Recent from talks
Contribute something
Nothing was collected or created yet.
Ebola
View on Wikipedia
| Ebola | |
|---|---|
| Other names | Ebola haemorrhagic fever (EHF), Ebola virus disease |
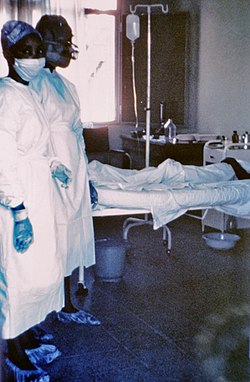 | |
| Two nurses standing near Mayinga N'Seka, a nurse with Ebola virus disease in the 1976 outbreak in Zaire. N'Seka died a few days later. | |
| Specialty | Infectious disease |
| Symptoms | Fever, sore throat, muscular pain, headaches, diarrhoea, bleeding[1] |
| Complications | Shock from fluid loss[2] |
| Usual onset | Two days to three weeks post exposure[1] |
| Causes | Ebolaviruses spread by direct contact[1] |
| Diagnostic method | Finding the virus, viral RNA, or antibodies in blood[1] |
| Differential diagnosis | Malaria, cholera, typhoid fever, meningitis, other viral haemorrhagic fevers[1] |
| Prevention | Coordinated medical services, careful handling of bushmeat[1] |
| Treatment | Supportive care[1] |
| Medication | Atoltivimab/maftivimab/odesivimab (Inmazeb) |
| Prognosis | 25–90% mortality[1] |
Ebola, also known as Ebola virus disease (EVD) and Ebola hemorrhagic fever (EHF), is a viral hemorrhagic fever in humans and other primates, caused by ebolaviruses.[1] Symptoms typically start anywhere between two days and three weeks after infection.[3] The first symptoms are usually fever, sore throat, muscle pain, and headaches.[1] These are usually followed by vomiting, diarrhoea, rash and decreased liver and kidney function,[1] at which point some people begin to bleed both internally and externally.[1] It kills between 25% and 90% of those infected – about 50% on average.[1] Death is often due to shock from fluid loss, and typically occurs between 6 and 16 days after the first symptoms appear.[2] Early treatment of symptoms increases the survival rate considerably compared to late start.[4] An Ebola vaccine was approved by the US FDA in December 2019.
The virus spreads through direct contact with body fluids, such as blood from infected humans or other animals,[1] or from contact with items that have recently been contaminated with infected body fluids.[1] There have been no documented cases, either in nature or under laboratory conditions, of spread through the air between humans or other primates.[5] After recovering from Ebola, semen or breast milk may continue to carry the virus for anywhere between several weeks to several months.[1][6][7] Fruit bats are believed to be the normal carrier in nature; they are able to spread the virus without being affected by it.[1] The symptoms of Ebola may resemble those of several other diseases, including malaria, cholera, typhoid fever, meningitis and other viral hemorrhagic fevers.[1] Diagnosis is confirmed by testing blood samples for the presence of viral RNA, viral antibodies or the virus itself.[1][8]
Control of outbreaks requires coordinated medical services and community engagement,[1] including rapid detection, contact tracing of those exposed, quick access to laboratory services, care for those infected, and proper disposal of the dead through cremation or burial.[1][9] Prevention measures involve wearing proper protective clothing and washing hands when in close proximity to patients and while handling potentially infected bushmeat, as well as thoroughly cooking bushmeat.[1] While there is no approved treatment for Ebola as of 2019[update],[10] two treatments (atoltivimab/maftivimab/odesivimab and ansuvimab) are associated with improved outcomes.[11] Supportive efforts also improve outcomes.[1] These include oral rehydration therapy (drinking slightly sweetened and salty water) or giving intravenous fluids, and treating symptoms.[1] In October 2020, atoltivimab/maftivimab/odesivimab (Inmazeb) was approved for medical use in the United States to treat the disease caused by Zaire ebolavirus.[12]
Signs and symptoms
[edit]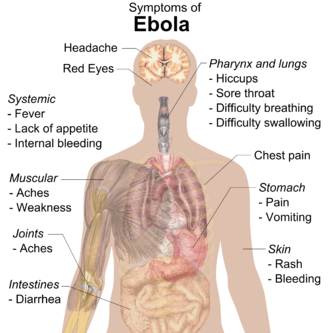
Onset
[edit]The length of time between exposure to the virus and the development of symptoms (incubation period) is between 2 and 21 days,[1][13] and usually between 4 and 10 days.[14] However, recent estimates based on mathematical models predict that around 5% of cases may take longer than 21 days to develop.[15]
Symptoms usually begin with a sudden influenza-like stage characterised by fatigue, fever, weakness, decreased appetite, muscular pain, joint pain, headache, and sore throat.[1][14][16][17] The fever is usually higher than 38.3 °C (101 °F).[18] This is often followed by nausea, vomiting, diarrhoea, abdominal pain, and sometimes hiccups.[17][19] The combination of severe vomiting and diarrhoea often leads to severe dehydration.[20] Next, shortness of breath and chest pain may occur, along with swelling, headaches, and confusion.[17] In about half of the cases, the skin may develop a maculopapular rash, a flat red area covered with small bumps, five to seven days after symptoms begin.[14][18]
Bleeding
[edit]In some cases, internal and external bleeding may occur.[1] This typically begins five to seven days after the first symptoms.[21] All infected people show some decreased blood clotting.[18] Bleeding from mucous membranes or from sites of needle punctures has been reported in 40–50% of cases.[22] This may cause vomiting blood, coughing up of blood, or blood in stool.[23] Bleeding into the skin may create petechiae, purpura, ecchymoses or haematomas (especially around needle injection sites).[24] Bleeding into the whites of the eyes may also occur.[25] Heavy bleeding is uncommon; if it occurs, it is usually in the gastrointestinal tract.[26] The incidence of bleeding into the gastrointestinal tract was reported to be ~58% in the 2001 outbreak in Gabon,[27] but in the 2014–15 outbreak in the US it was ~18%,[28] possibly due to improved prevention of disseminated intravascular coagulation.[20]
Recovery or death
[edit]Recovery may begin between seven and 14 days after first symptoms.[17] Death, if it occurs, follows typically six to sixteen days from first symptoms and is often due to shock from fluid loss.[2] In general, bleeding often indicates a worse outcome, and blood loss may result in death.[16] People are often in a coma near the end of life.[17]
Those who survive often have ongoing muscular and joint pain, liver inflammation, and decreased hearing, and may have continued tiredness, continued weakness, decreased appetite, and difficulty returning to pre-illness weight.[17][29] Problems with vision may develop.[30] It is recommended that survivors of EVD wear condoms for at least twelve months after initial infection or until the semen of a male survivor tests negative for Ebola virus on two separate occasions.[31]
Survivors develop antibodies against Ebola that last at least 10 years, but it is unclear whether they are immune to additional infections.[32]
Cause
[edit]EVD in humans is caused by four of six viruses of the genus Ebolavirus. The four are Bundibugyo virus (BDBV), Sudan virus (SUDV), Taï Forest virus (TAFV) and one simply called Ebola virus (EBOV, formerly Zaire Ebola virus).[33] EBOV, species Zaire ebolavirus, is the most dangerous of the known EVD-causing viruses, and is responsible for the largest number of outbreaks.[34] The fifth and sixth viruses, Reston virus (RESTV) and Bombali virus (BOMV),[35] are not thought to cause disease in humans, but have caused disease in other primates.[36][37] All six viruses are closely related to marburgviruses.[33]
Virology
[edit]
Ebolaviruses contain single-stranded, non-infectious RNA genomes.[38] Ebolavirus genomes contain seven genes including 3'-UTR-NP-VP35-VP40-GP-VP30-VP24-L-5'-UTR.[24][39] The genomes of the five different ebolaviruses (BDBV, EBOV, RESTV, SUDV and TAFV) differ in sequence and the number and location of gene overlaps. As with all filoviruses, ebolavirus virions are filamentous particles that may appear in the shape of a shepherd's crook, of a "U" or of a "6," and they may be coiled, toroid or branched.[39][40] In general, ebolavirions are 80 nanometers (nm) in width and may be as long as 14,000 nm.[41]
Their life cycle is thought to begin with a virion attaching to specific cell-surface receptors such as C-type lectins, DC-SIGN, or integrins, which is followed by fusion of the viral envelope with cellular membranes.[42] The virions taken up by the cell then travel to acidic endosomes and lysosomes where the viral envelope glycoprotein GP is cleaved.[42] This processing appears to allow the virus to bind to cellular proteins enabling it to fuse with internal cellular membranes and release the viral nucleocapsid.[42] The Ebolavirus structural glycoprotein (known as GP1,2) is responsible for the virus' ability to bind to and infect targeted cells.[43] The viral RNA polymerase, encoded by the L gene, partially uncoats the nucleocapsid and transcribes the genes into positive-strand mRNAs, which are then translated into structural and nonstructural proteins. The most abundant protein produced is the nucleoprotein, whose concentration in the host cell determines when L switches from gene transcription to genome replication. Replication of the viral genome results in full-length, positive-strand antigenomes that are, in turn, transcribed into genome copies of negative-strand virus progeny.[44] Newly synthesised structural proteins and genomes self-assemble and accumulate near the inside of the cell membrane. Virions bud off from the cell, gaining their envelopes from the cellular membrane from which they bud. The mature progeny particles then infect other cells to repeat the cycle. The genetics of the Ebola virus are difficult to study because of EBOV's virulent characteristics.[45]
Transmission
[edit]

It is believed that between people, Ebola disease spreads only by direct contact with the blood or other body fluids of a person who has developed symptoms of the disease.[46][47][48] Body fluids that may contain Ebola viruses include saliva, mucus, vomit, feces, sweat, tears, breast milk, urine and semen.[6][32] The WHO states that only people who are very sick are able to spread Ebola disease in saliva, and the virus has not been reported to be transmitted through sweat. Most people spread the virus through blood, feces and vomit.[49] Entry points for the virus include the nose, mouth, eyes, open wounds, cuts and abrasions.[32] Ebola may be spread through large droplets; however, this is believed to occur only when a person is very sick.[50] This contamination can happen if a person is splashed with droplets.[50] Contact with surfaces or objects contaminated by the virus, particularly needles and syringes, may also transmit the infection.[51][52] The virus is able to survive on objects for a few hours in a dried state, and can survive for a few days within body fluids outside of a person.[32][53]
The Ebola virus may be able to persist for more than three months in the semen after recovery, which could lead to infections via sexual intercourse.[6][54][55] Virus persistence in semen for over a year has been recorded in a national screening programme.[56] Ebola may also occur in the breast milk of women after recovery, and it is not known when it is safe to breastfeed again.[7] The virus was also found in the eye of one patient, in 2014, two months after it was cleared from his blood.[57] Otherwise, people who have recovered are not infectious.[51]
The potential for widespread infections in countries with medical systems capable of observing correct medical isolation procedures is considered low.[58] Usually when someone has symptoms of the disease, they are unable to travel without assistance.[59]
Dead bodies remain infectious; thus, people handling human remains in practices such as traditional burial rituals or more modern processes such as embalming are at risk.[58] Of the cases of Ebola infections in Guinea during the 2014 outbreak, 69% are believed to have been contracted via unprotected (or unsuitably protected) contact with infected corpses during certain Guinean burial rituals.[60][61]
Health-care workers treating people with Ebola are at greatest risk of infection.[51] The risk increases when they do not have appropriate protective clothing such as masks, gowns, gloves and eye protection; do not wear it properly; or handle contaminated clothing incorrectly.[51] This risk is particularly common in parts of Africa where the disease mostly occurs and health systems function poorly.[62] There has been transmission in hospitals in some African countries that reuse hypodermic needles.[63][64] Some health-care centres caring for people with the disease do not have running water.[65] In the United States the spread to two medical workers treating infected patients prompted criticism of inadequate training and procedures.[66]
Human-to-human transmission of EBOV through the air has not been reported to occur during EVD outbreaks,[5] and airborne transmission has only been demonstrated in very strict laboratory conditions, and then only from pigs to primates, but not from primates to primates.[46][52] Spread of EBOV by water, or food other than bushmeat, has not been observed.[51][52] No spread by mosquitos or other insects has been reported.[51] Other possible methods of transmission are being studied.[53]
Airborne transmission among humans is theoretically possible due to the presence of Ebola virus particles in saliva, which can be discharged into the air with a cough or sneeze, but observational data from previous epidemics suggests the actual risk of airborne transmission is low.[67] A number of studies examining airborne transmission broadly concluded that transmission from pigs to primates could happen without direct contact because, unlike humans and primates, pigs with EVD get very high ebolavirus concentrations in their lungs, and not their bloodstream.[68] Therefore, pigs with EVD can spread the disease through droplets in the air or on the ground when they sneeze or cough.[69] By contrast, humans and other primates accumulate the virus throughout their body and specifically in their blood, but not very much in their lungs.[69] It is believed that this is the reason researchers have observed pig to primate transmission without physical contact, but no evidence has been found of primates being infected without actual contact, even in experiments where infected and uninfected primates shared the same air.[68][69]
Initial case
[edit]
Although it is not entirely clear how Ebola initially spreads from animals to humans, the spread is believed to involve direct contact with an infected wild animal or fruit bat.[51] Besides bats, other wild animals that are sometimes infected with EBOV include several species of monkeys such as baboons, great apes (chimpanzees and gorillas), and duikers (a species of antelope).[73]
Animals may become infected when they eat fruit partially eaten by bats carrying the virus.[74] Fruit production, animal behavior and other factors may trigger outbreaks among animal populations.[74]
Evidence indicates that both domestic dogs and pigs can also be infected with EBOV.[75] Dogs do not appear to develop symptoms when they carry the virus, and pigs appear to be able to transmit the virus to at least some primates.[75] Although some dogs in an area in which a human outbreak occurred had antibodies to EBOV, it is unclear whether they played a role in spreading the disease to people.[75]
Reservoir
[edit]The natural reservoir for Ebola has yet to be confirmed; however, bats are considered to be the most likely candidate.[52] Three types of fruit bats (Hypsignathus monstrosus, Epomops franqueti and Myonycteris torquata) were found to possibly carry the virus without getting sick.[76] As of 2013[update], whether other animals are involved in its spread is not known.[75] Plants, arthropods, rodents, and birds have also been considered possible viral reservoirs.[1][20]
Bats were known to roost in the cotton factory in which the first cases of the 1976 and 1979 outbreaks were observed, and they have also been implicated in Marburg virus infections in 1975 and 1980.[77] Of 24 plant and 19 vertebrate species experimentally inoculated with EBOV, only bats became infected.[78] The bats displayed no clinical signs of disease, which is considered evidence that these bats are a reservoir species of EBOV. In a 2002–2003 survey of 1,030 animals including 679 bats from Gabon and the Republic of the Congo, immunoglobulin G (IgG) immune defense molecules indicative of Ebola infection were found in three bat species; at various periods of study, between 2.2 and 22.6% of bats were found to contain both RNA sequences and IgG molecules indicating Ebola infection.[79] Antibodies against Zaire and Reston viruses have been found in fruit bats in Bangladesh, suggesting that these bats are also potential hosts of the virus and that the filoviruses are present in Asia.[80]
Between 1976 and 1998, in 30,000 mammals, birds, reptiles, amphibians and arthropods sampled from regions of EBOV outbreaks, no Ebola virus was detected apart from some genetic traces found in six rodents (belonging to the species Mus setulosus and Praomys) and one shrew (Sylvisorex ollula) collected from the Central African Republic.[77][81] However, further research efforts have not confirmed rodents as a reservoir.[82] Traces of EBOV were detected in the carcasses of gorillas and chimpanzees during outbreaks in 2001 and 2003, which later became the source of human infections. However, the high rates of death in these species resulting from EBOV infection make it unlikely that these species represent a natural reservoir for the virus.[77]
Deforestation has been mentioned as a possible contributor to recent outbreaks, including the West African Ebola virus epidemic. Index cases of EVD have often been close to recently deforested lands.[83][84]
Pathophysiology
[edit]
Like other filoviruses, EBOV replicates very efficiently in many cells, producing large amounts of virus in monocytes, macrophages, dendritic cells and other cells including liver cells, fibroblasts, and adrenal gland cells.[85] Viral replication triggers high levels of inflammatory chemical signals and leads to a septic state.[29]
EBOV is thought to infect humans through contact with mucous membranes or skin breaks.[46] After infection, endothelial cells (cells lining the inside of blood vessels), liver cells, and several types of immune cells such as macrophages, monocytes, and dendritic cells are the main targets of attack.[46] Following infection, immune cells carry the virus to nearby lymph nodes where further reproduction of the virus takes place.[46] From there the virus can enter the bloodstream and lymphatic system and spread throughout the body.[46] Macrophages are the first cells infected with the virus, and this infection results in programmed cell death.[41] Other types of white blood cells, such as lymphocytes, also undergo programmed cell death leading to an abnormally low concentration of lymphocytes in the blood.[46] This contributes to the weakened immune response seen in those infected with EBOV.[46]
Endothelial cells may be infected within three days after exposure to the virus.[41] The breakdown of endothelial cells leading to blood vessel injury can be attributed to EBOV glycoproteins. This damage occurs due to the synthesis of Ebola virus glycoprotein (GP), which reduces the availability of specific integrins responsible for cell adhesion to the intercellular structure and causes liver damage, leading to improper clotting. The widespread bleeding that occurs in affected people causes swelling and shock due to loss of blood volume.[86] The dysfunctional bleeding and clotting commonly seen in EVD has been attributed to increased activation of the extrinsic pathway of the coagulation cascade due to excessive tissue factor production by macrophages and monocytes.[14]
After infection, a secreted glycoprotein, small soluble glycoprotein (sGP or GP) is synthesised. EBOV replication overwhelms protein synthesis of infected cells and the host immune defences. The GP forms a trimeric complex, which tethers the virus to the endothelial cells. The sGP forms a dimeric protein that interferes with the signalling of neutrophils, another type of white blood cell. This enables the virus to evade the immune system by inhibiting early steps of neutrophil activation.[medical citation needed] Furthermore, the virus is capable of hijacking cellular metabolism. Studies have shown that Ebola virus-like particles can reprogram metabolism in both vascular and immune cells.[87]
Immune system evasion
[edit]Filoviral infection also interferes with proper functioning of the innate immune system.[42][44] EBOV proteins blunt the human immune system's response to viral infections by interfering with the cells' ability to produce and respond to interferon proteins such as interferon-alpha, interferon-beta, and interferon gamma.[43][88]
The VP24 and VP35 structural proteins of EBOV play a key role in this interference. When a cell is infected with EBOV, receptors located in the cell's cytosol (such as RIG-I and MDA5) or outside of the cytosol (such as Toll-like receptor 3 (TLR3), TLR7, TLR8 and TLR9) recognise infectious molecules associated with the virus.[43] On TLR activation, proteins including interferon regulatory factor 3 and interferon regulatory factor 7 trigger a signalling cascade that leads to the expression of type 1 interferons.[43] The type 1 interferons are then released and bind to the IFNAR1 and IFNAR2 receptors expressed on the surface of a neighbouring cell.[43] Once interferon has bound to its receptors on the neighbouring cell, the signalling proteins STAT1 and STAT2 are activated and move to the cell's nucleus.[43] This triggers the expression of interferon-stimulated genes, which code for proteins with antiviral properties.[43] EBOV's V24 protein blocks the production of these antiviral proteins by preventing the STAT1 signalling protein in the neighbouring cell from entering the nucleus.[43] The VP35 protein directly inhibits the production of interferon-beta.[88] By inhibiting these immune responses, EBOV may quickly spread throughout the body.[41]
Diagnosis
[edit]When EVD is suspected, travel, work history, and exposure to wildlife are important factors with respect to further diagnostic efforts.[89]
Laboratory testing
[edit]Possible non-specific laboratory indicators of EVD include a low platelet count; an initially decreased white blood cell count followed by an increased white blood cell count; elevated levels of the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST); and abnormalities in blood clotting often consistent with disseminated intravascular coagulation (DIC) such as a prolonged prothrombin time, partial thromboplastin time, and bleeding time.[90] Filovirions such as EBOV may be identified by their unique filamentous shapes in cell cultures examined with electron microscopy.[91]
The specific diagnosis of EVD is confirmed by isolating the virus, detecting its RNA or proteins, or detecting antibodies against the virus in a person's blood.[92] Isolating the virus by cell culture, detecting the viral RNA by polymerase chain reaction (PCR)[8][14] and detecting proteins by enzyme-linked immunosorbent assay (ELISA) are methods best used in the early stages of the disease and also for detecting the virus in human remains.[8][92] Detecting antibodies against the virus is most reliable in the later stages of the disease and in those who recover.[92] IgM antibodies are detectable two days after symptom onset and IgG antibodies can be detected six to 18 days after symptom onset.[14] During an outbreak, isolation of the virus with cell culture methods is often not feasible. In field or mobile hospitals, the most common and sensitive diagnostic methods are real-time PCR and ELISA.[93] In 2014, with new mobile testing facilities deployed in parts of Liberia, test results were obtained 3–5 hours after sample submission.[94] In 2015, a rapid antigen test which gives results in 15 minutes was approved for use by WHO.[95] It is able to confirm Ebola in 92% of those affected and rule it out in 85% of those not affected.[95]
Differential diagnosis
[edit]Early symptoms of EVD may be similar to those of other diseases common in Africa, including malaria and dengue fever.[16] The symptoms are also similar to those of other viral haemorrhagic fevers such as Marburg virus disease, Crimean–Congo haemorrhagic fever, and Lassa fever.[96][97]
The complete differential diagnosis is extensive and requires consideration of many other infectious diseases such as typhoid fever, shigellosis, rickettsial diseases, cholera, sepsis, borreliosis, EHEC enteritis, leptospirosis, scrub typhus, plague, Q fever, candidiasis, histoplasmosis, trypanosomiasis, visceral leishmaniasis, measles, and viral hepatitis among others.[98]
Non-infectious diseases that may result in symptoms similar to those of EVD include acute promyelocytic leukaemia, haemolytic uraemic syndrome, snake envenomation, clotting factor deficiencies/platelet disorders, thrombotic thrombocytopenic purpura, hereditary haemorrhagic telangiectasia, Kawasaki disease, and warfarin poisoning.[93][99][100][101]
Prevention
[edit]Vaccines
[edit]An Ebola vaccine, rVSV-ZEBOV, was approved in the United States in December 2019.[102] It appears to be fully effective ten days after being given.[102] It was studied in Guinea between 2014 and 2016.[102] More than 100,000 people have been vaccinated against Ebola as of 2019[update].[103] The WHO reported that approximately 345,000 people were given the vaccine during the Kivu Ebola epidemic from 2018 to 2020.[104]
Infection control
[edit]
Community awareness of the benefits on survival chances of admitting cases early is important for the infected and infection control [4]
Caregivers
[edit]
People who care for those infected with Ebola should wear protective clothing including masks, gloves, gowns and goggles.[105] The U.S. Centers for Disease Control (CDC) recommend that the protective gear leaves no skin exposed.[106] These measures are also recommended for those who may handle objects contaminated by an infected person's body fluids.[107] In 2014, the CDC began recommending that medical personnel receive training on the proper suit-up and removal of personal protective equipment (PPE); in addition, a designated person, appropriately trained in biosafety, should be watching each step of these procedures to ensure they are done correctly.[106] In Sierra Leone, the typical training period for the use of such safety equipment lasts approximately 12 days.[108] In 2022 in Uganda, lighter personal protection equipment has become available as well as possibilities to monitor and communicate with patients from windows in the treatment tents until it is necessary to enter if e.g. a patient's oxygen levels drop.[4]
Patients and household members
[edit]The infected person should be in barrier-isolation from other people.[105] All equipment, medical waste, patient waste and surfaces that may have come into contact with body fluids need to be disinfected.[107] During the 2014 outbreak, kits were put together to help families treat Ebola disease in their homes, which included protective clothing as well as chlorine powder and other cleaning supplies.[109] Education of caregivers in these techniques, and providing such barrier-separation supplies has been a priority of Doctors Without Borders.[110]
Disinfection
[edit]Ebolaviruses can be eliminated with heat (heating for 30 to 60 minutes at 60 °C or boiling for five minutes). To disinfect surfaces, some lipid solvents such as some alcohol-based products, detergents, sodium hypochlorite (bleach) or calcium hypochlorite (bleaching powder), and other suitable disinfectants may be used at appropriate concentrations.[73][111]
General population
[edit]Education of the general public about the risk factors for Ebola infection and of the protective measures individuals may take to prevent infection is recommended by the World Health Organization.[1] These measures include avoiding direct contact with infected people and regular hand washing using soap and water.[112]
Bushmeat
[edit]Bushmeat, an important source of protein in the diet of some Africans, should be handled and prepared with appropriate protective clothing and thoroughly cooked before consumption.[1] Some research suggests that an outbreak of Ebola disease in the wild animals used for consumption may result in a corresponding human outbreak. Since 2003, such animal outbreaks have been monitored to predict and prevent Ebola outbreaks in humans.[113]
Corpses, burial
[edit]If a person with Ebola disease dies, direct contact with the body should be avoided.[105] Certain burial rituals, which may have included making various direct contacts with a dead body, require reformulation so that they consistently maintain a proper protective barrier between the dead body and the living.[114][115][116] Social anthropologists may help find alternatives to traditional rules for burials.[117]
Transport, travel, contact
[edit]Transportation crews are instructed to follow a certain isolation procedure, should anyone exhibit symptoms resembling EVD.[118] As of August 2014[update], the WHO does not consider travel bans to be useful in decreasing spread of the disease.[59] In October 2014, the CDC defined four risk levels used to determine the level of 21-day monitoring for symptoms and restrictions on public activities.[119] In the United States, the CDC recommends that restrictions on public activity, including travel restrictions, are not required for the following defined risk levels:[119]
- having been in a country with widespread Ebola disease transmission and having no known exposure (low risk); or having been in that country more than 21 days ago (no risk)
- encounter with a person showing symptoms; but not within three feet of the person with Ebola without wearing PPE; and no direct contact with body fluids
- having had brief skin contact with a person showing symptoms of Ebola disease when the person was believed to be not very contagious (low risk)
- in countries without widespread Ebola disease transmission: direct contact with a person showing symptoms of the disease while wearing PPE (low risk)
- contact with a person with Ebola disease before the person was showing symptoms (no risk).
