Recent from talks
Nothing was collected or created yet.
Preterm birth
View on Wikipedia
| Preterm birth | |
|---|---|
| Other names | Premature birth, preemies, premmies |
 | |
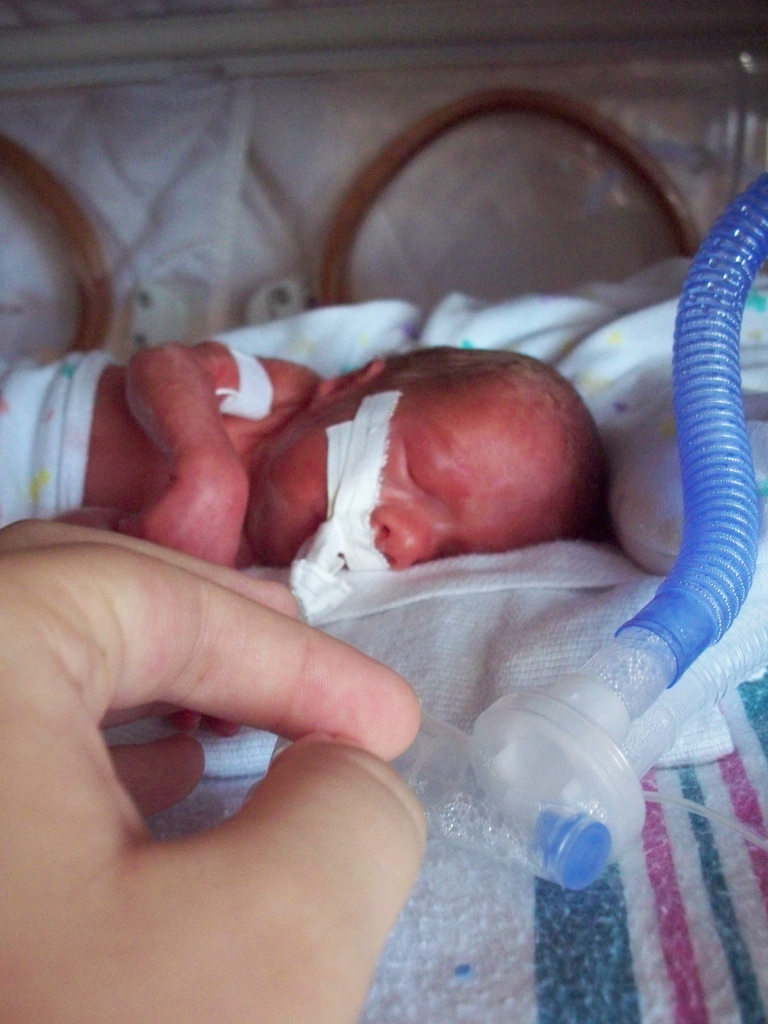
| Intubated preterm baby in an incubator | |
| Specialty | Neonatology, Pediatrics, Obstetrics |
| Symptoms | Birth of a baby at younger than 37 weeks' gestational age[1] |
| Complications | Cerebral palsy, delays in development, hearing problems, sight problems[1] |
| Causes | Often unknown[2] |
| Risk factors | Diabetes, high blood pressure, Multiple gestation, obesity or underweight, a number of vaginal infections, celiac disease, tobacco smoking, psychological stress[2][3][4] |
| Prevention | Progesterone[5] |
| Treatment | Corticosteroids, keeping the baby warm through skin-to-skin contact, supporting breastfeeding, treating infections, supporting breathing[2][6] |
| Frequency | ~15 million a year (12% of deliveries)[2] |
| Deaths | 805,800[7] |
Preterm birth, also known as premature birth, is the birth of a baby at fewer than 37 weeks gestational age, as opposed to full-term delivery at approximately 40 weeks.[1] Extreme preterm[2] is less than 28 weeks, very early preterm birth is between 28 and 32 weeks, early preterm birth occurs between 32 and 34 weeks, late preterm birth is between 34 and 36 weeks' gestation.[8] These babies are also known as premature babies or colloquially preemies (American English)[9] or premmies (Australian English).[10] Symptoms of preterm labor include uterine contractions which occur more often than every ten minutes and/or the leaking of fluid from the vagina before 37 weeks.[11][12] Premature infants are at greater risk for cerebral palsy, delays in development, hearing problems and problems with their vision.[1] The earlier a baby is born, the greater these risks will be.[1]
The cause of spontaneous preterm birth is often not known.[2] Risk factors include diabetes, high blood pressure, multiple gestation (being pregnant with more than one baby), being either obese or underweight, vaginal infections, air pollution exposure, tobacco smoking, and psychological stress.[2][3][13] For a healthy pregnancy, medical induction of labor or cesarean section are not recommended before 39 weeks unless required for other medical reasons.[2] There may be certain medical reasons for early delivery such as preeclampsia.[14]
Preterm birth may be prevented in those at risk if the hormone progesterone is taken during pregnancy.[5] Evidence does not support the usefulness of bed rest to prevent preterm labor.[5][15] Of the approximately 900,000 preterm deaths globally in 2019, it is estimated that at least 75% of these preterm infants would have survived with appropriate cost-effective treatment, and the survival rate is highest among the infants born the latest in gestation.[2] In women who might deliver between 24 and 37 weeks, corticosteroid treatment may improve outcomes.[6][16] A number of medications, including nifedipine, may delay delivery so that a mother can be moved to where more medical care is available and the corticosteroids have a greater chance to work.[17] Once the baby is born, care includes keeping the baby warm through skin-to-skin contact or incubation, supporting breastfeeding and/or formula feeding, treating infections, and supporting breathing.[2] Preterm babies sometimes require intubation.[2]
Preterm birth is the most common cause of death among infants worldwide.[1] About 15 million babies are preterm each year (5% to 18% of all deliveries).[2] Late preterm birth accounts for 75% of all preterm births.[18] This rate is inconsistent across countries. In the United Kingdom 7.9% of babies are born pre-term and in the United States 12.3% of all births are before 37 weeks gestation.[19][20] Approximately 0.5% of births are extremely early periviable births (20–25 weeks of gestation), and these account for most of the deaths.[21] In many countries, rates of premature births have increased between the 1990s and 2010s.[2] Complications from preterm births resulted globally in 0.81 million deaths in 2015, down from 1.57 million in 1990.[7][22] The chance of survival at 22 weeks is about 6%, while at 23 weeks it is 26%, 24 weeks 55% and 25 weeks about 72%.[23] The chances of survival without any long-term difficulties are lower.[24]
Signs and symptoms
[edit]
Signs and symptoms of preterm labor include four or more uterine contractions in one hour. In contrast to false labour, true labor is accompanied by cervical dilation and effacement. Also, vaginal bleeding in the third trimester, heavy pressure in the pelvis, or abdominal or back pain could be indicators that a preterm birth is about to occur. A watery discharge from the vagina may indicate premature rupture of the membranes that surround the baby. While the rupture of the membranes may not be followed by labor, usually delivery is indicated as infection (chorioamnionitis) is a serious threat to both fetus and mother. In some cases, the cervix dilates prematurely without pain or perceived contractions, so that the mother may not have warning signs until very late in the birthing process.
Causes
[edit]Preterm labor can be caused by medical conditions including infections, environmental or drug exposures, cervix insufficiency, uterine abnormalities, or amniotic fluid problems.[25] Some women require preterm labor induction for medical conditions.[25] Other preterm births are spontaneous. In some cases, preterm labor occurs spontaneously and there is no identifiable cause.[25] There are maternal and pregnancy-related risk factors that increase the risk of a women experiencing preterm labor.
Risk factors of preterm labor
[edit]The exact cause of spontaneous preterm birth is difficult to determine and it may be caused by many different factors at the same time as labor is a complex process.[26][27] The research available is limited with regard to the cervix and therefore is limited in discerning what is or is not normal.[12] Four different pathways have been identified that can result in preterm birth and have considerable evidence: precocious fetal endocrine activation, uterine overdistension (placental abruption), decidual bleeding, and intrauterine inflammation or infection.[28]
Identifying women at high risk of giving birth early would enable the health services to provide specialized care for these women and their babies, for example a hospital with a special care baby unit such as a neonatal intensive care unit (NICU). In some instances, it may be possible to delay the birth. Risk scoring systems have been suggested as an approach to identify those at higher risk; however, there is no strong research in this area so it is unclear whether the use of risk scoring systems for identifying mothers would prolong pregnancy and reduce the numbers of preterm births or not.[29]
Maternal factors
[edit]| Risk factor | Relative risk[30] | 95% confidence interval[30] |
|---|---|---|
| Fetal fibronectin | 4.0 | 2.9–5.5 |
| Short cervical length | 2.9 | 2.1–3.9 |
| Prenatal Care Absent[31] | 2.9 | 2.8–3.0 |
| Chlamydia | 2.2 | 1.0–4.8 |
| Low socio-economic status | 1.9 | 1.7–2.2 |
| Large or small pregnancy weight gain | 1.8 | 1.5–2.3 |
| Short maternal height | 1.8 | 1.3–2.5 |
| Periodontitis | 1.6 | 1.1–2.3 |
| Celiac disease | 1.4[32] | 1.2–1.6[32] |
| Asymptomatic bacteriuria | 1.1 | 0.8–1.5 |
| High or low BMI | 0.96 | 0.66–1.4 |
| odds ratio | ||
| History of spontaneous preterm birth | 3.6 | 3.2–4.0 |
| Bacterial vaginosis | 2.2 | 1.5–3.1 |
| Black ethnicity/race | 2.0 | 1.8–2.2 |
| Filipino ancestry[33] | 1.7 | 1.5–2.1 |
| Unwanted pregnancy[34]: 1 | 1.5 | 1.41–1.61 |
| Unintended pregnancy[34]: 1 | 1.31 | 1.09–1.58 |
| Being single/unmarried[35] | 1.2 | 1.03–1.28 |

Risk factors in the mother have been identified that are linked to a higher risk of a preterm birth. These include age (either very young or older),[36] high or low body mass index (BMI),[37][38] length of time between pregnancies,[39] endometriosis,[40] previous spontaneous (i.e., miscarriage) or surgical abortions,[41][42] unintended pregnancies,[34] untreated or undiagnosed celiac disease,[32][4] fertility difficulties, heat exposure,[43] and genetic variables.[44]
Studies on type of work and physical activity have given conflicting results, but it is opined that stressful conditions, hard labor, and long hours are probably linked to preterm birth.[36] Obesity does not directly lead to preterm birth;[45] however, it is associated with diabetes and hypertension which are risk factors by themselves.[36] To some degree those individuals may have underlying conditions (i.e., uterine malformation, hypertension, diabetes) that persist. Couples who have tried more than one year versus those who have tried less than one year before achieving a spontaneous conception have an adjusted odds ratio of 1.35 (95% confidence interval 1.22–1.50) of preterm birth.[46] Pregnancies after IVF confers a greater risk of preterm birth than spontaneous conceptions after more than one year of trying, with an adjusted odds ratio of 1.55 (95% CI 1.30–1.85).[46]
Certain ethnicities may have a higher risk as well. For example, in the U.S. and the UK, Black women have preterm birth rates of 15–18%, more than double than that of the white population. Many Black women have higher preterm birth rates due to multiple factors but the most common is high amounts of chronic stress, which can eventually lead to premature birth.[47] Adult chronic disease is not always the case with premature birth in Black women, which makes the main factor of premature birth challenging to identify.[47] Filipinos are also at high risk of premature birth, and it is believed that nearly 11–15% of Filipinos born in the U.S. (compared to other Asians at 7.6% and whites at 7.8%) are premature.[48] Filipinos being a big risk factor is evidenced with the Philippines being the eighth-highest ranking in the world for preterm births, the only non-African country in the top 10.[49] This discrepancy is not seen in comparison to other Asian groups or Hispanic immigrants and remains unexplained.[36] Genetic make-up is a factor in the causality of preterm birth. Genetics has been a big factor into why Filipinos have a high risk of premature birth as the Filipinos have a large prevalence of mutations that help them be predisposed to premature births.[48] An intra- and transgenerational increase in the risk of preterm delivery has been demonstrated.[44] No single gene has been identified.
