperatures ranging from 25 to 29 °C (77 to 84 °F), but it can tolerate temperatures as low as 12 °C (54 °F) and as high as 40 °C (104 °F).[31] These conditions are found, among other places, in the northern part of the Gulf Coastal Plain in Mexico.[29] In this part of Mexico the following soil types have been shown to be good for cassava cultivation: phaeozem, regosol, arenosol, andosol and luvisol.[29]
-
Stakes
Harvesting
[edit]Before harvest, the leafy stems are removed. The harvest is gathered by pulling up the base of the stem and cutting off the tuberous roots.[30]
Handling and storage
[edit]Cassava deteriorates after harvest, when the tuberous roots are first cut. The healing mechanism produces coumaric acid, which oxidizes and blackens the roots, making them inedible after a few days. This deterioration is related to the accumulation of reactive oxygen species initiated by cyanide release during mechanical harvesting. Cassava shelf life may be increased up to three weeks by overexpressing a cyanide-insensitive alternative oxidase, which suppressed ROS by 10-fold.[32] Post-harvest deterioration is a major obstacle to the export of cassava. Fresh cassava can be preserved like potato, using thiabendazole or bleach as a fungicide, then wrapping in plastic, freezing, or applying a wax coating.[33]
While alternative methods for controlling post-harvest deterioration have been proposed, such as preventing reactive oxygen species effects by using plastic bags during storage and transport, coating the roots with wax, or freezing roots, such strategies have proved to be economically or technically impractical, leading to breeding of cassava varieties with improved durability after harvest, achieved by different mechanisms.[34][35] One approach used gamma rays to try to silence a gene involved in triggering deterioration; another strategy selected for plentiful carotenoids, antioxidants which may help to reduce oxidization after harvest.[35]
-
Starch processing
-
Starch flour
-
Starch wet-processing
-
Spreading Casabe burrero (cassava bread) to dry, Venezuela
-
Starch being prepared for packaging
-
Starch noodles packaged for shipping
-
Frozen leaves in a Los Angeles market
-
Picked buds
Pests and diseases
[edit]
Cassava is subject to pests from multiple taxonomic groups, including nematodes, and insects, as well as diseases caused by viruses, bacteria, and fungi. All cause reductions in yield, and some cause serious losses of crops.[36]
Viruses
[edit]Several viruses cause enough damage to cassava crops to be of economic importance. The African cassava mosaic virus causes the leaves of the cassava plant to wither, limiting the growth of the root.[37] An outbreak of the virus in Africa in the 1920s led to a major famine.[38] The virus is spread by the whitefly and by the transplanting of diseased plants into new fields. Sometime in the late-1980s, a mutation occurred in Uganda that made the virus even more harmful, causing the complete loss of leaves. This mutated virus spread at a rate of 80 kilometres (50 miles) per year, and as of 2005 was found throughout Uganda, Rwanda, Burundi, the Democratic Republic of the Congo and the Republic of the Congo.[39] Viruses are a severe production limitation in the tropics. They are the primary reason for the complete lack of yield increases in the 25 years up to 2021[update].[40] Cassava brown streak virus disease is a major threat to cultivation worldwide.[38] Cassava mosaic virus (CMV) is widespread in Africa, causing cassava mosaic disease (CMD).[41] Bredeson et al. 2016 find the M. esculenta cultivars most widely used on that continent have M. carthaginensis subsp. glaziovii genes of which some appear to be CMD resistance genes.[41] Although the ongoing CMD pandemic affects both East and Central Africa, Legg et al. found that these two areas have two distinct subpopulations of the vector, Bemisia tabaci whiteflies.[42][43] Genetically engineered cassava offers opportunities for the improvement of virus resistance, including CMV and CBSD resistance.[44]
Bacteria
[edit]Among the most serious bacterial pests is Xanthomonas axonopodis pv. manihotis, which causes bacterial blight of cassava. This disease originated in South America and has followed cassava around the world.[45] Bacterial blight has been responsible for near catastrophic losses and famine in past decades, and its mitigation requires active management practices.[45] Several other bacteria attack cassava, including the related Xanthomonas campestris pv. cassavae, which causes bacterial angular leaf spot.[46]
Fungi and oomycetes
[edit]Several fungi and oomycetes bring about significant crop losses, one of the most serious being cassava root rot; the pathogens involved are species of Phytophthora, the genus which causes potato blight. Cassava root rot can result in losses of as much as 80 percent of the crop.[36] A major pest is a rust caused by Uromyces manihotis.[47] Superelongation disease, caused by Elsinoë brasiliensis, can cause losses of over 80 percent of young cassava in Latin America and the Caribbean when temperature and rainfall are high.[36][48][49]
Nematodes
[edit]Nematode pests of cassava are thought to cause harms ranging from negligible to seriously damaging,[50][51][52] making the choice of management methods difficult.[53] A wide range of plant parasitic nematodes have been reported associated with cassava worldwide. These include Pratylenchus brachyurus, Rotylenchulus reniformis, Helicotylenchus spp., Scutellonema spp. and Meloidogyne spp., of which Meloidogyne incognita and Meloidogyne javanica are the most widely reported and economically important.[54] Meloidogyne spp. feeding produces physically damaging galls with eggs inside them. Galls later merge as the females grow and enlarge, and they interfere with water and nutrient supply.[52] Cassava roots become tough with age and restrict the movement of the juveniles and the egg release. It is therefore possible that extensive galling can be observed even at low densities following infection.[53] Other pests and diseases can gain entry through the physical damage caused by gall formation, leading to rots. They have not been shown to cause direct damage to the enlarged tuberous roots, but plant height can be reduced if the root system is reduced.[55] Nematicides reduce the numbers of galls per feeder root, along with fewer rots in the tuberous roots.[56] The organophosphorus nematicide fenamiphos does not reduce crop growth or harvest yield. Nematicide use in cassava does not increase harvested yield significantly, but lower infestation at harvest and lower subsequent storage loss provide a higher effective yield. The use of tolerant and resistant cultivars is the most practical management method in most locales.[57][53][58]
Insects
[edit]
Insects such as stem borers and other beetles, moths including Chilomima clarkei, scale insects, fruit flies, shootflies, burrower bugs, grasshoppers, leafhoppers, gall midges, leafcutter ants, and termites contribute to losses of cassava in the field,[36] while others contribute to serious losses, between 19% and 30%, of dried cassava in storage.[59] In Africa, a previous issue was the cassava mealybug (Phenacoccus manihoti) and cassava green mite (Mononychellus tanajoa). These pests can cause up to 80 percent crop loss, which is extremely detrimental to the production of subsistence farmers. These pests were rampant in the 1970s and 1980s but were brought under control following the establishment of the Biological Control Centre for Africa of the International Institute of Tropical Agriculture (IITA) under the leadership of Hans Rudolf Herren.[60] The Centre investigated biological control for cassava pests; two South American natural enemies Anagyrus lopezi (a parasitoid wasp) and Typhlodromalus aripo (a predatory mite) were found to effectively control the cassava mealybug and the cassava green mite, respectively.[61]
Production
[edit]| Cassava production – 2022 | |
|---|---|
| Country | millions of tonnes |
| 60.8 | |
| 48.8 | |
| 34.1 | |
| 25.6 | |
| 17.7 | |
| 17.6 | |
| World | 330 |
| Source: FAOSTAT of the United Nations[62] | |
In 2022, world production of cassava root was 330 million tonnes, led by Nigeria with 18% of the total (table). Other major growers were Democratic Republic of the Congo and Thailand.