The CDC recommends monitoring for the symptoms of Ebola disease for those both at "low risk" and at higher risk.[119]
Laboratory
[edit]In laboratories where diagnostic testing is carried out, biosafety level 4-equivalent containment is required.[120] Laboratory researchers must be properly trained in BSL-4 practices and wear proper PPE.[120]
Isolation
[edit]Isolation refers to separating those who are sick from those who are not. Quarantine refers to separating those who may have been exposed to a disease until they either show signs of the disease or are no longer at risk.[121] Quarantine, also known as enforced isolation, is usually effective in decreasing spread.[122][123] Governments often quarantine areas where the disease is occurring or individuals who may transmit the disease outside of an initial area.[124] In the United States, the law allows quarantine of those infected with ebolaviruses.[125][126]
Contact tracing
[edit]Contact tracing is considered important to contain an outbreak. It involves finding everyone who had close contact with infected individuals and monitoring them for signs of illness for 21 days. If any of these contacts comes down with the disease, they should be isolated, tested and treated. Then the process is repeated, tracing the contacts' contacts.[127][128]
Management
[edit]As of 2019[update] two treatments (atoltivimab/maftivimab/odesivimab and ansuvimab) are associated with improved outcomes.[10][11] The U.S. Food and Drug Administration (FDA) advises people to be careful of advertisements making unverified or fraudulent claims of benefits supposedly gained from various anti-Ebola products.[129][130]
In October 2020, the U.S. Food and Drug Administration (FDA) approved atoltivimab/maftivimab/odesivimab with an indication for the treatment of infection caused by Zaire ebolavirus.[12]
Standard support
[edit]
Treatment is primarily supportive in nature.[131] Early supportive care with rehydration and symptomatic treatment improves survival.[1] Rehydration may be via the oral or intravenous route.[131] These measures may include pain management, and treatment for nausea, fever, and anxiety.[131] The World Health Organization (WHO) recommends avoiding aspirin or ibuprofen for pain management, due to the risk of bleeding associated with these medications.[132]
Blood products such as packed red blood cells, platelets, or fresh frozen plasma may also be used.[131] Other regulators of coagulation have also been tried including heparin in an effort to prevent disseminated intravascular coagulation and clotting factors to decrease bleeding.[131] Antimalarial medications and antibiotics are often used before the diagnosis is confirmed,[131] though there is no evidence to suggest such treatment helps. Several experimental treatments are being studied.[133]
Where hospital care is not possible, the WHO's guidelines for home care have been relatively successful. Recommendations include using towels soaked in a bleach solution when moving infected people or bodies and also applying bleach on stains. It is also recommended that the caregivers wash hands with bleach solutions and cover their mouth and nose with a cloth.[134]
Intensive care
[edit]Intensive care is often used in the developed world.[24] This may include maintaining blood volume and electrolytes (salts) balance as well as treating any bacterial infections that may develop.[24] Dialysis may be needed for kidney failure, and extracorporeal membrane oxygenation may be used for lung dysfunction.[24]
Prognosis
[edit]EVD has a risk of death in those infected of between 25% and 90%.[1][135] As of September 2014[update], the average risk of death among those infected is 50%.[1] The highest risk of death was 90% in the 2002–2003 Republic of the Congo outbreak.[136] Early admission significantly increases survival rates [4]
Death, if it occurs, follows typically six to sixteen days after symptoms appear and is often due to low blood pressure from fluid loss.[2] Early supportive care to prevent dehydration may reduce the risk of death.[133]
Post-Ebola virus syndrome
[edit]If an infected person survives, recovery may be quick and complete.[14][137] However, a large portion of survivors develop post-Ebola virus syndrome after the acute phase of the infection.[138]
Prolonged cases are often complicated by the occurrence of long-term problems, such as inflammation of the testicles, joint pains, fatigue, hearing loss, mood and sleep disturbances, muscular pain, abdominal pain, menstrual abnormalities, miscarriages, skin peeling, or hair loss.[14][137] Inflammation and swelling of the uveal layer of the eye is the most common eye complication in survivors of Ebola virus disease.[137] Eye symptoms, such as light sensitivity, excess tearing, and vision loss have been described.[139]
Ebola can stay in some body parts like the eyes,[140] breasts, and testicles after infection.[6][141] Sexual transmission after recovery has been suspected.[142][143] If sexual transmission occurs following recovery, it is believed to be a rare event.[144] One case of a condition similar to meningitis has been reported many months after recovery, as of October 2015[update].[145]
Epidemiology
[edit]The disease typically occurs in outbreaks in tropical regions of Sub-Saharan Africa.[1] From 1976 (when it was first identified) through 2013, the WHO reported 2,387 confirmed cases with 1,590 overall fatalities.[1][146] The largest outbreak to date was the Ebola virus epidemic in West Africa, which caused a large number of deaths in Guinea, Sierra Leone, and Liberia.[147][148]
1976
[edit]Sudan
[edit]
The first known outbreak of EVD was identified only after the fact. It occurred between June and November 1976, in Nzara, South Sudan[33][149] (then part of Sudan), and was caused by Sudan virus (SUDV). The Sudan outbreak infected 284 people and killed 151. The first identifiable case in Sudan occurred on 27 June in a storekeeper in a cotton factory in Nzara, who was hospitalised on 30 June and died on 6 July.[24][150] Although the WHO medical staff involved in the Sudan outbreak knew that they were dealing with a heretofore unknown disease, the actual "positive identification" process and the naming of the virus did not occur until some months later in Zaire.[150]
Zaire
[edit]
On 26 August 1976, the second outbreak of EVD began in Yambuku, a small rural village in Mongala District in northern Zaire (now known as the Democratic Republic of the Congo).[151][152] This outbreak was caused by EBOV, formerly designated Zaire ebolavirus, a different member of the genus Ebolavirus than in the first Sudan outbreak. The first person infected with the disease was the village school's headmaster Mabalo Lokela, who began displaying symptoms on 26 August 1976.[153] Lokela had returned from a trip to Northern Zaire near the border of the Central African Republic, after visiting the Ebola River between 12 and 22 August. He was originally believed to have malaria and was given quinine. However, his symptoms continued to worsen, and he was admitted to Yambuku Mission Hospital on 5 September. Lokela died on 8 September 14 days after he began displaying symptoms.[154][155]
Soon after Lokela's death, others who had been in contact with him also died, and people in Yambuku began to panic. The country's Minister of Health and Zaire President Mobutu Sese Seko declared the entire region, including Yambuku and the country's capital, Kinshasa, a quarantine zone. No-one was permitted to enter or leave the area, and roads, waterways, and airfields were placed under martial law. Schools, businesses and social organisations were closed.[156] The initial response was led by Congolese doctors, including Jean-Jacques Muyembe-Tamfum, one of the discoverers of Ebola. Muyembe took a blood sample from a Belgian nun; this sample would eventually be used by Peter Piot to identify the previously unknown Ebola virus.[157] Muyembe was also the first scientist to come into direct contact with the disease and survive.[158] Researchers from the Centers for Disease Control and Prevention (CDC), including Piot, co-discoverer of Ebola, later arrived to assess the effects of the outbreak, observing that "the whole region was in panic."[159][160][161]
Piot concluded that Belgian nuns had inadvertently started the epidemic by giving unnecessary vitamin injections to pregnant women without sterilizing the syringes and needles. The outbreak lasted 26 days and the quarantine lasted two weeks. Researchers speculated that the disease disappeared due to the precautions taken by locals, the quarantine of the area, and discontinuing of the injections.[156]
During this outbreak, Ngoy Mushola recorded the first clinical description of EVD in Yambuku, where he wrote the following in his daily log: "The illness is characterised with a high temperature of about 39 °C (102 °F), haematemesis, diarrhoea with blood, retrosternal abdominal pain, prostration with 'heavy' articulations, and rapid evolution death after a mean of three days."[162]
The virus responsible for the initial outbreak, first thought to be the Marburg virus, was later identified as a new type of virus related to the genus Marburgvirus. Virus strain samples isolated from both outbreaks were named "Ebola virus" after the Ebola River, near the first-identified viral outbreak site in Zaire.[24] Reports conflict about who initially coined the name: either Karl Johnson of the American CDC team[163] or Belgian researchers.[164] Subsequently, a number of other cases were reported, almost all centred on the Yambuku mission hospital or close contacts of another case.[153] In all, 318 cases and 280 deaths (an 88% fatality rate) occurred in Zaire.[165] Although the two outbreaks were at first believed connected, scientists later realised that they were caused by two distinct ebolaviruses, SUDV and EBOV.[152]
1995–2014
[edit]
The second major outbreak occurred in Zaire (now the Democratic Republic of the Congo, DRC), in 1995, affecting 315 and killing 254.[1]
In 2000, Uganda had an outbreak infecting 425 and killing 224; in this case, the Sudan virus was found to be the Ebola species responsible for the outbreak.[1]
In 2003, an outbreak in the DRC infected 143 and killed 128, a 90% death rate, the highest of a genus Ebolavirus outbreak to date.[166]
In 2004, a Russian scientist died from Ebola after sticking herself with an infected needle.[167]
Between April and August 2007, a fever epidemic[168] in a four-village region[169] of the DRC was confirmed in September to have been cases of Ebola.[170] Many people who attended the recent funeral of a local village chief died.[169] The 2007 outbreak eventually infected 264 individuals and killed 187.[1]
On 30 November 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District in Western Uganda. After confirming samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization (WHO) confirmed the presence of a new species of genus Ebolavirus, which was tentatively named Bundibugyo.[171] The WHO reported 149 cases of this new strain and 37 of those led to deaths.[1]
The WHO confirmed two small outbreaks in Uganda in 2012, both caused by the Sudan variant. The first outbreak affected seven people, killing four, and the second affected 24, killing 17.[1]
On 17 August 2012, the Ministry of Health of the DRC reported an outbreak of the Ebola-Bundibugyo variant[172] in the eastern region.[173][174] Other than its discovery in 2007, this was the only time that this variant has been identified as responsible for an outbreak. The WHO revealed that the virus had sickened 57 people and killed 29. The probable cause of the outbreak was tainted bush meat hunted by local villagers around the towns of Isiro and Viadana.[1][175]
In 2014, an outbreak occurred in the DRC. Genome-sequencing showed that this outbreak was not related to the 2014–15 West Africa Ebola virus outbreak, but was the same EBOV species, the Zaire species.[176] It began in August 2014, and was declared over in November with 66 cases and 49 deaths.[177] This was the 7th outbreak in the DRC, three of which occurred during the period when the country was known as Zaire.[178]
2013–2016 West Africa
[edit]
In March 2014, the World Health Organization (WHO) reported a major Ebola outbreak in Guinea, a West African nation.[179] Researchers traced the outbreak to a one-year-old child who died in December 2013.[180][181] The disease rapidly spread to the neighbouring countries of Liberia and Sierra Leone. It was the largest Ebola outbreak ever documented, and the first recorded in the region.[179] On 8 August 2014, the WHO declared the epidemic an international public health emergency. Urging the world to offer aid to the affected regions, its Director-General said, "Countries affected to date simply do not have the capacity to manage an outbreak of this size and complexity on their own. I urge the international community to provide this support on the most urgent basis possible."[182] By mid-August 2014, Doctors Without Borders reported the situation in Liberia's capital, Monrovia, was "catastrophic" and "deteriorating daily". They reported that fears of Ebola among staff members and patients had shut down much of the city's health system, leaving many people without medical treatment for other conditions.[183] In a 26 September statement, WHO said, "The Ebola epidemic ravaging parts of West Africa is the most severe acute public health emergency seen in modern times. Never before in recorded history has a biosafety level four pathogen infected so many people so quickly, over such a broad geographical area, for so long."[184]
Intense contact tracing and strict isolation largely prevented further spread of the disease in the countries that had imported cases.
It caused significant mortality, with a considerable case fatality rate.[185][186][187][note 1] By the end of the epidemic, 28,616 people had been infected; of these, 11,310 had died, for a case-fatality rate of 40%.[188] As of 8 May 2016[update], 28,646 suspected cases and 11,323 deaths were reported;[189][190] however, the WHO said that these numbers may be underestimated.[191] Because they work closely with the body fluids of infected patients, healthcare workers were especially vulnerable to infection; in August 2014, the WHO reported that 10% of the dead were healthcare workers.[192]

In September 2014, it was estimated that the countries' capacity for treating Ebola patients was insufficient by the equivalent of 2,122 beds; by December there were a sufficient number of beds to treat and isolate all reported Ebola cases, although the uneven distribution of cases was causing serious shortfalls in some areas.[193] On 28 January 2015, the WHO reported that for the first time since the week ending 29 June 2014, there had been fewer than 100 new confirmed cases reported in a week in the three most-affected countries. The response to the epidemic then moved to a second phase, as the focus shifted from slowing transmission to ending the epidemic.[194] On 8 April 2015, the WHO reported only 30 confirmed cases, the lowest weekly total since the third week of May 2014.[195]
On 29 December 2015, 42 days after the last person tested negative for a second time, Guinea was declared free of Ebola transmission.[196] At that time, a 90-day period of heightened surveillance was announced by that agency. "This is the first time that all three countries – Guinea, Liberia and Sierra Leone – have stopped the original chains of transmission ...", the organisation stated in a news release.[197] A new case was detected in Sierra Leone on 14 January 2016.[198] However, the outbreak was declared no longer an emergency on 29 March 2016.[199]
2014 spread outside West Africa
[edit]On 19 September, Eric Duncan flew from his native Liberia to Texas; five days later he began showing symptoms and visited a hospital but was sent home. His condition worsened and he returned to the hospital on 28 September, where he died on 8 October. Health officials confirmed a diagnosis of Ebola on 30 September – the first case in the United States.[200]
In early October, Teresa Romero, a 44-year-old Spanish nurse, contracted Ebola after caring for a priest who had been repatriated from West Africa. This was the first transmission of the virus to occur outside Africa.[201] Romero tested negative for the disease on 20 October, suggesting that she may have recovered from Ebola infection.[202]
On 12 October, the Centers for Disease Control and Prevention (CDC) confirmed that a nurse in Texas, Nina Pham, who had treated Duncan tested positive for the Ebola virus, the first known case of transmission in the United States.[203] On 15 October, a second Texas health-care worker who had treated Duncan was confirmed to have the virus.[66][204] Both of these people recovered.[205] An unrelated case involved a doctor in New York City, who returned to the United States from Guinea after working with Médecins Sans Frontières and tested positive for Ebola on 23 October.[206] The person recovered and was discharged from Bellevue Hospital on 11 November.[205] On 24 December 2014, a laboratory in Atlanta, Georgia reported that a technician had been exposed to Ebola.[207]
On 29 December 2014, Pauline Cafferkey, a British nurse who had just returned to Glasgow from Sierra Leone, was diagnosed with Ebola at Glasgow's Gartnavel General Hospital.[208] After initial treatment in Glasgow, she was transferred by air to RAF Northolt, then to the specialist high-level isolation unit at the Royal Free Hospital in London for longer-term treatment.[209]
2017 Democratic Republic of the Congo
[edit]On 11 May 2017, the DRC Ministry of Public Health notified the WHO about an outbreak of Ebola. Four people died, and four people survived; five of these eight cases were laboratory-confirmed. A total of 583 contacts were monitored. On 2 July 2017, the WHO declared the end of the outbreak.[210]
2018 Équateur province
[edit]On 14 May 2018, the World Health Organization reported that "the Democratic Republic of Congo reported 39 suspected, probable or confirmed cases of Ebola between 4 April and 13 May, including 19 deaths."[211] Some 393 people identified as contacts of Ebola patients were being followed up. The outbreak centred on the Bikoro, Iboko, and Wangata areas in Equateur province,[211] including in the large city of Mbandaka. The DRC Ministry of Public Health approved the use of an experimental vaccine.[212][213][214] On 13 May 2018, WHO Director-General Tedros Adhanom Ghebreyesus visited Bikoro.[215] Reports emerged that maps of the area were inaccurate, not so much hampering medical providers as epidemiologists and officials trying to assess the outbreak and containment efforts.[216] The 2018 outbreak in the DRC was declared over on 24 July 2018.[217]
2018–2020 Kivu
[edit]On 1 August 2018, the world's 10th Ebola outbreak was declared in North Kivu province of the Democratic Republic of the Congo. It was the first Ebola outbreak in a military conflict zone, with thousands of refugees in the area.[218][219] By November 2018, nearly 200 Congolese had died of Ebola, about half of them from the city of Beni, where armed groups are fighting over the region's mineral wealth, impeding medical relief efforts.[220]
By March 2019, this became the second largest Ebola outbreak ever recorded, with more than 1,000 cases and insecurity continuing to be the major resistance to providing an adequate response.[221][222] As of 4 June 2019[update], the WHO reported 2025 confirmed and probable cases with 1357 deaths.[223] In June 2019, two people died of Ebola in neighbouring Uganda.[224]
In July 2019, an infected man travelled to Goma, home to more than two million people.[225] One week later, on 17 July 2019, the WHO declared the Ebola outbreak a global health emergency, the fifth time such a declaration has been made by the organisation.[226] A government spokesman said that half of the Ebola cases are unidentified, and he added that the current outbreak could last up to three years.[227]
On 25 June 2020, the second biggest EVD outbreak ever was declared over.[228]
2020 Équateur province
[edit]On 1 June 2020, the Congolese health ministry announced a new DRC outbreak of Ebola in Mbandaka, Équateur Province, a region along the Congo River. Genome sequencing suggests that this outbreak, the 11th outbreak since the virus was first discovered in the country in 1976, is unrelated to the one in North Kivu Province or the previous outbreak in the same area in 2018. It was reported that six cases had been identified; four of the people had died. It is expected that more people will be identified as surveillance activities increase.[229] By 15 June the case count had increased to 17 with 11 deaths, with more than 2,500 people having been vaccinated.[230] The 11th EVD outbreak was officially declared over on 19 November 2020.[231] By the time the Équateur outbreak ended, it had 130 confirmed cases with 75 recoveries and 55 deaths.