Marital status has long been associated with risks for preterm birth. A 2005 study of 25,373 pregnancies in Finland revealed that unmarried mothers had more preterm deliveries than married mothers (P=0.001).[35] Pregnancy outside of marriage was associated overall with a 20% increase in total adverse outcomes, even at a time when Finland provided free maternity care. A study in Quebec of 720,586 births from 1990 to 1997 revealed less risk of preterm birth for infants with legally married mothers compared with those with common-law wed or unwed parents.[50][needs update] A study conducted in Malaysia in 2015 showed a similar trend, with marital status being significantly associated with preterm birth.[51] However, the result of a study conducted in the US showed that between 1989 and 2006, marriage became less protective of preterm births which was attributed to the changing social norms and behaviors surrounding marriage.[52]
Factors during pregnancy
[edit]Medications during pregnancy, living conditions, air pollution, smoking, illicit drugs or alcohol, infection, or physical trauma may also cause a preterm birth.
Air pollution: Living in an area with a high concentration of air pollution is a major risk factor for preterm labor, including living near major roadways or highways where vehicle emissions are high from traffic congestion or are a route for diesel trucks that tend to emit more pollution.[53][54][13]
The use of fertility medication that stimulates the ovary to release multiple eggs and of IVF with embryo transfer of multiple embryos has been implicated as a risk factor for preterm birth. Often labor has to be induced for medical reasons; such conditions include high blood pressure,[55] pre-eclampsia,[56] maternal diabetes,[57] asthma, thyroid disease, and heart disease.
Certain medical conditions in the pregnant mother may also increase the risk of preterm birth. Some women have anatomical problems that prevent the baby from being carried to term. These include a weak or short cervix (the strongest predictor of premature birth).[58][59][60][55] Women with vaginal bleeding during pregnancy are at higher risk for preterm birth. While bleeding in the third trimester may be a sign of placenta previa or placental abruption—conditions that occur frequently preterm—even earlier bleeding that is not caused by these conditions is linked to a higher preterm birth rate.[61] Women with abnormal amounts of amniotic fluid, whether too much (polyhydramnios) or too little (oligohydramnios), are also at risk.[36] Anxiety and depression have been linked as risk factors for preterm birth.[36][62]
The use of tobacco, cocaine, and excessive alcohol during pregnancy increases the chance of preterm delivery. Tobacco is the most commonly used drug during pregnancy and contributes significantly to low birth weight delivery.[63] Babies with birth defects are at higher risk of being born preterm.[64]
Passive smoking and/or smoking before the pregnancy influences the probability of a preterm birth. The World Health Organization published an international study in March 2014.[65]
Presence of anti-thyroid antibodies is associated with an increased risk preterm birth with an odds ratio of 1.9 and 95% confidence interval of 1.1–3.5.[66]
Intimate violence against the mother is another risk factor for preterm birth.[67]
Physical trauma may case a preterm birth. The Nigerian cultural method of abdominal massage has been shown to result in 19% preterm birth among women in Nigeria, plus many other adverse outcomes for the mother and baby.[68] This ought not be confused with massage therapy conducted by a fully trained and certified/licensed massage therapist or by significant others trained to provide massage during pregnancy, which—in a study involving pregnant females with prenatal depression—has been shown to have numerous positive results during pregnancy, including the reduction of preterm birth, less depression, lower cortisol, and reduced anxiety.[69] In healthy women, however, no effects have been demonstrated in a controlled study.
Infection
[edit]The frequency of infection in preterm birth is inversely related to the gestational age. Mycoplasma genitalium infection is associated with increased risk of preterm birth, and spontaneous abortion.[70]
Infectious microorganisms can be ascending, hematogenous, iatrogenic by a procedure, or retrograde through the fallopian tubes. From the deciduae they may reach the space between the amnion and chorion, the amniotic fluid, and the fetus. A chorioamnionitis also may lead to sepsis of the mother. Fetal infection is linked to preterm birth and to significant long-term disability including cerebral palsy.[71]
It has been reported that asymptomatic colonization of the decidua occurs in up to 70% of women at term using a DNA probe suggesting that the presence of micro-organism alone may be insufficient to initiate the infectious response.
As the condition is more prevalent in black women in the U.S. and the UK, it has been suggested to be an explanation for the higher rate of preterm birth in these populations. It is opined that bacterial vaginosis before or during pregnancy may affect the decidual inflammatory response that leads to preterm birth. The condition known as aerobic vaginitis can be a serious risk factor for preterm labor; several previous studies failed to acknowledge the difference between aerobic vaginitis and bacterial vaginosis, which may explain some of the contradiction in the results.[72]
Untreated yeast infections are associated with preterm birth.[73]
A review into prophylactic antibiotics (given to prevent infection) in the second and third trimester of pregnancy (13–42 weeks of pregnancy) found a reduction in the number of preterm births in women with bacterial vaginosis. These antibiotics also reduced the number of waters breaking before labor in full-term pregnancies, reduced the risk of infection of the lining of the womb after delivery (endometritis), and rates of gonococcal infection. However, the women without bacterial vaginosis did not have any reduction in preterm births or pre-labor preterm waters breaking. Much of the research included in this review lost participants during follow-up so did not report the long-term effects of the antibiotics on mothers or babies. More research in this area is needed to find the full effects of giving antibiotics throughout the second and third trimesters of pregnancy.[74]
A number of maternal bacterial infections are associated with preterm birth including pyelonephritis, asymptomatic bacteriuria, pneumonia, and appendicitis. A review into giving antibiotics in pregnancy for asymptomatic bacteriuria (urine infection with no symptoms) found the research was of very low quality but that it did suggest that taking antibiotics reduced the numbers of preterm births and babies with low birth weight.[75] Another review found that one dose of antibiotics did not seem as effective as a course of antibiotics but fewer women reported side effects from one dose.[76] This review recommended that more research is needed to discover the best way of treating asymptomatic bacteriuria.[75]
A different review found that preterm births happened less for pregnant women who had routine testing for low genital tract infections than for women who only had testing when they showed symptoms of low genital tract infections.[77] The women being routinely tested also gave birth to fewer babies with a low birth weight. Even though these results look promising, the review was only based on one study so more research is needed into routine screening for low genital tract infections.[77]
Also periodontal disease has been shown repeatedly to be linked to preterm birth.[78][79] In contrast, viral infections, unless accompanied by a significant febrile response, are considered not to be a major factor in relation to preterm birth.[36]
Genetics
[edit]There is believed to be a maternal genetic component in preterm birth.[80] Estimated heritability of timing-of-birth in women was 34%. However, the occurrence of preterm birth in families does not follow a clear inheritance pattern, thus supporting the idea that preterm birth is a non-Mendelian trait with a polygenic nature.[81]
Prenatal care
[edit]The absence of prenatal care has been associated with higher rates of preterm births. Analysis of 15,627,407 live births in the United States in 1995–1998 concluded that the absence of prenatal care carried a 2.9 (95%CI 2.8, 3.0) times higher risk of preterm births.[31] This same study found statistically significant relative risks of maternal anemia, intrapartum fever, unknown bleeding, renal disease, placental previa, hydramnios, placenta abruption, and pregnancy-induced hypertension with the absence of prenatal care. All these prenatal risks were controlled for other high-risk conditions, maternal age, gravidity, marital status, and maternal education. The absence of prenatal care prior to and during the pregnancy is primarily a function of socioeconomic factors (low family income and education), access to medical consultations (large distance from the place of residence to the healthcare unit and transportation costs), quality of healthcare, and social support.[82] Efforts to decrease rates of preterm birth should aim to increase the deficits posed by the aforementioned barriers and to increase access to prenatal care.
Diagnosis
[edit]Placental alpha microglobulin-1
[edit]Placental alpha microglobulin-1 (PAMG-1) has been the subject of several investigations evaluating its ability to predict imminent spontaneous preterm birth in women with signs, symptoms, or complaints suggestive of preterm labor.[83][84][85][86][87][88] In one investigation comparing this test to fetal fibronectin testing and cervical length measurement via transvaginal ultrasound, the test for PAMG-1 (commercially known as the PartoSure test) has been reported to be the single best predictor of imminent spontaneous delivery within 7 days of a patient presenting with signs, symptoms, or complaints of preterm labor. Specifically, the PPV, or positive predictive value, of the tests were 76%, 29%, and 30% for PAMG-1, fFN and CL, respectively (P < 0.01).[89]
Fetal fibronectin
[edit]Fetal fibronectin (fFN) has become an important biomarker—the presence of this glycoprotein in the cervical or vaginal secretions indicates that the border between the chorion and decidua has been disrupted. A positive test indicates an increased risk of preterm birth, and a negative test has a high predictive value.[36] It has been shown that only 1% of women in questionable cases of preterm labor delivered within the next week when the test was negative.[90]
Ultrasound
[edit]Obstetric ultrasound has become useful in the assessment of the cervix in women at risk for premature delivery. A short cervix preterm is undesirable: A cervical length of less than 25 mm (0.98 in) at or before 24 weeks of gestational age is the most common definition of cervical incompetence.[91]
Emerging technologies
[edit]Technologies under research and development to facilitate earlier diagnosis of preterm births include sanitary pads that identify biomarkers such as fFN and PAMG-1 in vaginal secretions. These devices then calculate a risk of preterm birth and send the findings to a smartphone.[92] The notion that risk-scoring systems are accurate in predicting preterm birth has been debated in multiple literature reviews.[93][94]
Classification
[edit]
In humans, the usual definition of preterm birth is birth before a gestational age of 37 complete weeks.[95] In the normal human fetus, several organ systems mature between 34 and 37 weeks, and the fetus reaches adequate maturity by the end of this period. One of the main organs greatly affected by premature birth is the lungs. The lungs are one of the last organs to mature in the womb; because of this, many premature babies spend the first days and weeks of their lives on ventilators. Therefore, a significant overlap exists between preterm birth and prematurity. Generally, preterm babies are premature and term babies are mature. Preterm babies born near 37 weeks often have no problems relating to prematurity if their lungs have developed adequate surfactant, which allows the lungs to remain expanded between breaths. Sequelae of prematurity can be reduced to a small extent by using drugs to accelerate maturation of the fetus, and to a greater extent by preventing preterm birth.
Prevention
[edit]Historically efforts have been primarily aimed to improve survival and health of preterm infants (tertiary intervention). Such efforts, however, have not reduced the incidence of preterm birth. Increasingly primary interventions that are directed at all women, and secondary intervention that reduce existing risks are looked upon as measures that need to be developed and implemented to prevent the health problems of premature infants and children.[96] Smoking bans are effective in decreasing preterm births.[97] Different strategies are used in the administration of prenatal care, and future studies need to determine if the focus can be on screening for high-risk women, or widened support for low-risk women, or to what degree these approaches can be merged.[96]
Before pregnancy
[edit]Adoption of specific professional policies can immediately reduce risk of preterm birth as the experience in assisted reproduction has shown when the number of embryos during embryo transfer was limited.[96] Many countries have established specific programs to protect pregnant women from hazardous or night-shift work and to provide them with time for prenatal visits and paid pregnancy-leave. The EUROPOP study showed that preterm birth is not related to type of employment, but to prolonged work (over 42 hours per week) or prolonged standing (over 6 hours per day).[98] Also, night work has been linked to preterm birth.[99] Health policies that take these findings into account can be expected to reduce the rate of preterm birth.[96] Preconceptional intake of folic acid is recommended to reduce birth defects. There is also some evidence that folic acid supplement preconceptionally (before becoming pregnant) may reduce premature birth.[100] Reducing smoking is expected to benefit pregnant women and their offspring.[96]
During pregnancy
[edit]Self-care methods to reduce the risk of preterm birth include proper nutrition, avoiding stress, seeking appropriate medical care, avoiding infections, and the control of preterm birth risk factors (e.g. working long hours while standing on feet, carbon monoxide exposure, domestic abuse, and other factors).[101] Reducing physical activity during pregnancy has not been shown to reduce the risk of a preterm birth.[102] Healthy eating can be instituted at any stage of the pregnancy including nutritional adjustments and consuming suggested vitamin supplements.[96] Calcium supplementation in women who have low dietary calcium may reduce the number of negative outcomes including preterm birth, pre-eclampsia, and maternal death.[103] The World Health Organization (WHO) suggests 1.5–2 g of calcium supplements daily, for pregnant women who have low levels of calcium in their diet.[104] Supplemental intake of C and E vitamins have not been found to reduce preterm birth rates.[105]
While periodontal infection has been linked with preterm birth, randomized trials have not shown that periodontal care during pregnancy reduces preterm birth rates.[96] Smoking cessation has also been shown to reduce the risk.[106] The use of personal at home uterine monitoring devices to detect contractions and possible preterm births in women at higher risk of having a preterm baby have been suggested.[107] These home monitors may not reduce the number of preterm births, however, using these devices may increase the number of unplanned antenatal visits and may reduce the number of babies admitted to special care when compared with women receiving normal antenatal care.[107] Support from medical professionals, friends, and family during pregnancy may be beneficial at reducing caesarean birth and may reduce prenatal hospital admissions; however, these social supports alone may not prevent preterm birth.[108]
Screening during pregnancy
[edit]Screening for asymptomatic bacteriuria followed by appropriate treatment reduces pyelonephritis and reduces the risk of preterm birth.[109] Extensive studies have been carried out to determine if other forms of screening in low-risk women followed by appropriate intervention are beneficial, including screening for and treatment of Ureaplasma urealyticum, group B streptococcus, Trichomonas vaginalis, and bacterial vaginosis did not reduce the rate of preterm birth.[96] Routine ultrasound examination of the length of the cervix may identify women at risk of preterm labour and tentative evidence suggests ultrasound measurement of the length of the cervix in those with preterm labor can help adjust management and results in the extension of pregnancy by about four days.[110] Screening for the presence of fibronectin in vaginal secretions is not recommended at this time in women at low risk of preterm birth.[medical citation needed]
Reducing existing risks
[edit]Women are identified to be at increased risk for preterm birth on the basis of their past obstetrical history or the presence of known risk factors. Preconception intervention can be helpful in selected patients in a number of ways. Patients with certain uterine anomalies may have a surgical correction (i.e. removal of a uterine septum), and those with certain medical problems can be helped by optimizing medical therapies prior to conception, be it for asthma, diabetes, hypertension, and others.