Cassava is the third-largest source of carbohydrates in food in the tropics, after rice and maize.[63][64][40] making it an important staple; more than 500 million people depend on it.[65] It offers the advantage of being exceptionally drought-tolerant, and able to grow productively on poor soil. Cassava grows well within 30° of the equator, where it can be produced at up to 2,000 m (7,000 ft) above sea level, and with 50 to 5,000 mm (2 to 200 in) of rain per year. These environmental tolerances suit it to conditions across much of South America and Africa.[66]
Cassava yields a large amount of food energy per unit area of land per day – 1,000,000 kJ/ha (250,000 kcal/ha), as compared with 650,000 kJ/ha (156,000 kcal/ha) for rice, 460,000 kJ/ha (110,000 kcal/ha) for wheat and 840,000 kJ/ha (200,000 kcal/ha) for maize.[67]
Cassava, yams (Dioscorea spp.), and sweet potatoes (Ipomoea batatas) are important sources of food in the tropics. The cassava plant gives the third-highest yield of carbohydrates per cultivated area among crop plants, after sugarcane and sugar beets.[68] Cassava plays a particularly important role in agriculture in developing countries, especially in sub-Saharan Africa, because it does well on poor soils and with low rainfall, and because it is a perennial that can be harvested as required. Its wide harvesting window allows it to act as a famine reserve and is invaluable in managing labor schedules. It offers flexibility to resource-poor farmers because it serves as either a subsistence or a cash crop.[69] Worldwide, 800 million people depend on cassava as their primary food staple.[70]
Toxicity
[edit]
Cassava roots, peels and leaves are dangerous to eat raw because they contain linamarin and lotaustralin, which are toxic cyanogenic glycosides. These are decomposed by the cassava enzyme linamarase, releasing poisonous hydrogen cyanide.[71] Cassava varieties are often categorized as either bitter (high in cyanogenic glycosides) or sweet (low in those bitter compounds). Sweet cultivars can contain as little as 20 milligrams of cyanide per kilogram of fresh roots, whereas bitter cultivars may contain as much as 1000 milligrams per kilogram. Cassavas grown during drought are especially high in these toxins.[72][73] A dose of 25 mg of pure cassava cyanogenic glucoside, which contains 2.5 mg of cyanide, is sufficient to kill a rat.[74] Excess cyanide residue from improper preparation causes goiters and acute cyanide poisoning, and is linked to ataxia (a neurological disorder affecting the ability to walk, also known as konzo).[75] It has also been linked to tropical fibrocalcific pancreatitis in humans, leading to chronic pancreatitis.[76][77]
Symptoms of acute cyanide intoxication appear four or more hours after ingesting raw or poorly processed cassava: vertigo, vomiting, goiter, ataxia, partial paralysis, collapse, and death.[78][79][80][81] It can be treated easily with an injection of thiosulfate (which makes sulfur available for the patient's body to detoxify by converting the poisonous cyanide into thiocyanate).[75]
Chronic, low-level exposure to cyanide may contribute to both goiter and tropical ataxic neuropathy, also called konzo, which can be fatal. The risk is highest in famines, when as many as 3 percent of the population may be affected.[82][83]
Like many other root and tuber crops, both bitter and sweet varieties of cassava contain antinutritional factors and toxins; the bitter varieties contain much larger amounts.[75] The more toxic varieties of cassava have been used in some places as famine food during times of food insecurity.[78][75] For example, during the shortages in Venezuela in the late 2010s, dozens of deaths were reported due to Venezuelans resorting to eating bitter cassava in order to curb starvation.[84][85] Cases of cassava poisoning were also documented during the famine accompanying the Great Leap Forward (1958–1962) in China.[86] Farmers may select bitter cultivars to reduce crop losses.[87]
Societies that traditionally eat cassava generally understand that processing (soaking, cooking, fermentation, etc.) is necessary to avoid getting sick. Brief soaking (four hours) of cassava is not sufficient, but soaking for 18–24 hours can remove up to half the level of cyanide. Drying may not be sufficient, either.[75]
In many West African regions, especially Nigeria, bitter cassava roots are traditionally detoxified in a lengthy process. The roots are peeled and grated. The moist pulp is soaked (or “retted”) in water for 48 to 72 hours to initiate spontaneous fermentation. During this period endogenous linamarase acts on linamarin and lotaustralin; the resulting hydrogen cyanide dissolves or volatilises, reducing the cyanogenic potential by 85 – 99 %.[88][89][90] After soaking, the mash is pressed to expel liquid and boiled, roasted, or toasted to make foods such as gari, fufu, and lafun, further lowering residual cyanide to within the WHO safe limit of 10 mg HCN kg⁻¹.[91]
For some smaller-rooted, sweet varieties, cooking is sufficient to eliminate all toxicity. The cyanide is carried away in the processing water and the amounts produced in domestic consumption are too small to have environmental impact.[71] The larger-rooted, bitter varieties used for production of flour or starch must be processed to remove the cyanogenic glucosides. The large roots are peeled and then ground into flour, which is then soaked in water, squeezed dry several times, and toasted. The starch grains that flow with the water during the soaking process are also used in cooking.[92] The flour is used throughout South America and the Caribbean. Industrial production of cassava flour, even at the cottage level, may generate enough cyanide and cyanogenic glycosides in the effluents to have a severe environmental impact.[71]
Uses
[edit]Food and drink
[edit]There are many ways of cooking cassava.[93] It has to be prepared correctly to remove its toxicity.[94] The root of the sweet variety is mild to the taste, like potatoes; Jewish households sometimes use it in cholent.[95] It can be made into a flour that is used in breads, cakes and cookies. In Brazil, farofa, a dry meal made from cooked powdered cassava, is roasted in butter, eaten as a side dish, or sprinkled on other food.[96] In Taiwanese culture, later spread to the United States, cassava "juices" are dried to a fine powder and used to make tapioca, a popular starch used to make bubbles, a chewy topping in bubble tea.[97]
Alcoholic beverages made from cassava include cauim (Brazil),[98] kasiri (Venezuela, Guyana, Suriname),[99] parakari or kari (Venezuela, Guyana, Surinam),[100] and nihamanchi (South America),[101]
-
Heavy cake
-
Bread
-
Noodles, Cambodia
Preparation of bitter cassava
[edit]An ancestral method used by the indigenous people of the Caribbean to detoxify cassava is by peeling, grinding, and mashing; filtering the mash through a basket tube (sebucan or tipiti) to remove the hydrogen cyanide; and drying and sieving the mash for flour. The poisonous filtrate water was boiled to release the hydrogen cyanide, and used as a base for stews.[102]
A safe processing method known as the "wetting method" is to mix the cassava flour with water into a thick paste, spread it in a thin layer over a basket and then let it stand for five hours at 30 °C in the shade.[103] In that time, about 83% of the cyanogenic glycosides are broken down by linamarase; the resulting hydrogen cyanide escapes to the atmosphere, making the flour safe for consumption the same evening.[103]
The traditional method used in West Africa is to peel the roots and put them into water for three days to ferment. The roots are then dried or cooked. In Nigeria and several other west African countries, including Ghana, Cameroon, Benin, Togo, Ivory Coast, and Burkina Faso, they are usually grated and lightly fried in palm oil to preserve them. The result is a foodstuff called garri. Fermentation is also used in other places such as Indonesia, such as Tapai. The fermentation process also reduces the level of antinutrients, making the cassava a more nutritious food.[104] The reliance on cassava as a food source and the resulting exposure to the goitrogenic effects of thiocyanate has been responsible for the endemic goiters seen in the Akoko area of southwestern Nigeria.[105][106]
-
Tuberous root, peeled and soaking to reduce toxicity
-
Filling a sebucan or tipiti filter
Bioengineering has been applied to grow cassava with lower cyanogenic glycosides combined with fortification of vitamin A, iron and protein to improve the nutrition of people in sub-Saharan Africa.[107][108]
In Guyana the traditional cassareep is made from bitter cassava juice.[109] The juice is boiled until it is reduced by half in volume,[110] to the consistency of molasses[111] and flavored with spices—including cloves, cinnamon, salt, sugar, and cayenne pepper.[112] Traditionally, cassareep was boiled in a soft pot, the actual "pepper pot", which would absorb the flavors and also impart them (even if dry) to foods such as rice and chicken cooked in it.[113] The poisonous but volatile hydrogen cyanide is evaporated by heating.[114] Nevertheless, improperly cooked cassava has been blamed for a number of deaths.[115] Amerindians from Guyana reportedly made an antidote by steeping chili peppers in rum.[111] The natives of Guyana traditionally brought the product to town in bottles,[116] and it is available on the US market in bottled form.[117]
Nutrition
[edit]| Nutritional value per 100 g (3.5 oz) | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy | 670 kJ (160 kcal) | ||||||||||||||||||||||||||||||||||||||
38.1 g | |||||||||||||||||||||||||||||||||||||||
| Sugars | 1.7 g | ||||||||||||||||||||||||||||||||||||||
| Dietary fiber | 1.8 g | ||||||||||||||||||||||||||||||||||||||
0.3 g | |||||||||||||||||||||||||||||||||||||||
1.4 g | |||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Other constituents | Quantity | ||||||||||||||||||||||||||||||||||||||
| Water | 60 g | ||||||||||||||||||||||||||||||||||||||
| †Percentages estimated using US recommendations for adults,[118] except for potassium, which is estimated based on expert recommendation from the National Academies.[119] | |||||||||||||||||||||||||||||||||||||||
Raw cassava is 60% water, 38% carbohydrates, 1% protein, and has negligible fat (table).[120] In a 100-gram (3+1⁄2-ounce) reference serving, raw cassava provides 670 kilojoules (160 kilocalories) of food energy and 23% of the Daily Value (DV) of vitamin C, but otherwise has no micronutrients in significant content (i.e., above 10% of the relevant DV).[120]
Biofuel
[edit]Cassava has been studied as a feedstock to produce ethanol as a biofuel, including to improve the efficiency of conversion from cassava flour,[121] and to convert crop residues such as stems and leaves as well as the more easily processed roots.[122] China has created facilities to produce substantial amounts of ethanol fuel from cassava roots.[123]
Animal feed
[edit]Cassava roots and hay are used worldwide as animal feed. Young cassava hay is harvested at three to four month, when it reaches about 30 to 45 cm (12 to 18 in) above ground; it is dried in the sun until its dry matter content approaches 85 percent. The hay contains 20–27 percent protein and 1.5–4 percent tannin. It is valued as a source of roughage for ruminants such as cattle.[124]
-
Grating of tuberous roots
-
A close-up of the product
-
Drying on road to be used for pig and chicken feed
Laundry starch
[edit]Cassava is used in laundry products, especially as starch to stiffen shirts and other garments.[125]
Folklore
[edit]Maní, a Tupí myth of origins, is the name of an indigenous girl with very fair complexion. The Amazonian legend of Maní is related to the cult of Manioc, the native staple that sprang from her grave.[126][127][128] Sometime later a crack opened on the earth and the people of the tribe found a fruit that resembled the white skin tone of the dead child's body. They picked up the fruit from the ground, peeled and cooked it, and for their surprise it tasted delicious. It even renewed their strength. They also prepared a drink which could easily put one to sleep. So, from this day on, they began using the root as their staple food and called it "mandioca", which in Tupy language means "house (oca, in Tupi–Guarani) of Mandi= Maní".[129]
In Java, a myth relates that food derives from the body of Dewi Teknowati, who killed herself rather than accept the advances of the god Batara Guru. She was buried, and her lower leg grew into a cassava plant.[130] In Trinidad, folk stories tell of a saapina or snake-woman; the word is related to sabada, meaning to pound, for what is traditionally a woman's work of pounding cassava.[131]
The identity of the Macushi people of Guyana is closely bound up with the growth and processing of cassava in their slash-and-burn subsistence lifestyle. A story tells that the great spirit Makunaima climbed a tree, cutting off pieces with his axe; when they landed on the ground, each piece became a type of animal. The opossum brought the people to the tree, where they found all the types of food, including bitter cassava. A bird told the people how to prepare the cassava safely.[132]
See also
[edit]References
[edit]- ^ a b "Manihot esculenta Crantz, Rei Herb. 1: 167 (1766)". Plants of the World Online. Board of Trustees of the Royal Botanic Gardens, Kew. 2022. Archived from the original on 11 November 2022. Retrieved 11 November 2022.
- ^ a b "Manihot esculenta Crantz". Singapore National Parks. Retrieved 7 July 2024.
Genus Manihot is from the Tupi-Guarani name "manioca" which means cassava. Species esculenta means edible by humans.
- ^ "manioc (n.)". Online Etymology Dictionary. Retrieved 7 July 2024.
- ^ "cassava (n.)". Online Etymology Dictionary. Retrieved 7 July 2024.
- ^ "yucca (n.)". Online Etymology Dictionary. Retrieved 7 July 2024.