2021
[edit]North Kivu
[edit]On 7 February 2021, the Congolese health ministry announced a new case of Ebola near Butembo, North Kivu detected a day before. The case was a 42-year-old woman who had symptoms of Ebola in Biena on 1 February 2021. A few days after, she died in a hospital in Butembo. The WHO said that more than 70 people with contact with the woman had been tracked.[232][233]
On 11 February 2021, another woman who had contact with the previous woman died in the same town, and the number of traced contacts increased to 100.[234] A day after, a third case was detected in Butembo.[235]
On 3 May 2021, the 12th EVD outbreak was declared over, resulting in 12 cases and six deaths.[236][237] Heightened surveillance will continue for 90 days after the declaration, in case of resurgence.[236]
Guinea
[edit]In February 2021, Sakoba Keita, head of Guinea's national health agency confirmed that three people had died of Ebola in the south-eastern region near the city of Nzérékoré. A further five people also tested positive. Keita also confirmed more testing was underway, and attempts to trace and isolate further cases had begun.[238] On 14 February, the Guinean government declared an Ebola epidemic.[239] The outbreak may have started following reactivation of a latent case in a survivor of an earlier outbreak.[240][241] As of 4 May 2021, 23 cases were reported, with no new cases or deaths since 3 April 2021.[236] A 42-day countdown period was started on 8 May 2021, and on 19 June, the outbreak was declared over.[236][242]
Ivory Coast
[edit]On 14 August 2021, The Ministry of Health of Cote d’Ivoire confirmed the country's first case of Ebola since 1994. This came after the Institut Pasteur in Cote d'Ivoire confirmed the Ebola Virus Disease in samples collected from a patient, who was hospitalized in the commercial capital of Abidjan, after arriving from Guinea.[243]
However, on 31 August 2021, the WHO found that, after further tests in a laboratory in Lyon, the patient did not have Ebola. The cause of her disease is still being analyzed.[244]
2022
[edit]On 23 April 2022, a case of Ebola was confirmed in the DRC in the Equateur province. The case was a 31-year-old man whose symptoms began on 5 April, but did not seek treatment for over a week. On 21 April, he was admitted to an Ebola treatment centre and died later that day.[245] By 24 May 2022, there were 5 recorded deaths in the DRC.[246] On 15 August, the fifth case was buried, and the outbreak was declared over, 42 days after, on 4 July 2022.[247]
In September 2022, Uganda reported 7 cases infected with the Ebola Sudan strain,[248] but by mid-October the count had increased to 63.[249] In November 2022, the outbreak in Uganda continued — still without a vaccine.[4] On 10 January 2023, the outbreak was considered over after no new cases had been reported for 42 days; the outbreak killed nearly 80 people.[250]
History
[edit]Ebola was first identified in 1976, in two simultaneous outbreaks, one in Nzara (a town in South Sudan) and the other in Yambuku (the Democratic Republic of the Congo), a village near the Ebola River, for which the disease was named.[1] Ebola outbreaks occur intermittently in tropical regions of sub-Saharan Africa.[1] Between 1976 and 2012, according to the World Health Organization, there were 24 outbreaks of Ebola resulting in a total of 2,387 cases, and 1,590 deaths.[1][146] The largest Ebola outbreak to date was an epidemic in West Africa from December 2013 to January 2016, with 28,646 cases and 11,323 deaths.[189][147][148] On 29 March 2016, it was declared to no longer be an emergency.[199] Other outbreaks in Africa began in the Democratic Republic of the Congo in May 2017,[251][252] and 2018.[253][217] In July 2019, the World Health Organization declared the Congo Ebola outbreak a world health emergency.[254]
Society and culture
[edit]Weaponisation
[edit]Ebolavirus is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention.[85][255] It has the potential to be weaponised for use in biological warfare,[256][257] and was investigated by Biopreparat for such use, but might be difficult to prepare as a weapon of mass destruction because the virus becomes ineffective quickly in open air.[258] Fake emails pretending to be Ebola information from the WHO or the Mexican government have, in 2014, been misused to spread computer malware.[259] The BBC reported in 2015 that "North Korean state media has suggested the disease was created by the U.S. military as a biological weapon."[260]
Literature
[edit]Richard Preston's 1995 best-selling book, The Hot Zone, dramatised the Ebola outbreak in Reston, Virginia.[261][262][263]
William Close's 1995 Ebola: A Documentary Novel of Its First Explosion[264][265] and 2002 Ebola: Through the Eyes of the People focused on individuals' reactions to the 1976 Ebola outbreak in Zaire.[266][267]
Tom Clancy's 1996 novel, Executive Orders, involves a Middle Eastern terrorist attack on the United States using an airborne form of a deadly Ebola virus strain named "Ebola Mayinga" (see Mayinga N'Seka).[268][269]
As the Ebola virus epidemic in West Africa developed in 2014, a number of popular self-published and well-reviewed books containing sensational and misleading information about the disease appeared in electronic and printed formats. The authors of some such books admitted that they lacked medical credentials and were not technically qualified to give medical advice. The World Health Organization and the United Nations stated that such misinformation had contributed to the spread of the disease.[270]
Other animals
[edit]Wild animals
[edit]Ebola has a high mortality rate among primates.[271] Frequent outbreaks of Ebola may have resulted in the deaths of 5,000 gorillas.[272] Outbreaks of Ebola may have been responsible for an 88% decline in tracking indices of observed chimpanzee populations in the 420 km2 Lossi Sanctuary between 2002 and 2003.[273] Transmission among chimpanzees through meat consumption constitutes a significant risk factor, whereas contact between the animals, such as touching dead bodies and grooming, is not.[274]
Recovered gorilla carcasses have contained multiple Ebola virus strains, suggesting multiple introductions of the virus. Bodies decompose quickly and carcasses are not infectious after three to four days. Contact between gorilla groups is rare, suggesting that transmission among gorilla groups is unlikely, and that outbreaks result from transmission between viral reservoirs and animal populations.[273]
Domestic animals
[edit]In 2012, it was demonstrated that the virus can travel without contact from pigs to nonhuman primates, although the same study failed to achieve transmission in that manner between primates.[75][275]
Dogs may become infected with EBOV but not develop symptoms. Dogs in some parts of Africa scavenge for food, and they sometimes eat EBOV-infected animals and also the corpses of humans. A 2005 survey of dogs during an EBOV outbreak found that although they remain asymptomatic, about 32 percent of dogs closest to an outbreak showed a seroprevalence for EBOV versus nine percent of those farther away.[276] The authors concluded that there were "potential implications for preventing and controlling human outbreaks."
Reston virus
[edit]In late 1989, Hazelton Research Products' Reston Quarantine Unit in Reston, Virginia, had an outbreak of fatal illness amongst certain lab monkeys. This lab outbreak was initially diagnosed as simian haemorrhagic fever virus (SHFV) and occurred amongst a shipment of crab-eating macaque monkeys imported from the Philippines. Hazelton's veterinary pathologist in Reston sent tissue samples from dead animals to the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) at Fort Detrick, Maryland, where an ELISA test indicated the antibodies present in the tissue were a response to Ebola virus and not SHFV.[277] An electron microscopist from USAMRIID discovered filoviruses similar in appearance, in crystalloid aggregates and as single filaments with a shepherd's hook, to Ebola in the tissue samples sent from Hazelton Research Products' Reston Quarantine Unit.[278]
A US Army team headquartered at USAMRIID euthanised the surviving monkeys, and brought all the dead monkeys to Fort Detrick for study by the Army's veterinary pathologists and virologists, and eventual disposal under safe conditions.[277] Blood samples were taken from 178 animal handlers during the incident.[279] Of those, six animal handlers eventually seroconverted, including one who had cut himself with a bloody scalpel.[86][280] Despite its status as a Level‑4 organism and its apparent pathogenicity in monkeys, when the handlers did not become ill, the CDC concluded that the virus had a very low pathogenicity to humans.[280][281]
The Philippines and the United States had no previous cases of Ebola infection, and upon further isolation, researchers concluded it was another strain of Ebola, or a new filovirus of Asian origin, which they named Reston ebolavirus (RESTV) after the location of the incident.[277] Reston virus (RESTV) can be transmitted to pigs.[75] Since the initial outbreak it has since been found in nonhuman primates in Pennsylvania, Texas, and Italy,[282] where the virus had infected pigs.[283] According to the WHO, routine cleaning and disinfection of pig (or monkey) farms with sodium hypochlorite or detergents should be effective in inactivating the Reston ebolavirus. Pigs that have been infected with RESTV tend to show symptoms of the disease.[284]
Research
[edit]Treatments
[edit]
As of July 2015[update], no medication has been proven safe and effective for treating Ebola. By the time the Ebola virus epidemic in West Africa began in 2013, there were at least nine different candidate treatments. Several trials were conducted in late 2014, and early 2015, but some were abandoned due to lack of efficacy or lack of people to study.[285]
As of August 2019[update], two experimental treatments known as atoltivimab/maftivimab/odesivimab and ansuvimab were found to be 90% effective.[286][287][288]
Diagnostic tests
[edit]The diagnostic tests currently available require specialised equipment and highly trained personnel. Since there are few suitable testing centres in West Africa, this leads to delay in diagnosis.[289]
On 29 November 2014, a new 15-minute Ebola test was reported that if successful, "not only gives patients a better chance of survival, but it prevents transmission of the virus to other people." The new equipment, about the size of a laptop and solar-powered, allows testing to be done in remote areas.[290]
On 29 December 2014, the U.S. Food and Drug Administration (FDA) approved the LightMix Ebola Zaire rRT-PCR test for patients with symptoms of Ebola.[291]
Disease models
[edit]Animal models and in particular non-human primates are being used to study different aspects of Ebola virus disease. Developments in organ-on-a-chip technology have led to a chip-based model for Ebola haemorrhagic syndrome.[292]
See also
[edit]Notes
[edit]- ^ The mortality (number of dead per number of healthy per time frame) recorded in Liberia up to 26 August 2014 was 70%.[187] However, due to the estimation method used, the estimated case fatality rate (70.8%) for this particular epidemic differs from the actual ratio between the number of deaths and the number of cases.
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar "Ebola virus disease, Fact sheet N°103, Updated September 2014". World Health Organization (WHO). September 2014. Archived from the original on 14 December 2014. Retrieved 15 December 2014.
- ^ a b c d Singh SK, Ruzek D, eds. (2014). Viral hemorrhagic fevers. Boca Raton: CRC Press, Taylor & Francis Group. p. 444. ISBN 978-1439884294. Archived from the original on 29 April 2016.
- ^ Modrow S, Falke D, Truyen U, Schätzl H (2013). "Viruses: Definition, Structure, Classification". In Modrow S, Falke D, Truyen U, Schätzl H (eds.). Molecular Virology. Berlin, Heidelberg: Springer. pp. 17–30. doi:10.1007/978-3-642-20718-1_2. ISBN 978-3-642-20718-1. S2CID 83235976.
- ^ a b c d e Ebola in Uganda: Guiliani R. "Our game-changing treatment centres will save more lives". msf.org.urk. Archived from the original on 15 November 2022. Retrieved 15 November 2022.
- ^ a b "2014 Ebola Virus Disease (EVD) outbreak in West Africa". World Health Organization (WHO). 21 April 2014. Archived from the original on 29 July 2014. Retrieved 3 August 2014.
- ^ a b c d "Preliminary study finds that Ebola virus fragments can persist in the semen of some survivors for at least nine months". Centers for Disease Control and Prevention (CDC). 14 October 2015. Archived from the original on 24 August 2017.
- ^ a b "Recommendations for Breastfeeding/Infant Feeding in the Context of Ebola". Centers for Disease Control and Prevention (CDC). 19 September 2014. Archived from the original on 24 October 2014. Retrieved 26 October 2014.
- ^ a b c Broadhurst MJ, Brooks TJ, Pollock NR (13 July 2016). "Diagnosis of Ebola Virus Disease: Past, Present, and Future". Clinical Microbiology Reviews. 29 (4). American Society for Microbiology: 773–793. doi:10.1128/cmr.00003-16. ISSN 0893-8512. LCCN 88647279. OCLC 38839512. PMC 5010747. PMID 27413095.
- ^ "Guidance for Safe Handling of Human Remains of Ebola Patients in U.S. Hospitals and Mortuaries". Centers for Disease Control and Prevention (CDC). Archived from the original on 9 October 2014. Retrieved 10 October 2014.
- ^ a b "Ebola Treatment Research". NIH: National Institute of Allergy and Infectious Diseases. Archived from the original on 20 August 2019. Retrieved 20 August 2019.
- ^ a b "Independent Monitoring Board Recommends Early Termination of Ebola Therapeutics Trial in DRC Because of Favorable Results with Two of Four Candidates". NIH: National Institute of Allergy and Infectious Diseases. 12 August 2019. Archived from the original on 19 August 2019. Retrieved 20 August 2019.
- ^ a b "FDA Approves First Treatment for Ebola Virus". U.S. Food and Drug Administration (FDA) (Press release). 14 October 2020. Archived from the original on 15 October 2020. Retrieved 14 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Ebola Hemorrhagic Fever Signs and Symptoms". Centers for Disease Control and Prevention (CDC). 28 January 2014. Archived from the original on 1 August 2014. Retrieved 2 August 2014.
- ^ a b c d e f g h Goeijenbier M, van Kampen JJ, Reusken CB, Koopmans MP, van Gorp EC (November 2014). "Ebola virus disease: a review on epidemiology, symptoms, treatment and pathogenesis". Neth J Med. 72 (9): 442–448. PMID 25387613. Archived from the original on 29 November 2014.
- ^ Haas CN (14 October 2014). "On the Quarantine Period for Ebola Virus". PLOS Currents Outbreaks. 6. doi:10.1371/currents.outbreaks.2ab4b76ba7263ff0f084766e43abbd89. ISSN 2157-3999. PMC 4205154. PMID 25642371.
- ^ a b c Gatherer D (August 2014). "The 2014 Ebola virus disease outbreak in West Africa". J Gen Virol. 95 (Pt 8): 1619–1624. doi:10.1099/vir.0.067199-0. PMID 24795448.
- ^ a b c d e f Magill A (2013). Hunter's tropical medicine and emerging infectious diseases (9th ed.). New York: Saunders. p. 332. ISBN 978-1416043904. Archived from the original on 20 March 2016.
- ^ a b c Hoenen T, Groseth A, Falzarano D, Feldmann H (May 2006). "Ebola virus: unravelling pathogenesis to combat a deadly disease". Trends in Molecular Medicine. 12 (5): 206–215. doi:10.1016/j.molmed.2006.03.006. PMID 16616875.
- ^ Brown CS, Mepham S, Shorten RJ (June 2017). "Ebola Virus Disease: An Update on Epidemiology, Symptoms, Laboratory Findings, Diagnostic Issues, and Infection Prevention and Control Issues for Laboratory Professionals". Clinical Laboratory Medicine (Review). 37 (2): 269–284. doi:10.1016/j.cll.2017.01.003. PMID 28457350.
- ^ a b c Sharma N, Cappell MS (September 2015). "Gastrointestinal and Hepatic Manifestations of Ebola Virus Infection". Digestive Diseases and Sciences (Review). 60 (9): 2590–2603. doi:10.1007/s10620-015-3691-z. PMID 25972150. S2CID 5674317.
- ^ Simpson DI (1977). Marburg and Ebola virus infections: a guide for their diagnosis, management, and control. World Health Organization. p. 10f. hdl:10665/37138. ISBN 924170036X. WHO offset publication; no. 36.
- ^ King JW, Rafeek H (14 January 2021). Chandrasekar PH (ed.). "Ebola Virus, Clinical Presentation". Medscape. Archived from the original on 1 January 2012. Retrieved 30 July 2012.
- ^ "Appendix A: Disease-Specific Chapters – Chapter: Hemorrhagic fevers caused by: i) Ebola virus and ii) Marburg virus and iii) Other viral causes including bunyaviruses, arenaviruses, and flaviviruses" (PDF). Ministry of Health and Long-Term Care. Archived (PDF) from the original on 15 October 2014. Retrieved 9 October 2014.
- ^ a b c d e f g Feldmann H, Geisbert TW (March 2011). "Ebola haemorrhagic fever". Lancet. 377 (9768): 849–862. doi:10.1016/S0140-6736(10)60667-8. PMC 3406178. PMID 21084112.
- ^ Shantha JG, Yeh S, Nguyen QD (November 2016). "Ebola virus disease and the eye". Current Opinion in Ophthalmology (Review). 27 (6): 538–544. doi:10.1097/ICU.0000000000000313. PMID 27585217. S2CID 34367099.
- ^ West TE, von Saint André-von Arnim A (November 2014). "Clinical presentation and management of severe Ebola virus disease". Annals of the American Thoracic Society (Review). 11 (9): 1341–1350. doi:10.1513/AnnalsATS.201410-481PS. PMID 25369317.
- ^ Sharma N, Cappell MS (September 2015). "Gastrointestinal and Hepatic Manifestations of Ebola Virus Infection". Digestive Diseases and Sciences. 60 (9): 2590–2603. doi:10.1007/s10620-015-3691-z. PMID 25972150. S2CID 5674317.
- ^ "Ebola virus disease Information for Clinicians in U.S. Healthcare Settings | For Clinicians | Ebola (Ebola Virus Disease) | Ebola Hemorrhagic Fever | CDC". www.cdc.gov. 6 January 2020. Archived from the original on 1 April 2020. Retrieved 22 March 2020.
- ^ a b Tosh PK, Sampathkumar P (December 2014). "What Clinicians Should Know About the 2014 Ebola Outbreak". Mayo Clin Proc. 89 (12): 1710–1717. doi:10.1016/j.mayocp.2014.10.010. PMID 25467644.
- ^ "An emergency within an emergency: caring for Ebola survivors". World Health Organization (WHO). 7 August 2015. Archived from the original on 13 August 2015. Retrieved 12 August 2015.
- ^ "Ebola Virus Disease". SRHD. Archived from the original on 15 September 2020. Retrieved 15 September 2020.
- ^ a b c d "Q&A on Transmission, Ebola". Centers for Disease Control and Prevention (CDC). September 2014. Archived from the original on 2 October 2014. Retrieved 3 October 2014.
- ^ a b c Hoenen T, Groseth A, Feldmann H (July 2012). "Current Ebola vaccines". Expert Opin Biol Ther. 12 (7): 859–872. doi:10.1517/14712598.2012.685152. PMC 3422127. PMID 22559078.
- ^ Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, et al. (December 2010). "Proposal for a revised taxonomy of the family Filoviridae: Classification, names of taxa and viruses, and virus abbreviations". Archives of Virology. 155 (12): 2083–2103. doi:10.1007/s00705-010-0814-x. PMC 3074192. PMID 21046175.
- ^ Branswell H (27 July 2018). "New Ebola species is reported for first time in a decade". Stat. Archived from the original on 27 July 2018. Retrieved 8 July 2022.
- ^ Spickler A. "Ebolavirus and Marburgvirus Infections" (PDF). Archived (PDF) from the original on 30 April 2015.
- ^ "About Ebola Virus Disease". Centers for Disease Control and Prevention (CDC). Archived from the original on 16 October 2014. Retrieved 18 October 2014.
- ^ Pringle CR (2005). "Order Mononegavirales". In Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds.). Virus Taxonomy – Eighth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier/Academic Press. pp. 609–614. ISBN 978-0-12-370200-5.
- ^ a b Stahelin RV (June 2014). "Membrane binding and bending in Ebola VP40 assembly and egress". Front Microbiol. 5: 300. doi:10.3389/fmicb.2014.00300. PMC 4061899. PMID 24995005.
- ^ Ascenzi P, Bocedi A, Heptonstall J, Capobianchi MR, Di Caro A, Mastrangelo E, et al. (June 2008). "Ebolavirus and Marburgvirus: insight the Filoviridae family" (PDF). Mol Aspects Med. 29 (3): 151–185. doi:10.1016/j.mam.2007.09.005. hdl:2434/53604. PMID 18063023. Archived (PDF) from the original on 8 March 2021. Retrieved 20 August 2019.
- ^ a b c d Chippaux JP (October 2014). "Outbreaks of Ebola virus disease in Africa: the beginnings of a tragic saga". J Venom Anim Toxins Incl Trop Dis. 20 (1): 44. doi:10.1186/1678-9199-20-44. PMC 4197285. PMID 25320574.
- ^ a b c d Misasi J, Sullivan NJ (October 2014). "Camouflage and Misdirection: The Full-On Assault of Ebola Virus Disease". Cell. 159 (3): 477–486. doi:10.1016/j.cell.2014.10.006. PMC 4243531. PMID 25417101.
- ^ a b c d e f g h Kühl A, Pöhlmann S (September 2012). "How Ebola virus counters the interferon system". Zoonoses Public Health. 59 (Supplement 2): 116–131. doi:10.1111/j.1863-2378.2012.01454.x. PMC 7165950. PMID 22958256.
- ^ a b Olejnik J, Ryabchikova E, Corley RB, Mühlberger E (August 2011). "Intracellular events and cell fate in filovirus infection". Viruses. 3 (8): 1501–1531. doi:10.3390/v3081501. PMC 3172725. PMID 21927676.
- ^ Feldmann H, Geisbert TW, Jahrling PB, Klenk H, Netesov SV, Peters CJ, Sanchez A, Swanepoel R, Volchkov VE (2005). Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds.). Virus Taxonomy – Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press. ISBN 978-0123702005.
- ^ a b c d e f g h Funk DJ, Kumar A (November 2014). "Ebola virus disease: an update for anesthesiologists and intensivists". Can J Anaesth. 62 (1): 80–91. doi:10.1007/s12630-014-0257-z. PMC 4286619. PMID 25373801.
- ^ "Ebola (Ebola Virus Disease) Transmission". Centers for Disease Control and Prevention (CDC). 5 November 2014. Archived from the original on 6 November 2014. Retrieved 7 November 2014.
- ^ Drazen JM, Kanapathipillai R, Campion EW, Rubin EJ, Hammer SM, Morrissey S, Baden LR (November 2014). "Ebola and quarantine". N Engl J Med. 371 (21): 2029–2030. doi:10.1056/NEJMe1413139. PMID 25347231.
- ^ McNeil Jr DG (3 October 2014). "Ask Well: How Does Ebola Spread? How Long Can the Virus Survive?". The New York Times. Archived from the original on 24 October 2014. Retrieved 24 October 2014.
- ^ a b "How Ebola Is Spread" (PDF). Centers for Disease Control and Prevention (CDC). 1 November 2014. Archived (PDF) from the original on 8 July 2017.
- ^ a b c d e f g "Transmission". Centers for Disease Control and Prevention (CDC). 17 October 2014. Archived from the original on 19 October 2014. Retrieved 18 October 2014.
- ^ a b c d Chowell G, Nishiura H (October 2014). "Transmission dynamics and control of Ebola virus disease (EVD): a review". BMC Med. 12 (1): 196. doi:10.1186/s12916-014-0196-0. PMC 4207625. PMID 25300956.
- ^ a b Osterholm MT, Moore KA, Kelley NS, Brosseau LM, Wong G, Murphy FA, et al. (19 February 2015). "Transmission of Ebola viruses: what we know and what we do not know". mBio. 6 (2) e00137. doi:10.1128/mBio.00137-15. PMC 4358015. PMID 25698835.
- ^ "Sexual transmission of the Ebola Virus : evidence and knowledge gaps". World Health Organization (WHO). 4 April 2015. Archived from the original on 14 April 2015. Retrieved 16 April 2015.
- ^ Wu B (2 May 2015). "Ebola Can Be Transmitted Through Sex". Science Times. Archived from the original on 2 May 2015. Retrieved 3 May 2015.
- ^ Soka MJ, Choi MJ, Baller A, White S, Rogers E, Purpura LJ, et al. (2016). "Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data". Lancet Global Health. 4 (10): e736 – e743. doi:10.1016/S2214-109X(16)30175-9. PMID 27596037.
- ^ Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. (7 May 2015). "Persistence of Ebola Virus in Ocular Fluid during Convalescence". The New England Journal of Medicine. 372 (25): 2423–2427. doi:10.1056/NEJMoa1500306. hdl:2328/35704. PMC 4547451. PMID 25950269.
- ^ a b "CDC Telebriefing on Ebola outbreak in West Africa". Centers for Disease Control and Prevention (CDC). 28 July 2014. Archived from the original on 2 August 2014. Retrieved 3 August 2014.
- ^ a b "Air travel is low-risk for Ebola transmission". World Health Organization (WHO). 14 August 2014. Archived from the original on 19 January 2015.
- ^ Chan M (September 2014). "Ebola virus disease in West Africa – no early end to the outbreak". N Engl J Med. 371 (13): 1183–1185. doi:10.1056/NEJMp1409859. PMID 25140856.
- ^ "Sierra Leone: a traditional healer and a funeral". World Health Organization (WHO). Archived from the original on 6 October 2014. Retrieved 6 October 2014.
- ^ Salaam-Blyther T (26 August 2014). "The 2014 Ebola Outbreak: International and U.S. Responses" (PDF). Archived (PDF) from the original on 3 September 2014. Retrieved 9 September 2014.
- ^ Lashley FR, Durham JD, eds. (2007). Emerging infectious diseases trends and issues (2nd ed.). New York: Springer. p. 141. ISBN 978-0826103505. Archived from the original on 9 May 2016.