Multiple pregnancies
[edit]In multiple pregnancies, which often result from use of assisted reproductive technology, there is a high risk of preterm birth. Selective reduction is used to reduce the number of fetuses to two or three.[111][112][113]
Reducing indicated preterm birth
[edit]A number of agents have been studied for the secondary prevention of indicated preterm birth. Trials using low-dose aspirin, fish oil, vitamin C and E, and calcium to reduce preeclampsia demonstrated some reduction in preterm birth only when low-dose aspirin was used.[96] Even if agents such as calcium or antioxidants were able to reduce preeclampsia, a resulting decrease in preterm birth was not observed.[96]
Reducing spontaneous preterm birth
[edit]Reduction in activity by the mother—pelvic rest, limited work, bed rest—may be recommended although there is no evidence it is useful with some concerns it is harmful.[114] Increasing medical care by more frequent visits and more education has not been shown to reduce preterm birth rates.[108] Use of nutritional supplements such as omega-3 polyunsaturated fatty acids is based on the observation that populations who have a high intake of such agents are at low risk for preterm birth, presumably as these agents inhibit production of proinflammatory cytokines. A randomized trial showed a significant decline in preterm birth rates,[115] and further studies are in the making.
Antibiotics
[edit]While antibiotics can get rid of bacterial vaginosis in pregnancy, this does not appear to change the risk of preterm birth.[116] It has been suggested that chronic chorioamnionitis is not sufficiently treated by antibiotics alone (and therefore they cannot ameliorate the need for preterm delivery in this condition).[96]
Progestogens
[edit]Progestogens—often given in the form of vaginal[117] progesterone or hydroxyprogesterone caproate—relax the uterine musculature, maintain cervical length, and possess anti-inflammatory properties; all of which invoke physiological and anatomical changes considered to be beneficial in reducing preterm birth. Two meta-analyses demonstrated a reduction in the risk of preterm birth in women with recurrent preterm birth by 40–55%.[118][119]
Progestogen supplementation also reduces the frequency of preterm birth in pregnancies where there is a short cervix.[120] A short cervix is one that is less than 25mm, as detected during a transvaginal cervical length assessment in the midtrimester.[121] However, progestogens are not effective in all populations, as a study involving twin gestations failed to see any benefit.[122] Despite extensive research related to progestogen effectiveness, uncertainties remain concerning types of progesterone and routes of administration.[123]
Cervical cerclage
[edit]In preparation for childbirth, the woman's cervix shortens. Preterm cervical shortening is linked to preterm birth and can be detected by ultrasonography. Cervical cerclage is a surgical intervention that places a suture around the cervix to prevent its shortening and widening. Numerous studies have been performed to assess the value of cervical cerclage and the procedure appears helpful primarily for women with a short cervix and a history of preterm birth.[120][124] Instead of a prophylactic cerclage, women at risk can be monitored during pregnancy by sonography, and when shortening of the cervix is observed, the cerclage can be performed.[96]
Treatment
[edit]
Tertiary interventions are aimed at women who are about to go into preterm labor, or rupture the membranes or bleed preterm. The use of the fibronectin test and ultrasonography improves the diagnostic accuracy and reduces false-positive diagnosis. While treatments to arrest early labor where there is progressive cervical dilatation and effacement will not be effective to gain sufficient time to allow the fetus to grow and mature further, it may defer delivery sufficiently to allow the mother to be brought to a specialized center that is equipped and staffed to handle preterm deliveries.[125] In a hospital setting women are hydrated via intravenous infusion (as dehydration can lead to premature uterine contractions).[126]
If a baby has cardiac arrest at birth and is less than 22 to 24 weeks gestational age, attempts at resuscitation are not generally indicated.[127]
Steroids
[edit]Severely premature infants may have underdeveloped lungs because they are not yet producing their own surfactant. This can lead directly to respiratory distress syndrome, also called hyaline membrane disease, in the neonate. To try to reduce the risk of this outcome, pregnant mothers with threatened premature delivery prior to 34 weeks are often administered at least one course of glucocorticoids, an antenatal steroid that crosses the placental barrier and stimulates the production of surfactant in the lungs of the baby.[16] Steroid use up to 37 weeks is also recommended by the American Congress of Obstetricians and Gynecologists.[16] Typical glucocorticoids that would be administered in this context are betamethasone or dexamethasone, often when the pregnancy has reached viability at 23 weeks.[citation needed]
In cases where premature birth is imminent, a second "rescue" course of steroids may be administered 12 to 24 hours before the anticipated birth. There are still some concerns about the efficacy and side effects of a second course of steroids, but the consequences of RDS are so severe that a second course is often viewed as worth the risk. A 2015 Cochrane review (updated in 2022) supports the use of repeat dose(s) of prenatal corticosteroids for women still at risk of preterm birth seven days or more after an initial course.[128]
A Cochrane review from 2020 recommends the use of a single course of antenatal corticosteroids to accelerate fetal lung maturation in women at risk of preterm birth. Treatment with antenatal corticosteroids reduces the risk of perinatal death, neonatal death and respiratory distress syndrome and probably reduces the risk of IVH.[129]
Concerns about adverse effects of prenatal corticosteroids include increased risk for maternal infection, difficulty with diabetic control, and possible long-term effects on neurodevelopmental outcomes for the infants. There is ongoing discussion about when steroids should be given (i.e. only antenatally or postnatally too) and for how long (i.e. single course or repeated administration). Despite these unknowns, there is a consensus that the benefits of a single course of prenatal glucocorticosteroids vastly outweigh the potential risks.[130][131][132]
Antibiotics
[edit]The routine administration of antibiotics to all women with threatened preterm labor reduces the risk of the baby being infected with group B streptococcus and has been shown to reduce related mortality rates.[133]
When membranes rupture prematurely, obstetrical management looks for development of labor and signs of infection. Prophylactic antibiotic administration has been shown to prolong pregnancy and reduced neonatal morbidity with rupture of membranes at less than 34 weeks.[134] Because of concern about necrotizing enterocolitis, amoxicillin or erythromycin has been recommended but not amoxicillin + clavulanic acid (co-amoxiclav).[134]
Tocolysis
[edit]A number of medications may be useful to delay delivery including: nonsteroidal anti-inflammatory drugs, calcium channel blockers, beta mimetics, and atosiban.[135] Tocolysis rarely delays delivery beyond 24–48 hours.[136] This delay, however, may be sufficient to allow the pregnant woman to be transferred to a center specialized for management of preterm deliveries and give administered corticosteroids to reduce neonatal organ immaturity. Meta-analyses indicate that calcium-channel blockers and an oxytocin antagonist can delay delivery by 2–7 days, and β2-agonist drugs delay by 48 hours but carry more side effects.[96][137] Magnesium sulfate does not appear to be useful to prevent preterm birth.[138] Its use before delivery, however, does appear to decrease the risk of cerebral palsy.[139]
Mode of delivery
[edit]The routine use of caesarean section for early delivery of infants expected to have very low birth weight is controversial,[140] and a decision concerning the route and time of delivery probably needs to be made on a case-by-case basis.
Neonatal care
[edit]
In developed countries premature infants are usually cared for in a neonatal intensive care unit (NICU). The physicians who specialize in the care of very sick or premature babies are known as neonatologists. In the NICU, premature babies are kept under radiant warmers or in incubators (also called isolettes), which are bassinets enclosed in plastic with climate control equipment designed to keep them warm and limit their exposure to germs. Modern neonatal intensive care involves sophisticated measurement of temperature, respiration, cardiac function, oxygenation, and brain activity. After delivery, plastic wraps or warm mattresses are useful to keep the infant warm on their way to the NICU.[141] Treatments may include fluids and nutrition through intravenous catheters, oxygen supplementation, mechanical ventilation support, and medications.[142] In developing countries where advanced equipment and even electricity may not be available or reliable, simple measures such as kangaroo care (skin-to-skin warming), encouraging breastfeeding, and basic infection control measures can significantly reduce preterm morbidity and mortality. Kangaroo mother care (KMC) can decrease the risk of neonatal sepsis, hypothermia, hypoglycemia and increase exclusive breastfeeding.[143] Bili lights may also be used to treat newborn jaundice (hyperbilirubinemia).