- ^ "Basic Report: 11134, Cassava, raw". National Nutrient Database for Standard Reference Release 28. Agricultural Research Service, US Department of Agriculture. May 2016. Archived from the original on 12 July 2017. Retrieved 7 December 2016.
- ^ Latif, Sajid; Müller, Joachim (2015). "Potential of cassava leaves in human nutrition: a review". Trends in Food Science & Technology. 44 (2): 147–158. doi:10.1016/j.tifs.2015.04.006.
- ^ Ravindran, Velmerugu (1992). "Preparation of cassava leaf products and their use as animal feeds" (PDF). FAO Animal Production and Health Paper (95): 111–125. Archived from the original (PDF) on 15 January 2012. Retrieved 13 August 2010.
- ^ a b c Qi, W.; Lim, Y.; Patrignani, A.; Schläpfer, P.; Bratus-Neuenschwander, A.; et al. (2022). "The haplotype-resolved chromosome pairs of a heterozygous diploid African cassava cultivar reveal novel pan-genome and allele-specific transcriptome features". GigaScience. 11. doi:10.1093/gigascience/giac028. PMC 8952263. PMID 35333302.
- ^ a b c McGregor, Andrew; Manley, M.; Tubuna, S.; Deo, R.; Bourke, Mike (2020). "Pacific Island food security: situation, challenges and opportunities". Pacific Economic Bulletin. Asia Pacific Press. hdl:1885/39234.
- ^ Zierer, Wolfgang; Rüscher, David; Sonnewald, Uwe; Sonnewald, Sophia (2021). "Tuber and Tuberous Root Development". Annual Review of Plant Biology. 72 (1). Annual Reviews: 551–580. Bibcode:2021AnRPB..72..551Z. doi:10.1146/annurev-arplant-080720-084456. PMID 33788583.
- ^ Olsen, K. M.; Schaal, B. A. (1999). "Evidence on the origin of cassava: phylogeography of Manihot esculenta". Proceedings of the National Academy of Sciences of the United States of America. 96 (10): 5586–5591. Bibcode:1999PNAS...96.5586O. doi:10.1073/pnas.96.10.5586. PMC 21904. PMID 10318928.
- ^ Pope, Kevin O.; Pohl, Mary E. D.; Jones, John G.; Lentz, David L.; von Nagy, Christopher; Vega, Francisco J.; Quitmyer, Irvy R. (2001). "Origin and Environmental Setting of Ancient Agriculture in the Lowlands of Mesoamerica". Science. 292 (5520): 1370–1373. Bibcode:2001Sci...292.1370P. doi:10.1126/science.292.5520.1370. PMID 11359011.
- ^ Carroll, Rory (23 August 2007). "CU team discovers Mayan crop system". The Guardian. Archived from the original on 31 July 2019. Retrieved 31 July 2019.
- ^ "Taino: History & Culture". Encyclopedia Britannica. Archived from the original on 1 September 2020. Retrieved 24 September 2020.
- ^ Berrin, Katherine & Larco Museum. The Spirit of Ancient Peru:Treasures from the Museo Arqueológico Rafael Larco Herrera. New York: Thames & Hudson, 1997.
- ^ Earle, Rebecca (2012) The Body of the Conquistador: Food, Race, and the Colonial Experience in Spanish America, 1492–1700. New York: Cambridge University Press. pp. 54–57, 151. ISBN 978-1107693296.
- ^ Long, Janet (2003). Conquest and food: consequences of the encounter of two worlds; page 75. UNAM. ISBN 978-9703208524. Archived from the original on 20 April 2023. Retrieved 24 August 2020.
- ^ Watkins, Thayer (2006). "The Economic History of Havana, Cuba: A City So Beautiful and Important It Was Once Worth More Than All of Florida". San José State University, Department of Economics. Archived from the original on 2 May 2016. Retrieved 20 August 2015.
- ^ Super, John C. (1984). "Spanish Diet in the Atlantic Crossing". Terrae Incognitae. 16: 60–63. doi:10.1179/008228884791016718.
- ^ Nweke, Felix I. (2005). "The cassava transformation in Africa". A review of cassava in Africa with country case studies on Nigeria, Ghana, the United Republic of Tanzania, Uganda and Benin. Proceedings of the Validation Forum on the Global Cassava Development Strategy. Vol. 2. Rome: The Food and Agriculture Organization of the United Nations. Archived from the original on 11 February 2019. Retrieved 1 January 2011.
- ^ Hershey, Clair; et al. (April 2000). "Cassava in Asia. Expanding the Competitive Edge in Diversified Markets". A review of cassava in Asia with country case studies on Thailand and Viet Nam. Rome: Food and Agriculture Organization of the United Nations. Archived from the original on 7 November 2017. Retrieved 28 January 2018.
- ^ Adams, C.; Murrieta, R.; Siqueira, A.; Neves, W.; Sanches, R. (2009). "Bread of the Land: The Invisibility of Manioc in the Amazon". Amazon Peasant Societies in a Changing Environment. pp. 281–305. doi:10.1007/978-1-4020-9283-1_13. ISBN 978-1-4020-9282-4.
- ^ Mota-Guttierez, Jatziri; O'Brien, Gerard Michael (September 2019). "Cassava consumption and the occurrence of cyanide in cassava in Vietnam, Indonesia and Philippines". Public Health Nutrition. 23 (13): 2410–2423. doi:10.1017/S136898001900524X. PMC 11374567. PMID 32438936.
- ^ Ofcansky, Thomas P.; Yeager, Rodger; Kurtz, Laura S. (1997). Historical dictionary of Tanzania. African historical dictionaries (2nd ed.). Lanham, Md: Scarecrow Press. p. 134. ISBN 978-0-8108-3244-2.
- ^ Nagarajan, Saraswathy (27 June 2019). "How tapioca came to Travancore". The Hindu. Archived from the original on 27 July 2020.
- ^ Ainslie, Whitelaw; Halford, Henry (1813). Materia medica of Hindoostan, and artisan's and agriculturalist's nomenclature. Madras State: Government Press.
- ^ "Kappa for all seasons - many avatars of the magic starch root..." Onmanorama. Kerala, India. 1 February 2018. Retrieved 11 May 2024.
- ^ a b c Del-Rosario-Arellano, José Luis; Aguilar-Rivera, Noe; Leyva-Ovalle, Otto Raúl; Andres-Meza, Pablo; Meneses-Marquez, Isaac; Bolio-López, Gloria Ivette (2022). "Zonificación edafoclimática de la yuca (Manihot esculenta Crantz) para la producción sostenible de bioproductos" [Edaphoclimatic zoning of cassava (manihot esculenta crantz) for sustainable production of bioproducts]. Norte Grande Geography Journal (in Spanish) (81): 361–383. doi:10.4067/S0718-34022022000100361. eISSN 0718-3402. S2CID 249657496.
- ^ a b Howeler, Reinhardt H. (2007). "Production techniques for sustainable cassava production in Asia" (PDF). Centro Internacional de Agricultura Tropical, Bangkok. Archived from the original (PDF) on 5 October 2016. Retrieved 3 May 2016.
- ^ Verheye, Willy H., ed. (2010). "Tropical Root and Tuber Crops". Soils, Plant Growth and Crop Production Volume II. EOLSS Publishers. p. 273. ISBN 978-1-84826-368-0. Archived from the original on 11 May 2021. Retrieved 29 December 2020.
- ^ Zidenga, T.; et al. (2012). "Extending cassava root shelf life via reduction of reactive oxygen species production". Plant Physiology. 159 (4): 1396–1407. doi:10.1104/pp.112.200345. PMC 3425186. PMID 22711743.
- ^ "Storage and processing of roots and tubers in the tropics". U.N. Food and Agriculture Organization. Archived from the original on 22 April 2016. Retrieved 4 May 2016.
- ^ Venturini, M.T.; Santos, L.R.; Vildoso, C. I; Santos, V. S; Oliveira, E.J. (2016). "Variation in cassava germplasm for tolerance to post-harvest physiological deterioration". Genetics and Molecular Research. 15 (2). doi:10.4238/gmr.15027818 (inactive 1 July 2025). PMID 27173317.
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ a b Morante, N.; Sánchez, T.; Ceballos, H.; et al. (2010). "Tolerance to Postharvest Physiological Deterioration in Cassava Roots". Crop Science. 50 (4): 1333–1338. doi:10.2135/cropsci2009.11.0666.
- ^ a b c d e Alvarez, Elizabeth; Llano, Germán Alberto; Mejía, Juan Fernando (2012). "Cassava diseases in Latin America, Africa and Asia". The Cassava Handbook (PDF). p. 258.[permanent dead link]
- ^ "Cassava (manioc)". Archived from the original on 30 June 2015. Retrieved 29 May 2015.
- ^ a b "Virus ravages cassava plants in Africa". The New York Times. 31 May 2010. Archived from the original on 16 March 2017. Retrieved 24 February 2017.
- ^ "Hungry African nations balk at biotech cassava". St. Louis Post-Dispatch. 31 August 2005. Archived from the original on 3 March 2012. Retrieved 11 August 2008.
- ^ a b Afedraru, Lominda (31 January 2019). "Uganda to launch innovative gene-edited cassava research". Alliance for Science. Archived from the original on 15 August 2021. Retrieved 15 August 2021.
- ^ a b Lebot, Vincent (2020). Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams And Aroids. Wallingford, Oxfordshire, UK; Boston, USA: CABI (Centre for Agriculture and Bioscience International). p. 541. ISBN 978-1-78924-336-9. OCLC 1110672215.
- ^ Legg, James P.; Kumar, P. Lava; Makeshkumar, T.; et al. (2015). "Cassava Virus Diseases: Biology, Epidemiology, and Management". In Loebenstein, Gad; Katis, Nikolaos I. (eds.). Advances in Virus Research. Control of Plant Virus Diseases: Vegetatively-Propagated Crops. Vol. 91. Academic Press. pp. 85–142. doi:10.1016/bs.aivir.2014.10.001. ISBN 9780128027622. ISSN 0065-3527. PMID 25591878.
- ^ Legg, James P.; Sseruwagi, Peter; Boniface, Simon; et al. (2014). "Spatio-temporal patterns of genetic change amongst populations of cassava Bemisia tabaci whiteflies driving virus pandemics in East and Central Africa". Virus Research. 186: 61–75. doi:10.1016/j.virusres.2013.11.018. PMID 24291251.