- ^ Magill AJ, Strickland GT, Maguire JH, Ryan ET, Solomon T, eds. (2013). Hunter's tropical medicine and emerging infectious disease (9th ed.). London; New York: Elsevier. pp. 170–172. ISBN 978-1455740437. OCLC 822525408. Archived from the original on 20 May 2016.
- ^ "Questions and Answers on Ebola Hemorrhagic Fever". Centers for Disease Control and Prevention (CDC). 22 May 2018. Archived from the original on 28 July 2017.
- ^ a b "Ebola in Texas: Second Health Care Worker Tests Positive". NBC News. 15 October 2014. Archived from the original on 15 October 2014.
- ^ Jones RM, Brosseau LM (May 2015). "Aerosol transmission of infectious disease". Journal of Occupational and Environmental Medicine (Review). 57 (5): 501–508. doi:10.1097/JOM.0000000000000448. PMID 25816216. S2CID 11166016.
- ^ a b "Transmission of Ebola virus". virology.ws. 27 September 2014. Archived from the original on 29 January 2016. Retrieved 22 January 2016.
- ^ a b c Weingartl HM, Embury-Hyatt C, Nfon C, Leung A, Smith G, Kobinger G (2012). "Transmission of Ebola virus from pigs to non-human primates". Scientific Reports. 2: 811. Bibcode:2012NatSR...2..811W. doi:10.1038/srep00811. PMC 3498927. PMID 23155478.
- ^ "Risk of Exposure". Centers for Disease Control and Prevention (CDC). 12 October 2014. Archived from the original on 16 October 2014. Retrieved 18 October 2014.
- ^ "FAO warns of fruit bat risk in West African Ebola epidemic". fao.org. 21 July 2014. Archived from the original on 13 October 2014. Retrieved 22 October 2014.
- ^ Williams E. "African monkey meat that could be behind the next HIV". The Independent. Archived from the original on 22 June 2017.
25 people in Bakaklion, Cameroon killed due to eating of ape
- ^ a b "Ebolavirus – Pathogen Safety Data Sheets". Public Health Agency of Canada. 17 September 2001. Archived from the original on 20 August 2014. Retrieved 22 August 2014.
- ^ a b Gonzalez JP, Pourrut X, Leroy E (2007). "Ebolavirus and Other Filoviruses". Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission. Current Topics in Microbiology and Immunology. Vol. 315. pp. 363–387. doi:10.1007/978-3-540-70962-6_15. ISBN 978-3540709619. PMC 7121322. PMID 17848072.
- ^ a b c d e f Weingartl HM, Nfon C, Kobinger G (May 2013). "Review of Ebola virus infections in domestic animals". Dev Biol (Basel). Developments in Biologicals. 135: 211–218. doi:10.1159/000178495. ISBN 978-3318023657. PMID 23689899.
- ^ Laupland KB, Valiquette L (May 2014). "Ebola virus disease". Can J Infect Dis Med Microbiol. 25 (3): 128–129. doi:10.1155/2014/527378. PMC 4173971. PMID 25285105.
- ^ a b c Pourrut X, Kumulungui B, Wittmann T, Moussavou G, Délicat A, Yaba P, et al. (June 2005). "The natural history of Ebola virus in Africa". Microbes Infect. 7 (7–8): 1005–1014. doi:10.1016/j.micinf.2005.04.006. PMID 16002313.
- ^ Swanepoel R, Leman PA, Burt FJ, Zachariades NA, Braack LE, Ksiazek TG, et al. (October 1996). "Experimental inoculation of plants and animals with Ebola virus". Emerg. Infect. Dis. 2 (4): 321–325. doi:10.3201/eid0204.960407. ISSN 1080-6040. PMC 2639914. PMID 8969248.
- ^ Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, et al. (December 2005). "Fruit bats as reservoirs of Ebola virus". Nature. 438 (7068): 575–576. Bibcode:2005Natur.438..575L. doi:10.1038/438575a. PMID 16319873. S2CID 4403209.
- ^ Olival KJ, Islam A, Yu M, Anthony SJ, Epstein JH, Khan SA, et al. (February 2013). "Ebola virus antibodies in fruit bats, Bangladesh". Emerg. Infect. Dis. 19 (2): 270–273. doi:10.3201/eid1902.120524. PMC 3559038. PMID 23343532.
- ^ Morvan JM, Deubel V, Gounon P, Nakouné E, Barrière P, Murri S, et al. (December 1999). "Identification of Ebola virus sequences present as RNA or DNA in organs of terrestrial small mammals of the Central African Republic". Microbes and Infection. 1 (14): 1193–1201. doi:10.1016/S1286-4579(99)00242-7. PMID 10580275.
- ^ Groseth A, Feldmann H, Strong JE (September 2007). "The ecology of Ebola virus". Trends Microbiol. 15 (9): 408–416. doi:10.1016/j.tim.2007.08.001. PMID 17698361.
- ^ "Did deforestation cause the Ebola outbreak?". New Internationalist. 10 April 2018. Archived from the original on 26 June 2018. Retrieved 10 July 2018.
- ^ Olivero J, Fa JE, Real R, Márquez AL, Farfán MA, Vargas JM, et al. (30 October 2017). "Recent loss of closed forests is associated with Ebola virus disease outbreaks". Scientific Reports. 7 (1): 14291. Bibcode:2017NatSR...714291O. doi:10.1038/s41598-017-14727-9. ISSN 2045-2322. PMC 5662765. PMID 29085050.
- ^ a b Ansari AA (September 2014). "Clinical features and pathobiology of Ebolavirus infection". J Autoimmun. 55: 1–9. doi:10.1016/j.jaut.2014.09.001. PMID 25260583.
- ^ a b Smith T (2005). Ebola (Deadly Diseases and Epidemics). Chelsea House Publications. ISBN 978-0791085059.
- ^ Tang H, Abouleila Y, Saris A, Shimizu Y, Ottenhoff TH, Mashaghi A (May 2023). "Ebola virus-like particles reprogram cellular metabolism". Journal of Molecular Medicine. 101 (5): 557–568. doi:10.1007/s00109-023-02309-4. PMC 10036248. PMID 36959259.
- ^ a b Ramanan P, Shabman RS, Brown CS, Amarasinghe GK, Basler CF, Leung DW (September 2011). "Filoviral immune evasion mechanisms". Viruses. 3 (9): 1634–1649. doi:10.3390/v3091634. PMC 3187693. PMID 21994800.
- ^ "Screening Patients | For Clinicians | Ebola Virus Disease | CDC". www.cdc.gov. 5 May 2021. Archived from the original on 20 September 2022. Retrieved 20 September 2022.
- ^ Kortepeter MG, Bausch DG, Bray M (November 2011). "Basic clinical and laboratory features of filoviral hemorrhagic fever". J Infect Dis. 204 (Supplement 3): S810–16. doi:10.1093/infdis/jir299. PMID 21987756.
- ^ Goldsmith CS, Miller SE (October 2009). "Modern uses of electron microscopy for detection of viruses". Clinical Microbiology Reviews (Review). 22 (4): 552–563. doi:10.1128/CMR.00027-09. PMC 2772359. PMID 19822888.
- ^ a b c "Ebola Hemorrhagic Fever Diagnosis". Centers for Disease Control and Prevention (CDC). 28 January 2014. Archived from the original on 24 September 2014. Retrieved 3 August 2014.
- ^ a b Grolla A, Lucht A, Dick D, Strong JE, Feldmann H (September 2005). "Laboratory diagnosis of Ebola and Marburg hemorrhagic fever". Bull Soc Pathol Exot. 98 (3): 205–209. PMID 16267962.
- ^ "Liberia: New Ebola mobile lab speeds up diagnosis and improves care". World Health Organization (WHO). October 2014. Archived from the original on 24 October 2014. Retrieved 26 October 2014.
- ^ a b "First Antigen Rapid Test for Ebola through Emergency Assessment and Eligible for Procurement". World Health Organization (WHO). Archived from the original on 20 February 2015. Retrieved 20 February 2015.
- ^ Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J, eds. (2011). "Chapter 197". Harrison's Principles of Internal Medicine (18th ed.). McGraw-Hill. ISBN 978-0071748896.
- ^ Beeching NJ, Fenech M, Houlihan CF (December 2014). "Ebola Virus Disease". BMJ (Review). 10 (349) g7438. doi:10.1136/bmj.g7348. PMC 4707720. PMID 25497512.
- ^ "Viral Hemorrhagic Fever". S.F. Dept Public Health – Infectious Disease Emergencies. July 2008. Archived from the original (PDF) on 20 April 2013. Retrieved 17 August 2014.
- ^ Gear JH (May–June 1989). "Clinical aspects of African viral hemorrhagic fevers". Rev Infect Dis. 11 (Supplement 4): S777–82. doi:10.1093/clinids/11.supplement_4.s777. PMID 2665013.
- ^ Gear JH, Ryan J, Rossouw E (February 1978). "A consideration of the diagnosis of dangerous infectious fevers in South Africa". South African Medical Journal. 53 (7): 235–237. PMID 565951.
- ^ Bogomolov BP (1998). "Differential diagnosis of infectious diseases with hemorrhagic syndrome". Terapevticheskii Arkhiv. 70 (4): 63–68. PMID 9612907.
- ^ a b c "First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response" (Press release). U.S. Food and Drug Administration (FDA). 20 December 2019. Archived from the original on 20 December 2019. Retrieved 22 December 2019.
- ^ Mahamba, Fiston; Nebehay, Stephanie (3 May 2019). "Congo Ebola deaths surpass 1,000 as attacks on treatment centers go on". Reuters. Archived from the original on 8 May 2019. Retrieved 8 May 2019.
- ^ "Ebola virus disease: Vaccines". www.who.int. Archived from the original on 29 March 2023. Retrieved 29 March 2023.
- ^ a b c "Ebola Hemorrhagic Fever Prevention". Centers for Disease Control and Prevention (CDC). 31 July 2014. Archived from the original on 4 September 2014. Retrieved 2 August 2014.
- ^ a b "Guidance on Personal Protective Equipment To Be Used by Healthcare Workers During Management of Patients with Ebola Virus Disease in U.S. Hospitals, Including Procedures for Putting On (Donning) and Removing (Doffing)". Centers for Disease Control and Prevention (CDC). 20 October 2014. Archived from the original on 22 October 2014. Retrieved 22 October 2014.
- ^ a b Peters CJ (December 1998). Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (PDF). Centers for Disease Control and Prevention (CDC). Archived (PDF) from the original on 8 July 2017.
- ^ Govan F (11 October 2014). "Ebola medics 'better trained in Sierra Leone than Spain'". The Telegraph. Archived from the original on 28 June 2020.
- ^ Sifferlin A (9 October 2014). "This Is How Ebola Patients Are Equipping Their Homes". Time Magazine. Archived from the original on 5 March 2015.
- ^ Sheri F, Nossiter A, Kanter J (10 October 2014). "Doctors Without Borders Evolves as It Forms the Vanguard in Ebola Fight". The New York Times. Archived from the original on 14 October 2014. Retrieved 12 October 2014.
- ^ Organization, World Health (August 2014). "Interim infection prevention and control guidance for care of patients with suspected or confirmed filovirus haemorrhagic fever in health-care settings, with focus on Ebola". WHO Technical Documents. World Health Organization (WHO). hdl:10665/130596. WHO/HIS/SDS/2014.4 Rev. 1.
- ^ "Ebola – 5 tips to avoid the deadly disease". Plan International. 6 September 2014. Archived from the original on 24 March 2015.
- ^ Rouquet P, Froment JM, Bermejo M, Kilbourn A, Karesh W, Reed P, et al. (February 2005). "Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003". Emerg. Infect. Dis. 11 (2): 283–290. doi:10.3201/eid1102.040533. ISSN 1080-6040. PMC 3320460. PMID 15752448.
- ^ Centers for Disease Control and Prevention and World Health Organization (1998). Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (PDF). Centers for Disease Control and Prevention (CDC). Archived (PDF) from the original on 11 April 2014. Retrieved 8 February 2013.
- ^ "Section 7: Use Safe Burial Practices" (PDF). Information resources on Ebola virus disease. World Health Organization (WHO). 1 June 2014. Archived (PDF) from the original on 26 February 2015.
- ^ Harden B (24 December 2000). "Ebola's Shadow". The New York Times. Archived from the original on 20 October 2014. Retrieved 12 October 2014.
- ^ Fassassi A (September 2014). "How anthropologists help medics fight Ebola in Guinea". SciDev.Net. Archived from the original on 6 October 2014. Retrieved 3 October 2014.
- ^ "West Africa – Ebola virus disease Update: Travel and transport". International travel and health. World Health Organization (WHO). Archived from the original on 29 July 2014.
- ^ a b c "Monitoring Symptoms and Controlling Movement to Stop Spread of Ebola". Centers for Disease Control and Prevention (CDC). 27 October 2014. Archived from the original on 8 July 2017.
- ^ a b "Ebola: Control and Prevention". Occupational Safety and Health Administration (OSHA). Archived from the original on 9 November 2014. Retrieved 9 November 2014.
- ^ "About Quarantine and Isolation". Centers for Disease Control and Prevention (CDC). 28 August 2014. Archived from the original on 20 October 2014. Retrieved 26 October 2014.
- ^ Sompayrac L (2002). How pathogenic viruses work (3rd ed.). Boston: Jones and Bartlett Publishers. p. 87. ISBN 978-0763720827.
- ^ Alazard-Dany N, Ottmann Terrangle M, Volchkov V (April 2006). "[Ebola and Marburg viruses: the humans strike back]" (PDF). Med Sci (Paris) (in French). 22 (4): 405–410. doi:10.1051/medsci/2006224405. PMID 16597410. Archived (PDF) from the original on 22 February 2020. Retrieved 20 August 2019.
- ^ "Ebola virus disease update – west Africa". World Health Organization (WHO). 19 August 2014. Archived from the original on 22 October 2014. Retrieved 26 October 2014.
- ^ Koenig K, Schultz C, eds. (2009). Koenig and Schultz's disaster medicine : comprehensive principles and practices. Cambridge: Cambridge University Press. p. 209. ISBN 978-0521873673. Archived from the original on 15 May 2016.
- ^ "Control of Communicable Diseases". Federal Register. 19 January 2017. Archived from the original on 6 August 2019. Retrieved 13 August 2019.
- ^ Frieden TR, Damon I, Bell BP, Kenyon T, Nichol S (25 September 2014). "Ebola 2014 – New Challenges, New Global Response and Responsibility". New England Journal of Medicine. 371 (13): 1177–1180. doi:10.1056/NEJMp1409903. PMID 25140858.
- ^ "What is Contact Tracing?" (PDF). Centers for Disease Control and Prevention (CDC). Archived (PDF) from the original on 12 September 2014. Retrieved 15 September 2014.
- ^ "FDA warns consumers about fraudulent Ebola treatment products" (Press release). Archived from the original on 19 August 2014. Retrieved 20 August 2014.
- ^ "Natural Solutions Foundation Warning Letter". U.S. Food and Drug Administration (FDA). 22 September 2014. Archived from the original on 8 October 2014. Retrieved 9 October 2014.
- ^ a b c d e f Clark DV, Jahrling PB, Lawler JV (September 2012). "Clinical management of filovirus-infected patients". Viruses. 4 (9): 1668–1686. doi:10.3390/v4091668. PMC 3499825. PMID 23170178.
- ^ "Ebola messages for the general public". World Health Organization (WHO). Archived from the original on 25 October 2014. Retrieved 26 October 2014.
- ^ a b "Ebola virus disease". www.who.int. Archived from the original on 16 February 2021. Retrieved 28 May 2020.
- ^ "Annex 18. Transmission risk reduction of filoviruses in home-care settings" (PDF). World Health Organization (WHO). Archived (PDF) from the original on 26 October 2014. Retrieved 26 October 2014.
- ^ Fauquet CM (2005). Virus taxonomy classification and nomenclature of viruses; 8th report of the International Committee on Taxonomy of Viruses. Oxford: Elsevier/Academic Press. p. 648. ISBN 978-0080575483. Archived from the original on 4 June 2016.
- ^ "More or Less behind the stats Ebola". BBC World Service. Archived from the original on 3 September 2014. Retrieved 8 October 2014.
- ^ a b c Shantha JG, Crozier I, Yeh S (November 2017). "An update on ocular complications of Ebola virus disease". Current Opinion in Ophthalmology. 28 (6): 600–606. doi:10.1097/ICU.0000000000000426. PMC 5988239. PMID 28872492.
- ^ Rojas M, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Ansari AA, Gershwin ME, Anaya JM (January 2020). "Ebola virus disease: An emerging and re-emerging viral threat". Journal of Autoimmunity. 106 102375. doi:10.1016/j.jaut.2019.102375. PMID 31806422.
- ^ Wiwanitkit S, Wiwanitkit V (2015). "Ocular problem in Ebola virus infection: A short review". Saudi Journal of Ophthalmology. 29 (3): 225–226. doi:10.1016/j.sjopt.2015.02.006. PMC 4487942. PMID 26155084.
- ^ Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. (2015). "Persistence of Ebola Virus in Ocular Fluid during Convalescence". New England Journal of Medicine. 372 (25): 2423–2427. doi:10.1056/NEJMoa1500306. hdl:2328/35704. ISSN 0028-4793. PMC 4547451. PMID 25950269.
- ^ Mackay IM, Arden KE (2015). "Ebola virus in the semen of convalescent men". The Lancet Infectious Diseases. 15 (2): 149–150. doi:10.1016/S1473-3099(14)71033-3. ISSN 1473-3099. PMID 25467652.
- ^ Rogstad KE, Tunbridge A (February 2015). "Ebola virus as a sexually transmitted infection". Current Opinion in Infectious Diseases. 28 (1): 83–85. doi:10.1097/qco.0000000000000135. PMID 25501666. S2CID 7538665.
- ^ Christie A, Davies-Wayne GJ, Cordier-Lassalle T, Blackley DJ, Laney AS, Williams DE, et al. (8 May 2015). "Possible sexual transmission of Ebola virus – Liberia, 2015". MMWR. Morbidity and Mortality Weekly Report. 64 (17): 479–481. PMC 4584553. PMID 25950255.
- ^ Sprecher, A (14 October 2015). "Handle Survivors with Care". The New England Journal of Medicine. 377 (15): 1480–1482. doi:10.1056/NEJMe1512928. hdl:10144/619038. PMID 26465064.
- ^ "Neuro complications cited in UK nurse's Ebola case". 15 October 2015. Archived from the original on 17 October 2015. Retrieved 19 October 2015.
- ^ a b Dixon MG, Schafer IJ (June 2014). "Ebola viral disease outbreak – West Africa, 2014" (PDF). MMWR Morb. Mortal. Wkly. Rep. 63 (25): 548–551. PMC 5779383. PMID 24964881. Archived (PDF) from the original on 23 October 2020. Retrieved 7 April 2020.
- ^ a b "CDC urges all US residents to avoid nonessential travel to Liberia, Guinea and Sierra Leone because of an unprecedented outbreak of Ebola". Centers for Disease Control and Prevention (CDC). 31 July 2014. Archived from the original on 9 August 2014. Retrieved 2 August 2014.
- ^ a b "2014 Ebola Outbreak in West Africa". Centers for Disease Control and Prevention (CDC). 4 August 2014. Archived from the original on 2 October 2014. Retrieved 5 August 2014.
- ^ Peterson AT, Bauer JT, Mills JN (2004). "Ecologic and Geographic Distribution of Filovirus Disease". Emerg. Infect. Dis. 10 (1): 40–47. doi:10.3201/eid1001.030125. PMC 3322747. PMID 15078595.
- ^ a b "Ebola haemorrhagic fever in Sudan, 1976" (PDF). Archived from the original (PDF) on 13 October 2014. Retrieved 8 October 2014.
- ^ Hewlett B, Hewlett B (2007). Ebola, Culture and Politics: The Anthropology of an Emerging Disease. Cengage Learning. p. 103. ISBN 978-1111797317. Archived from the original on 21 May 2024. Retrieved 31 July 2014.
- ^ a b Feldmann H, Jones S, Klenk HD, Schnittler HJ (August 2003). "Ebola virus: from discovery to vaccine". Nature Reviews Immunology. 3 (8): 677–685. doi:10.1038/nri1154. PMID 12974482. S2CID 27486878.
- ^ a b Report of an International Commission (1978). "Ebola haemorrhagic fever in Zaire, 1976" (PDF). Bull. World Health Organ. 56 (2): 271–293. PMC 2395567. PMID 307456. Archived from the original (PDF) on 8 August 2014. Retrieved 14 August 2014.
- ^ Centers for Disease Control Prevention (CDC) (1995). "Outbreak of Ebola Viral Hemorrhagic Fever – Zaire, 1995". Morbidity and Mortality Weekly Report. 44 (19): 381–382. PMID 7739512. Archived from the original on 25 June 2017.
- ^ Elezra M. "Ebola: The Truth Behind The Outbreak (Video) l". Mabalo Lokela Archives – Political Mol. Archived from the original on 12 July 2015. Retrieved 18 October 2014.
- ^ a b Stimola A (2011). Ebola (1st ed.). New York: Rosen Pub. pp. 31, 52. ISBN 978-1435894334.
- ^ "This Congolese Doctor Discovered Ebola But Never Got Credit For It – Until Now". NPR.org. Archived from the original on 5 November 2019. Retrieved 5 November 2019.
- ^ McNeish H (24 March 2017). "He Treated The Very First Ebola Cases 40 Years Ago. Then He Watched The World Forget". HuffPost. Archived from the original on 25 July 2019. Retrieved 5 November 2019.
- ^ Piot P, Marshall R (2012). No time to lose: a life in pursuit of deadly viruses (1st ed.). New York: W.W. Norton & Co. pp. 30, 90. ISBN 978-0393063165.
- ^ Piot P (11 August 2014). "Part one: A virologist's tale of Africa's first encounter with Ebola". ScienceInsider. Archived from the original on 9 October 2014.
- ^ Piot P (13 August 2014). "Part two: A virologist's tale of Africa's first encounter with Ebola". ScienceInsider. Archived from the original on 16 November 2014.
- ^ Bardi JS. "Death Called a River". The Scripps Research Institute. Archived from the original on 2 August 2014. Retrieved 9 October 2014.
- ^ Preston R (20 July 1995). The Hot Zone. Anchor Books (Random House). p. 117.
Karl Johnson named it Ebola
- ^ von Bredow R, Hackenbroch V (4 October 2014). "In 1976 I Discovered Ebola – Now I Fear an Unimaginable Tragedy". The Guardian. Archived from the original on 3 January 2017.
- ^ King JW (2 April 2008). "Ebola Virus". eMedicine. WebMD. Archived from the original on 28 September 2008. Retrieved 6 October 2008.
- ^ Formenty P, Libama F, Epelboin A, Allarangar Y, Leroy E, Moudzeo H, et al. (2003). "[Outbreak of Ebola hemorrhagic fever in the Republic of the Congo, 2003: a new strategy?]". Med Trop (Mars) (in French). 63 (3): 291–295. PMID 14579469.
- ^ Miller J (25 May 2004). "Russian Scientist Dies in Ebola Accident at Former Weapons Lab". The New York Times. Archived from the original on 17 October 2014. Retrieved 12 October 2014.
- ^ "Ebola outbreak in Congo". CBC News. 12 September 2007. Archived from the original on 19 November 2014.