Water can be carefully provided to prevent dehydration but not so much to increase risks of side effects.[144]
Breathing support
[edit]In terms of respiratory support, there may be little or no difference in the risk of death or chronic lung disease between high flow nasal cannulae (HFNC) and continuous positive airway pressure (CPAP) or nasal intermittent positive pressure ventilation (NPPV).[145] For extremely preterm babies (born before 28 weeks' gestation), targeting a higher versus a lower oxygen saturation range makes little or no difference overall to the risk of death or major disability.[146] Babies born before 32 weeks have been shown to have a lower risk of death from bronchopulmonary dysplasia if they have CPAP immediately after being born, compared to receiving either supportive care or assisted ventilation.[147]
There is insufficient evidence for or against placing preterm stable twins in the same cot or incubator (co-bedding).[148]
Nutrition
[edit]Meeting the appropriate nutritional needs of preterm infants is important for long-term health. Optimal care may require a balance of meeting nutritional needs and preventing complications related to feeding. The ideal growth rate is not known; however, preterm infants usually require a higher energy intake compared to babies who are born at term.[149] The recommended amount of milk is often prescribed based on approximated nutritional requirements of a similar aged fetus who is not compromised.[150] An immature gastrointestinal tract (GI tract), medical conditions (or co-morbidities), risk of aspirating milk, and necrotizing enterocolitis may lead to difficulties in meeting this high nutritional demand and many preterm infants have nutritional deficits that may result in growth restrictions.[150] In addition, very small preterm infants cannot coordinate sucking, swallowing, and breathing.[151] Tolerating a full enteral feeding (the prescribed volume of milk or formula) is a priority in neonatal care as this reduces the risks associated with venous catheters including infection, and may reduce the length of time the infant requires specialized care in the hospital.[150] Different strategies can be used to optimize feeding for preterm infants. The type of milk/formula and fortifiers, route of administration (by mouth, tube feeding, venous catheter), timing of feeding, quantity of milk, continuous or intermittent feeding, and managing gastric residuals are all considered by the neonatal care team when optimizing care. The evidence in the form of high quality randomized trials is generally fairly weak in this area, and for this reason different neonatal intensive care units may have different practices and this results in a fairly large variation in practice. The care of preterm infants also varies in different countries and depends on resources that are available.[150]
Human breast milk and formula
[edit]The American Academy of Pediatrics recommended feeding preterm infants human milk, finding "significant short- and long-term beneficial effects," including lower rates of necrotizing enterocolitis (NEC).[152] In the absence of evidence from randomised controlled trials about the effects of feeding preterm infants with formula compared with mother's own breast milk, data collected from other types of studies suggest that mother's own breast milk is likely to have advantages over formula in terms of the baby's growth and development.[153][149] Milk from human donors also reduces the risk of NEC by half in very low birth rate infants and very preterm infants.[23]
Breast milk or formula alone may not be sufficient to meet the nutritional needs of some preterm infants. Fortification of breast milk or formula by adding extra nutrients is an approach often taken for feeding preterm infants, with the goal of meeting the high nutritional demand.[149] High quality randomized controlled trials are needed in this field to determine the effectiveness of fortification.[154] It is unclear if fortification of breast milk improves outcomes in preterm babies, though it may speed growth.[154] Supplementing human milk with extra protein may increase short-term growth but the longer-term effects on body composition, growth and brain development are uncertain.[155][156] Higher protein formula (between 3 and 4 grams of protein per kilo of body weight) may be more effective than low protein formula (less than 3 grams per kilo per day) for weight gain in formula-fed low-birth-weight infants.[157] There is insufficient evidence about the effect on preterm babies' growth of supplementing human milk with carbohydrate,[158] fat,[159][160] and branched-chain amino acids.[161] Conversely, there is some indication that preterm babies who cannot breastfeed may do better if they are fed only with diluted formula compared to full strength formula but the clinical trial evidence remains uncertain.[162]
Individualizing the nutrients and quantities used to fortify enteral milk feeds in infants born with very low birth weight may lead to better short-term weight gain and growth but the evidence is uncertain for longer term outcomes and for the risk of serious illness and death.[163] This includes targeted fortification (adjusting the level of nutrients in response to the results of a test on the breast milk) and adjustable fortification (adding nutrients based on testing the infant).[163]
Multi-nutrient fortifier used to fortify human milk and formula has traditionally been derived from bovine milk.[164] Fortifier derived from humans is available; however, the evidence from clinical trials is uncertain and it is not clear if there are any differences between human-derived fortifier and bovine-derived fortifier in terms of neonatal weight gain, feeding intolerance, infections, or the risk of death.[164]
Timing of feeds
[edit]For very preterm infants, most neonatal care centres start milk feeds gradually, rather than starting with a full enteral feeding right away; however, it is not clear if starting full enteral feeding early affects the risk of necrotising enterocolitis.[150] In these cases, the preterm infant would be receiving the majority of their nutrition and fluids intravenously. The milk volume is usually gradually increased over the following weeks.[150] Research into the ideal timing of enteral feeding and whether delaying enteral feeding or gradually introducing enteral feeds is beneficial at improving growth for preterm infants or low birth weight infants is needed.[150] In addition, the ideal timing of enteral feeds to prevent side effects such as necrotising enterocolitis or mortality in preterm infants who require a packed red blood cell transfusion is not clear.[165] Potential disadvantages of a more gradual approach to feeding preterm infants associated with less milk in the gut and include slower GI tract secretion of hormones and gut motility and slower microbial colonization of the gut.[150]
Regarding the timing of starting fortified milk, preterm infants are often started on fortified milk/formula once they are fed 100 mL/kg of their body weight. Other some neonatal specialists feel that starting to feed a preterm infant fortified milk earlier is beneficial to improve intake of nutrients.[166] The risks of feeding intolerance and necrotising enterocolitis related to early versus later fortification of human milk are not clear.[166] Once the infant is able to go home from the hospital there is limited evidence to support prescribing a preterm (fortified) formula.[167]
Intermittent feeding versus continuous feeding
[edit]For infants who weigh less than 1500 grams, tube feeding is usually necessary.[151] Most often, neonatal specialists feed preterm babies intermittently with a prescribed amount of milk over a short period of time. For example, a feed could last 10–20 minutes and be given every 3 hours. This intermittent approach is meant to mimic conditions of normal bodily functions involved with feeding and allow for a cyclic pattern in the release of gastrointestinal tract hormones to promote development of the gastrointestinal system.[151] In certain cases, continuous nasogastric feeding is sometimes preferred. There is low to very low certainty evidence to suggest that low birth weight babies who receive continuous nasogastic feeding may reach the benchmark of tolerating full enteral feeding later than babies fed intermittently and it is not clear if continuous feeding has any effect on weight gain or the number of interruptions in feedings.[151] Continuous feeding may have little to no effect on length of body growth or head circumference and the effects of continuous feeding on the risk of developing necrotising enterocolitis is not clear.[151]
Since preterm infants with gastro-oesophageal reflux disease do not have a fully developed antireflux mechanism, deciding on the most effective approach for nutrition is important. It is not clear if continuous bolus intragastric tube feeding is more effective compared to intermittent bolus intragastric tube feeding for feeding preterm infants with gastroesophageal reflux disease.[168]
For infants who would benefit from intermittent bolus feeding, some infants may be fed using the "push feed" method using a syringe to gently push the milk or formula into the stomach of the infant. Others may be fed using a gravity feeding system where the syringe is attached directly to a tube and the milk or formula drips into the infant's stomach. It is not clear from medical studies which approach to intermittent bolus feeding is more effective or reduces adverse effects such as apnea, bradycardia, or oxygen desaturation episodes.[169][170]
High volume feeds
[edit]High-volume (more than 180 mL per kilogram per day) enteral feeds of fortified or non-fortified human breast milk or formula may improve weight gain while the pre-term infant is hospitalized, however, there is insufficient evidence to determine if this approach improves growth of the neonate and other clinical outcomes including length of hospital stay.[149] The risks or adverse effects associated with high-volume enteral feeding of preterm infants including aspiration pneumonia, reflux, apnea, and sudden oxygen desaturation episodes have not been reported in the trials considered in a 2021 systematic review.[149]
Parenteral (intraveneous) nutrition
[edit]For preterm infants who are born after 34 weeks of gestation ("late preterm infants") who are critically ill and cannot tolerate milk, there is some weak evidence that the infant may benefit from including amino acids and fats in the intravenous nutrition at a later time point (72 hours or longer from hospital admission) versus early (less than 72 hours from admission to hospital), however further research is required to understand the ideal timing of starting intravenous nutrition.[171]
Gastric residuals
[edit]For preterm infants in neonatal intensive care on gavage feeds, monitoring the volume and colour of gastric residuals, the milk and gastrointestinal secretions that remain in the stomach after a set amount of time, is common standard of care practice.[172] Gastric residual often contains gastric acid, hormones, enzymes, and other substances that may help improve digestion and mobility of the gastrointestinal tract.[172] Analysis of gastric residuals may help guide timing of feeds.[172] Increased gastric residual may indicate feeding intolerance or it may be an early sign of necrotizing enterocolitis.[172] Increased gastric residual may be caused by an underdeveloped gastrointestinal system that leads to slower gastric emptying or movement of the milk in the intestinal tract, reduced hormone or enzyme secretions from the gastrointestinal tract, duodenogastric reflux, formula, medications, and/or illness.[172] The clinical decision to discard the gastric residuals (versus re-feeding) is often individualized based on the quantity and quality of the residual.[172] Some experts also suggest replacing the fresh milk or curded milk and bile-stained aspirates, but not replacing haemorrhagic residual.[172] Evidence to support or refute the practice of re-feeding preterm infants with gastric residuals is lacking.[172]
Hyponatraemia and hypernatraemia
[edit]Imbalances of sodium (hyponatraemia and hypernatraemia) are common in babies born preterm.[173] Hypernatraemia (sodium levels in the serum of more than 145-150 mmol/L) is common early on in preterm babies and the risk of hyponatraemia (sodium levels of less than 135 nmol/L) increases after about a week of birth if left untreated and prevention approaches are not used.[173] Preventing complications associated with sodium imbalances is part of standard of care for preterm infants and includes careful monitoring of water and sodium given to the infant.[173] The optimal sodium dose given immediately after birth (first day) is not clear and further research is needed to understand the idea management approach.[173]
Hearing assessment
[edit]The Joint Committee on Infant Hearing (JCIH) state that for preterm infants who are in the neonatal intensive care unit (NICU) for a prolonged time should have a diagnostic audiologic evaluation before they are discharged from the hospital.[174] Well babies follow a 1-2-3-month benchmark timeline where they are screened, diagnosed, and receiving intervention for a hearing loss. However, for very premature babies, it might not be possible to complete a hearing screen at one month of age due to several factors. Once the baby is stable, an audiologic evaluation should be performed. For premature babies in the NICU, auditory brainstem response (ABR) testing is recommended. If the infant does not pass the screen, they should be referred for an audiologic evaluation by an audiologist.[174] If the infant is on aminoglycosides such as gentamicin for less than five days they should be monitored and have a follow-up 6–7 months of being discharged from the hospital to ensure there is no late onset hearing loss due to the medication.[174]
Outcomes and prognosis
[edit]
Preterm births can result in a range of problems including mortality and physical and mental delays.[181][182]
Mortality and morbidity
[edit]In the U.S. where many neonatal infections and other causes of neonatal death have been markedly reduced, prematurity is the leading cause of neonatal mortality at 25%.[183] Prematurely born infants are also at greater risk for having subsequent serious chronic health problems as discussed below.
The earliest gestational age at which the infant has at least a 50% chance of survival is referred to as the limit of viability. As NICU care has improved over the last 40 years, the limit of viability has reduced to approximately 24 weeks.[184][185] Most newborns who die, and 40% of older infants who die, were born between 20 and 25.9 weeks (gestational age), during the second trimester.[21]
As risk of brain damage and developmental delay is significant at that threshold even if the infant survives, there are ethical controversies over the aggressiveness of the care rendered to such infants. The limit of viability has also become a factor in the abortion debate.[186]
Specific risks for the preterm neonate
[edit]Preterm infants usually show physical signs of prematurity in reverse proportion to the gestational age. As a result, they are at risk for numerous medical problems affecting different organ systems.
- Neurological problems can include apnea of prematurity, hypoxic-ischemic encephalopathy (HIE), retinopathy of prematurity (ROP),[187] developmental disability, transient hyperammonemia, cerebral palsy, and intraventricular hemorrhage, the latter affecting 25% of babies born preterm, usually before 32 weeks of pregnancy.[188] Mild brain bleeds usually leave no or few lasting complications, but severe bleeds often result in brain damage or even death.[188] Neurodevelopmental problems have been linked to lack of maternal thyroid hormones, at a time when their own thyroid is unable to meet postnatal needs.[189]
- Cardiovascular complications may arise from the failure of the ductus arteriosus to close after birth: patent ductus arteriosus (PDA).
- Respiratory problems are common, specifically respiratory distress syndrome (RDS or IRDS) (previously called hyaline membrane disease). Another problem can be bronchopulmonary dysplasia or BPD, a form of chronic lung disease.
- Gastrointestinal and metabolic issues can arise from neonatal hypoglycemia, feeding difficulties, rickets of prematurity, hypocalcemia, inguinal hernia, and necrotizing enterocolitis (NEC).
- Hematologic complications include anemia of prematurity, thrombocytopenia, and hyperbilirubinemia (jaundice) that can lead to kernicterus.