- ^ Rey, Chrissie; Vanderschuren, Hervé (2017). "Cassava Mosaic and Brown Streak Diseases: Current Perspectives and Beyond". Annual Review of Virology. 4 (1). Annual Reviews: 429–452. doi:10.1146/annurev-virology-101416-041913. ISSN 2327-056X. PMID 28645239. S2CID 25767024.
- ^ a b Lozano, J. Carlos (September 1986). "Cassava bacterial blight: a manageable disease" (PDF). Plant Disease. 70 (12). American Phytopathological Society: 1089–1093. Bibcode:1986PlDis..70.1089L. doi:10.1094/PD-70-1089. hdl:10568/43244. Archived (PDF) from the original on 14 January 2023. Retrieved 14 January 2023.
- ^ Zárate-Chaves, Carlos A.; Gómez de la Cruz, Diana; Verdier, Valérie; López, Camilo E.; Bernal, Adriana; Szurek, Boris (2021). "Cassava diseases caused by Xanthomonas phaseoli pv. manihotis and Xanthomonas cassavae". Molecular Plant Pathology. 22 (12): 1520–1537. Bibcode:2021MolPP..22.1520Z. doi:10.1111/mpp.13094. ISSN 1464-6722. PMC 8578842. PMID 34227737.
- ^ "Uromyces manihotis (rust of cassava)". Invasive Species Compendium. CABI (Centre for Agriculture and Bioscience International). 2019. Archived from the original on 9 November 2022. Retrieved 27 October 2022.
- ^ Alleyne, A.T.; Gilkes, J.M.; Briggs, G. (1 January 2015). "Early detection of Super-elongation disease in Manihot esculenta Crantz (cassava) using molecular markers for gibberellic acid biosynthesis". European Journal of Plant Pathology. 141 (1): 27–34. Bibcode:2015EJPP..141...27A. doi:10.1007/s10658-014-0517-3.
- ^ Alleyne, Angela; Mason, Shanice; Vallès, Yvonne (2023). "Characterization of the Cassava Mycobiome in Symptomatic Leaf Tissues Displaying Cassava Superelongation Disease". Journal of Fungi. 9 (12): 1130. doi:10.3390/jof9121130. PMC 10743849. PMID 38132731.
- ^ Coyne, D. L.; Talwana, L. A. H. (2000). "Reaction of cassava cultivars to root-knot nematode (Meloidogyne spp.) in pot experiments and farmer-managed field trials in Uganda". International Journal of Nematology. 10: 153–158. S2CID 83213308. Retrieved 22 September 2018.
- ^ Makumbi-Kidza, N. N.; Speijer, P. R.; Sikora, R. A. (2000). "Effects of Meloidogyne incognita on growth and storage-root formation of cassava (Manihot esculenta)". Journal of Nematology. 32 (4S): 475–477. PMC 2620481. PMID 19270997.
- ^ a b Gapasin, R. M. (1980). "Reaction of golden yellow cassava to Meloidogyne spp. Inoculation". Annals of Tropical Research. 2: 49–53.
- ^ a b c Coyne, D. L. (1994). "Nematode pests of cassava". African Crop Science Journal. 2 (4): 355–359. Archived from the original on 22 September 2018. Retrieved 22 September 2018.
- ^ McSorley, R.; Ohair, S. K.; Parrado, J. L. (1983). "Nematodes of Cassava, Manihot esculenta Crantz". Nematropica. 13: 261–287. Archived from the original on 3 June 2016. Retrieved 4 May 2016.
- ^ Caveness, F. E. (1982). "Root-knot nematodes as parasites of cassava". IITA Research Briefs. 3 (2): 2–3.
- ^ Coyne, D. L.; Kagoda, F.; Wambugu, E.; Ragama, P. (2006). "Response of cassava to nematicide application and plant-parasitic nematode infection in East Africa, with emphasis on root-knot nematode". International Journal of Pest Management. 52 (3): 215–223. doi:10.1080/09670870600722959. S2CID 84771539.
- ^ Coyne, Danny L.; Cortada, Laura; Dalzell, Johnathan J.; Claudius-Cole, Abiodun O.; Haukeland, Solveig; Luambano, Nessie; Talwana, Herbert (25 August 2018). "Plant-Parasitic Nematodes and Food Security in Sub-Saharan Africa". Annual Review of Phytopathology. 56 (1). Annual Reviews: 381–403. Bibcode:2018AnRvP..56..381C. doi:10.1146/annurev-phyto-080417-045833. ISSN 0066-4286. PMC 7340484. PMID 29958072. S2CID 49615468.
- ^ Uchechukwumgemezu, Chidinma (21 December 2020). "Nigeria to introduce new cassava varieties". Todayng. Archived from the original on 21 December 2020. Retrieved 21 December 2020.
- ^ Osipitan, A. A.; Sangowusi, V. T.; Lawal, O. I.; Popoola, K. O. (2015). "Correlation of Chemical Compositions of Cassava Varieties to Their Resistance to Prostephanus truncatus Horn (Coleoptera: Bostrichidae)". Journal of Insect Science. 15 (1): 13. doi:10.1093/jisesa/ieu173. PMC 4535132. PMID 25700536.
- ^ "1995: Herren". The World Food Prize Foundation. Archived from the original on 11 July 2015. Retrieved 29 May 2015.
- ^ "1995: Herren". The World Food Prize Foundation. Archived from the original on 11 July 2015. Retrieved 29 May 2015.
- ^ "Cassava production in 2022, Crops/World Regions/Production Quantity/Year from pick lists". UN Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). 2024. Retrieved 29 May 2024.
- ^ "Cassava". Food and Agriculture Organization of the United Nations (FAO). Archived from the original on 18 November 2016. Retrieved 24 November 2011.
- ^ Fauquet Claude; Fargette Denis (1990). "African Cassava Mosaic Virus: Etiology, Epidemiology, and Control" (PDF). Plant Disease. 74 (6). American Phytopathological Society (APS): 404–411. Bibcode:1990PlDis..74..404F. doi:10.1094/pd-74-0404. Archived (PDF) from the original on 9 August 2017. Retrieved 10 January 2011.
- ^ "Dimensions of Need: An atlas of food and agriculture". United Nations Food and Agriculture Organization (FAO). 1995. Archived from the original on 24 November 2016. Retrieved 23 November 2011.
- ^ Cock, James H. (September 1980). "Cassava". The Crop Productivity Symposium, IRRI, los Banos, Philippines: 1–33. reprinted as a chapter in Crop physiology case histories for major crops. Academic Press, 2021, pages 588-633.
- ^ El-Sharkawy, Mabrouk A. (1 August 1993). "Drought-tolerant Cassava for Africa, Asia, and Latin America". BioScience. 43 (7): 441–451. doi:10.2307/1311903. ISSN 1525-3244. JSTOR 1311903. Archived from the original on 21 January 2022. Retrieved 19 April 2020.
- ^ "Nutrition per Hectare for Staple Crops". GardeningPlaces.com. Archived from the original on 9 June 2016.
- ^ Stone, G. D. (2002). "Both Sides Now". Current Anthropology. 43 (4): 611–630. doi:10.1086/341532. S2CID 18867515.
- ^ Save and Grow: Cassava (PDF). Rome: Food and Agriculture Organization. 2013. p. iii. ISBN 978-92-5-107641-5. Archived (PDF) from the original on 23 November 2016. Retrieved 27 October 2016.
- ^ a b c d Cereda, M. P.; Mattos, M. C. Y. (1996). "Linamarin: the Toxic Compound of Cassava". Journal of Venomous Animals and Toxins. 2: 6–12. doi:10.1590/S0104-79301996000100002. hdl:11449/64711.
- ^ Aregheore E. M.; Agunbiade O. O. (1991). "The toxic effects of cassava (Manihot esculenta Crantz) diets on humans: a review". Veterinary and Human Toxicology. 33 (3): 274–275. PMID 1650055.
- ^ White W. L. B.; Arias-Garzon D. I.; McMahon J. M.; Sayre R. T. (1998). "Cyanogenesis in Cassava, The Role of Hydroxynitrile Lyase in Root Cyanide Production". Plant Physiol. 116 (4): 1219–1225. doi:10.1104/pp.116.4.1219. PMC 35028. PMID 9536038.
- ^ "Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on hydrocyanic acid in flavourings and other food ingredients with flavouring properties". EFSA Journal. 105: 1–28. 2004. Archived from the original on 29 September 2015. Retrieved 6 April 2013.
- ^ a b c d e "Ch. 7 Toxic substances and antinutritional factors". Roots, tubers, plantains and bananas in human nutrition. Rome: Food and Agriculture Organization of the United Nations (FAO). 1990. ISBN 9789251028629.
- ^ Bhatia E (2002). "Tropical calcific pancreatitis: strong association with SPINK1 trypsin inhibitor mutations". Gastroenterology. 123 (4): 1020–1025. doi:10.1053/gast.2002.36028. PMID 12360463.
- ^ Harford, Tim (4 September 2019). "How do people learn to cook a poisonous plant safely?". BBC News. Archived from the original on 4 September 2019. Retrieved 4 September 2019.
- ^ a b "CASSAVA POISONING – VENEZUELA". ProMED-mail. 29 January 2017. Archived from the original on 2 February 2017. Retrieved 29 January 2017.
- ^ "Cassava poisoning was integral to Episode 177 of Series 17 of the BBC drama 'Doctors'". BBC. 5 February 2016. Archived from the original on 8 February 2016. Retrieved 13 February 2018.
- ^ Soto-Blanco, Benito; Górniak, Silvana Lima (1 July 2010). "Toxic effects of prolonged administration of leaves of cassava (Manihot esculenta Crantz) to goats". Experimental and Toxicologic Pathology. 62 (4): 361–366. Bibcode:2010EToxP..62..361S. doi:10.1016/j.etp.2009.05.011. ISSN 0940-2993. PMID 19559583.
- ^ Suharti, Sri; Oktafiani, Hafni; Sudarman, Asep; Baik, Myunggi; Wiryawan, Komang Gede (1 December 2021). "Effect of cyanide-degrading bacteria inoculation on performance, rumen fermentation characteristics of sheep fed bitter cassava (Manihot esculenta Crantz) leaf meal". Annals of Agricultural Sciences. 66 (2): 131–136. doi:10.1016/j.aoas.2021.09.001. ISSN 0570-1783. S2CID 244191058.