- ^ a b "Mystery DR Congo fever kills 100". BBC News Online. 31 August 2007. Archived from the original on 11 November 2007.
- ^ "Ebola Outbreak Confirmed in Congo". NewScientist.com. 11 September 2007. Archived from the original on 18 June 2015.
- ^ "Uganda: Deadly Ebola Outbreak Confirmed – UN". UN News Service. 30 November 2007. Archived from the original on 3 March 2008. Retrieved 25 February 2008.
- ^ "DRC Confirms Ebola Outbreak". Voice of America. 21 August 2012. Archived from the original on 15 September 2012. Retrieved 15 April 2013.
- ^ "WHO – Ebola outbreak in Democratic Republic of Congo". World Health Organization (WHO). 17 August 2012. Archived from the original on 27 October 2013. Retrieved 15 April 2013.
- ^ "WHO – Ebola outbreak in Democratic Republic of Congo – update". World Health Organization (WHO). 21 August 2012. Archived from the original on 16 December 2012. Retrieved 15 April 2013.
- ^ Castillo M (2012). "Ebola virus claims 31 lives in Democratic Republic of the Congo". CBS News. United States. Archived from the original on 14 September 2012. Retrieved 14 September 2012.
- ^ "Virological Analysis: no link between Ebola outbreaks in west Africa and Democratic Republic of Congo". World Health Organization (WHO). 2 September 2014. Archived from the original on 21 November 2014.
- ^ "Congo declares its Ebola outbreak over". Reuters. 15 November 2014. Archived from the original on 29 November 2014. Retrieved 15 November 2014.
- ^ "Democratic Republic of the Congo: The country that knows how to beat Ebola". World Health Organization (WHO). December 2014. Archived from the original on 26 February 2015. Retrieved 26 February 2015.
- ^ a b "Guidelines for Evaluation of US Patients Suspected of Having Ebola Virus Disease". Centers for Disease Control and Prevention (CDC). 1 August 2014. Archived from the original on 8 August 2014. Retrieved 5 August 2014.
- ^ Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, et al. (October 2014). "Emergence of Zaire Ebola Virus Disease in Guinea" (PDF). New England Journal of Medicine. 371 (15): 1418–1425. doi:10.1056/NEJMoa1404505. PMID 24738640. S2CID 34198809. Archived from the original (PDF) on 21 February 2019.
- ^ "The first cases of this Ebola outbreak traced by WHO" (png). World Health Organization (WHO). 2014. Archived from the original on 6 October 2014.
- ^ "WHO raises global alarm over Ebola outbreak". CBS News. Archived from the original on 8 August 2014. Retrieved 2 August 2014.
- ^ Fulton D (18 August 2014). "In Liberia's Ebola-Stricken Villages, Residents Face 'Stark' Choices". Common Dreams. Archived from the original on 20 August 2014. Retrieved 20 August 2014.
- ^ "Experimental therapies: growing interest in the use of whole blood or plasma from recovered Ebola patients (convalescent therapies)" (Press release). World Health Organization (WHO). 26 September 2014. Archived from the original on 28 September 2014. Retrieved 28 September 2014.
- ^ WHO Ebola Response Team (23 September 2014). "Ebola virus disease in West Africa – the first 9 months of the epidemic and forward projections". The New England Journal of Medicine. 371 (16): 1481–1495. doi:10.1056/NEJMoa1411100. PMC 4235004. PMID 25244186.
... we estimate that the case fatality rate is 70.8% (95% confidence interval [CI], 69 to 73) among persons with known clinical outcome of infection.
- ^ Ebola response roadmap situation report (PDF) (Report). WHO. 31 December 2014. Archived from the original (PDF) on 31 December 2014. Retrieved 1 January 2015.
The reported case fatality rate in the three intense-transmission countries among all cases for whom a definitive outcome is known is 71%.
- ^ a b "Case Fatality Rate for ebolavirus". University of Edinburgh. 2015. Archived from the original on 29 August 2014. Retrieved 28 January 2015.
- ^ Wappes J. "US health worker monitored as DRC Ebola nears 600 cases". CIDRAP. Retrieved 23 January 2019.
- ^ a b Ebola virus disease (Report). World Health Organization. Retrieved 6 June 2019.
- ^ "2014 Ebola Outbreak in West Africa – Case Counts". Centers for Disease Control and Prevention (CDC). 2014. Archived from the original on 6 August 2017.
- ^ "Ebola Response Roadmap Situation Report" (PDF). World Health Organization (WHO). 1 October 2014. Archived (PDF) from the original on 2 October 2014.
- ^ "Unprecedented number of medical staff infected with Ebola". World Health Organization (WHO). 25 August 2014. Archived from the original on 28 August 2014. Retrieved 29 August 2014.
- ^ "Ebola response roadmap – Situation report" (PDF). World Health Organization (WHO). 10 December 2014. Archived (PDF) from the original on 10 December 2014. Retrieved 11 December 2014.
- ^ "Ebola Situation Report". World Health Organization (WHO). 28 January 2015. Archived from the original on 3 February 2015. Retrieved 5 February 2015.
- ^ "Ebola Situation Report" (PDF). World Health Organization (WHO). 8 April 2015. Archived (PDF) from the original on 14 April 2015. Retrieved 14 April 2015.
- ^ "Ebola gone from Guinea". CBC News. Thomson Reuters. 29 December 2015. Archived from the original on 30 December 2015. Retrieved 30 December 2015.
- ^ "UN declares end to Ebola virus transmission in Guinea; first time all three host countries free" (Press release). United Nations. Archived from the original on 1 January 2016. Retrieved 30 December 2015.
- ^ "New Ebola case in Sierra Leone. WHO continues to stress risk of more flare-ups". World Health Organization (WHO). 15 January 2016. Archived from the original on 27 January 2016. Retrieved 25 January 2016.
- ^ a b "Statement on the 9th meeting of the IHR Emergency Committee regarding the Ebola outbreak in West Africa". World Health Organization (WHO). 29 March 2016. Archived from the original on 31 March 2016. Retrieved 30 March 2016.
- ^ Botelho G, Wilson J (8 October 2014). "Thomas Eric Duncan: First Ebola death in U.S." CNN. Archived from the original on 29 May 2016. Retrieved 8 August 2017.
- ^ "Second US Ebola diagnosis 'deeply concerning', admits CDC chief". MSN. Archived from the original on 14 October 2014. Retrieved 20 February 2020.archive-url= October 2013
- ^ "Ebola crisis: Tests show Spanish nurse Teresa Romero no longer has the virus". ABC News. 20 October 2014. Archived from the original on 29 October 2014.
- ^ Fernandez M (12 October 2014). "Texas Health Worker Tests Positive for Ebola". The New York Times. Archived from the original on 12 October 2014. Retrieved 12 October 2014.
- ^ "Ebola in Texas: Second Health Care Worker Tests Positive". Archived from the original on 15 October 2014.
- ^ a b "Cases of Ebola Diagnosed in the United States". 22 May 2018. Archived from the original on 26 February 2017.
- ^ Sanchez R, Prokupecz S (23 October 2014). "N.Y. doctor positive for Ebola had no symptoms until Thursday, officials say". CNN. Archived from the original on 24 October 2014. Retrieved 23 October 2014.
- ^ Sun LH, Achenbach J (24 December 2014). "CDC reports potential Ebola exposure in Atlanta lab". The Washington Post. Archived from the original on 25 December 2014. Retrieved 25 December 2014.
- ^ "Ebola case confirmed in Glasgow hospital". BBC News Online. 29 December 2014. Archived from the original on 29 December 2014.
- ^ "Ebola nurse Pauline Cafferkey transferred to London unit". BBC News Online. 30 December 2014. Archived from the original on 30 December 2014.
- ^ "Ebola Epidemiology". www.ebolavirusnet.com. Archived from the original on 31 May 2020. Retrieved 26 May 2020.
- ^ a b "WHO says 19 dead, 39 infected so far in Congo Ebola outbreak". MSN. Archived from the original on 15 May 2018. Retrieved 14 May 2018.
- ^ "WHO planning for 'worst case scenario' over DRC Ebola outbreak". Al Jazeera. Archived from the original on 13 May 2018. Retrieved 14 May 2018.
- ^ "19 dead in latest Congo Ebola outbreak: WHO". Reuters. Archived from the original on 15 May 2018. Retrieved 14 May 2018.
- ^ "Update 1 – WHO gets approval to use Ebola vaccine in Democratic…". Reuters. 14 May 2018. Archived from the original on 5 August 2018. Retrieved 5 August 2018.
- ^ "WHO Director General visits Ebola-affected areas in DR Congo". World Health Organization (WHO). Archived from the original on 10 September 2020. Retrieved 14 May 2018.
- ^ Yong E (21 May 2018). "Most Maps of the New Ebola Outbreak Are Wrong: Villages, and sometimes whole regions of the Congo, are misplaced – but the ministry of health and a team of cartographers are racing to get better data". The Atlantic. Archived from the original on 30 July 2020. Retrieved 26 May 2018.
- ^ a b "Ebola outbreak in DRC ends: WHO calls for international efforts to stop other deadly outbreaks in the country". World Health Organization (WHO). Archived from the original on 25 July 2018. Retrieved 26 July 2018.
- ^ Beaubien J (2 August 2018). "Ebola In A Conflict Zone". NPR. National Public Radio (NPR). Archived from the original on 5 August 2018. Retrieved 5 August 2018.
- ^ Specia M (2 August 2018). "The Latest Ebola Outbreak Is Centered in a War Zone". The New York Times. Archived from the original on 4 August 2018. Retrieved 5 August 2018.
- ^ "DRC: Ebola Outbreak Worst in Country's History, Kills Almost 200". Democracy Now. 13 November 2018. Archived from the original on 13 November 2018. Retrieved 14 November 2018.
- ^ "Ebola outbreak in eastern Democratic Republic of Congo tops 1,000 cases" (Press release). Centers for Disease Control and Prevention (CDC). 29 March 2019. Archived from the original on 15 June 2019. Retrieved 12 May 2019.
- ^ "Ebola virus disease – Democratic Republic of the Congo". World Health Organization (WHO). Archived from the original on 5 May 2019. Retrieved 12 May 2019.
- ^ "Ebola virus disease – Democratic Republic of the Congo – Disease outbreak news: Update 6 June 2019". World Health Organization (WHO). 6 June 2019. Archived from the original on 7 June 2019. Retrieved 7 June 2019.
- ^ "Ebola outbreak: Grandmother dies in Uganda". BBC News Online. 13 June 2019. Archived from the original on 14 June 2019. Retrieved 15 June 2019.
- ^ Larson K (15 July 2019). "Congo tracing contacts of first Ebola case in eastern city". AP News. Archived from the original on 16 July 2019. Retrieved 17 July 2019.
- ^ Cheng M, Keaten J (17 July 2019). "Ebola outbreak in Congo declared a global health emergency". AP News. Archived from the original on 17 July 2019. Retrieved 17 July 2019.
- ^ "Half of Ebola cases in DR Congo 'unidentified'". BBC News Online. 2 August 2019. Archived from the original on 3 August 2019. Retrieved 3 August 2019.
- ^ "DR Congo's deadliest Ebola outbreak declared over". BBC News. 25 June 2020. Archived from the original on 31 December 2020. Retrieved 25 June 2020.
- ^ "Ebola virus disease case in Equateur Province, DRC is a new spillover". Virological.org. 5 June 2020. Archived from the original on 8 June 2020. Retrieved 8 June 2020.
- ^ "17 infected, 11 dead in new Ebola outbreak in DR Congo". www.aljazeera.com. Archived from the original on 15 June 2020. Retrieved 15 June 2020.
- ^ "Democratic Republic of the Congo declares the end to its 11th Ebola outbreak". www.cnn.com. 19 November 2020. Archived from the original on 19 November 2020. Retrieved 19 November 2020.
- ^ "New Ebola case detected in eastern DRC". www.aljazeera.com. Archived from the original on 16 February 2021. Retrieved 17 February 2021.
- ^ "Outbreak of Ebola virus disease in North Kivu – Democratic Republic of the Congo – 2021". European Centre for Disease Prevention and Control. 15 February 2021. Archived from the original on 17 February 2021. Retrieved 17 February 2021.
- ^ "DRC confirms two Ebola deaths in resurgence of outbreak". www.aljazeera.com. Archived from the original on 17 February 2021. Retrieved 17 February 2021.
- ^ "DRC confirms third Ebola case in North Kivu province". www.aljazeera.com. Archived from the original on 17 February 2021. Retrieved 17 February 2021.
- ^ a b c d "Communicable Disease Threats Report" (PDF). European Centre for Disease Prevention and Control. 7 May 2021. Archived (PDF) from the original on 7 May 2021. Retrieved 10 May 2021.
- ^ "Latest deadly Ebola virus outbreak in DR Congo declared over". UN News. 3 May 2021. Archived from the original on 10 May 2021. Retrieved 10 May 2021.
- ^ "Guinea records first Ebola deaths since 2016". BBC News. 14 February 2021. Archived from the original on 14 February 2021. Retrieved 14 February 2021.
- ^ "Guinea declares Ebola epidemic after three deaths". www.aljazeera.com. Archived from the original on 14 February 2021. Retrieved 15 February 2021.
- ^ Kupferschmidt, K (March 2021). "Ebola virus may lurk in survivors for many years". Science. 371 (6535): 1188. Bibcode:2021Sci...371.1188K. doi:10.1126/science.371.6535.1188. ISSN 0036-8075. PMID 33737465. S2CID 232303082.
- ^ Keita AK, Düx A, Diallo H, Calvignac-Spencer S, Sow MS, Keita MB, et al. (12 March 2021). "Resurgence of Ebola virus in Guinea after 5 years calls for careful attention to survivors without creating further stigmatization". virological.org. Archived from the original on 25 March 2021. Retrieved 23 March 2021.
- ^ @WHOAFRO (19 June 2021). "The #Ebola outbreak in #Guinea was declared over today! Here is a look back at the hard work of communities, health workers, partners & Guinea's health authorities to bring this outbreak to an end while also fighting #COVID19" (Tweet) – via Twitter.
- ^ "Cote d'Ivoire declares first Ebola outbreak in more than 25 years". afro.who.int. 14 August 2021. Archived from the original on 15 August 2021. Retrieved 15 August 2021.
- ^ "New test finds no evidence of Ebola virus in Cote d'Ivoire case". WHO | Regional Office for Africa. Archived from the original on 3 October 2021. Retrieved 3 October 2021.
- ^ "New case of Ebola hemorrhagic fever found in DR Congo". Al Jazeera. 23 April 2022. Archived from the original on 24 April 2022. Retrieved 24 April 2022.
- ^ "All reported DR Congo Ebola patients dead: WHO". Peoples Gazette. 24 May 2022. Archived from the original on 25 May 2022. Retrieved 25 May 2022.
- ^ "Ebola virus disease – Democratic Republic of the Congo", World Health Organization, 4 July 2022, archived from the original on 22 September 2022, retrieved 15 September 2022
- ^ Biryabarema E (22 September 2022). "Uganda has confirmed seven Ebola cases so far, one death". Reuters. Archived from the original on 27 September 2022. Retrieved 23 September 2022.
- ^ "Uganda is battling Ebola again – and the world doesn't have a vaccine | Devi Sridhar". the Guardian. 10 October 2022. Archived from the original on 10 October 2022. Retrieved 12 October 2022.
- ^ "Ebola outbreak in Uganda declared over". BNO News. 10 January 2023. Archived from the original on 26 March 2023. Retrieved 12 January 2023.
- ^ "Statement on Ebola in the Democratic Republic of the Congo". World Health Organization (WHO). Archived from the original on 12 May 2017. Retrieved 12 May 2017.
- ^ Hodal K (12 May 2017). "Ebola outbreak declared in Democratic Republic of the Congo after three die". The Guardian. United Kingdom. ISSN 1756-3224. OCLC 60623878. Archived from the original on 12 May 2017. Retrieved 12 May 2017.
- ^ Scutti S, Goldschmidt D. "Ebola outbreak declared in Democratic Republic of Congo". CNN. Archived from the original on 8 May 2018. Retrieved 9 May 2018.
- ^ Grady D (17 July 2019). "Congo's Ebola Outbreak Is Declared a Global Health Emergency". The New York Times. ISSN 1553-8095. OCLC 1645522. Archived from the original on 17 July 2019. Retrieved 17 July 2019.
- ^ MacNeil A, Rollin PE (June 2012). "Ebola and Marburg hemorrhagic fevers: neglected tropical diseases?". PLOS Negl Trop Dis. 6 (6) e1546. doi:10.1371/journal.pntd.0001546. PMC 3385614. PMID 22761967.
- ^ Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, et al. (May 2002). "Hemorrhagic fever viruses as biological weapons: medical and public health management". Journal of the American Medical Association. 287 (18): 2391–2405. doi:10.1001/jama.287.18.2391. PMID 11988060.
- ^ Salvaggio MR, Baddley JW (July 2004). "Other viral bioweapons: Ebola and Marburg hemorrhagic fever". Dermatologic Clinics. 22 (3): 291–302, vi. doi:10.1016/j.det.2004.03.003. PMID 15207310.
- ^ Zubray G (2013). Agents of Bioterrorism: Pathogens and Their Weaponization. New York: Columbia University Press. pp. 73–74. ISBN 978-0231518130. Archived from the original on 4 May 2016.
- ^ "Malicious Ebola-Themed Emails Are on the Rise". The New York Times. 24 October 2014. Archived from the original on 7 July 2017. Retrieved 26 October 2014.
- ^ "North Korea bans foreigners from Pyongyang marathon over Ebola". BBC News Online. 23 February 2015. Archived from the original on 24 February 2015. Retrieved 23 February 2015.
- ^ Preston R (1995). The Hot Zone, A Terrifying True Story. Anchor Books. ISBN 978-0385479561. OCLC 32052009. Archived from the original on 13 May 2016.
- ^ "Best Sellers: 4 June 1995". The New York Times Book Review. 4 June 1995. Archived from the original on 8 October 2014. Retrieved 10 September 2014.
- ^ "About The Hot Zone". Random House. Archived from the original on 7 October 2014. Retrieved 10 September 2014.
- ^ Close WT (1995). Ebola: A Documentary Novel of Its First Explosion. New York: Ivy Books. ISBN 978-0804114325. OCLC 32753758.
- ^ Grove R (2 June 2006). "More about the people than the virus". Review of Close, William T., Ebola: A Documentary Novel of Its First Explosion. Archived from the original on 21 October 2014. Retrieved 17 September 2014.
- ^ Close WT (2002). Ebola: Through the Eyes of the People. Marbleton, Wyoming: Meadowlark Springs Productions. ISBN 978-0970337115. OCLC 49193962. Archived from the original on 17 June 2016.
- ^ Pink B (24 June 2008). "A fascinating perspective". Review of Close, William T., Ebola: Through the Eyes of the People. Archived from the original on 21 October 2014. Retrieved 17 September 2014.
- ^ Clancy T (1996). Executive Orders. New York: Putnam. ISBN 978-0399142185. OCLC 34878804.
- ^ Stone O (2 September 1996). "Who's That in the Oval Office?". The New York Times. Archived from the original on 10 April 2009. Retrieved 10 September 2014.
- ^ Dewey C (2 October 2014). "Popular on Amazon: Wildly misleading self-published books about Ebola, by random people without medical degrees". The Washington Post. Archived from the original on 22 October 2014. Retrieved 27 October 2014.
- ^ Choi JH, Croyle MA (December 2013). "Emerging targets and novel approaches to Ebola virus prophylaxis and treatment". BioDrugs. 27 (6): 565–583. doi:10.1007/s40259-013-0046-1. PMC 3833964. PMID 23813435.
- ^ "Ebola 'kills over 5,000 gorillas'". BBC News Online. 8 December 2006. Archived from the original on 29 March 2009. Retrieved 31 May 2009.
- ^ a b Leroy EM, Rouquet P, Formenty P, Souquière S, Kilbourne A, Froment JM, et al. (January 2004). "Multiple Ebola virus transmission events and rapid decline of central African wildlife". Science. 303 (5656): 387–390. Bibcode:2004Sci...303..387L. doi:10.1126/science.1092528. PMID 14726594. S2CID 43305484.
- ^ Formenty P, Boesch C, Wyers M, Steiner C, Donati F, Dind F, et al. (February 1999). "Ebola virus outbreak among wild chimpanzees living in a rain forest of Côte d'Ivoire". The Journal of Infectious Diseases. 179 (Suppl 1): S120 – S126. CiteSeerX 10.1.1.484.5782. doi:10.1086/514296. PMID 9988175. S2CID 18658117.
- ^ Weingartl HM, Embury-Hyatt C, Nfon C, Leung A, Smith G, Kobinger G (November 2012). "Transmission of Ebola virus from pigs to non-human primates". Sci Rep. 2: 811. Bibcode:2012NatSR...2..811W. doi:10.1038/srep00811. PMC 3498927. PMID 23155478.
- ^ Allela L, Boury O, Pouillot R, Délicat A, Yaba P, Kumulungui B, et al. (March 2005). "Ebola virus antibody prevalence in dogs and human risk". Emerg. Infect. Dis. 11 (3): 385–390. doi:10.3201/eid1103.040981. PMC 3298261. PMID 15757552.
- ^ a b c Preston R (1994). The Hot Zone. New York: Random House. p. 300. ISBN 978-0679437840.
- ^ McCormick, Fisher-Hoch & Horvitz 1999, pp. 277–279
- ^ Waterman T (1999). Ebola Reston Outbreaks. Stanford University. Archived from the original on 16 June 2008. Retrieved 2 August 2008.
- ^ a b McCormick, Fisher-Hoch & Horvitz 1999, pp. 298–299
- ^ McCormick, Fisher-Hoch & Horvitz 1999, p. 300
- ^ "Outbreaks Chronology: Ebola Virus Disease". Centers for Disease Control and Prevention (CDC).gov. Archived from the original on 26 October 2014. Retrieved 26 October 2014.
- ^ McNeil Jr DG (24 January 2009). "Pig-to-Human Ebola Case Suspected in Philippines". The New York Times. Archived from the original on 10 March 2009. Retrieved 26 January 2009.
- ^ Lee J (2009). "Ebola-Reston virus in pigs". Microbiology Australia. 30 (4): 140. doi:10.1071/ma09140. ISSN 1324-4272.
- ^ "Final trial results confirm Ebola vaccine provides high protection against disease". World Health Organization (WHO). Archived from the original on 1 April 2017. Retrieved 29 March 2017.
- ^ Maxmen A (12 August 2019). "Two Ebola drugs show promise amid ongoing outbreak". Nature. doi:10.1038/d41586-019-02442-6. ISSN 0028-0836. PMID 32778704. S2CID 201975390.
- ^ Hoenen T, Groseth A, Feldmann H (24 July 2019). "Therapeutic strategies to target the Ebola virus life cycle". Nature Reviews. Microbiology. 17 (10): 593–606. doi:10.1038/s41579-019-0233-2. ISSN 1740-1526. PMID 31341272.
- ^ "Investigational Monoclonal Antibody to Treat Ebola Is Safe in Adults" (Press release). National Institute of Allergy and Infectious Diseases (NIAID). 24 January 2019. Archived from the original on 13 August 2019. Retrieved 12 August 2019.