- Infection, including sepsis, pneumonia, and urinary tract infection.[1]
Survival
[edit]The chance of survival at 22 weeks is about 6%, while at 23 weeks it is 26%, 24 weeks 55% and 25 weeks about 72% as of 2016.[190] With extensive treatment up to 30% of those who survive birth at 22 weeks survive longer term as of 2019.[191] The chances of survival without long-term difficulties is less.[24] Of those who survive following birth at 22 weeks 33% have severe disabilities.[191] In the developed world, overall survival is about 90% while in low-income countries survival rates are about 10%.[192]
Some children will adjust well during childhood and adolescence,[181] although disability is more likely nearer the limits of viability. A large study followed children born between 22 and 25 weeks until the age of 6 years old. Of these children, 46% had moderate to severe disabilities such as cerebral palsy, vision or hearing loss and learning disabilities, 34% had mild disabilities, and 20% had no disabilities; 12% had disabling cerebral palsy.[193] Up to 15% of premature infants have significant hearing loss.[194]
As survival has improved, the focus of interventions directed at the newborn has shifted to reduce long-term disabilities, particularly those related to brain injury.[181] Some of the complications related to prematurity may not be apparent until years after the birth. A long-term study demonstrated that the risks of medical and social disabilities extend into adulthood and are higher with decreasing gestational age at birth and include cerebral palsy, intellectual disability, disorders of psychological development, behavior, and emotion, disabilities of vision and hearing, and epilepsy.[195] Standard intelligence tests showed that 41% of children born between 22 and 25 weeks had moderate or severe learning disabilities when compared to the test scores of a group of similar classmates who were born at full term.[193] It is also shown that higher levels of education were less likely to be obtained with decreasing gestational age at birth.[195] People born prematurely may be more susceptible to developing depression as teenagers.[196] Some of these problems can be described as being within the executive domain and have been speculated to arise due to decreased myelinization of the frontal lobes.[197] Studies of people born premature and investigated later with MRI brain imaging, demonstrate qualitative anomalies of brain structure and grey matter deficits within temporal lobe structures and the cerebellum that persist into adolescence.[198] Throughout life they are more likely to require services provided by physical therapists, occupational therapists, or speech therapists.[181] They are more likely to develop type 1 diabetes (roughly 1.2 times the rate) and type 2 diabetes (1.5 times).[199]
Despite the neurosensory, mental and educational problems studied in school age and adolescent children born extremely preterm, the majority of preterm survivors born during the early years of neonatal intensive care are found to do well and to live fairly normal lives in young adulthood.[200] Young adults born preterm seem to acknowledge that they have more health problems than their peers, yet feel the same degree of satisfaction with their quality of life.[201]
Beyond the neurodevelopmental consequences of prematurity, infants born preterm have a greater risk for many other health problems. For instance, children born prematurely have an increased risk for developing chronic kidney disease.[202]
Epidemiology
[edit]
Preterm birth complicates 5–18% of births worldwide.[73] In Europe and many developed countries the preterm birth rate is generally 5–9%,[204] while in the U.S. from 2007 to 2022 the rate fluctuated from 9.6 to 10.5 per cent.[205]
As weight is easier to determine than gestational age, the World Health Organization tracks rates of low birth weight (< 2,500 grams), which occurred in 16.5% of births in less developed regions in 2000.[206] It is estimated that one third of these low birth weight deliveries are due to preterm delivery. Weight generally correlates to gestational age; however, infants may be underweight for other reasons than a preterm delivery. Neonates of low birth weight (LBW) have a birth weight of less than 2,500 g (5 lb 8 oz) and are mostly but not exclusively preterm babies as they also include small for gestational age (SGA) babies. Weight-based classification further recognizes Very Low Birth Weight (VLBW) which is less than 1,500 g, and Extremely Low Birth Weight (ELBW) which is less than 1,000 g.[207] Almost all neonates in these latter two groups are born preterm.
About 75% of nearly a million deaths due to preterm delivery would survive if provided warmth, breastfeeding, treatments for infection, and breathing support.[192] Complications from preterm births resulted in 740,000 deaths in 2013, down from 1.57 million in 1990.[22]
Society and culture
[edit]Economics
[edit]Preterm birth is a significant cost factor in healthcare, not even considering the expenses of long-term care for individuals with disabilities due to preterm birth. A 2003 study in the U.S. determined neonatal costs to be $224,400 for a newborn at 500–700 g versus $1,000 at over 3,000 g. The costs increase exponentially with decreasing gestational age and weight.[208] The 2007 Institute of Medicine report Preterm Birth[209] found that the 550,000 premature babies born each year in the U.S. run up about $26 billion in annual costs, mostly related to care in neonatal intensive care units, but the real tab may top $50 billion.[210]
Notable cases
[edit]James Elgin Gill (born on 20 May 1987 in Ottawa, Ontario, Canada) was the earliest premature baby in the world, until that record was broken in 2004. He was 128 days premature, 21 weeks 5 days gestation, and weighed 624 g (1 lb 6 oz). He survived.[211][212]
In 2014, Lyla Stensrud, born in San Antonio, Texas, U.S., became the youngest premature baby in the world. She was born at 21 weeks 4 days and weighed 410 grams (less than a pound). Kaashif Ahmad resuscitated the baby after she was born. As of November 2018, Lyla was attending preschool. She had a slight delay in speech, but no other known medical issues or disabilities.[213]
Amillia Taylor is also often cited as the most premature baby.[214] She was born on 24 October 2006 in Miami, Florida, U.S., at 21 weeks and 6 days' gestation.[215] This report has created some confusion as her gestation was measured from the date of conception (through in vitro fertilization) rather than the date of her mother's last menstrual period, making her appear 2 weeks younger than if gestation was calculated by the more common method. At birth, she was 23 cm (9 in) long and weighed 280 g (10 oz).[214] She had digestive and respiratory problems, together with a brain hemorrhage. She was discharged from the Baptist Children's Hospital on 20 February 2007.[214]
The record for the smallest premature baby to survive was held for a considerable amount of time by Madeline Mann, who was born in 1989 at 26 weeks, weighing 280.0 g (9.875 oz) and measuring 24 cm (9.5 in) long.[216] This record was broken in September 2004 by Rumaisa Rahman, who was born in the same hospital, Loyola University Medical Center in Maywood, Illinois.[217] at 25 weeks' gestation. At birth, she was 20 cm (8 in) long and weighed 261 g (9.2 oz).[218] Her twin sister was also a small baby, weighing 563 g (1 lb 3.9 oz) at birth. During pregnancy their mother had pre-eclampsia, requiring birth by caesarean section. The larger twin left the hospital at the end of December, while the smaller remained there until 10 February 2005 by which time her weight had increased to 1.18 kg (2 lb 10 oz).[219] Generally healthy, the twins had to undergo laser eye surgery to correct vision problems, a common occurrence among premature babies.
In May 2019, Sharp Mary Birch Hospital for Women & Newborns in San Diego announced that a baby nicknamed "Saybie" had been discharged almost five months after being born at 23 weeks' gestation and weighing 244 g (8.6 oz). Saybie was confirmed by Dr. Edward Bell of the University of Iowa, which keeps the Tiniest Babies Registry, to be the new smallest surviving premature baby in that registry.[220]
Born in February 2009, at Children's Hospitals and Clinics of Minnesota, Jonathon Whitehill was just 25 weeks' gestation with a weight of 310 g (11 oz). He was hospitalized in a neonatal intensive care unit for five months, and then discharged.[221]
Richard Hutchinson was born at Children's Hospitals and Clinics of Minnesota in Minneapolis, Minnesota, on June 5, 2020, at 21 weeks 2 days gestation. At birth he weighed 340 g (12 oz). He remained hospitalized until November 2020, when he was then discharged.[222][223]
On 5 July 2020 Curtis Means was born at the University of Alabama at Birmingham hospital at 21 weeks 1 day, and weighed 420 g (15 oz). He was discharged in April 2021.[224]
Born on 5 July 2024 at 21st week of gestation with a weight of 10 ounces, Nash Keen currently holds a title of the world's most premature child according to Guinness World Records. He was discharged from the hospital at the age of six months.[225]
Historical figures who were born prematurely include Johannes Kepler (born in 1571 at seven months' gestation), Isaac Newton (born in 1642, small enough to fit into a quart mug, according to his mother), Winston Churchill (born in 1874 at seven months' gestation), and Anna Pavlova (born in 1885 at seven months' gestation).[226]
Effect of the coronavirus pandemic
[edit]During the COVID-19 pandemic, a drastic drop in the rate of premature births was reported in many countries, ranging from a 20% reduction to a 90% drop in the starkest cases. Studies in Ireland and Denmark first noticed the phenomenon, and it has been confirmed elsewhere. There is no universally accepted explanation for this drop as of August 2020[update]. Hypotheses include additional rest and support for expectant mothers staying at home, less air pollution due to shutdowns and reduced car fumes, and reduced likelihood of catching other diseases and viruses in general due to the lockdowns.[227][needs update]
Research
[edit]Brain injury is common among preterms, ranging from white matter injury to intraventricular and cerebellar haemorrhages.[228] The characteristic neuropathology of preterms has been described as the "encephalopathy of prematurity".[229] The number of preterms that receive special education is doubled compared to the general population. School marks are lower and so are verbal learning, executive function, language skills, and memory performance scores,[230][231][232][233] as well as IQ scores.[231][233][234][235][236][237][238] Behaviourally, adolescents who were born very preterm and/or very low birth weight have similar self-reports of quality of life, health status and self-esteem as term controls.[239][240][241][242]
Various structural magnetic resonance studies found consistent reductions in whole brain volume.[233][234][236][237][243] The extensive list of particular regions with smaller volumes compared to controls includes many cortical areas (temporal, frontal, parietal, occipital and cingulate), the hippocampal regions, thalamus, basal ganglia, amygdala, brain stem, internal capsule, corpus callosum, and cerebellum. Brain volume reduction seems to be present throughout the whole brain. In contrast, larger volumes were found in some of the same areas including medial/anterior frontal, parietal and temporal cortex, cerebellum, middle temporal gyrus, parahippocampal gyrus, and fusiform gyrus, as well as larger lateral ventricles on average.[244] The cause of these inconsistencies are unknown. Additionally, reductions in cortical surface area/cortical thickness were found in the temporal lobes bilaterally and in left frontal and parietal areas.[235][245] Thicker cortex was found bilaterally in the medial inferior and anterior parts of the frontal lobes and in the occipital lobes. Gestational age was positively correlated with volumes of the temporal and fusiform gyri and sensorimotor cortex bilaterally, left inferior parietal lobule, brain stem, and various white matter tracts, as well as specific positive associations with the cerebellum and thalamus. Several structural brain alterations have been linked back to cognitive and behavioural outcome measures. For example, total brain tissue volume explained between 20 and 40% of the IQ and educational outcome differences between extremely preterm born adolescents and control adolescents.[236][237] In another study, a 25% quartile decrease in white matter values in middle temporal gyrus was associated with a 60% increase in the risk of cognitive impairment.[230] Nosarti and colleagues previously hypothesised that maturational patterns in preterm brains were consistent with the age-related stages typically observed in younger subjects. Their most recent study suggests, however, that their trajectory may not only be delayed but also fundamentally distinctive. Since both smaller and larger regional volumes were found in very preterm individuals compared to controls.[231]
The evidence to support the use of osteopathic manipulations to provide benefit in neonatal care is weak.[246][247]
See also
[edit]References
[edit]- ^ a b c d e f "Preterm Labor and Birth: Condition Information". National Institutes of Health. 3 November 2014. Archived from the original on 2 April 2015. Retrieved 7 March 2015.
- ^ a b c d e f g h i j k l m World Health Organization (November 2014). "Preterm birth Fact sheet N°363". Archived from the original on 7 March 2015. Retrieved 6 March 2015.
- ^ a b "What are the risk factors for preterm labor and birth?". National Institutes of Health. 3 November 2014. Archived from the original on 5 April 2015. Retrieved 7 March 2015.
- ^ a b Saccone G, Berghella V, Sarno L, Maruotti GM, Cetin I, Greco L, et al. (February 2016). "Celiac disease and obstetric complications: a systematic review and metaanalysis". American Journal of Obstetrics and Gynecology. 214 (2): 225–234. doi:10.1016/j.ajog.2015.09.080. hdl:11369/330101. PMID 26432464.
- ^ a b c "What treatments are used to prevent preterm labor and birth?". National Institutes of Health. 3 November 2014. Archived from the original on 2 April 2015. Retrieved 7 March 2015.
- ^ a b "What treatments can reduce the chances of preterm labor & birth?". National Institutes of Health. 11 June 2013. Archived from the original on 2 April 2015. Retrieved 7 March 2015.
- ^ a b Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (BD 2015 Mortality and Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ Brown HK, Speechley KN, Macnab J, Natale R, Campbell MK (June 2014). "Neonatal morbidity associated with late preterm and early term birth: the roles of gestational age and biological determinants of preterm birth". International Journal of Epidemiology. 43 (3): 802–814. doi:10.1093/ije/dyt251. PMC 4052131. PMID 24374829.