- ^ Wagner, Holly. "Cassava's cyanide-producing abilities can cause neuropathy". cidpusa.org. Archived from the original on 24 September 2010. Retrieved 21 June 2010.
- ^ Siritunga D; Sayre RT (September–October 2007). "Transgenic approaches for cyanogen reduction in cassava". J AOAC Int. 90 (5): 1450–1455. doi:10.1093/jaoac/90.5.1450. PMID 17955993.
- ^ Castro, Maolis (6 March 2017). "La yuca amarga alimenta la muerte en Venezuela". El País (in Spanish). Archived from the original on 12 February 2018. Retrieved 25 February 2018.
- ^ "Estragos de la crisis: Ocho niños han muerto en Aragua por consumir yuca amarga". La Patilla (in European Spanish). 22 February 2018. Archived from the original on 23 February 2018. Retrieved 25 February 2018.
- ^ Zhou Xun (2012). "Ch. 3 Seasons of death". The Great Famine in China, 1958-1962: A Documentary History. Yale University Press.
- ^ Chiwona-Karltun, Linley; Katundu, Chrissie; Ngoma, James; Chipungu, Felistus; Mkumbira, Jonathan; Simukoko, Sidney; Jiggins, Janice (2002). "Bitter cassava and women: an intriguing response to food security". LEISA Magazine. Vol. 18, no. 4. Archived from the original on 22 September 2018. Retrieved 22 September 2018.
- ^ "Cyanide in Cassava: A Review". Journal of Food Research: 15–29. 2024.
- ^ "When knowledge is not enough: barriers to recommended cassava processing". BMC Public Health: 1–13. 2023.
- ^ "Processing techniques to reduce toxicity and antinutrients of cassava". Comprehensive Review of Food Science and Food Safety: 17–27. 2008.
- ^ "Cyanides reduction and pasting properties of cassava flours fermented 72 h". Food Science and Nutrition: 332–340. 2017.
- ^ Padmaja, G.; Steinkraus, K. H. (1995). "Cyanide detoxification in cassava for food and feed uses". Critical Reviews in Food Science and Nutrition. 35 (4): 299–339. doi:10.1080/10408399509527703. PMID 7576161.
- ^ Opie, Frederick Douglass (2008). Hog and Hominy: Soul Food from Africa to America. Columbia University Press. chapters 1–2.
- ^ "Cassava: Benefits, toxicity, and how to prepare". www.medicalnewstoday.com. 9 February 2021. Archived from the original on 30 March 2022. Retrieved 30 March 2022.
- ^ "Manioc Root - Cargo Handbook - the world's largest cargo transport guidelines website". cargohandbook.com. Archived from the original on 20 May 2022. Retrieved 30 March 2022.
- ^ Zeldes, Leah A. (3 February 2010). "Eat this! Hearty Brazilian feijoada, just in time for Carnival!". Dining Chicago. Chicago's Restaurant & Entertainment Guide. Archived from the original on 12 February 2010. Retrieved 5 February 2010.
- ^ Sweenie, Jennifer (18 April 2023). "What Is Tapioca And How Do You Cook It?". Tasting Table. Retrieved 12 October 2024.
- ^ Schwan, Rosane F.; Almeida, Euziclei G.; Souza-dias, Maria Aparecida G.; Jespersen, Lene (September 2007). "Yeast diversity in rice-cassava fermentations produced by the indigenous Tapirapé people of Brazil". FEMS Yeast Research. 7 (6): 966–972. doi:10.1111/j.1567-1364.2007.00241.x. PMID 17697080.
- ^ van Vark, Manon (28 August 1999). "Tribal cures for modern ailments, Surinam". BBC News.
Their staple food is cassava, from which they make cassava bread and brew kasiri, 'cassava beer'.
- ^ Henkel, Terry W. (1 March 2005). "Parakari, an indigenous fermented beverage using amylolytic Rhizopus in Guyana". Mycologia. 97 (1): 1–11. doi:10.1080/15572536.2006.11832833. PMID 16389951. S2CID 218588548.
- ^ Howell, Edward (1995). Enzyme Nutrition: The Food Enzyme Concept. Avery Publishing Group. p. 49. ISBN 978-0895292216.
- ^ Keegan, William; Carlson, Lisbeth (2008). Talking Taino: Caribbean Natural History from a Native Perspective (Caribbean Archaeology and Ethnohistory). Fire Ant Books. p. 74. ISBN 978-0817355081.
- ^ a b Bradbury, J.H. (2006). "Simple wetting method to reduce cyanogen content of cassava flour" (PDF). Journal of Food Composition and Analysis. 19 (4): 388–393. doi:10.1016/j.jfca.2005.04.012. Archived (PDF) from the original on 5 February 2015. Retrieved 23 March 2018.
- ^ Oboh, G.; Oladunmoye, M.K. (2007). "Biochemical Changes in Micro-Fungi Fermented Cassava Flour Produced from Low- and Medium-Cyanide Variety of Cassava Tubers". Nutrition and Health. 18 (4): 355–367. doi:10.1177/026010600701800405. ISSN 0260-1060. PMID 18087867.
- ^ Akindahunsi, A. A.; Grissom, F. E.; Adewusi, S. R.; Afolabi, O. A.; Torimiro, S. E.; Oke, O. L. (1998). "Parameters of thyroid function in the endemic goitre of Akungba and Oke-Agbe villages of Akoko area of southwestern Nigeria". African Journal of Medicine and Medical Sciences. 27 (3–4): 239–242. ISSN 0309-3913. PMID 10497657.
- ^ Bumoko, G.M.-M.; Sadiki, N.H.; Rwatambuga, A.; Kayembe, K.P.; Okitundu, D.L.; Mumba Ngoyi, D.; Muyembe, J.-J.T.; Banea, J.-P.; Boivin, M.J.; Tshala-Katumbay, D. (2015). "Lower serum levels of selenium, copper, and zinc are related to neuromotor impairments in children with konzo". Journal of the Neurological Sciences. 349 (1–2): 149–153. doi:10.1016/j.jns.2015.01.007. PMC 4323625. PMID 25592410.
- ^ Sayre, R.; Beeching, J. R.; Cahoon, E. B.; Egesi, C.; Fauquet, C.; Fellman, J.; Fregene, M.; Gruissem, W.; Mallowa, S.; Manary, M.; Maziya-Dixon, B.; Mbanaso, A.; Schachtman, D. P.; Siritunga, D.; Taylor, N.; Vanderschuren, H.; Zhang, P. (2011). "The BioCassava Plus Program: Biofortification of Cassava for Sub-Saharan Africa". Annual Review of Plant Biology. 62 (1): 251–272. Bibcode:2011AnRPB..62..251S. doi:10.1146/annurev-arplant-042110-103751. PMID 21526968.
- ^ "BioCassava Plus". St. Louis, Missouri, USA: Donald Danforth Plant Science Center. 2018. Archived from the original on 27 March 2016. Retrieved 23 March 2018.
- ^ Aregheore, E. M.; Agunbiade, O. O. (1991). "The toxic effects of cassava (manihot esculenta grantz) diets on humans: a review". Vet. Hum. Toxicol. 33 (3): 274–275. PMID 1650055.
- ^ Jackson, J. R. (1872). "New Edibles". Food Journal. 2: 372-378 [375].
- ^ a b Nicholls, Henry Alfred Alford (1906). A text-book of tropical agriculture. Macmillan. p. 278.
- ^ Harris, Dunstan A. (2003). Island Cooking: Recipes from the Caribbean. Ten Speed Press. p. 138. ISBN 978-1-58008-501-4.
- ^ Wood, John George (1886). Man and his handiwork. Society for promoting Christian knowledge. pp. 455–456.
- ^ Meehans' monthly: a magazine of horticulture, botany and kindred subjects, Volumes 11-12. Thomas Meehan & Sons. 1901. p. 108.
- ^ White, W. L. B.; Arias-Garzon, D. I.; McMahon, J. M.; Sayre R. T. (1998). "Cyanogenesis in Cassava : The Role of Hydroxynitrile Lyase in Root Cyanide Production". Plant Physiology. 116 (4): 1219–1225. doi:10.1104/pp.116.4.1219. PMC 35028. PMID 9536038.
- ^ Dalton, Henry G. (2005). The History of British Guiana: Comprising a General Description of the Colony (1855). Adamant Media Corporation (reprint). p. 185. ISBN 978-1-4021-8865-7.
- ^ Herbst, Sharon Tyler (2001). The new food lover's companion: comprehensive definitions of nearly 6,000 food, drink, and culinary terms. Barron's Educational Series. p. 105. ISBN 978-0-7641-1258-4.
- ^ United States Food and Drug Administration (2024). "Daily Value on the Nutrition and Supplement Facts Labels". FDA. Archived from the original on 27 March 2024. Retrieved 28 March 2024.
- ^ "TABLE 4-7 Comparison of Potassium Adequate Intakes Established in This Report to Potassium Adequate Intakes Established in the 2005 DRI Report". p. 120. In: Stallings, Virginia A.; Harrison, Meghan; Oria, Maria, eds. (2019). "Potassium: Dietary Reference Intakes for Adequacy". Dietary Reference Intakes for Sodium and Potassium. pp. 101–124. doi:10.17226/25353. ISBN 978-0-309-48834-1. PMID 30844154. NCBI NBK545428.
- ^ a b Tewe, Olumide O. (2004). "The Global Cassava Development Strategy". U.N. Food and Agriculture Organization. Archived from the original on 19 January 2012. Retrieved 24 November 2011.
- ^ Bakky, Aa; Hoque, Mr; Islam, Ms (11 February 2021). "Production of Biofuel from Cassava". Journal of Environmental Science and Natural Resources. 12 (1–2): 171–174. doi:10.3329/jesnr.v12i1-2.52032. ISSN 2408-8633.