- ^ Butler D. "Ebola Experts Seek to Expand Testing". Scientific American. Archived from the original on 13 December 2014. Retrieved 11 December 2014.
- ^ Buchanan RT (29 November 2014). "Ebola outbreak: New 15-minute test offers hope for thousands". The Independent. Archived from the original on 1 December 2014. Retrieved 1 December 2014.
- ^ Falconi M (29 December 2014). "Roche Secures Emergency Approval by U.S. Regulators for Ebola Test". The Wall Street Journal. Archived from the original on 29 December 2014. Retrieved 29 December 2014.
- ^ Junaid A, Tang H, van Reeuwijk A, Abouleila Y, Wuelfroth P, van Duinen V, et al. (January 2020). "Ebola Hemorrhagic Shock Syndrome-on-a-Chip". iScience. 23 (1) 100765. Bibcode:2020iSci...23j0765J. doi:10.1016/j.isci.2019.100765. PMC 6941864. PMID 31887664.
- The article uses public domain text from the CDC as cited.
Bibliography
[edit]- McCormick J, Fisher-Hoch S, Horvitz LA (1999) [1996]. Level 4: Virus Hunters of the CDC (Limited preview) (Updated [3rd] ed.). Barnes & Noble. ISBN 978-0760712085. Archived from the original on 21 May 2024. Retrieved 3 September 2020.
Further reading
[edit]- Wilson F (2014). CDC Guidance on Ebola Virus (EVD). International Publications Media Group. ISBN 978-1632670113.
- Ebola Virus: New Insights for the Healthcare Professional: 2011 Edition: ScholarlyPaper. Scholarly Editions. 2012. ISBN 978-1464914935.
- Klenk HD (January 1999). Marburg and Ebola Viruses. Current Topics in Microbiology and Immunology 235. Berlin: Springer-Verlag Telos. ISBN 978-3540647294.
- Klenk HD, Feldmann H (2004). Ebola and Marburg viruses: molecular and cellular biology (Limited preview). Wymondham, Norfolk: Horizon Bioscience. ISBN 978-0954523237. Archived from the original on 21 May 2024. Retrieved 3 September 2020.
- Kuhn JH (2008). Filoviruses: A Compendium of 40 Years of Epidemiological, Clinical, and Laboratory Studies. Archives of Virology Supplement (Limited preview). Vol. 20. Vienna: SpringerWienNewYork. ISBN 978-3211206706. Archived from the original on 21 May 2024. Retrieved 3 September 2020.
- Pattyn SR (1978). Ebola Virus Haemorrhagic Fever (1st ed.). Amsterdam: Elsevier/North-Holland Biomedical Press. ISBN 978-0444800602. Archived from the original (Full free text) on 11 December 2010.
- Ryabchikova EI, Price BB (2004). Ebola and Marburg Viruses: A View of Infection Using Electron Microscopy. Columbus, Ohio: Battelle Press. ISBN 978-1574771312.
External links
[edit]- WHO fact sheet on Ebola
- Ebola (Ebola Virus Disease) – Centers for Disease Control and Prevention, Viral Special Pathogens Branch.
- Videos: Ebola outbreak response – World Health Organization.
- "Ebola Preparedness and Response". U.S. Food and Drug Administration (FDA). 13 January 2021. Archived from the original on 8 July 2019.
- Ebola: What You Need to Know – Scientific American articles related to Ebola; note these are general reading articles, they are not scientific peer-reviewed research articles.
Ebola
View on GrokipediaEbola virus disease (EVD) is a severe, often fatal illness caused by infection with orthoebolaviruses of the Filoviridae family, first identified in 1976 during outbreaks near the Ebola River in what is now the Democratic Republic of the Congo and in Sudan.[1][2] These viruses, including the highly virulent Zaire ebolavirus species, primarily affect humans and nonhuman primates in sub-Saharan Africa, with natural reservoirs believed to be fruit bats.[3] Transmission occurs via direct contact with the bodily fluids of infected individuals or contaminated surfaces, or initially through zoonotic spillover from handling infected bushmeat or primates, but not through airborne routes or casual contact.[4][5] Symptoms typically emerge 2 to 21 days post-exposure and include fever, severe headache, muscle pain, vomiting, diarrhea, and in advanced cases, internal and external bleeding leading to multi-organ failure.[6][7] Case fatality rates average around 50% but have ranged from 25% to 90% across outbreaks, influenced by viral strain, access to supportive care, and more recently, vaccines and monoclonal antibody treatments like mAb114.[1][2] The 2014–2016 West Africa epidemic, driven by Zaire ebolavirus, marked the largest recorded outbreak with over 28,000 cases and 11,000 deaths, highlighting challenges in containment amid dense populations and weak health infrastructure, though subsequent responses have improved with rapid vaccination deployment.[2][8] Despite advances, recurrent outbreaks in Central Africa underscore the persistent zoonotic threat and the need for sustained surveillance in high-risk areas.[2]
Clinical Presentation
Initial Onset
The incubation period for Ebola virus disease ranges from 2 to 21 days following exposure to the virus, with symptoms typically manifesting after a median of 8 to 10 days.[6] Initial clinical presentation is characterized by abrupt onset of non-specific symptoms that closely resemble those of common infections such as influenza or malaria, including high fever, severe headache, muscle pain (myalgia), profound fatigue, and sore throat.[9] [1] Within 3 to 5 days of fever onset, gastrointestinal manifestations often emerge, such as vomiting, watery diarrhea, and abdominal pain, further complicating early differentiation from enteric pathogens.[6] These early symptoms reflect the virus's initial systemic dissemination but lack pathognomonic features, leading to frequent misdiagnosis in endemic regions where surveillance relies on syndromic recognition.[1] Surveillance data from major outbreaks, including the 2014–2016 West Africa epidemic, indicate that fever was the presenting symptom in approximately 89% of laboratory-confirmed cases, underscoring its near-universal role in initial onset while highlighting variability in other prodromal signs across patient cohorts. This empirical pattern, derived from prospective cohort analyses, emphasizes the critical window for isolation and testing within the first few days of illness to mitigate secondary transmission.Hemorrhagic and Systemic Progression
As Ebola virus disease advances, severe cases manifest coagulopathy with petechial or maculopapular rash progressing to ecchymoses, conjunctival injection, and mucosal bleeding from sites such as gums or venipuncture locations.[10] Overt external hemorrhage occurs in approximately 18-40% of patients, varying by outbreak and viral strain, though internal bleeding—evidenced by autopsy-detected gastrointestinal and retroperitoneal hemorrhages—is more prevalent but often subclinical during life.[11] [12] Systemic progression involves multi-organ failure, prominently featuring hepatic dysfunction with marked transaminitis and hepatocellular necrosis, alongside acute kidney injury characterized by oliguria, elevated creatinine, and tubular necrosis on histopathology.[12] [7] Dehydration from ongoing fluid losses via vomiting and diarrhea constitutes an immediate lethal threat, precipitating hypovolemic shock; clinical data document associated electrolyte imbalances including hypokalemia (prevalent in over 80% of cases), hyponatremia, and hypocalcemia, exacerbating cardiac and neuromuscular instability.[13] [14] Strain-specific differences modulate hemorrhagic intensity; Zaire ebolavirus infections correlate with higher overall severity and coagulopathic features compared to Sudan ebolavirus, though both elicit similar patterns of organ involvement absent targeted strain data on hemorrhage frequency.[15] Autopsy series from multiple outbreaks consistently highlight diffuse lymphoid depletion, adrenal necrosis, and splenic white pulp inactivation as hallmarks of terminal systemic collapse.[12]Recovery or Fatal Outcomes
Fatal cases of Ebola virus disease typically progress to death 6 to 16 days after symptom onset, driven by hypovolemic shock, disseminated intravascular coagulation, and multi-organ failure resulting from severe vascular leakage and tissue hypoperfusion.[16] [17] In cohort studies from the 2014 West African outbreak, the median time from onset to death was 8 days (interquartile range 7–11 days), with most fatalities occurring within the first two weeks due to uncontrolled viremia and cytokine storm overwhelming host physiology.[16] High initial viral loads, often exceeding 10^5–10^7 RNA copies per milliliter of blood, strongly predict this trajectory, as evidenced by multivariate analyses in treatment center cohorts where elevated viremia correlated independently with mortality risk after adjusting for age and admission delay.[18] Survivors generally achieve viral clearance through adaptive immune responses around days 10–14 post-onset, marked by declining viremia and emergence of neutralizing antibodies that facilitate resolution before irreversible organ damage.[19] In non-human primate models mirroring human kinetics, protective antibody titers appear by day 14 in surviving animals, aligning with human cohort data showing immune-mediated control in cases where peak viral loads remain below fatal thresholds.[20] Early admission to care, within 1–3 days of symptoms, enhances survival odds by enabling fluid resuscitation and monitoring to mitigate shock, as retrospective analyses from 2014–2016 outbreaks demonstrate reduced progression to terminal phases when interventions precede viremia escalation.[21] Case-fatality ratios (CFRs) for untreated Ebola range from 25% to 90% across outbreaks, reflecting strain variability and host factors, with historical averages around 50–70% absent supportive measures.[8] During the 2014–2016 West African epidemic, implementation of basic supportive care in Ebola treatment units lowered observed CFRs to approximately 40% overall, per epidemiological modeling of over 28,000 cases, though this still exceeded rates in high-resource settings with advanced monitoring (under 20%).[22] Viral load at presentation remains the dominant causal determinant, with cohorts showing CFRs approaching 90% for loads over 10^6 copies/mL versus under 30% for lower levels, underscoring immune competence as key to clearance over viral burden.[18] Rare neurological and ocular manifestations can emerge in the terminal phase, including encephalitis or uveitis precursors verified by neuroimaging and fundoscopy in outbreak autopsies, though these contribute minimally to mortality compared to systemic collapse.[23]Causative Agent
Virology and Species
Ebolaviruses belong to the family Filoviridae and genus Ebolavirus, featuring enveloped, filamentous virions with surface glycoprotein spikes that facilitate host cell entry. The viral genome consists of a non-segmented, linear, negative-sense single-stranded RNA molecule approximately 19 kb in length, encoding seven structural proteins and four non-structural proteins.[24][25] Five species of ebolavirus are recognized: Zaire ebolavirus (EBOV), Sudan ebolavirus (SUDV), Bundibugyo ebolavirus (BDBV), Taï Forest ebolavirus (TAFV), and Reston ebolavirus (RESTV). These species are differentiated primarily through genomic sequencing, revealing sequence divergences of 30-40% between species and 10-20% within species.[25][26] Zaire ebolavirus, responsible for the most severe outbreaks, exhibits the highest case fatality rates (CFRs) of 69-88% across documented epidemics, compared to approximately 50% for SUDV and 34% for BDBV. RESTV is non-pathogenic in humans despite causing lethal disease in nonhuman primates. Virulence differences correlate with variations in glycoprotein and VP24 proteins, as identified in comparative genomic analyses.[27][28][29] As an RNA virus lacking proofreading mechanisms, ebolaviruses have a relatively high mutation rate, estimated at 0.5-8 × 10⁻⁴ nucleotide substitutions per site per year across species, enabling intra-outbreak genetic diversity but constrained by purifying selection in phylogenetic reconstructions.[26][30]Transmission Dynamics
Ebola virus disease (EVD) transmits primarily through direct contact with blood, secretions, organs, or other bodily fluids of symptomatic infected individuals, or indirectly via contaminated surfaces or materials such as bedding and clothing soaked in these fluids. Transmission occurs almost exclusively among close contacts, including family members providing care, healthcare workers without proper personal protective equipment (PPE), and participants in funeral rituals involving body handling. Contact tracing during outbreaks, such as the 2014–2016 West Africa epidemic, has mapped infection chains to these verifiable fluid exposures, with infectiousness peaking during the acute symptomatic phase when viral loads in fluids are highest.[1][31] Epidemiological data refute sustained airborne transmission, as no outbreak clusters have been linked to respiratory spread despite dense population exposures in households and hospitals; instead, all documented superspreading events trace to fluid contact. Experimental aerosol studies in nonhuman primates demonstrate transmission only under high-dose, artificial conditions requiring proximity and ventilation mimicking direct exposure, underscoring inefficiency in natural aerosols compared to fluid routes. The basic reproduction number (R0) in untreated settings ranges from 1.5 to 2.5, indicating modest contagiousness reliant on intimate contacts rather than casual airborne dissemination, with R0 falling below 1 under basic interventions like isolation.[32][33] Nosocomial settings and funeral rites have historically amplified outbreaks via repeated fluid exposures. In the 2014 West Africa epidemic, healthcare workers accounted for approximately 3% of 28,616 confirmed cases (881 infections, 531 fatal) despite comprising a small population fraction, with rates reaching up to 50% in some under-equipped facilities before PPE scaling; this reflected lapses in barrier nursing rather than inherent aerosol risk. Traditional funerals, involving washing and touching corpses, fueled surges, as evidenced by one Sierra Leone ceremony linking to 85 confirmed cases, including 62 within a week. Laboratory-acquired infections remain rare, with documented percutaneous exposures like the 1976 UK needle-stick case confirming fluid routes even in controlled environments, but no epidemic-scale aerosol incidents.[34][35][36]Reservoirs and Zoonotic Origins
Fruit bats of the family Pteropodidae, particularly species such as Eidolon helvum and Hypsignathus monstrosus, are the leading candidates for the natural reservoir of ebolaviruses based on serological and molecular evidence from Central and West Africa.[37] [38] Studies have detected antibodies against Ebola virus antigens in these bats, with seroprevalence rates ranging from 1.2% to 10.8% depending on testing criteria and locations sampled during and after outbreaks.[39] Viral RNA fragments have also been identified in bat tissues, supporting exposure to ebolaviruses, though no viable virus has been isolated from wild-caught bats despite extensive sampling efforts.[40] [41] Ecological modeling further corroborates this role, as bat distributions overlap with historical spillover sites, and experimental inoculations demonstrate asymptomatic infection and shedding in fruit bats without severe disease.[42] [43] While bats likely serve as the primary reservoir, zoonotic spillovers to humans typically occur through intermediate amplification in susceptible wildlife, such as non-human primates, which experience high mortality rather than persistent carriage.[44] Index cases in multiple outbreaks have been associated with handling bushmeat from infected primates; for instance, during the 1994–1996 outbreaks in Gabon and the Democratic Republic of Congo, initial human infections traced to hunters butchering chimpanzee carcasses found dead in forests.[45] [46] In the 1995 Kikwit outbreak, the chain began with exposure to forest fauna, consistent with patterns of direct contact with deceased animals in tropical settings.[44] These events underscore that primates act as dead-end hosts, facilitating virus transfer to humans via butchering or consumption without sustaining the reservoir.[44] Spillover incidents cluster in tropical forest ecotones where human activities encroach on wildlife habitats, with empirical studies linking recent deforestation to elevated outbreak risk.[47] Habitat fragmentation and loss of closed-canopy forests increase human-wildlife interfaces, amplifying contact rates between bushmeat hunters and infected animals; statistical models show that sites with deforestation in the preceding years exhibit higher probabilities of Ebola virus disease emergence. [47] This causal pathway is evidenced by geospatial analyses of outbreak locations, where forest disturbance correlates with spillover events independent of other variables like population density. Such dynamics highlight deforestation not as a mere correlate but as a direct amplifier of zoonotic transmission through disrupted ecosystems and intensified resource extraction.[48]Pathogenesis
Viral Replication and Spread
Ebola virus initiates infection by attaching to host cells, primarily macrophages and dendritic cells, through interactions between its glycoprotein (GP) and surface receptors such as TIM-1 and C-type lectins, followed by endocytosis.[49] Within the endosome, cathepsin-mediated cleavage of GP exposes the receptor-binding domain, which binds to the Niemann-Pick C1 (NPC1) cholesterol transporter protein, triggering conformational changes necessary for membrane fusion and viral genome release into the cytoplasm.[50] This entry mechanism is critical for infection of mononuclear phagocytes and endothelial cells, as demonstrated in in vitro models using human cell lines and primary isolates.[51] Upon release, the negative-sense RNA genome is transcribed and replicated by the viral RNA-dependent RNA polymerase in the cytoplasm, producing high levels of viral proteins and progeny genomes.[52] Initial replication occurs rapidly in draining lymph nodes, where infected monocytes and macrophages disseminate the virus, leading to systemic viremia within days of exposure, as observed in nonhuman primate models.[53] From lymphoid tissues, the virus spreads to the liver and spleen, sites of peak replication, where viral loads can exceed 10^7 RNA copies per mL in plasma, correlating with disease severity and fatal outcomes in human cases.[54] Empirical data from outbreaks show that viremia thresholds above 10^7 copies/mL are associated with mortality rates exceeding 80%, reflecting unchecked dissemination and tissue tropism.[55] Strain-specific variations in GP structure influence replication efficiency and endothelial tropism; for instance, Zaire ebolavirus GP undergoes more efficient furin-like cleavage than less pathogenic species like Reston ebolavirus, enhancing endothelial cell activation, barrier disruption, and vascular damage in vitro and in animal models.[56] These differences arise from sequence variations in the GP cleavage site and mucin-like domain, which modulate shedding and cytotoxicity without altering core entry via NPC1.[57] In susceptible hosts, this targeted spread to endothelium contributes to coagulopathy, though direct viral replication drives the process independently of secondary immune effects.[58]Immune Evasion and Host Damage
The Ebola virus employs multiple strategies to evade the host innate immune response, primarily through its VP35 and VP24 proteins, which disrupt type I interferon (IFN) signaling pathways. VP35 acts as an IFN antagonist by binding double-stranded RNA and sequestering interferon regulatory factor 3 (IRF3), thereby inhibiting RIG-I-like receptor signaling and preventing the transcription of IFN-β and other antiviral genes.[59] VP24 further blocks IFN signaling by interacting with karyopherin-α proteins, impeding the nuclear import of tyrosine-phosphorylated STAT1 and STAT2, which are essential for IFN-stimulated gene expression.[60] These mechanisms delay the activation of adaptive immunity, allowing unchecked viral replication in monocytes, macrophages, and dendritic cells, as evidenced by transcriptomic analyses showing suppressed IFN-related gene expression in early infection stages.[61] This evasion contributes to profound host damage, particularly lymphopenia, a hallmark observed in autopsies and clinical cases where CD4+ and CD8+ T lymphocytes undergo massive depletion in lymphoid tissues.[62] Histological examinations of nonhuman primate models reveal destruction of lymph node architecture and follicular loss, correlating with peripheral blood lymphopenia in human patients, which impairs antigen presentation and cytotoxic responses.[63] Transcriptomic profiling from fatal Ebola virus disease (EVD) cases confirms downregulation of lymphocyte survival genes, underscoring how initial IFN suppression leads to apoptotic loss of immune effectors, sustaining high viremia levels that exceed 10^7 plaque-forming units per milliliter in severe cases.[64] Paradoxically, following evasion, a dysregulated hyperinflammatory response emerges, characterized by a cytokine storm that exacerbates tissue damage. Elevated levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-8, detected in plasma from 2014 West Africa outbreak patients, drive endothelial dysfunction and vascular leakage, contributing to hypovolemic shock and multi-organ failure.[65] Shed soluble glycoprotein from infected cells further amplifies this by triggering TLR4-mediated cytokine release and increased vascular permeability in vitro.[66] In survivor cohorts from the 2014 epidemic, lower peak cytokine concentrations distinguished non-fatal outcomes from fatalities, where unchecked inflammation correlated with case fatality rates (CFR) approaching 70%.[67] Host genetic factors modulate susceptibility to this evasion and damage, with certain HLA class I alleles influencing T-cell recognition and viral control. Studies of EVD survivors identified KIR-HLA ligand mismatches associated with reduced mortality, as these enhance natural killer cell activity against infected cells.[68] Experimental models using genetically diverse mice demonstrated heritable variation in survival, with loci linked to IFN pathways explaining up to 25% of outcome differences, highlighting why CFR remains elevated despite supportive interventions by underscoring virus-host genetic mismatches in immune priming.[69]Diagnosis
Laboratory Confirmation
Reverse transcription polymerase chain reaction (RT-PCR) targeting Ebola virus RNA in blood, serum, or other body fluids serves as the gold-standard diagnostic method, capable of detecting viral genetic material within 1-3 days of symptom onset with high sensitivity and specificity.[70][71] This quantitative or qualitative assay identifies species-specific targets like the nucleoprotein or glycoprotein genes, achieving limits of detection as low as 10^3 genomic equivalents per milliliter, though performance varies by primer set and viral load.[72] Serological assays, including IgM-capture enzyme-linked immunosorbent assay (ELISA) using Zaire ebolavirus antigens, detect acute immune responses but typically yield positive results only after a median of 10 days post-onset, limiting their utility for early confirmation.[73][74] Laboratory procedures demand stringent biosafety measures, with RT-PCR on clinical specimens requiring biosafety level 3 (BSL-3) containment due to aerosol risks, while virus isolation or culture necessitates BSL-4 facilities equipped for full-body positive-pressure suits.[75][76] Point-of-care rapid diagnostic tests (RDTs), such as the ReEBOV antigen RDT, provide field-deployable alternatives with reported sensitivities of 91-100% and specificities of 92-97% against RT-PCR reference standards in symptomatic patients, yet they exhibit limitations including false negatives during the initial days of infection when viremia is low or in cases of non-Zaire species.61042-X/fulltext)[77][78] These RDTs fall short of World Health Organization targets for >98% sensitivity in high-risk settings, underscoring the need for confirmatory RT-PCR.00133-0/fulltext) Post-mortem confirmation protocols prioritize minimally invasive techniques to reduce exposure risks, involving collection of oral or nasal swabs, conjunctival fluids, or skin snips fixed in 10% buffered formalin for RT-PCR or immunohistochemistry on formalin-fixed tissues.[73][79] Viral RNA remains detectable in such samples for up to three weeks postmortem if viremia was present at death, enabling retrospective diagnosis while adhering to safe burial practices.[80][81]Differential Considerations
Ebola virus disease (EVD) must be differentiated from other causes of acute febrile illness in tropical settings, where symptoms such as high fever, myalgia, headache, and vomiting overlap with endemic infections. Clinical algorithms emphasize epidemiological risk factors—like recent exposure in endemic zones or contact with confirmed cases—to elevate pretest probability, applying Bayesian principles wherein low prior likelihood for EVD (outside outbreaks) favors common alternatives unless exposure history shifts odds significantly.[1][82] Malaria, the most frequent mimic, typically features cyclical fevers with chills and rigors every 48-72 hours, contrasting EVD's irregular high fever without periodicity; relative bradycardia and splenomegaly may favor malaria, while EVD progresses to asthenia and dehydration without initial parasitemia signs. Typhoid fever presents with sustained fever, relative bradycardia, and abdominal tenderness, often lacking EVD's rapid onset of severe prostration or early conjunctival injection. Lassa fever shares hemorrhagic potential but is distinguished by prominent pharyngitis, retrosternal pain, and milder early bleeding, with geographic overlap in West Africa necessitating risk-based prioritization.[83][84] Other viral hemorrhagic fevers, such as dengue or yellow fever, may simulate EVD's capillary leak and shock, but dengue often shows myalgias with rash and petechiae responsive to fluids, whereas EVD entails diffuse endothelial damage without early leukopenia reversal. In resource-poor settings, retrospective analyses of outbreaks reveal initial misdiagnosis rates exceeding 10% for EVD cases mistaken as bacterial sepsis or arboviral illness, driven by absent early hemorrhage (present in <50% at onset) and delayed recognition of exposure.[85][86] Advanced differentiation employs imaging: dengue hemorrhagic fever yields pleural effusions and ascites from plasma leakage, while EVD correlates with acalculous cholecystitis or multiorgan hypoperfusion on ultrasound, guiding triage without confirmatory assays. Yellow fever adds jaundice and higher transaminitis early, reducing overlap likelihood in non-vaccinated travelers. Overall, algorithms integrate symptom progression—EVD's escalation to neurological signs or bleeding by day 5-7—against static mimics, with outbreak contexts inverting defaults toward EVD suspicion.[87]Prevention