- ^ "preemie noun – Definition, pictures, pronunciation and usage notes | Oxford Advanced Learner's Dictionary at". Oxford Learners Dictionaries. Retrieved 6 May 2022.
- ^ "Premmie".
- ^ "What are the symptoms of preterm labor?". National Institutes of Health. 11 June 2013. Archived from the original on 2 April 2015. Retrieved 7 March 2015.
- ^ a b Vink J, Myers K (October 2018). "Cervical alterations in pregnancy". Best Practice & Research. Clinical Obstetrics & Gynaecology. Biological Basis and Prevention of Preterm Birth Treatment. 52: 88–102. doi:10.1016/j.bpobgyn.2018.03.007. PMC 6282836. PMID 30314740.
- ^ a b Korten I, Ramsey K, Latzin P (January 2017). "Air pollution during pregnancy and lung development in the child". Paediatric Respiratory Reviews. 21: 38–46. doi:10.1016/j.prrv.2016.08.008. PMID 27665510.
- ^ "What causes preterm labor and birth?". National Institutes of Health. 3 November 2014. Archived from the original on 2 April 2015. Retrieved 7 March 2015.
- ^ Sosa CG, Althabe F, Belizán JM, Bergel E (March 2015). "Bed rest in singleton pregnancies for preventing preterm birth". The Cochrane Database of Systematic Reviews. 2015 (3) CD003581. doi:10.1002/14651858.CD003581.pub3. PMC 7144825. PMID 25821121.
- ^ a b c "Antenatal Corticosteroid Therapy for Fetal Maturation". ACOG. October 2016. Archived from the original on 29 September 2016. Retrieved 27 September 2016.
- ^ Haram K, Mortensen JH, Morrison JC (March 2015). "Tocolysis for acute preterm labor: does anything work". The Journal of Maternal-Fetal & Neonatal Medicine. 28 (4): 371–378. doi:10.3109/14767058.2014.918095. PMID 24990666. S2CID 20078137.
- ^ Trilla CC, Medina MC, Ginovart G, Betancourt J, Armengol JA, Calaf J (August 2014). "Maternal risk factors and obstetric complications in late preterm prematurity". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 179: 105–109. doi:10.1016/j.ejogrb.2014.05.030. PMID 24965989.
- ^ Chow YH, Dattani N (26 February 2009). "Estimating conception statistics using gestational age information from NHS Numbers for Babies data". Health Statistics Quarterly. 41 (1): 21–27. doi:10.1057/hsq.2009.5. PMID 19320250. S2CID 23996035.
- ^ Mathews TJ, Miniño AM, Osterman MJ, Strobino DM, Guyer B (January 2011). "Annual summary of vital statistics: 2008". Pediatrics. 127 (1): 146–157. doi:10.1542/peds.2010-3175. PMC 4079290. PMID 21173001.
- ^ a b Ecker JL, Kaimal A, Mercer BM, Blackwell SC, deRegnier RA, Farrell RM, et al. (American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine) (October 2017). "Obstetric Care consensus No. 6: Periviable Birth". Obstetrics and Gynecology. 130 (4): e187 – e199. doi:10.1097/AOG.0000000000002352. PMID 28937572.
- ^ a b Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. (GBD 2013 Mortality and Causes of Death Collaborators) (January 2015). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–171. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
- ^ a b Quigley, Maria; Embleton, Nicholas D.; Meader, Nicholas; McGuire, William (6 September 2024). "Donor human milk for preventing necrotising enterocolitis in very preterm or very low-birthweight infants". The Cochrane Database of Systematic Reviews. 2024 (9) CD002971. doi:10.1002/14651858.CD002971.pub6. ISSN 1469-493X. PMC 11378496. PMID 39239939.
- ^ a b Jarjour IT (February 2015). "Neurodevelopmental outcome after extreme prematurity: a review of the literature". Pediatric Neurology. 52 (2): 143–152. doi:10.1016/j.pediatrneurol.2014.10.027. PMID 25497122.
- ^ a b c "What are the risk factors for preterm labor and birth? | NICHD - Eunice Kennedy Shriver National Institute of Child Health and Human Development". www.nichd.nih.gov. 9 May 2023. Retrieved 8 April 2025.
- ^ "Preterm birth". World Health Organization. 19 February 2018. Retrieved 20 May 2020.
- ^ Frey HA, Klebanoff MA (April 2016). "The epidemiology, etiology, and costs of preterm birth". Seminars in Fetal & Neonatal Medicine. 21 (2): 68–73. doi:10.1016/j.siny.2015.12.011. PMID 26794420.
- ^ Behrman RE, Butler AS, et al. (Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes) (2007). Biological Pathways Leading to Preterm Birth. National Academies Press (US).
- ^ Davey MA, Watson L, Rayner JA, Rowlands S (October 2015). "Risk-scoring systems for predicting preterm birth with the aim of reducing associated adverse outcomes". The Cochrane Database of Systematic Reviews. 2015 (10) CD004902. doi:10.1002/14651858.CD004902.pub5. PMC 7388653. PMID 26490698.
- ^ a b Unless otherwise given in boxes, reference is: Van Os M, Van Der Ven J, Kazemier B, Haak M, Pajkrt E, Mol BW, De Groot C (2013). "Individualizing the risk for preterm birth: An overview of the literature". Expert Review of Obstetrics & Gynecology. 8 (5): 435–442. doi:10.1586/17474108.2013.825481. S2CID 8036202.
- ^ a b Vintzileos AM, Ananth CV, Smulian JC, Scorza WE, Knuppel RA (November 2002). "The impact of prenatal care in the United States on preterm births in the presence and absence of antenatal high-risk conditions". American Journal of Obstetrics and Gynecology. 187 (5): 1254–1257. doi:10.1067/mob.2002.127140. PMID 12439515.
- ^ a b c Tersigni C, Castellani R, de Waure C, Fattorossi A, De Spirito M, Gasbarrini A, et al. (2014). "Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms". Human Reproduction Update. 20 (4): 582–593. doi:10.1093/humupd/dmu007. hdl:10807/56796. PMID 24619876.
- ^ "Premature Birth Fact Sheet" (PDF). Archived (PDF) from the original on 8 August 2014. Retrieved 8 August 2014.
- ^ a b c Shah PS, Balkhair T, Ohlsson A, Beyene J, Scott F, Frick C (February 2011). "Intention to become pregnant and low birth weight and preterm birth: a systematic review". Maternal and Child Health Journal. 15 (2): 205–216. doi:10.1007/s10995-009-0546-2. PMID 20012348. S2CID 20441901.
- ^ a b Raatikainen K, Heiskanen N, Heinonen S (October 2005). "Marriage still protects pregnancy". BJOG. 112 (10): 1411–1416. doi:10.1111/j.1471-0528.2005.00667.x. PMID 16167946. S2CID 13193685.
- ^ a b c d e f g h Goldenberg RL, Culhane JF, Iams JD, Romero R (January 2008). "Epidemiology and causes of preterm birth". Lancet. 371 (9606): 75–84. doi:10.1016/S0140-6736(08)60074-4. PMC 7134569. PMID 18177778.
- ^ Moldenhauer JS. "Risk factors present before pregnancy". Merck Manual Home Edition. Merck Sharp & Dohme. Archived from the original on 17 August 2010.
- ^ Hendler I, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. (March 2005). "The Preterm Prediction Study: association between maternal body mass index and spontaneous and indicated preterm birth". American Journal of Obstetrics and Gynecology. 192 (3): 882–886. doi:10.1016/j.ajog.2004.09.021. PMID 15746686.
- ^ Smith GC, Pell JP, Dobbie R (August 2003). "Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study". BMJ. 327 (7410): 313–0. doi:10.1136/bmj.327.7410.313. PMC 169644. PMID 12907483.
- ^ Berlac, Janne Foss; Hartwell, Dorthe; Skovlund, Charlotte Wessel; Langhoff-Roos, Jens; Lidegaard, Øjvind (June 2017). "Endometriosis increases the risk of obstetrical and neonatal complications". Acta Obstetricia et Gynecologica Scandinavica. 96 (6): 751–760. doi:10.1111/aogs.13111. PMID 28181672.
- ^ "The Care of Women Requesting Induced Abortion" (PDF). Evidence-based Clinical Guideline No. 7. Royal College of Obstetricians and Gynaecologists. November 2011. pp. 44, 45. Archived from the original (PDF) on 29 May 2012. Retrieved 31 May 2013.
- ^ Virk J, Zhang J, Olsen J (August 2007). "Medical abortion and the risk of subsequent adverse pregnancy outcomes". The New England Journal of Medicine. 357 (7): 648–653. doi:10.1056/NEJMoa070445. PMID 17699814. S2CID 14975701.
- ^ Barreca A, Schaller J (2020). "The impact of high ambient temperatures on delivery timing and gestational lengths". Nature Climate Change. 10: 77–82. doi:10.1038/s41558-019-0632-4. ISSN 1758-6798. S2CID 208538820.
- ^ a b Bhattacharya S, Amalraj Raja E, Ruiz Mirazo E, Campbell DM, Lee AJ, Norman JE, Bhattacharya S (June 2010). "Inherited predisposition to spontaneous preterm delivery". Obstetrics and Gynecology. 115 (6): 1125–1133. doi:10.1097/AOG.0b013e3181dffcdb. hdl:2164/2233. PMID 20502281. S2CID 10113798.
- Lay summary in: "Premature birth risk is genetic, researchers suspect". BBC News. 25 May 2010.
- ^ Tsur A, Mayo JA, Wong RJ, Shaw GM, Stevenson DK, Gould JB (October 2017). "'The obesity paradox': a reconsideration of obesity and the risk of preterm birth". Journal of Perinatology. 37 (10): 1088–1092. doi:10.1038/jp.2017.104. PMID 28749482. S2CID 25566593.
- ^ a b Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström-Anttila V, et al. (2012). "Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis". Human Reproduction Update. 19 (2): 87–104. doi:10.1093/humupd/dms044. PMID 23154145.
- ^ a b Braveman P, Heck K, Egerter S, Dominguez TP, Rinki C, Marchi KS, Curtis M (11 October 2017). Ryckman KK (ed.). "Worry about racial discrimination: A missing piece of the puzzle of Black-White disparities in preterm birth?". PLOS ONE. 12 (10) e0186151. Bibcode:2017PLoSO..1286151B. doi:10.1371/journal.pone.0186151. PMC 5636124. PMID 29020025.
- ^ a b "Preterm birth by Filipino women linked to genetic mutational change". 14 January 2014. Archived from the original on 11 August 2014. Retrieved 8 August 2014.
- ^ "Smart Parenting: The Filipino Parenting Authority". Archived from the original on 14 August 2014. Retrieved 9 August 2014.
- ^ Luo ZC, Wilkins R, Kramer MS (June 2004). "Disparities in pregnancy outcomes according to marital and cohabitation status". Obstetrics and Gynecology. 103 (6): 1300–1307. doi:10.1097/01.AOG.0000128070.44805.1f. PMID 15172868. S2CID 43892340.
- ^ Zain, Norhasmah Mohd; Low, Wah-Yun; Othman, Sajaratulnisah (2015). "Impact of Maternal Marital Status on Birth Outcomes Among Young Malaysian Women: A Prospective Cohort Study". Asia-Pacific Journal of Public Health. 27 (3): 335–347. doi:10.1177/1010539514537678. ISSN 1010-5395. JSTOR 26725703. PMID 25005933.
- ^ El-Sayed, Abdulrahman M.; Galea, Sandro (September 2011). "Changes in the Relationship between Marriage and Preterm Birth, 1989–2006". Public Health Reports. 126 (5): 717–725. doi:10.1177/003335491112600514. ISSN 0033-3549. PMC 3151189. PMID 21886332.
- ^ Currie J (October 2009). "Traffic Congestion and Infant Health: Evidence from E-ZPass" (PDF). National Bureau of Economic Research.
- ^ Chung E (30 October 2019). "Harmful air pollution 'definitely too high for the public' near city roads, study suggests". CBC News. Retrieved 2 November 2019.
- ^ a b Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad AH, Copper RL, et al. (February 1998). "The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network". American Journal of Public Health. 88 (2): 233–238. doi:10.2105/AJPH.88.2.233. PMC 1508185. PMID 9491013.