- ^ Sivamani, Selvaraju; Chandrasekaran, Arun Pandian; Balajii, Muthusamy; Shanmugaprakash, Muthusamy; Hosseini-Bandegharaei, Ahmad; Baskar, Rajoo (2018). "Evaluation of the potential of cassava-based residues for biofuels production". Reviews in Environmental Science and Bio/Technology. 17 (3): 553–570. Bibcode:2018RESBT..17..553S. doi:10.1007/s11157-018-9475-0. ISSN 1569-1705.
- ^ Anderson-Sprecher, Andrew; Ji, James. "China Biofuel Industry Faces Uncertain Future" (PDF). USDA Foreign Agriculture Service. Archived (PDF) from the original on 27 July 2020. Retrieved 8 November 2019.
- ^ R. Lunsin; M. Wanapat; P. Rowlinson (October 2012). "Effect of cassava hay and rice bran oil supplementation on rumen fermentation, milk yield and milk composition in lactating dairy cows". Asian-Australasian Journal of Animal Sciences. 25 (10): 1364–1373. doi:10.5713/ajas.2012.12051. PMC 4093022. PMID 25049491.
- ^ "Tapioca or Cassava". www.botanischetuinen.nl. Archived from the original on 20 April 2023. Retrieved 30 March 2022.
- ^ Livia de Almeida, Ana Portella, Margaret Read MacDonald, Brazilian folktales, g. xi, Libraries Unlimited (2006), ISBN 1-56308-930-0
- ^ Yara Roberts, Richard Roberts, The Brazilian Table, p. 40, Gibbs M. Smith Inc (2009), ISBN 1-4236-0315-X
- ^ Hartley Burr Alexander, Latin-American [Mythology], p. 186, General Books (2009), ISBN 1-150-14877-2
- ^ Merrian Webster Dictionary. "Definition of Manioc". First Known Use: circa 1554. mw4.m-w.com. Archived from the original on 14 July 2011. Retrieved 13 September 2010.
- ^ Sudardi, Bani; Widyastuti, Hesti (2016). "The Folklore about Food Sustainability according Javanese Culture" (PDF). Journal of Education and Social Science (3): 8–11.
- ^ Provost, M. C. L. (2011). "Where Asian Indian folklore meets Arawak and Kalinago folklore, 'Sound' Symmetry and Asymmetry can make you jump!". Lokoratna Journal of Folklore. 6.
- ^ Schacht, Ryan N. (2013). "Cassava and the Makushi: a shared history of resiliency and transformation". Food and Identity in the Caribbean: 15–29. doi:10.5040/9781350042162.ch-001. ISBN 978-1-350-04216-2.
External links
[edit]Cassava
View on GrokipediaTaxonomy and Etymology
Botanical Classification
Cassava, scientifically known as Manihot esculenta Crantz, belongs to the plant kingdom Plantae, phylum Tracheophyta, class Magnoliopsida, order Malpighiales, family Euphorbiaceae, and genus Manihot.[6][7] The species was first described by the Austrian botanist Heinrich Johann Nepomuk von Crantz in 1766.[8] Within the Euphorbiaceae family, which comprises over 6,000 species of flowering plants known for their milky latex and diverse tropical distributions, the genus Manihot includes about 100 species, primarily shrubs and herbs native to the Americas.[9] M. esculenta is distinguished as a perennial woody shrub originating from tropical South America, characterized by its tuberous roots that serve as the primary edible storage organ and its monoecious reproductive strategy, with male and female flowers borne on the same plant.[10][9][11] Evolutionarily, M. esculenta is domesticated from wild relatives in the genus Manihot, with its closest progenitor being M. esculenta subsp. flabellifolia, alongside other wild species such as M. glaziovii and M. dichotoma, from which gene flow and introgression have occurred, contributing to its genetic diversity.[1][12][13] Historical botanical synonyms for M. esculenta include Manihot aypi Pohl, Manihot utilissima Pohl, Jatropha manihot L., and Janipha manihot (L.) Cass., reflecting early taxonomic revisions and nomenclatural shifts.[14][15] Common names vary regionally, such as manioc in French-speaking areas, yuca in Spanish-speaking regions, and tapioca derived from its processed form, underscoring its widespread cultivation.[15][16]Origin of the Name
The name "cassava" derives from the Taíno word kasabi (or caçabi), which referred to the flour or bread made from the plant's roots, entering European languages through Spanish cazabe during early colonial encounters in the Caribbean.[17] This Taíno term, part of the Arawakan language family spoken by indigenous peoples of the Greater Antilles, was documented by Spanish explorers in the late 15th century as they encountered the crop in the Americas.[18] In parallel, the Portuguese adopted mandioca from the Tupi-Guarani languages of Brazil, where mani means "house" or "spirit" and oca refers to a container or the manioc plant itself, reflecting indigenous mythological associations with the crop's origins.[18] Regional nomenclature for cassava shows significant variation tied to Arawak roots and subsequent adaptations. In Spanish-speaking Latin America, yuca—a direct borrowing from Taíno—predominates, often distinguishing the fresh root from processed forms.[18] West African languages have incorporated terms influenced by colonial introductions, such as gari in Yoruba and other Niger-Congo languages, which specifically denotes the fermented, granular product made from cassava and has become a generic reference to the crop in countries like Nigeria and Ghana. In Southeast Asia, particularly Indonesia, the Javanese term singkong emerged as the common name, adapted from Dutch colonial influences during the 19th century.[19] The historical evolution of cassava's nomenclature was profoundly shaped by 16th-century European colonial trade, which disseminated both the plant and its names across continents. Portuguese traders, transporting cassava from Brazil to West Africa around 1550, introduced mandioca alongside the crop, leading to hybrid terms in African languages that blended Portuguese with local words for tubers.[20] Similarly, Spanish expeditions spread yuca to the Philippines and other Asian outposts, where it intermixed with indigenous terms, fostering linguistic distortions like singkong through phonetic adaptations in Austronesian languages.[18] This colonial diffusion not only globalized the crop but also standardized certain names in international commerce, such as "cassava" in English botanical texts by the 17th century.[18]Description
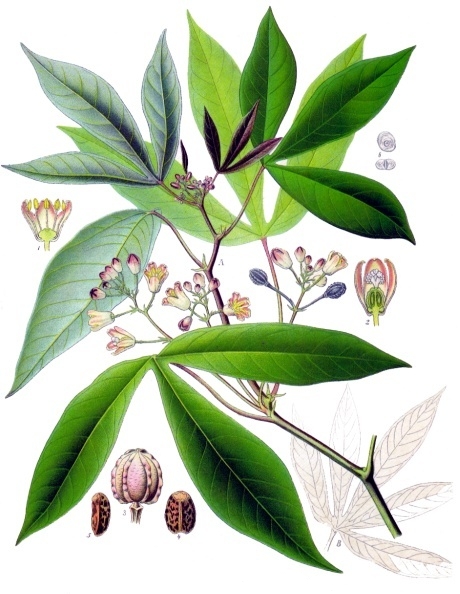
Morphology
Cassava (Manihot esculenta) is a perennial woody shrub typically grown as an annual crop, reaching heights of 1 to 4 meters under optimal conditions.[21] The plant exhibits a semi-woody growth habit with cylindrical stems that are sparingly branched, often light green to reddish in color, and feature sympodial branching where the main stem divides dichotomously, trichotomously, or tetrachotomously to produce secondary branches.[21][2] These stems can develop a smooth outer bark ranging from light brown to yellowish gray, with an inner cream-green layer and soft, creamy wood beneath.[2] The leaves are simple and alternate, arranged in a 2/5 phyllotaxy spiral around the stem, with petioles measuring 5 to 40 cm in length that vary from light greenish to red.[21] The leaf blades are palmate, deeply lobed with 3 to 9 lobes (typically 5 to 7), glabrous, and shiny with a waxy upper surface; the lobes are narrow, often 2.9 to 12.5 times longer than wide, and the blade is dark green above and pale greenish-gray below, sometimes with variegation.[21][2] Stomata are abundant on the lower leaf surface, numbering 278 to 700 per square millimeter.[21] The root system consists of a cluster of 4 to 8 tuberous roots emerging from the base of the stem, serving as the primary storage organs for starch.[2] These roots are cylindrical and tapered, typically 20 to 38 cm long and 2.5 to 10 cm in diameter, encased in a thin reddish-brown fibrous bark with a pure white, starch-rich interior parenchyma containing xylem vessels.[21][2] Beyond these enlarged storage roots, the plant develops a fibrous root network for anchorage and nutrient uptake.[22] Cassava varieties are broadly classified as sweet or bitter based on cyanogenic glycoside content in the roots, primarily linamarin and lotaustralin, which release hydrogen cyanide (HCN) upon hydrolysis. Sweet varieties contain less than 100 ppm HCN equivalents in fresh roots, allowing consumption with minimal processing, while bitter varieties exceed 100 ppm (often 500 ppm or more), necessitating detoxification methods like soaking or fermentation for safe use.[23] Morphological differences between types are subtle, with no consistent traits directly tied to cyanide levels, though bitter types may show variations in root shape, skin thickness, or leaf coloration influenced by environmental factors.[24] Flowering occurs in axillary positions, producing small white to pinkish flowers in racemes, but fruiting is rare in cultivation, yielding trilocular dehiscent capsules that seldom set viable seed due to reliance on vegetative propagation.[22] Cassava is propagated vegetatively using 20- to 30-cm stem cuttings from mature woody portions, which sprout new shoots and roots within weeks, enabling rapid establishment.[21] The growth cycle spans 6 to 24 months from planting to harvest, with roots maturing in 10 to 12 months under tropical conditions, after which the plant is typically harvested as an annual crop, though it can persist for 1 to 3 years as a perennial shrub before senescence.[22][2] During dry periods, the plant may enter a 2- to 3-month dormancy, resuming vegetative growth with renewed rainfall.[2]Genome and Genetics
The cassava genome is diploid with a size of approximately 1.5–1.7 Gb, consisting of 18 chromosome pairs (2n=36), and is characterized by high heterozygosity levels ranging from 0.7% to 1.4%, which complicates assembly and breeding efforts due to structural variations and repetitive sequences.[25][26][27] Cassava is considered to have an allotetraploid origin, with a base chromosome number of x=9, contributing to its genetic complexity and adaptability.[28] This polyploid structure, combined with elevated heterozygosity, results in significant allelic diversity that influences traits like yield and stress tolerance, though it poses challenges for genomic studies.[29] The first draft of the cassava genome was generated in 2009 through a collaborative effort led by the U.S. Department of Energy Joint Genome Institute (DOE-JGI) as part of the Community Sequencing Program, producing a 454-based whole-genome shotgun assembly covering about 69% of the estimated genome size and 96% of protein-coding genes.[29] A chromosome-scale assembly followed in 2014, integrating a high-resolution genetic linkage map from 10 populations to anchor scaffolds onto chromosomes, enabling comparative analyses between wild ancestors (Manihot flabellifolia) and domesticated varieties.[30][31] Subsequent updates in the 2020s have included haplotype-resolved assemblies, such as the 2022 telomere-to-telomere reference for the cultivar Yuxi 6-7, which resolved repetitive regions and improved contiguity.[26] In 2025, further advancements included chromosome-level assemblies of Thai cassava ecotypes, a pan-genome and haplotype map of cultivars and wild ancestors, and BAC-guided haplotype assemblies, providing deeper insights into adaptive evolution, domestication, and breeding targets.