Vaccines and Immunoprophylaxis
The rVSV-ZEBOV vaccine, marketed as Ervebo, demonstrated 100% efficacy (95% CI 68.9–100.0) against Ebola virus disease in a phase 3 ring vaccination trial conducted in Guinea from April to July 2015, with no cases occurring ≥10 days post-vaccination among 2,014 immediate-vaccination participants versus four cases in the delayed arm.32621-6/fulltext) This single-dose, replication-competent vesicular stomatitis virus-vectored vaccine targets the Zaire ebolavirus glycoprotein and has been deployed in ring vaccination strategies during outbreaks, such as the 2018–2020 Democratic Republic of Congo epidemic, where effectiveness ≥10 days post-vaccination was estimated at 84% (95% credible interval 70–92%).00419-5/fulltext) The heterologous two-dose regimen of Ad26.ZEBOV (Zabdeno) followed by MVA-BN-Filo (Mvabea), both targeting Zaire ebolavirus glycoprotein, was authorized by the European Medicines Agency in July 2020 and WHO-prequalified in 2021 based on immunogenicity data from over 3,300 participants across five phase 1–3 trials, showing durable antibody responses comparable to Ervebo but without direct human efficacy trials.[88] This regimen is recommended for preventive vaccination in lower-risk areas or contacts, with nonhuman primate challenge studies indicating protection when doses are spaced 8 weeks apart.[89] For Sudan ebolavirus, no licensed vaccine exists as of October 2025; however, a phase 3 ring vaccination trial of an experimental IAVI-developed candidate launched in Uganda on February 3, 2025, evaluating safety and efficacy among contacts of confirmed cases during the ongoing outbreak.[90] Preemptive ring vaccination with available Zaire-targeted vaccines has shown potential to interrupt transmission chains by reducing secondary cases among high-risk contacts, though cross-protection against non-Zaire species remains unproven.00038-2/fulltext) Global stockpiling under the International Coordinating Group reached 500,000 Ervebo doses by 2022 for outbreak response, yet deployment in endemic African regions has faced delays due to varying national regulatory approvals, limited preventive use beyond emergencies, and insufficient integration into routine health systems, as highlighted in Gavi's 2025 market shaping roadmap and African health summits.[91][92] These access barriers, including slower registration in some African countries compared to Western markets, have constrained equitable rollout despite WHO prequalification.[93]Infection Control Protocols
Infection control protocols for Ebola virus disease (EVD) prioritize barrier precautions to interrupt direct contact and droplet transmission in healthcare, laboratory, and transport settings.[94] Core measures include the use of full personal protective equipment (PPE) ensembles, rigorous hand hygiene, patient isolation, and biosafety level 4 (BSL-4) handling for specimens, with evidence from outbreak analyses showing that compliance failures, such as improper PPE doffing, contributed to early nosocomial clusters in the 2014 West Africa epidemic.[95] [96] PPE protocols mandate impermeable full-body coveralls or gowns, double gloving with nitrile or latex gloves, goggles or face shields for eye protection, respiratory masks or powered air-purifying respirators (PAPRs) in high-risk aerosol-generating procedures, and boot covers, all donned and doffed under supervision by a trained observer to minimize self-contamination risks.[97] [95] Hand hygiene, using alcohol-based sanitizers or soap-and-water washing immediately before and after PPE contact, has been modeled to reduce nosocomial transmission by over 80% in simulated EVD scenarios by eliminating viral residues on surfaces and skin.[98] [99] Compliance lapses, including inadequate training, led to healthcare worker infections comprising up to 10% of cases in uncontrolled settings, underscoring the causal role of procedural adherence in outbreak containment.[100] Patient isolation in dedicated facilities with negative-pressure rooms or cohort wards, combined with contact quarantine, demonstrated efficacy in the 2014 outbreak, where intensified isolation reduced secondary household transmissions by 82%, from a serial interval attack rate of 9.3% to 1.7%, effectively halting approximately 70-80% of projected cases through rapid segregation.[101] [102] Laboratory processing requires BSL-4 containment, including positive-pressure suits and Class III biosafety cabinets, while transport of suspect specimens follows triple packaging with UN 3373 labeling and chain-of-custody documentation to designated reference labs, preventing iatrogenic exposures documented in prior incidents.[103] [104] Waste decontamination via autoclaving or incineration further reinforces these protocols, with modeling indicating that integrated adherence averts exponential spread.[105]Community and Behavioral Measures
Traditional funeral practices, involving direct contact with deceased bodies, have facilitated Ebola transmission, with at least 20% of new infections occurring during such burials.[106] A single traditional ceremony in Sierra Leone linked to 85 confirmed cases, including 62 reported in one week.[35] Modifying these to safe and dignified burials (SDB), where trained teams use protective equipment and limit handling, reduced incidence by up to 40% in adjacent areas during outbreaks.[107] Such interventions, when timely and successful, cut transmission risks by 7-40%, though full epidemic control requires combining with isolation.[108] Handling and consumption of bushmeat from wildlife reservoirs like bats and primates pose zoonotic spillover risks, with empirical studies linking outbreaks to these practices despite low perceived danger among hunters.[109] Bans on bushmeat hunting and trade, enforced during epidemics, alongside education on risks, have curtailed potential introductions, though compliance varies due to economic reliance.[110] No direct Ebola cases from imported bushmeat in the EU have been documented, but wild meat handling increases cross-species transmission potential.[111] Contact tracing identifies and monitors exposed individuals, reducing community transmission time and yielding an 84% lower risk among traced contacts during Sudan virus outbreaks.[112] In the Democratic Republic of Congo's 2018-2020 epidemic, large-scale tracing faced barriers from community resistance and conflict, limiting completeness despite unprecedented implementation.[113] Community-based isolation strategies in insecure areas have shown promise in curbing spread when trusted leaders promote adherence.[114] Travel screening at borders, focusing on symptomatic individuals, offers limited efficacy against Ebola due to its post-symptomatic transmission pattern and potential for pre-symptomatic travel, delaying outbreaks by an average of 3.5 days in models but failing to prevent importation in over 50% of scenarios.[115] Asymptomatic travelers pose no infection risk, rendering broad restrictions on them ineffective for containment.[116]Treatment
Supportive Care
Supportive care for Ebola virus disease focuses on maintaining hydration, correcting electrolyte imbalances, and providing nutritional support to mitigate the effects of severe fluid loss from vomiting, diarrhea, and fever, which are primary contributors to hypovolemic shock and multi-organ failure.31795-6/fulltext) Intravenous administration of balanced crystalloid solutions, such as Ringer's lactate, is recommended empirically to replace losses, with protocols emphasizing 3–5 liters per day in the initial phase for hemodynamically unstable patients, alongside monitoring for renal function and acid-base status.[117] Electrolyte disturbances, including hypokalemia and hyponatremia, are common and addressed through targeted supplementation to prevent arrhythmias and seizures.[13] Facility-based care incorporating these measures has demonstrated reduced case-fatality rates compared to home management, where dehydration often progresses unchecked; observational data from the 2014–2015 West Africa outbreak indicate that admission to Ebola treatment units was associated with approximately a 50% lower hazard of death, attributable in part to aggressive fluid resuscitation unavailable in community settings.[118] This aligns with broader evidence that early intensive supportive interventions can lower overall mortality by addressing reversible complications, though randomized trials isolating supportive care effects are limited due to ethical constraints.[119] Nutritional support via oral rehydration solutions when tolerated, or nasogastric/enteral feeding in severe cases, alongside symptom control with analgesics and antipyretics, further aids recovery by preserving lean body mass and reducing metabolic stress.31795-6/fulltext) In the 2018–2020 Democratic Republic of Congo outbreak, empirical implementation of enhanced protocols—including optimized fluid management and nutrition—correlated with improved survival rates in treated cohorts, contributing to a decline in case-fatality to around 40–50% in centers with robust supportive infrastructure, versus higher rates in prior epidemics lacking such interventions.30242-9/fulltext)[1]Antiviral and Monoclonal Therapies
Antiviral and monoclonal antibody therapies for Ebola virus disease (EVD) caused by Zaire ebolavirus have advanced through clinical evaluation, with monoclonal antibodies demonstrating superior efficacy in randomized trials. Remdesivir, a nucleotide analogue antiviral, was investigated in the PALM trial (NCT03719586) conducted during the 2018-2019 Democratic Republic of Congo outbreak, where it yielded a 28-day mortality rate of 53% among treated patients, performing worse than the control monoclonal antibody ZMapp (49.7% mortality), leading to early termination of its arm.[120][121] In contrast, the single monoclonal antibody mAb114 (ansuvimab) achieved a 35.1% mortality rate, and the REGN-EB3 cocktail (atoltivimab, maftivimab, odesivimab) reduced mortality to 29%, both significantly outperforming ZMapp (p<0.05 for REGN-EB3; p=0.007 for mAb114).[120][122] These monoclonal therapies neutralize the virus by targeting its glycoprotein, preventing host cell entry. The U.S. Food and Drug Administration approved REGN-EB3 (marketed as Inmazeb) in October 2020 and mAb114 (Ebanga) in December 2020 for treating Zaire ebolavirus infections in adults and children, based on PALM trial data showing 66-71% survival rates versus approximately 50% in controls.[123][124] Prior compassionate use of investigational antivirals, including remdesivir and early monoclonal candidates like ZMapp from 2014-2016, involved small cohorts with reported survival in some cases (e.g., 12.5% mortality in eight ZMapp recipients), but lacked randomized controls and showed inconsistent outcomes amid high baseline fatality.[125][126] Deployment challenges persist despite efficacy gains, as these therapies require cold-chain logistics, specialized infusion capabilities, and high costs—estimated in the tens of thousands per dose—limiting scalability in resource-poor African settings where outbreaks occur.[127] Stockpiles exist in Western nations for emergency response, but transfer to endemic regions faces delays from regulatory hurdles, infrastructure deficits, and supply chain vulnerabilities, resulting in underutilization during cycles like 2017-2022.[128] The World Health Organization's 2022 guidelines prioritize these agents for confirmed cases, yet access inequities highlight systemic barriers beyond trial-proven benefits.[129]Prognosis
Fatality Rates and Predictors
The case fatality rate (CFR) for Ebola virus disease (EVD), calculated as deaths among confirmed cases with known outcomes, has varied across outbreaks and ebolavirus species, with meta-analyses estimating a pooled global CFR of 60.6% (95% CI: 51.6–69.4%) from 1976 to 2022 based on 34,936 cases and 15,409 deaths.[130] Untreated cases in early outbreaks without supportive care or antivirals typically exhibited CFRs exceeding 50%, though rigorous meta-analyses incorporating historical data show higher averages due to limited ascertainment of mild or survived cases.[131] Significant variance exists by ebolavirus species, with Zaire ebolavirus (EBOV) demonstrating the highest lethality in meta-analyses, with CFRs ranging 66.6–79% across outbreaks, compared to Sudan ebolavirus (SUDV) at 48.5–54%.[130][28] Bundibugyo ebolavirus has shown lower rates, around 34%, while Taï Forest ebolavirus cases remain too few for robust estimation but align with intermediate severity.[28] These differences persist after adjusting for outbreak-specific factors like healthcare access, reflecting inherent viral pathogenicity variations confirmed in animal models and genomic analyses.[132] Multivariate analyses from clinical cohorts identify key independent predictors of mortality, including advanced age over 40 years (adjusted odds ratio [aOR] up to 2.5), high initial viral load exceeding 10^7 copies/mL (aOR >3), and delayed presentation beyond 5 days from symptom onset (aOR 1.5–2.0), which correlate with advanced organ dysfunction and cytokine storm.[133][134] Other factors like hemorrhagic manifestations (aOR 2–4) and severe admission status (e.g., disorientation or shock) further elevate risk in logistic regression models, with viral load emerging as the strongest biomarker proxy for poor prognosis.[135][136] CFRs have declined in outbreaks with enhanced supportive care, fluid resuscitation, and monoclonal antibodies like mAb114, dropping to 30–40% in treated cohorts versus 70–90% historically, though elevated rates persist in remote or resource-limited settings due to barriers in rapid diagnosis and intervention.[137][22] These trends underscore the modifiable nature of outcomes through timely access, independent of strain-specific virulence.[138]Post-Infection Sequelae
Survivors of Ebola virus disease (EVD) frequently experience persistent multi-systemic morbidities, with longitudinal cohort studies documenting symptoms that endure for months to years post-discharge. In a 2025 analysis of Sudan ebolavirus (SUDV) survivors followed for up to two decades, 50% reported ongoing issues including arthralgia (26.5%), muscular pain (14.5%), and neurological symptoms like numbness and confusion, mirroring patterns in Zaire ebolavirus (EBOV) cohorts where musculoskeletal complaints affected 18–87%. These findings underscore underreported chronicity, as early post-outbreak assessments often overlooked long-term follow-up, revealing immune dysregulation and tissue damage as causal drivers rather than transient recovery phases.[139][140] Ocular sequelae, particularly uveitis, manifest in 13.5–34% of survivors, leading to vision impairment in subsets requiring intervention; a Guinea cohort of 340 EBOV survivors identified uveitis in 13.5%, with 15 cases progressing to 20/40 or worse acuity. Auditory deficits, including hearing loss, occur alongside, with somatic symptom surveys reporting prevalence in frequent clusters with arthralgia and fatigue. Musculoskeletal involvement, such as arthritis and joint pain, affects 20–50% across studies, with 49.7% of treated survivors exhibiting these in a recent evaluation, often linked to viral-induced inflammation persisting beyond acute viremia clearance.[141][142][143] Psychological sequelae include post-traumatic stress disorder (PTSD) at rates of approximately 20–24% in West African 2014–2016 cohorts, with one Liberian study documenting 21% possible cases via standardized screening, associated with peritraumatic distress and stigmatization rather than direct neuroinvasion. Neurological persistence, like cranial nerve deficits, compounds this, as evidenced in 61.7% of survivors reporting such symptoms years later.[144][145][146] EBOV RNA persistence in immune-privileged sites, notably semen, extends beyond blood clearance, with cohort data showing median detection duration of 204 days and 75% positivity at 6 months post-onset; isolated cases document RNA up to 40 months, though infectious virus viability declines over time. Sexual transmission post-recovery remains rare, with only three documented instances amid thousands of survivors, and no evidence of vertical transmission or fertility impairment after seminal clearance, as pregnancy outcomes in recovered women show low reactivation risk without adverse infant effects.[147][148][149][150][151]Epidemiology
Discovery and Initial Outbreaks
The Ebola virus was first detected in 1976 during two concurrent outbreaks in Sudan and Zaire (now the Democratic Republic of the Congo). In Sudan, the outbreak began in the Nzara region, where a cotton factory worker fell ill on June 27, 1976, and died five days later; the virus likely spread through contact at the factory and subsequently amplified at Maridi Hospital due to inadequate infection control. This resulted in 284 confirmed cases, including 67 in Nzara and 213 in Maridi, with 151 deaths, yielding a case fatality rate of 53%.[152] [2] Simultaneously, in Zaire, the outbreak emerged on September 1, 1976, in Yambuku village, centered around a rural clinic where contaminated needle reuse facilitated rapid nosocomial transmission; a Belgian nun who prepared injections was among the early victims. By October 24, 1976, it had caused 318 cases and 280 deaths, with an 88% case fatality rate.[153] [2] Virus identification posed significant challenges, as initial symptoms mimicked known hemorrhagic fevers like Marburg virus disease; blood samples from patients were shipped to the Institute of Tropical Medicine in Antwerp and then to the U.S. Centers for Disease Control in Atlanta, where electron microscopy revealed thread-like virions distinct from Marburg, confirming a novel filovirus by mid-September 1976. The virus was named Ebolavirus after the nearby Ebola River, highlighting diagnostic limitations in resource-poor settings without prior serological reagents.[154] [2] The next major initial outbreak occurred in Kikwit, Zaire, in 1995, with 315 cases and 254 deaths (81% fatality); it traced to a charcoal miner who handled a chimpanzee carcass in a forest logging area, where dead primates were reported, and postmortem testing confirmed Ebola virus in one chimpanzee sample, underscoring zoonotic spillover risks in bushmeat handling.[2] [155]Intermittent Epidemics (1976–2013)
From 1976 to 2013, Ebola virus disease outbreaks remained sporadic and contained, primarily in rural areas of Central and East Africa, with a total of approximately 24 recorded events involving around 2,387 laboratory-confirmed or probable cases and 1,590 deaths, yielding an overall case fatality rate (CFR) of about 67%.[156] These epidemics were characterized by rapid onset in isolated communities, often linked to initial cases involving bushmeat handling or direct contact with infected wildlife, such as fruit bats or non-human primates, followed by human-to-human transmission through bodily fluids in healthcare or funeral settings.[157] The majority occurred in countries including the Democratic Republic of the Congo (then Zaire), Sudan (now South Sudan), Gabon, Uganda, and the Republic of the Congo, with outbreak sizes typically ranging from a few to several hundred cases, allowing for containment through isolation, contact tracing, and barrier nursing.[158] The inaugural outbreaks in 1976 established the pattern: simultaneous emergences of Sudan ebolavirus in Nzara, Sudan (284 cases, 151 deaths, CFR 53%) near a cotton factory where workers handled rodents, and Zaire ebolavirus in Yambuku, Zaire (318 cases, 280 deaths, CFR 88%), traced to a clinic injection using contaminated needles.[156] Subsequent epidemics, such as those in Gabon (1994–1997, multiple waves totaling over 60 cases with CFRs around 57–74%) often initiated via chimpanzee consumption, and Uganda (e.g., 2000–2001, 425 cases, 224 deaths, CFR 53% from Sudan virus), highlighted recurrent involvement of forest-adjacent populations.[157] CFRs varied by ebolavirus species—higher for Zaire (up to 90%) than Sudan (around 50%)—and showed no significant decline over time despite improving diagnostics, averaging 71% in 1976–1986 versus 62% post-1990, potentially reflecting better supportive care in later responses though confounded by strain differences and small sample sizes.[158] Underreporting likely inflated CFR estimates and understated true incidence, as remote locations, cultural burial practices delaying detection, and limited laboratory capacity in affected regions led to missed mild or community cases; retrospective analyses suggest surveillance captured only a fraction of transmissions in forested zones.[159] Parallel to African human outbreaks, Reston ebolavirus—a non-pathogenic strain in humans—was detected outside Africa, first in 1989–1990 when cynomolgus macaques imported from the Philippines to the United States exhibited hemorrhagic fever, with subsequent serologic evidence of asymptomatic human exposure among animal handlers; further animal outbreaks in Philippine pigs (2008) and bats confirmed endemic circulation without human disease.[160][161] These events underscored zoonotic origins but reinforced that only African ebolaviruses caused severe human illness during this era.West Africa Epidemic (2014–2016)
The epidemic originated in Guinea in December 2013, with the index case linked to a 2-year-old child in Guéckédou near the borders with Liberia and Sierra Leone; WHO confirmed Ebola virus disease on March 23, 2014, after initial cases of hemorrhagic fever.[163] Transmission rapidly crossed porous borders, reaching Liberia by March 30 and Sierra Leone by May 25, 2014, fueled by familial and travel links. By July 2014, cases had infiltrated urban centers such as Conakry, Monrovia, and Freetown, where high population densities and inadequate infrastructure accelerated community spread through contact with infected bodily fluids during care and funerals.[163] The outbreak escalated to 28,652 confirmed, probable, and suspected cases, with 11,325 deaths across Guinea (3,814 cases, 2,544 deaths), Liberia (10,678 cases, 4,810 deaths), and Sierra Leone (14,124 cases, 3,956 deaths), representing over 99% of the global toll.00129-3/fulltext) The reported case-fatality rate stood at approximately 40%, varying by access to care; untreated cases in remote areas approached 70%, while improved supportive measures in later phases reduced lethality in facilities. Health systems collapsed under the strain, with over 800 health workers infected and 518 fatalities among them, highlighting vulnerabilities in personal protective equipment adherence and training.00129-3/fulltext) International response lagged despite early alerts from Médecins Sans Frontières (MSF), which by June 2014 warned of an uncontrollable crisis; WHO delayed declaring a Public Health Emergency of International Concern until August 8, 2014, after cases surpassed 1,000, citing insufficient evidence of cross-border threat despite mounting data.[164] This hesitation stemmed from bureaucratic inertia and reluctance to alarm economies, allowing exponential growth; MSF attributed the delay to WHO's underestimation, while a subsequent independent panel criticized organizational failures in surveillance and leadership.[164] [165] Only after peak transmission did scaled interventions—contact tracing, safe burial teams, and treatment centers—curb the epidemic, with Liberia declared free of transmission on May 9, 2015, followed by Sierra Leone on November 7, 2015, and Guinea on June 1, 2016.[163] Exported cases remained limited despite international air travel, with four air-transmitted infections reaching the United States (two, including one death), United Kingdom (one), and Italy (one), alongside treated evacuees; Spain reported one secondary case in a nurse exposed during repatriation care.[166] Containment succeeded through rapid isolation, contact monitoring, and enhanced protocols, preventing secondary chains beyond isolated instances like the U.S. cluster from index patient Thomas Eric Duncan in September 2014. These events underscored effective risk mitigation in high-resource settings, contrasting with West Africa's systemic gaps.[166]Democratic Republic of Congo Cycles (2017–2022)
The Democratic Republic of the Congo (DRC) faced recurrent Ebola virus disease (EVD) outbreaks from 2017 to 2020, with four distinct episodes in remote and conflict-affected regions, totaling over 3,500 cases and underscoring how armed violence impeded containment efforts such as contact tracing and safe burials. These cycles involved the Zaire ebolavirus species and were characterized by initial zoonotic spillovers, often linked to handling bushmeat or funeral practices, but prolonged by logistical barriers in unstable areas. Unlike prior isolated incidents, the eastern outbreaks were exacerbated by militia activities, which increased transmission risk through disrupted healthcare access and community mistrust of interventions.[167][168][169] The eighth outbreak began in April 2017 in Bas-Uélé province, a remote northern area bordering the Central African Republic, with the index case traced to a motorcycle driver who prepared bushmeat from infected animals. By June 2, 2017, when the World Health Organization (WHO) declared it over, there were 8 cases (5 laboratory-confirmed, 3 probable) and 4 deaths, yielding a case-fatality ratio of approximately 50%. Response efforts relied on rapid isolation, contact tracing of over 400 individuals, and supportive care, containing spread without vaccines or advanced therapeutics. Genetic analysis confirmed a distinct lineage from prior DRC strains, suggesting an independent zoonotic event rather than human-to-human reintroduction.[167][170] In May 2018, the ninth outbreak emerged in Equateur province's Bikoro health zone, western DRC, declared on May 8 after 21 suspected deaths prompted investigation. It involved 54 cases (38 confirmed, 16 probable) and 33 deaths by its end on July 24, with a case-fatality ratio of 61%. This episode marked the first deployment of the recombinant vesicular stomatitis virus-based Zaire ebolavirus glycoprotein (rVSV-ZEBOV) vaccine in ring vaccination strategy, immunizing over 3,300 contacts and high-risk individuals, which contributed to swift containment alongside improved diagnostics and mobile labs. No cross-border spread occurred despite proximity to urban centers like Mbandaka.[171][172][173] The tenth outbreak, declared August 1, 2018, in North Kivu province and extending to Ituri, represented the largest and most protracted in DRC history up to that point, ending June 25, 2020, after 3,481 cases (3,323 confirmed, 158 probable) and 2,299 deaths across 29 health zones. Originating near Beni, it amplified due to dense populations, porous borders, and ongoing armed conflict involving groups like the Allied Democratic Forces (ADF), which conducted over 300 attacks on health facilities and workers, killing at least 6 responders and displacing communities. Conflict dynamics causally prolonged the epidemic by restricting safe burial teams—essential for breaking transmission chains—and fostering vaccine hesitancy amid rumors of Western bioweapons; empirical models estimate violence doubled transmission rates in affected zones by hindering 20-30% of interventions. Over 300,000 rVSV-ZEBOV doses were administered in ring and frontline vaccination, reducing incidence by up to 97% in vaccinated clusters per cluster-randomized trials, though logistical sabotage limited reach. South Kivu saw spillover cases, but no sustained chains.[174][2][168][175] These cycles demonstrated that while virological and therapeutic advances like vaccination curbed smaller outbreaks, endemic insecurity in eastern DRC—rooted in resource disputes and weak governance—functioned as a force multiplier for EVD persistence, with on-ground data from responders indicating conflict-related delays accounted for excess mortality beyond baseline fatality rates.[169][176]Recent Outbreaks (2023–2025)