- ^ Bánhidy F, Acs N, Puhó EH, Czeizel AE (2007). "Pregnancy complications and birth outcomes of pregnant women with urinary tract infections and related drug treatments". Scandinavian Journal of Infectious Diseases. 39 (5): 390–397. doi:10.1080/00365540601087566. PMID 17464860. S2CID 5159387.
- ^ Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA (September 2005). "Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups". American Journal of Public Health. 95 (9): 1545–1551. doi:10.2105/AJPH.2005.065680. PMC 1449396. PMID 16118366.
- ^ To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH (April 2006). "Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study". Ultrasound in Obstetrics & Gynecology. 27 (4): 362–367. doi:10.1002/uog.2773. PMID 16565989. S2CID 24970386.
- ^ Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH (August 2007). "Progesterone and the risk of preterm birth among women with a short cervix". The New England Journal of Medicine. 357 (5): 462–469. doi:10.1056/NEJMoa067815. PMID 17671254. S2CID 14884358.
- ^ Romero R (October 2007). "Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment". Ultrasound in Obstetrics & Gynecology. 30 (5): 675–686. doi:10.1002/uog.5174. PMID 17899585. S2CID 46366053.
- ^ Krupa FG, Faltin D, Cecatti JG, Surita FG, Souza JP (July 2006). "Predictors of preterm birth". International Journal of Gynaecology and Obstetrics. 94 (1): 5–11. doi:10.1016/j.ijgo.2006.03.022. PMID 16730012. S2CID 41368575.
- ^ Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P (January 2003). "Maternal stress and preterm birth". American Journal of Epidemiology. 157 (1): 14–24. doi:10.1093/aje/kwf176. PMID 12505886. S2CID 44325654. Archived from the original on 8 October 2007.
- ^ Parazzini F, Chatenoud L, Surace M, Tozzi L, Salerio B, Bettoni G, Benzi G (October 2003). "Moderate alcohol drinking and risk of preterm birth". European Journal of Clinical Nutrition. 57 (10): 1345–1349. doi:10.1038/sj.ejcn.1601690. PMID 14506499. S2CID 27688375.
- ^ Dolan SM, Gross SJ, Merkatz IR, Faber V, Sullivan LM, Malone FD, et al. (August 2007). "The contribution of birth defects to preterm birth and low birth weight". Obstetrics and Gynecology. 110 (2 Pt 1): 318–324. doi:10.1097/01.AOG.0000275264.78506.63. PMID 17666606. S2CID 32544532.
- ^ The Lancet 28. März 2014: Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. This study is registered with PROSPERO, number CRD42013003522
- ^ van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH (2011). "Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review". Human Reproduction Update. 17 (5): 605–619. doi:10.1093/humupd/dmr024. PMID 21622978.
- ^ Boy A, Salihu HM (2004). "Intimate partner violence and birth outcomes: a systematic review". International Journal of Fertility and Women's Medicine. 49 (4): 159–164. PMID 15481481.
- ^ Ugboma HA, Akani CI (2004). "Abdominal massage: another cause of maternal mortality". Nigerian Journal of Medicine. 13 (3): 259–262. PMID 15532228.
- ^ Field T, Deeds O, Diego M, Hernandez-Reif M, Gauler A, Sullivan S, et al. (October 2009). "Benefits of combining massage therapy with group interpersonal psychotherapy in prenatally depressed women". Journal of Bodywork and Movement Therapies. 13 (4): 297–303. doi:10.1016/j.jbmt.2008.10.002. PMC 2785018. PMID 19761951.
- ^ Lis R, Rowhani-Rahbar A, Manhart LE (August 2015). "Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis". Clinical Infectious Diseases. 61 (3): 418–426. doi:10.1093/cid/civ312. hdl:1773/26479. PMID 25900174.
- ^ Schendel DE (2001). "Infection in pregnancy and cerebral palsy". Journal of the American Medical Women's Association. 56 (3): 105–108. PMID 11506145.
- ^ Donders G, Bellen G, Rezeberga D (September 2011). "Aerobic vaginitis in pregnancy". BJOG. 118 (10): 1163–1170. doi:10.1111/j.1471-0528.2011.03020.x. PMID 21668769. S2CID 7789770.
- ^ a b Roberts CL, Algert CS, Rickard KL, Morris JM (March 2015). "Treatment of vaginal candidiasis for the prevention of preterm birth: a systematic review and meta-analysis". Systematic Reviews. 4 (1) 31. doi:10.1186/s13643-015-0018-2. PMC 4373465. PMID 25874659.
- ^ Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E (June 2015). Thinkhamrop J (ed.). "Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity". The Cochrane Database of Systematic Reviews. 2015 (6) CD002250. doi:10.1002/14651858.CD002250.pub3. PMC 7154219. PMID 26092137.
- ^ a b Smaill FM, Vazquez JC (November 2019). "Antibiotics for asymptomatic bacteriuria in pregnancy". The Cochrane Database of Systematic Reviews. 2019 (11). doi:10.1002/14651858.CD000490.pub4. PMC 6953361. PMID 31765489.
- ^ Widmer M, Lopez I, Gülmezoglu AM, Mignini L, Roganti A (November 2015). "Duration of treatment for asymptomatic bacteriuria during pregnancy". The Cochrane Database of Systematic Reviews. 2015 (11) CD000491. doi:10.1002/14651858.CD000491.pub3. PMC 7043273. PMID 26560337.
- ^ a b Sangkomkamhang US, Lumbiganon P, Prasertcharoensuk W, Laopaiboon M (February 2015). "Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery". The Cochrane Database of Systematic Reviews. 2015 (2) CD006178. doi:10.1002/14651858.CD006178.pub3. PMC 8498019. PMID 25922860.
- ^ Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC (July 2001). "Periodontal infection and preterm birth: results of a prospective study". Journal of the American Dental Association. 132 (7): 875–880. doi:10.14219/jada.archive.2001.0299. PMID 11480640.
- ^ "Pregnancy and Oral Health - United Concordia Dental". Archived from the original on 20 January 2015. Retrieved 19 January 2015.
- ^ Kistka ZA, DeFranco EA, Ligthart L, Willemsen G, Plunkett J, Muglia LJ, Boomsma DI (July 2008). "Heritability of parturition timing: an extended twin design analysis". American Journal of Obstetrics and Gynecology. 199 (1): 43.e1–43.e5. doi:10.1016/j.ajog.2007.12.014. PMID 18295169.
- ^ Zhang G, Feenstra B, Bacelis J, Liu X, Muglia LM, Juodakis J, et al. (September 2017). "Genetic Associations with Gestational Duration and Spontaneous Preterm Birth". The New England Journal of Medicine. 377 (12): 1156–1167. doi:10.1056/NEJMoa1612665. PMC 5561422. PMID 28877031.
- ^ Rosa CQ, Silveira DS, Costa JS (December 2014). "Factors associated with lack of prenatal care in a large municipality". Revista de Saude Publica. 48 (6): 977–984. doi:10.1590/S0034-8910.2014048005283. PMC 4285828. PMID 26039401.
- ^ Lee SE, Park JS, Norwitz ER, Kim KW, Park HS, Jun JK (March 2007). "Measurement of placental alpha-microglobulin-1 in cervicovaginal discharge to diagnose rupture of membranes". Obstetrics and Gynecology. 109 (3): 634–640. doi:10.1097/01.AOG.0000252706.46734.0a. PMID 17329514. S2CID 20732037.
- ^ Lee SM, Lee J, Seong HS, Lee SE, Park JS, Romero R, Yoon BH (April 2009). "The clinical significance of a positive Amnisure test in women with term labor with intact membranes". The Journal of Maternal-Fetal & Neonatal Medicine. 22 (4): 305–310. doi:10.1080/14767050902801694. PMC 2744034. PMID 19350444.
- ^ Lee SM, Yoon BH, Park CW, Kim SM, Park JW (2011). "Intra-amniotic inflammation in patients with a positive Amnisure test in preterm labor and intact membranes". Am J Obstet Gynecol. 204 (1): S209. doi:10.1016/j.ajog.2010.10.543.
- ^ Lee SM, Romero R, Park JW, Kim SM, Park CW, Korzeniewski SJ, et al. (September 2012). "The clinical significance of a positive Amnisure test in women with preterm labor and intact membranes". The Journal of Maternal-Fetal & Neonatal Medicine. 25 (9): 1690–1698. doi:10.3109/14767058.2012.657279. PMC 3422421. PMID 22280400.
- ^ Sukchaya K, Phupong V (August 2013). "A comparative study of positive rate of placental α-microglobulin-1 test in pre-term pregnant women with and without uterine contraction". Journal of Obstetrics and Gynaecology. 33 (6): 566–568. doi:10.3109/01443615.2013.807786. PMID 23919851. S2CID 20265539.
- ^ Nikolova T, Bayev O, Nikolova N, Di Renzo GC (July 2014). "Evaluation of a novel placental alpha microglobulin-1 (PAMG-1) test to predict spontaneous preterm delivery". Journal of Perinatal Medicine. 42 (4): 473–477. doi:10.1515/jpm-2013-0234. PMID 24334429. S2CID 6547430.
- ^ Nikolova T, Bayev O, Nikolova N, Di Renzo GC. Comparison of a novel test for placental alpha microglobulin-1 with fetal fibronectin and cervical length measurement for the prediction of imminent spontaneous preterm delivery in patients with threatened preterm labor. J Perinat Med. 2015 Jan 6.
- ^ Lu GC, Goldenberg RL, Cliver SP, Kreaden US, Andrews WW (February 2001). "Vaginal fetal fibronectin levels and spontaneous preterm birth in symptomatic women". Obstetrics and Gynecology. 97 (2): 225–228. doi:10.1016/S0029-7844(00)01130-3. PMID 11165586. S2CID 34818112.
- ^ Cervical incompetence Archived 7 March 2014 at the Wayback Machine from Radiopaedia. Authors: Dr Praveen Jha and Dr Laughlin Dawes et al. Retrieved Feb 2014
- ^ "Rea - a smart bandage for pregnant women". Innosuisse-Swiss Innovation Agency. Archived from the original on 5 September 2023. Retrieved 5 September 2023.
- ^ Ferreira, Amaro; Bernardes, João; Gonçalves, Hernâni (January 2023). "Risk Scoring Systems for Preterm Birth and Their Performance: A Systematic Review". Journal of Clinical Medicine. 12 (13): 4360. doi:10.3390/jcm12134360. ISSN 2077-0383. PMC 10342801. PMID 37445395.
- ^ Davey, Mary-Ann; Watson, Lyndsey; Rayner, Jo Anne; Rowlands, Shelley (20 January 2010), Davey, Mary-Ann (ed.), "Risk scoring systems for predicting preterm birth with the aim of reducing associated adverse outcomes", Cochrane Database of Systematic Reviews, Chichester, UK: John Wiley & Sons, Ltd, doi:10.1002/14651858.cd004902.pub2
- ^ Steer P (March 2005). "The epidemiology of preterm labour". BJOG. 112 (Suppl 1): 1–3. doi:10.1111/j.1471-0528.2005.00575.x. PMID 15715585. S2CID 33738952.
- ^ a b c d e f g h i j k l m Iams JD, Romero R, Culhane JF, Goldenberg RL (January 2008). "Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth". Lancet. 371 (9607): 164–175. doi:10.1016/S0140-6736(08)60108-7. PMID 18191687. S2CID 8204299.
- ^ Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A (May 2014). "Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis". Lancet. 383 (9928): 1549–1560. doi:10.1016/S0140-6736(14)60082-9. PMID 24680633. S2CID 8532979.
- ^ Saurel-Cubizolles MJ, Zeitlin J, Lelong N, Papiernik E, Di Renzo GC, Bréart G (May 2004). "Employment, working conditions, and preterm birth: results from the Europop case-control survey". Journal of Epidemiology and Community Health. 58 (5): 395–401. doi:10.1136/jech.2003.008029. PMC 1732750. PMID 15082738.
- ^ Pompeii LA, Savitz DA, Evenson KR, Rogers B, McMahon M (December 2005). "Physical exertion at work and the risk of preterm delivery and small-for-gestational-age birth". Obstetrics and Gynecology. 106 (6): 1279–1288. doi:10.1097/01.AOG.0000189080.76998.f8. PMID 16319253. S2CID 19518460.