[27][32][33] Recent advancements incorporate CRISPR-Cas9 editing, as demonstrated in 2023 studies targeting susceptibility genes like eIF4E isoforms to confer resistance to cassava brown streak disease and bacterial blight, enhancing precision breeding.[34][35] Key genetic loci in cassava include those involved in cyanogenesis, the process producing toxic hydrocyanic acid, primarily the cytochrome P450 genes CYP79D1 and CYP79D2, which catalyze the first committed step in linamarin and lotaustralin biosynthesis.[36] These genes are highly expressed in leaves, directing cyanogenic glycoside transport to storage roots, and have been targeted for downregulation via RNA interference or CRISPR mutagenesis to reduce cyanide levels by up to 99% without compromising yield.[37] For drought tolerance breeding, loci such as MeZFP (encoding a zinc finger protein) and MeALDH (aldehyde dehydrogenase) have been identified as critical regulators, up-regulated under water stress to maintain photosynthesis and osmotic balance; marker-assisted selection incorporating these has accelerated development of resilient varieties.[38] Cassava's genetic diversity is highest in its center of origin in southern Brazil and adjacent regions, where landraces exhibit broad allelic variation shaped by pre-Columbian domestication and farmer selection.[39] This diversity underpins global adaptation but faces erosion from clonal propagation and climate pressures, prompting 2020s conservation initiatives like the comprehensive genotyping of over 7,000 Brazilian accessions by Embrapa and CIAT genebanks to identify core collections and purge duplicates.[40][41] These efforts support ex situ preservation and inform genomic selection for traits like drought tolerance, ensuring sustainable breeding amid narrowing diversity in African and Asian cultivars.[42]History and Domestication
Origins in the Americas
Cassava (Manihot esculenta) originated through domestication in the southwestern Amazon basin approximately 10,000 years ago, specifically in the border regions encompassing modern-day western Brazil, eastern Bolivia, and adjacent areas of Peru and Colombia.[43] The earliest direct archaeological evidence consists of phytoliths recovered from forest islands in the Llanos de Moxos savanna of Bolivia, dated to 10,350 calibrated years before present (cal yr BP), marking the onset of systematic cultivation by indigenous groups alongside other crops like squash.[44] By around 4,750 cal yr BP, phytoliths and starch grains from manioc processing appear at the Real Alto site in coastal Ecuador, indicating the crop's early dispersal and integration into mixed agricultural economies of the Valdivia culture, where it was prepared both raw and cooked using stone tools.[45] In pre-Columbian Amazonian societies, cassava served as a staple, with cultivation evidence from horticultural sites like Teotonio in western Brazil, where phytoliths on grinding stones dated to 6,000 cal BP reveal its processing into flour or bread within terra preta (anthropogenic dark earth) soils enriched by human activity.[46] Processing tools, including wooden or ceramic graters tipped with sharp stone fragments like silex, were essential for shredding tubers to extract toxic cyanogens through washing and pressing, a technique archaeologically attested from 300 BCE onward in Brazilian Amazon sites.[46] Domestication involved selective breeding from the wild progenitor M. esculenta subsp. flabellifolia, which produces small, fibrous roots, toward varieties with enlarged, unbranched storage tubers yielding higher starch content and easier propagation via stem cuttings.[47] This human-mediated shift, evident in morphological changes like thicker stems and reduced branching documented through genetic and archaeobotanical analysis, occurred primarily in the southern Amazon and enhanced cassava's resilience and caloric value for pre-Columbian communities.[43] Genetic diversity peaks in this domestication center, reflecting ongoing selection pressures (as explored in the Genome and Genetics section). Recent genomic studies, including analyses of historic manioc genomes and pan-genome mapping as of 2025, further confirm domestication in the southwestern Amazon around 10,000 years ago and provide insights into genetic adaptations for toxicity management and yield improvement.[45][48]Global Spread and Cultivation History
Cassava's global dissemination began during the Columbian Exchange, when Portuguese traders introduced the crop from Brazil to Africa in the 16th century, recognizing its potential as a reliable food source for their trading posts and colonies.[49] The Portuguese initially transported cassava to islands such as São Tomé and Fernando Po in the Gulf of Guinea, where it was cultivated to support enslaved populations and explorers. By around 1558, it had reached the Congo basin, spreading inland from coastal enclaves and gradually integrating into local farming systems as a resilient staple amid diverse ecological conditions.[50] In Asia, cassava arrived through parallel colonial routes, with Spanish traders carrying it from the Americas to the Philippines in the 17th century via the Manila galleon trade, while Portuguese settlers planted it in regions like Goa and Malacca.[51] Dutch colonizers further expanded its cultivation in Indonesia during the 18th century, promoting it for starch production and as a famine buffer.[51] These introductions facilitated etymological adaptations, such as the adoption of terms like "kamoteng kahoy" in the Philippines, reflecting local linguistic integrations during colonial encounters. By the 19th century, cassava's expansion accelerated in Africa, particularly as a famine-relief crop; in the Congo region during the 1880s, colonial administrators and missionaries distributed it to mitigate food shortages caused by conflicts and environmental stresses, solidifying its role in food security.[49] In Asia, its importance surged during World War II, when disrupted rice supplies in occupied territories like Indonesia and the Philippines elevated cassava to a critical reserve, supporting populations under wartime scarcities.[52] In the late 20th century, the Green Revolution's influence in Africa boosted cassava through breeding programs that developed higher-yielding, disease-resistant varieties, enhancing its productivity and adoption among smallholders in sub-Saharan regions.[53] Initiatives like the Alliance for a Green Revolution in Africa (AGRA) have since focused on cassava to address nutritional needs and climate vulnerabilities, distributing improved seeds that tolerate drought and poor soils.[53] Entering the 2020s, climate adaptation programs in sub-Saharan Africa, such as those led by the International Center for Tropical Agriculture (CIAT), emphasize genomic tools and resilient cultivars to sustain cassava production amid rising temperatures and erratic rainfall, aiming to bolster food systems for millions of farmers.[54]Cultivation Practices
Environmental Requirements
Cassava thrives in tropical and subtropical climates, where mean annual temperatures range from 25°C to 29°C, with soil temperatures around 30°C; growth halts below 10°C.[55] The crop requires well-distributed annual rainfall of 1,000 to 1,500 mm for optimal development, though it exhibits notable drought tolerance, enduring periods of up to six months with minimal water and adapting to semi-arid conditions with as little as 500 mm of precipitation per year.[55][38] For soil conditions, cassava prefers well-drained sandy loam or light-textured soils with a pH between 4.5 and 6.5, which support root expansion without impeding aeration.[55] While it demonstrates resilience to low-fertility and acidic soils, it cannot tolerate waterlogging or heavy clays, as prolonged moisture leads to root rot and reduced yields.[55][2] Cassava cultivation is feasible from sea level up to altitudes of 1,800 meters, beyond which cooler temperatures and reduced oxygen availability limit productivity.[55] Typical planting densities range from 10,000 plants per hectare, achieved through spacings of 1 meter by 1 meter, to balance competition for resources and maximize tuber yield.[56] In response to climate change, research in the 2020s has focused on breeding cassava varieties with enhanced tolerance to saline and highly acidic soils, enabling cultivation in marginal lands affected by rising salinity and soil degradation.[57] These adaptations aim to sustain production amid increasing environmental stresses, such as erratic rainfall and soil salinization.[58]Planting and Harvesting
Cassava is primarily propagated vegetatively using stem cuttings harvested from healthy, disease-free plants aged 8-12 months. These cuttings should measure 20-30 cm in length and contain 5-8 nodes, with a diameter of at least 2 cm to ensure vigorous sprouting and higher yields.[59][56] Cuttings from the lower, more mature portions of the stem are preferred, as they exhibit better establishment rates compared to those from younger sections.[59] Prior to planting, cuttings can be treated by soaking in a mixture of boiling and cold water for 5-10 minutes to reduce fungal infections, and they should be stored upright in shaded, moist soil for no more than 5 days.[59] Varietal selection influences propagation and overall crop timing, with sweet varieties (low cyanogenic glycoside content) typically maturing earlier at 6-12 months, suitable for direct consumption, while bitter varieties (higher cyanogenic content) often require 12-24 months for optimal root development and are preferred for industrial processing.[60][61] For both types, select certified, high-yielding varieties resistant to local stresses to maximize productivity.[56] Planting occurs during the onset of the rainy season to align with optimal soil moisture levels of 500-1000 mm annually. Cuttings are inserted 5-10 cm deep, either vertically or at a 45-degree angle for better anchorage, in rows spaced 1 m apart with 1 m between plants, accommodating about 10,000 plants per hectare.[59][56] In areas prone to waterlogging, plant on ridges or mounds raised 20-30 cm high to improve drainage and reduce root rot risk, whereas flat planting suits well-drained, upland soils.[59][60] Maintenance begins shortly after planting and focuses on weed control, nutrient supply, and compatible companion crops. Weeding is essential in the first 4-6 months when competition is highest; manual weeding at 3-4, 8, 12, and 20-24 weeks after planting can increase yields by up to 90% compared to unweeded fields.[59][56] Fertilization typically involves applying NPK in split doses, such as 50-100 kg N/ha, 10-20 kg P/ha, and 65-120 kg K/ha, with the first application at planting and subsequent ones at 2-3 and 4-5 months to support root bulking without excessive vegetative growth.[59][56] Intercropping with short-duration crops like maize, cowpeas, or groundnuts is common, enhancing land use efficiency and providing additional income, provided cassava density remains at 10,000 plants/ha to avoid competition.[59][56] Harvesting is labor-intensive and timed based on varietal maturity, generally 8-24 months after planting, when roots reach peak starch content. The process involves cutting stems 20-30 cm above ground level two weeks prior to extraction to facilitate access, followed by manual uprooting using hoes or forks to loosen soil and pull roots intact, minimizing breakage.[59][60] Yields of fresh roots typically range from 10-40 tons per hectare under good management, with higher figures achieved through improved varieties and fertilization.[59][56]Post-Harvest Handling and Storage