In September 2022, an outbreak of Sudan ebolavirus disease began in Uganda, with cases reported primarily in the Mubende and Kassanda districts, extending into 2023 across nine districts including Kampala.[177] By its declaration of end on January 11, 2023, health authorities recorded 164 cases (142 laboratory-confirmed and 22 probable) and 77 deaths (55 among confirmed cases and 22 among probable cases), yielding a case-fatality rate of approximately 47%.[177] [178] The outbreak was contained through rigorous contact tracing, isolation of cases, safe burial practices, and community engagement, despite the absence of a licensed vaccine specific to the Sudan strain; supportive care and experimental monoclonal antibodies were deployed in select cases, contributing to 87 recoveries among confirmed patients.[177] On September 4, 2025, the Democratic Republic of the Congo declared an outbreak of Zaire ebolavirus disease in the Kasai Province, centered in the Bulape health zone and spreading to adjacent areas including Mweka.[179] As of October 12, 2025, cumulative figures stood at 64 cases (53 confirmed and 11 probable) and 45 deaths (34 confirmed and 11 probable) across six affected health zones, with a case-fatality rate exceeding 70%; at least four health workers were among the fatalities in the initial phase.[180] Response efforts included deployment of the Ervebo vaccine in ring vaccination strategies, monoclonal antibody treatments such as REGN-EB3, and intensified surveillance, though challenges persisted due to the region's limited infrastructure and cross-border proximity to Angola and seven other provinces.[180] [181] The outbreak remained active as of late October 2025, with no secondary exportations reported beyond the province.[180]Societal Impacts
Economic and Infrastructure Burdens
The 2014–2016 West Africa Ebola epidemic imposed significant economic costs, with World Bank analyses estimating GDP losses of approximately $2.2 billion across Guinea, Liberia, and Sierra Leone in 2014 under a low-impact scenario, escalating to $7.4 billion in a high-impact projection; cumulative losses through 2015 reached up to $25.2 billion regionally when accounting for broader trade and labor disruptions.[182] These impacts equated to 2–4% annual GDP contractions in the hardest-hit nations, driven by halted commerce, reduced agricultural output, and investor flight, with one study pegging total regional economic damage at $32.6 billion over two years, or 3.3% of subregional GDP.[183] Broader assessments, including indirect effects like lost productivity and supply chain interruptions, elevated the figure to $53.19 billion for the three core countries alone.[184] Healthcare infrastructure faced acute overload, as existing facilities in low-resource settings proved insufficient for surge capacity, leading to widespread closures or conversions of hospitals and clinics solely for Ebola isolation; this diverted resources from routine care, contributing to excess deaths from treatable conditions like malaria and childbirth complications.[185] In Liberia, for instance, over 80% of hospital beds were repurposed by mid-2014, paralyzing non-Ebola services and amplifying systemic vulnerabilities in underfunded public health systems.[186] In the Democratic Republic of Congo's recurrent outbreaks from 2018 to 2020, economic disruptions centered on conflict-affected eastern provinces, where mining—accounting for much of local revenue—and cross-border trade halted amid quarantines and fear-driven market shutdowns, exacerbating poverty in mineral-dependent communities.[187] These cycles compounded baseline fragility, with GDP declines in outbreak zones reaching modeled peaks of up to 36% by the third year in sub-Saharan contexts, as export commodities like cobalt and gold faced export bans and logistics breakdowns.[188] Repeated epidemics have entrenched aid dependency patterns, wherein influxes of international funding for containment—totaling billions globally since 2014—bolster short-term responses but undermine incentives for domestic investment in resilient infrastructure, perpetuating reliance on external donors for surveillance and capacity building in affected African nations.[189] This dynamic, evident in post-outbreak reconstructions, delays economic recovery by prioritizing reactive aid over preventive health system fortification, as seen in Sierra Leone's pre-existing 78% donor-funded health sector prior to 2014.[190]Response Efficacy and Criticisms
Ring vaccination strategies using the rVSV-ZEBOV vaccine demonstrated high efficacy in containing Ebola transmission chains during outbreaks. In a 2015 cluster-randomized trial in Guinea, immediate vaccination of contacts reduced Ebola cases by over 80% compared to delayed vaccination, with vaccine effectiveness estimated at 100% (95% CI: 64.6-100%) in the interim analysis.61117-5/fulltext) 32621-6/fulltext) Similar ring vaccination in the Democratic Republic of Congo (DRC) from 2018-2020 helped limit spread in accessible areas, averting an estimated 80% or more of potential secondary infections in vaccinated clusters.[175] These empirical successes highlight targeted, rapid deployment as a causal factor in outbreak control, contrasting with broader systemic delays. The World Health Organization (WHO) faced substantial criticism for its sluggish initial response to the 2014 West Africa outbreak, delaying declaration of a Public Health Emergency of International Concern until August 8, 2014, despite cases emerging in March.[191] [192] Independent panels attributed this lag to leadership failures and inadequate surveillance, exacerbating the epidemic's scale to over 28,000 cases and 11,000 deaths.[193] Such delays stemmed from over-reliance on centralized decision-making, which overlooked early empirical signals from local health reports, allowing unchecked community transmission. In DRC outbreaks from 2018-2020, armed conflicts in North Kivu and Ituri provinces significantly impeded response efficacy, correlating with a 2-3 fold increase in reported cases per conflict event.[194] Vaccine hesitancy, fueled by mistrust in foreign aid and rumors of infertility or poisoning, reduced uptake rates to below 50% in some communities, despite high acceptance among healthcare workers.[195] [196] These local barriers, compounded by attacks on response teams, underscored how political instability and cultural skepticism amplified risks beyond technical interventions. Aid coordination suffered from documented mismanagement and corruption, undermining resource allocation. In Sierra Leone, audits revealed over $6 million in unaccounted Ebola funds by 2015, linked to procurement fraud and ghost workers, prolonging dependency on external support.[197] [198] In DRC, investigations exposed embezzlement in aid contracts during the 2018-2020 response, with up to 20% of funds diverted through inflated pricing and kickbacks, eroding local capacity building.[199] Critics argue this over-centralized model fostered inefficiency and elite capture, contrasting with calls for decentralizing authority to bolster indigenous health systems for sustained resilience.[200] Proponents of international aid counter that without such inflows, outbreaks would have been deadlier, though evidence of persistent losses questions net efficacy.[201]Cultural Practices and Risk Amplification
Traditional funeral rituals in affected West African communities often involve washing, dressing, and prolonged physical contact with the deceased's body, practices that expose participants to highly infectious bodily fluids containing peak viral loads postmortem. During the 2014–2016 epidemic, each unsafe burial generated an average of 2.58 secondary Ebola cases, effectively amplifying transmission by a factor of approximately 2–3 times compared to standard chains without such events.[202] [203] A single traditional funeral ceremony in Sierra Leone was epidemiologically linked to 85 confirmed cases, illustrating how these rituals can ignite superspreading clusters.[35] The World Health Organization estimated that at least 20% of new infections during the outbreak stemmed directly from burial-related exposures.[106] Bushmeat hunting and preparation, a longstanding dietary practice in Central and West African forest regions, perpetuates zoonotic spillovers by necessitating direct handling of infected animal carcasses, particularly fruit bats and nonhuman primates that serve as reservoirs. Transmission risks peak during butchering and processing, where contact with contaminated blood, tissues, and feces occurs without protective measures, rather than from cooked consumption alone.[204] Index cases in multiple outbreaks, including the 2014 epidemic, have been traced to these activities, with serological data confirming elevated filovirus exposure among bushmeat hunters.[205] [206] This reliance on wild game as a protein staple in rural economies sustains recurrent introduction events, as habitat encroachment and market trade heighten human-wildlife interfaces without altering core handling behaviors. Community-level education on transmission risks and promotion of protective protocols during burials and funerals mitigated amplification effects; rapid response interventions, including safe burial teams, reduced secondary transmissions from these practices by up to 50% in implemented districts through decreased unsafe contacts.[118] Similarly, awareness campaigns highlighting handling hazards have curbed bushmeat-related spillovers in targeted areas, though entrenched cultural dietary norms limit broader cessation without viable alternatives.[207] These practices underscore how human behaviors interfacing with infectious sources can exponentially escalate outbreak scales absent behavioral shifts.Zoonosis and Animal Involvement
Wildlife Reservoirs
Fruit bats of the family Pteropodidae, particularly species such as Eidolon helvum and Rousettus aegyptiacus, exhibit serological evidence of exposure to ebolaviruses, including antibodies detected in surveys across Central and West Africa.[37] [40] Multiple studies from 2012 to 2023 report EBOV-specific IgG antibodies in these bats, with prevalence rates up to 20-30% in some E. helvum populations in Gabon and the Democratic Republic of Congo, though viral RNA detection remains infrequent and no live virus isolation has confirmed asymptomatic persistence.[208] [209] R. aegyptiacus, a cave-roosting species, showed antibodies in samples from Uganda and the Central African Republic, linking to regional filovirus circulation without evidence of clinical disease in infected bats.[40] These findings stem from enzyme-linked immunosorbent assays (ELISA) and indirect immunofluorescence tests on blood and tissue samples, prioritizing frugivorous bats due to their dietary overlap with human foraging areas.[210] Bat ecology facilitates potential spillover through environmental contamination rather than direct contact. E. helvum forms large, migratory colonies in tree roosts, depositing guano and urine that can persist in foraging sites, while partially eaten fruit contaminated with saliva or feces serves as a vector for indirect exposure.[211] R. aegyptiacus roosts in dense cave clusters, amplifying guano accumulation, with serological positives correlating to high-density habitats in forested regions.[212] Experimental inoculations in 2024 demonstrated selective replication and vertical transmission in Egyptian fruit bats without overt pathology, supporting maintenance potential via shedding in saliva, urine, and feces.[43] Ecological models integrate bat distribution, habitat suitability, and anthropogenic factors to forecast spillover hotspots, predominantly in Central Africa's Congo Basin.[42] Species richness and population density of pteropodid bats correlate with outbreak indices, with forest fragmentation increasing overlap risks in areas like Gabon, Republic of Congo, and DRC borders, where 80% of historical spillovers align with predicted zones. [213] These models, validated against 1976-2020 events, emphasize seasonal bat birthing and migration pulses as amplifiers, projecting annual spillover probabilities based on deforestation rates exceeding 1% yearly in high-risk grids.[214]Domestic Animal Risks
Domestic pigs are susceptible to experimental infection with Zaire ebolavirus, developing mild respiratory symptoms, viremia, and the ability to transmit the virus to co-housed non-human primates via direct contact or aerosolized particles.[215] In these studies, infected pigs shed high viral loads from respiratory and oral secretions for up to 14 days post-infection, highlighting their potential as amplifiers despite lower pathogenicity compared to primates.[216] Natural infections in pigs remain rare, with serological surveys in West African countries like Guinea detecting antibodies in a subset of samples but no confirmed outbreaks or clinical cases.[217] [218] Non-human primates, including captive or farm-adjacent populations, serve as significant amplifiers due to their high susceptibility and mortality. In wild great ape populations, Ebola outbreaks have inflicted 90-98% fatality rates on gorillas and 77% on chimpanzees, decimating local groups and reducing global numbers by approximately one-third since the 1990s.[219] [220] [221] These events underscore primates' role in sustaining viral circulation near human settlements, though domestic confinement amplifies containment challenges. Culling of infected pigs has proven effective in experimental models by halting transmission chains, preventing onward spread to humans or other animals through rapid depopulation and biosecure disposal.[215] [222] Such measures, informed by swine disease control precedents, emphasize surveillance and prompt slaughter in endemic regions to mitigate farm-based amplification risks, as pigs' asymptomatic or mild presentation could delay detection.[223] Dogs, the other identified domestic host, show serological evidence of exposure but typically remain asymptomatic, posing lower amplification threats warranting monitoring rather than routine culling.[224]Reston Variant Distinctions
The Reston virus (RESTV), first detected in 1989 in cynomolgus macaques imported from the Philippines to a research facility in Reston, Virginia, represents the only ebolavirus species endemic to Asia rather than Africa.[225] Subsequent isolations occurred in 1990 and 1996 from similar monkey shipments originating in the Philippines, with a notable 2008 emergence in domestic pigs on four farms in Bulacan and Laguna provinces, where the virus caused respiratory symptoms and high mortality in swine co-infected with porcine reproductive and respiratory syndrome virus.[160] In humans, exposure during these events—such as among laboratory workers handling infected monkeys or pig farm personnel—has resulted in serological evidence of infection, including IgG antibodies detectable via enzyme-linked immunosorbent assay, yet no clinical disease or viremia has been reported, indicating asymptomatic seropositivity without progression to Ebola virus disease.[225][160] Genomically, RESTV shares a phylogenetic clade with Sudan ebolavirus, with nucleotide identities exceeding 80% across the ~19 kb non-segmented negative-sense RNA genome, but diverges significantly from human-pathogenic species like Zaire ebolavirus (case fatality rates up to 90%), Bundibugyo ebolavirus, and Taï Forest ebolavirus.[226] These distinctions are most pronounced in the glycoprotein (GP) precursor, which undergoes furin-mediated cleavage into GP1 (receptor-binding subunit) and GP2 (fusion subunit); RESTV's GP exhibits 30-40% amino acid divergence from Zaire GP, including fixed substitutions in the receptor-binding domain (e.g., residues influencing NPC1 receptor interaction) and heavily glycosylated mucin-like domain that hinder efficient binding to human endothelial cells and macrophages, thereby blocking viral entry, replication, and cytokine dysregulation central to hemorrhagic pathogenesis in other strains.[227][228] In silico modeling of 196 ebolavirus genomes confirms these GP-specific determinants as primary barriers to human tropism, with RESTV's adaptations favoring non-human primate and porcine hosts while rendering it avirulent in humans, though not excluding rare spillover or evolutionary shifts via recombination.[227][225]Research Frontiers
Vaccine Innovations
Following the approval of Ervebo (rVSV-ZEBOV) in 2019 for Zaire ebolavirus disease (EBOV), vaccine research has shifted toward candidates offering protection against multiple ebolavirus species, including Sudan ebolavirus (SUDV) and Bundibugyo ebolavirus (BDBV), to address the limitations of species-specific immunity.[88] Multivalent platforms, such as recombinant vesicular stomatitis virus (rVSV)-vectored vaccines incorporating glycoproteins from SUDV, have advanced to clinical stages; for instance, IAVI's rVSV-based SUDV candidate entered Phase 1 trials in 2023 and progressed to vaccination of participants in Uganda during the 2025 SUDV outbreak.[229] This trial, launched on February 3, 2025, by the World Health Organization and partners, represents the first evaluation of a SUDV-specific vaccine in an active outbreak setting, assessing safety, tolerability, and immunogenicity in a double-blinded, placebo-controlled design.[90] [230] Pan-ebolavirus vaccine candidates aim to elicit cross-protective responses against all five known ebolavirus species pathogenic to humans via multivalent antigen presentation. Preclinical studies have demonstrated efficacy of nanoparticle-based multivalent vaccines, which protected rodents from lethal challenges with adapted Zaire and Sudan viruses, highlighting potential for single-dose formulations targeting EBOV, SUDV, and BDBV glycoproteins.[231] Intranasal delivery platforms, such as human parainfluenza virus type 3 vectors expressing multiple ebolavirus glycoproteins, have shown promise in nonhuman primates for broad immunogenicity without requiring adjuvants.[232] These innovations build on viral vector and subunit technologies but face logistical hurdles, including stringent cold-chain requirements (-60°C to -80°C for many live-vectored candidates) that strain infrastructure in tropical outbreak zones with unreliable electricity and transport.[89] [233] Ongoing challenges underscore the need for thermostable formulations to enable rapid deployment; while some adenovirus-vectored regimens like Zabdeno/Mvabea require two doses and cold storage, emerging nanoparticle and particle-based designs seek to mitigate decay during last-mile distribution in resource-limited areas.[234] Clinical progression of these candidates remains contingent on demonstrating durable cross-species protection in human trials, with preclinical data indicating variable efficacy against heterologous strains due to glycoprotein sequence divergence.[231]Treatment Pipeline
The Ebola treatment pipeline emphasizes investigational antivirals, including small-molecule inhibitors targeting viral entry and replication, alongside next-generation monoclonal antibodies (mAbs). Small molecules such as toremifene inhibit EBOV fusion with host cells (EC50 = 0.162 μM), while MBX2270 disrupts NPC1 binding essential for entry.[235] Compounds like K11777 target proteolytic cleavage during entry (EC50 = 0.87 nM).[235] Broad-spectrum nucleoside analogs, including galidesivir (EC50 = 11.8 μM against EBOV), demonstrate potent in vitro activity but lack dedicated human EVD trials.[236][235] Opaganib, a host-targeted sphingosine kinase inhibitor, showed preclinical antiviral effects against Ebola and received BARDA funding in October 2024 to advance development.[237] Combination therapies enhance efficacy by addressing multiple viral stages; for instance, toremifene paired with mefloquine and posaconazole synergistically blocks entry and replication in cell cultures using FDA-approved drugs.[236] Such approaches leverage known pharmacokinetics to expedite deployment. For mAbs, MBP134AF—a bispecific cocktail—is undergoing clinical trials for Sudan ebolavirus disease in Uganda, providing cross-protection against EBOV, SUDV, and Bundibugyo ebolaviruses in animal models.[236] WHO-facilitated protocols in January 2025 enabled access to candidate mAbs and antivirals during the Uganda outbreak via trial mechanisms.[238] Scalability challenges hinder pipeline translation, with mAbs requiring specialized bioreactors and stringent cold-chain storage, limiting production in resource-poor settings. Small molecules offer advantages through straightforward chemical synthesis, ambient stability, and oral bioavailability, facilitating mass production.[235] Access inequities exacerbate risks: U.S. stockpiles hold approved mAb treatments like Inmazeb and Ebanga, registered post-2020 PALM trial, yet these remain unavailable or unregistered in African nations prone to outbreaks.[239] As of 2023, fragmented R&D prioritizes Western stockpiling over equitable global access, underscoring needs for localized manufacturing and African-led trials.[239]Modeling and Surveillance Advances
Phylodynamic models integrate phylogenetic data from viral genomes with epidemiological parameters to estimate transmission dynamics, including the basic reproduction number (R0). For Ebola Zaire virus, meta-analyses of outbreak data yield a pooled mean R0 of 1.94 (95% CI 1.73–2.15), while species-specific estimates for Bundibugyo virus reach 2.0 (95% CI 1.25–2.76).[240] Bayesian phylodynamic inference, applied to the 2014 Sierra Leone outbreak, forecasted R0 values ranging from 2.40 (95% HPD 1.54–3.87) assuming a 5.3-day latent period to 3.81 (95% HPD 2.47–6.3) for shorter latencies, enabling retrospective validation of superspreading events and forward projections of epidemic trajectories.[241] These models leverage serial interval distributions and coalescent processes to predict outbreak scale from genomic sampling, with posterior predictive simulations assessing model adequacy against observed phylogenies.[242] Genomic surveillance has advanced through portable, real-time sequencing protocols deployable in outbreak settings, achieving near-complete genome coverage to trace interhost variants and intrahost evolution. During the 2014–2016 epidemics, Illumina and MinION platforms sequenced hundreds of Ebola genomes at depths exceeding 2000×, revealing rapid mutation accumulation and single introductions seeding regional spread.[243] Pre-emptive assays combining 31 parallel PCRs with nanopore sequencing enable generic ebolavirus detection, facilitating early identification of emerging lineages before symptomatic surges.[244] In-country laboratories, such as those established in Liberia, support ongoing monitoring by generating sequences for phylogenetic reconstruction, distinguishing zoonotic spillovers from human-to-human chains with resolutions down to days.[245] Predictive models for ebolavirus spillover incorporate environmental covariates like forest loss and human population density to forecast annual outbreak probabilities. One such framework estimates spillover likelihood by integrating remote sensing data on habitat disruption, projecting heightened risks in Central African hotspots where bat reservoirs overlap with bushmeat hunting.[205] Multivariate logistic regressions using clinical-epidemiological features, such as fever onset and exposure history, achieve high specificity for early EVD case prediction, aiding triage in low-resource surveillance.[246] Machine learning extensions, including decision trees and neural networks trained on historical incidence, outperform traditional SIR models in anticipating case surges, with applications validated against 2014–2016 data for subnational forecasting.[247] Wastewater-based surveillance remains exploratory for Ebola, given the virus's persistence in untreated sewage for hours to days under simulated conditions, but lacks routine implementation due to sporadic outbreaks and infrastructural gaps in endemic regions. Pilot efforts in Uganda target multi-pathogen detection via CRISPR-Cas systems in urban effluents, potentially extending to Ebola for non-invasive early signals, though sensitivity thresholds require validation against clinical metrics.[248][249] Post-2020, AI-enhanced contact tracing draws from COVID-19 lessons to optimize Ebola responses, using graph neural networks to prioritize high-risk networks and reduce tracing delays by analyzing mobility data alongside genomic clusters. In DRC outbreaks, digital tools integrated with community reporting improved follow-up completeness, curbing secondary transmission risks by 84% among traced contacts, though scalability hinges on data privacy and connectivity in remote areas.[250][251]References
- https://www.cdc.gov/vhf/ebola/history/[chronology](/page/Chronology).html