Cassava roots are highly perishable after harvest, primarily due to post-harvest physiological deterioration (PPD), an endogenous process triggered by mechanical wounding that leads to latex exudation and subsequent oxidation, generating reactive oxygen species (ROS) and causing enzymatic browning and vascular discoloration. This deterioration typically begins within 24-72 hours at ambient temperatures of 20-30°C, rendering roots unmarketable within 3-4 days for most varieties, though some resistant genotypes may last up to 7-11 days.[62] The process involves starch hydrolysis to sugars, accumulation of cyanogenic glucosides, and secondary metabolite buildup, such as scopoletin, which exacerbates tissue breakdown and limits fresh root transport and marketing.[63] To mitigate PPD, immediate post-harvest handling emphasizes minimizing mechanical damage during uprooting and transport, followed by rapid processing into value-added products like flour or chips within hours of harvest.[64] A key technique is curing, which promotes wound healing and periderm (skin) formation to reduce moisture loss and pathogen entry; this involves storing roots at 30-35°C and 80-95% relative humidity for 7-9 days, allowing suberization of damaged tissues and delaying discoloration onset.[65] Pre-harvest practices, such as pruning foliage 2-3 weeks before harvest, can further enhance curing efficacy by reducing latex flow and improving root skin integrity.[62] Storage methods for fresh roots are limited but include traditional heap or clamp storage, where 300-500 kg of roots are piled in conical heaps covered with moist straw, leaves, or soil to maintain humidity and extend viability for 2-3 days up to 2 months under optimal conditions, though losses increase in hot, dry seasons.[62][65] For longer preservation, solar drying reduces root moisture to 8-12% by slicing into thin chips spread on mats or trays, preventing microbial growth while retaining usability for flour milling; alternatively, roots can be converted to dried chips or flour immediately post-harvest for indefinite storage in cool, dry conditions.[62] Refrigerated storage at 0-4°C inhibits PPD for exports, maintaining quality for 2-4 weeks, though it risks chilling injury and requires careful humidity control to avoid fungal issues.[62] Global post-harvest losses for cassava are estimated at 10-25%, with regional variations—9.5% in Africa, 6.3% in Asia, and 14% in the Americas—largely attributable to PPD and inadequate handling infrastructure in smallholder systems.[62][66] In the 2020s, innovations like wax coatings applied post-harvest have shown promise in extending shelf life to 14 days or more by sealing surfaces against oxygen and moisture, reducing weight loss and rot while improving marketability in African supply chains.[67]Pests and Diseases
Insect Pests
Cassava is highly susceptible to several key insect pests that inflict substantial damage, particularly in sub-Saharan Africa, where the crop supports the livelihoods of millions. Among the most destructive are the cassava green mite (Mononychellus tanajoa), the cassava mealybug (Phenacoccus manihoti), and the whitefly (Bemisia tabaci), which collectively contribute to yield reductions through direct feeding and, in the case of whiteflies, virus transmission.[68][69] These pests thrive in tropical conditions and have been inadvertently introduced from the Americas, exacerbating losses in non-native regions.[70] The cassava green mite (Mononychellus tanajoa), a tetranychid mite native to the Americas and introduced to Africa in 1971, primarily targets the growing points and undersides of young leaves.[68] Its life cycle accelerates in dry conditions, enabling rapid population buildup during the dry season, with feeding causing stippling, yellowing, mottling, and bronzing of leaves that severely impairs photosynthesis.[71] This damage leads to leaf drop and reduced root yields, with reported losses ranging from 13% to 80% depending on variety susceptibility and environmental factors.[72] The cassava mealybug (Phenacoccus manihoti), another invasive pest from South America introduced to Africa in the 1970s, reproduces parthenogenetically and feeds on phloem sap from leaves, buds, and stems.[68] Its life cycle averages 49.5 days for females, comprising eggs that hatch in 6-8 days followed by four nymphal instars, allowing continuous breeding in warm, humid tropics.[68] Feeding induces leaf curling, yellowing, necrosis, and defoliation, often resulting in distorted stems and yield reductions up to 80% in affected areas.[73] Whiteflies (Bemisia tabaci), a polyphagous hemipteran with a global tropical distribution, pose dual threats through direct sap-feeding and as vectors for devastating viruses like cassava mosaic disease.[74] The life cycle from egg to adult spans 19-29 days at optimal temperatures around 28°C, with females laying 4-5 eggs daily on leaf undersides and up to 11-12 generations per year.[74] Nymphs and adults extract phloem sap, causing chlorosis, leaf rolling, and sooty mold from honeydew excretion, which can directly reduce yields by up to 40% while amplifying viral epidemics.[74] Control strategies emphasize biological methods to minimize environmental impact and resistance development. For the green mite, predatory mites such as Typhlodromalus aripo have been introduced, achieving 35-60% population reductions and yield increases of up to one-third through natural spread via wind or infested cuttings.[68][75] Mealybug populations are effectively suppressed by the parasitoid Apoanagyrus lopezi, which has restored yields to pre-infestation levels across Africa following continent-wide releases.[76] Whitefly management benefits from parasitoids like Encarsia species, attaining up to 58% parasitism rates, alongside natural enemies such as Eretmocerus mundus.[74] Chemical controls, including neonicotinoid insecticides like 1% Rogor applied via stem-cutting dips, provide targeted suppression but are used judiciously to preserve beneficial insects.[76] Integrated pest management (IPM) programs, pioneered by the International Institute of Tropical Agriculture (IITA) since the 1970s, integrate these biological agents with cultural practices like intercropping (e.g., with cowpea or mung bean to disrupt pest habitats), timely planting at the rainy season's onset, and deployment of resistant varieties such as TMS 30572 for green mites.[68][76] These approaches have mitigated losses exceeding 50% in Africa, with ongoing efforts including a 2024 international partnership for whitefly biocontrol to enhance smallholder resilience.[77][78] Some cassava genotypes exhibit partial genetic resistance to these pests, complementing IPM without replacing it.[79]Pathogens and Diseases
Cassava is susceptible to a range of pathogens, including viruses, bacteria, fungi, and nematodes, which collectively pose significant threats to global production, particularly in tropical regions where the crop is a staple. These diseases can lead to substantial yield losses, with viral infections like cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) causing up to 50-100% reductions in some areas, while bacterial and fungal pathogens exacerbate damage under favorable environmental conditions. Management strategies emphasize the use of resistant varieties, sanitation practices, and integrated approaches to mitigate spread.[80][81] Viral DiseasesCassava mosaic disease (CMD), caused by geminiviruses such as African cassava mosaic virus (ACMV) and East African cassava mosaic virus (EACMV), is one of the most devastating viral pathogens, transmitted primarily by the whitefly vector Bemisia tabaci. Symptoms include leaf mottling, mosaic patterns, leaf distortion, and stunted growth, leading to reduced tuber yield; the disease has historically caused pandemic-level outbreaks in sub-Saharan Africa since the 1990s.[82][83]
Cassava brown streak disease (CBSD), induced by ipomoviruses including Cassava brown streak virus (CBSV) and Ugandan cassava brown streak virus (UCBSV), primarily affects East and Central Africa, with symptoms manifesting as chlorotic lesions on leaves and necrotic lesions in roots and stems, resulting in up to 70% yield loss through root rot. Transmission occurs via whiteflies and infected planting material, with the disease's spread accelerated by climate-driven vector proliferation.[84][85] Bacterial Diseases
Cassava bacterial blight, caused by Xanthomonas axonopodis pv. manihotis (Xam), is a systemic vascular disease prevalent in Africa and Latin America, characterized by angular leaf spots, wilting, stem cankers, and vascular discoloration, which can reduce yields by 20-50% in severe cases. The pathogen spreads through rain splash, tools, and infected cuttings, thriving in warm, humid conditions.[86][87] Fungal Diseases
Anthracnose, primarily caused by Colletotrichum gloeosporioides and related species in the C. gloeosporioides complex, affects leaves, stems, and roots, producing sunken lesions, dieback, and stem cankers that lead to plant lodging and yield losses of up to 60% in humid environments. The fungus overwinters in crop debris and spreads via rain and wind, with infections favored by high rainfall and temperatures above 25°C.[88][89] Nematode Diseases
Root-knot nematodes, mainly Meloidogyne incognita and M. javanica, are significant below-ground pathogens that induce galls on roots, impair nutrient uptake, and predispose plants to secondary infections, causing 30-50% yield reductions in infested soils across sub-Saharan Africa and Asia. These sedentary endoparasites penetrate roots as juveniles and form syncytia, with populations building up over multiple cropping cycles in sandy soils.[90][91] Management of these pathogens relies on deploying resistant varieties, such as Tropical Manihot Series (TMS) cultivars like TMS 30572 for CMD and newer 2020s releases incorporating CBSD resistance through conventional breeding and genomics-assisted selection at institutions like the International Institute of Tropical Agriculture (IITA). Key practices include using virus-indexed clean planting material, crop rotation, quarantine to prevent introduction, and early detection via molecular diagnostics to limit spread.[92][35][93]
Emerging threats include the spread of Sri Lankan cassava mosaic virus (SLCMV) strains in Southeast Asia, reported in 2023, potentially linked to climate-induced shifts in vector dynamics and trade, alongside re-emerging fungal diseases such as cassava witches' broom, caused by Ceratobasidium sp., which has spread from Southeast Asia to South America as of 2025 and could amplify losses if not monitored.[83][94][95][96]