- ^ Li B, Zhang X, Peng X, Zhang S, Wang X, Zhu C (2019). "Folic Acid and Risk of Preterm Birth: A Meta-Analysis". Frontiers in Neuroscience. 13 1284. doi:10.3389/fnins.2019.01284. PMC 6892975. PMID 31849592.
- ^ Lamont RF, Jaggat AN (March 2007). "Emerging drug therapies for preventing spontaneous preterm labor and preterm birth". Expert Opinion on Investigational Drugs. 16 (3): 337–345. doi:10.1517/13543784.16.3.337. PMID 17302528. S2CID 11591970.
- ^ Johnston M, Landers S, Noble L, Szucs K, Viehmann L, et al. (Section on Breastfeeding) (August 2021). "Prediction and Prevention of Spontaneous Preterm Birth: ACOG Practice Bulletin Summary, Number 234". Obstetrics and Gynecology. 138 (2): 320–323. doi:10.1097/AOG.0000000000004480. PMID 34293768. S2CID 236200411.
- ^ Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR (October 2018). "Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems". The Cochrane Database of Systematic Reviews. 2018 (10) CD001059. doi:10.1002/14651858.CD001059.pub5. PMC 6517256. PMID 30277579.
- ^ Guideline: Calcium supplementation in pregnant women. Geneva: World Health Organization. 2013.
- ^ Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS (April 2006). "Vitamins C and E and the risks of preeclampsia and perinatal complications". The New England Journal of Medicine. 354 (17): 1796–1806. doi:10.1056/NEJMoa054186. hdl:2440/23161. PMID 16641396.
- ^ Avşar TS, McLeod H, Jackson L (March 2021). "Health outcomes of smoking during pregnancy and the postpartum period: an umbrella review". BMC Pregnancy and Childbirth. 21 (1) 254. doi:10.1186/s12884-021-03729-1. PMC 7995767. PMID 33771100.
- ^ a b Urquhart C, Currell R, Harlow F, Callow L (February 2017). "Home uterine monitoring for detecting preterm labour". The Cochrane Database of Systematic Reviews. 2017 (2) CD006172. doi:10.1002/14651858.CD006172.pub4. PMC 6464057. PMID 28205207.
- ^ a b East CE, Biro MA, Fredericks S, Lau R (April 2019). "Support during pregnancy for women at increased risk of low birthweight babies". The Cochrane Database of Systematic Reviews. 2019 (4) CD000198. doi:10.1002/14651858.CD000198.pub3. PMC 6443020. PMID 30933309.
- ^ Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M (April 1989). "Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight". Obstetrics and Gynecology. 73 (4): 576–582. PMID 2927852.
- ^ Berghella V, Saccone G (September 2019). "Cervical assessment by ultrasound for preventing preterm delivery". The Cochrane Database of Systematic Reviews. 2019 (9) CD007235. doi:10.1002/14651858.CD007235.pub4. PMC 6760928. PMID 31553800.
- ^ "Opinion Number 719: Multifetal Pregnancy Reduction". American College of Obstetricians and Gynecologists' Committee on Ethics. September 2017. Archived from the original on 4 April 2019. Retrieved 26 October 2018.
- ^ Zipori Y, Haas J, Berger H, Barzilay E (September 2017). "Multifetal pregnancy reduction of triplets to twins compared with non-reduced triplets: a meta-analysis". Reproductive Biomedicine Online. 35 (3): 296–304. doi:10.1016/j.rbmo.2017.05.012. PMID 28625760.
- ^ Evans MI, Andriole S, Britt DW (2014). "Fetal reduction: 25 years' experience". Fetal Diagnosis and Therapy. 35 (2): 69–82. doi:10.1159/000357974. PMID 24525884.
- ^ McCall CA, Grimes DA, Lyerly AD (June 2013). ""Therapeutic" bed rest in pregnancy: unethical and unsupported by data". Obstetrics and Gynecology. 121 (6): 1305–1308. doi:10.1097/aog.0b013e318293f12f. PMID 23812466. S2CID 9069311.
- ^ Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C (March 2000). "Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish Oil Trials In Pregnancy (FOTIP) Team". BJOG. 107 (3): 382–395. doi:10.1111/j.1471-0528.2000.tb13235.x. PMID 10740336. S2CID 30837582.
- ^ Brocklehurst P, Gordon A, Heatley E, Milan SJ (January 2013). "Antibiotics for treating bacterial vaginosis in pregnancy". The Cochrane Database of Systematic Reviews. 1 (1) CD000262. doi:10.1002/14651858.CD000262.pub4. PMC 4164464. PMID 23440777.
- ^ "Progesterone: Use in the second and third trimester of pregnancy for the prevention of preterm birth" (PDF). The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. July 2017. Retrieved 29 January 2021.
- ^ Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA (July 2013). "Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth". The Cochrane Database of Systematic Reviews. 2015 (7) CD004947. doi:10.1002/14651858.CD004947.pub3. PMC 11035916. PMID 23903965. S2CID 43862120.
- ^ Mackenzie R, Walker M, Armson A, Hannah ME (May 2006). "Progesterone for the prevention of preterm birth among women at increased risk: a systematic review and meta-analysis of randomized controlled trials". American Journal of Obstetrics and Gynecology. 194 (5): 1234–1242. doi:10.1016/j.ajog.2005.06.049. PMID 16647905.
- ^ a b Iams JD (January 2014). "Clinical practice. Prevention of preterm parturition". The New England Journal of Medicine. 370 (3): 254–261. doi:10.1056/NEJMcp1103640. PMID 24428470. S2CID 29480873.
- ^ Romero R, Nicolaides KH, Conde-Agudelo A, O'Brien JM, Cetingoz E, Da Fonseca E, et al. (September 2016). "Vaginal progesterone decreases preterm birth ≤ 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study". Ultrasound in Obstetrics & Gynecology. 48 (3): 308–317. doi:10.1002/uog.15953. PMC 5053235. PMID 27444208.
- ^ Caritis S, Rouse D (2006). "A randomized controlled trial of 17-hydroxyprogesterone caproate (17-OHPC) for the prevention of preterm birth in twins". American Journal of Obstetrics & Gynecology. 195 (6): S2. doi:10.1016/j.ajog.2006.10.003.
- ^ Stewart LA, Simmonds M, Duley L, Dietz KC, Harden M, Hodkinson A, et al. (November 2017). "Evaluating progestogens for prevention of preterm birth international collaborative (EPPPIC) individual participant data (IPD) meta-analysis: protocol". Systematic Reviews. 6 (1) 235. doi:10.1186/s13643-017-0600-x. PMC 5706301. PMID 29183399.
- ^ Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM (July 2005). "Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data". Obstetrics and Gynecology. 106 (1): 181–189. doi:10.1097/01.AOG.0000168435.17200.53. PMID 15994635. S2CID 22742373.
- ^ Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH (May 2007). "Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants". The New England Journal of Medicine. 356 (21): 2165–2175. doi:10.1056/NEJMsa065029. PMID 17522400. S2CID 8083107.
- ^ Stan CM, Boulvain M, Pfister R, Hirsbrunner-Almagbaly P (November 2013). "Hydration for treatment of preterm labour". The Cochrane Database of Systematic Reviews (11) CD003096. doi:10.1002/14651858.CD003096.pub2. PMC 11751767. PMID 24190310.
- ^ Aziz K, Lee HC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, et al. (October 2020). "Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 142 (16_suppl_2): S524 – S550. doi:10.1161/CIR.0000000000000902. PMID 33081528. S2CID 224826008.
- ^ Walters A, McKinlay C, Middleton P, Harding JE, Crowther CA (April 2022). "Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes". The Cochrane Database of Systematic Reviews. 2022 (4) CD003935. doi:10.1002/14651858.CD003935.pub5. PMC 8978608. PMID 35377461.
- ^ McGoldrick E, Stewart F, Parker R, Dalziel SR (December 2020). "Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth". The Cochrane Database of Systematic Reviews. 12 (2) CD004454 (published 25 December 2020). doi:10.1002/14651858.CD004454.pub4. PMC 8094626. PMID 33368142.
- ^ "The National Institutes of Health (NIH) Consensus Development Program: The Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes". Archived from the original on 9 July 2017. Retrieved 18 July 2017.
- ^ "The National Institutes of Health (NIH) Consensus Development Program: Antenatal Corticosteroids Revisited: Repeat Courses". Archived from the original on 18 January 2017. Retrieved 18 July 2017.
- ^ Shepherd E, Salam RA, Middleton P, Makrides M, McIntyre S, Badawi N, Crowther CA (August 2017). "Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews". The Cochrane Database of Systematic Reviews. 2017 (8) CD012077. doi:10.1002/14651858.CD012077.pub2. PMC 6483544. PMID 28786098.
- ^ Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A (August 2002). "Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC". MMWR. Recommendations and Reports. 51 (RR-11): 1–22. PMID 12211284.
- ^ a b Kenyon SL, Taylor DJ, Tarnow-Mordi W (March 2001). "Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group". Lancet. 357 (9261): 989–994. doi:10.1016/S0140-6736(00)04233-1. PMID 11293641. S2CID 205936902.
- ^ Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ (October 2012). "Tocolytic therapy for preterm delivery: systematic review and network meta-analysis". BMJ. 345 e6226. doi:10.1136/bmj.e6226. PMC 4688428. PMID 23048010.
- ^ Simhan HN, Caritis SN (August 2007). "Prevention of preterm delivery". The New England Journal of Medicine. 357 (5): 477–487. doi:10.1056/NEJMra050435. PMID 17671256.
- ^ Li X, Zhang Y, Shi Z (February 2005). "Ritodrine in the treatment of preterm labour: a meta-analysis". The Indian Journal of Medical Research. 121 (2): 120–127. PMID 15756046.
- ^ Crowther CA, Brown J, McKinlay CJ, Middleton P (August 2014). "Magnesium sulphate for preventing preterm birth in threatened preterm labour". The Cochrane Database of Systematic Reviews. 2014 (8) CD001060. doi:10.1002/14651858.CD001060.pub2. PMC 10838393. PMID 25126773.
- ^ Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, et al. (October 2017). "Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis". PLOS Medicine. 14 (10) e1002398. doi:10.1371/journal.pmed.1002398. PMC 5627896. PMID 28976987.
- ^ Alfirevic Z, Milan SJ, Livio S (September 2013). "Caesarean section versus vaginal delivery for preterm birth in singletons". The Cochrane Database of Systematic Reviews. 2013 (9) CD000078. doi:10.1002/14651858.CD000078.pub3. PMC 7052739. PMID 24030708.
- ^ McCall EM, Alderdice F, Halliday HL, Vohra S, Johnston L (February 2018). "Interventions to prevent hypothermia at birth in preterm and/or low birth weight infants". The Cochrane Database of Systematic Reviews. 2018 (2) CD004210. doi:10.1002/14651858.CD004210.pub5. PMC 6491068. PMID 29431872.
- ^ Bruschettini, Matteo; Moberg, Tilda; O'Donnell, Colm Pf; Davis, Peter G.; Morley, Colin J.; Moja, Lorenzo; Calevo, Maria Grazia; Vermont Oxford Network; supported by the Cochrane Neonatal Review Group, and Cochrane Sweden (18 July 2025). "Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes". The Cochrane Database of Systematic Reviews. 7 (7) CD004953. doi:10.1002/14651858.CD004953.pub5. ISSN 1469-493X. PMC 12272818. PMID 40678985.
- ^ Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. (January 2016). "Kangaroo Mother Care and Neonatal Outcomes: A Meta-analysis". Pediatrics. 137 (1) e20152238. doi:10.1542/peds.2015-2238. PMC 4702019. PMID 26702029.
- ^ Bell EF, Acarregui MJ (4 December 2014). "Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants". The Cochrane Database of Systematic Reviews. 2014 (12) CD000503. doi:10.1002/14651858.CD000503.pub3. PMC 7038715. PMID 25473815.
- ^ Hodgson KA, Wilkinson D, De Paoli AG, Manley BJ (May 2023). "Nasal high flow therapy for primary respiratory support in preterm infants". The Cochrane Database of Systematic Reviews. 2023 (5) CD006405. doi:10.1002/14651858.CD006405.pub4. PMC 10161968. PMID 37144837.
- ^ Askie LM, Darlow BA, Davis PG, Finer N, Stenson B, Vento M, Whyte R (April 2017). "Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants". The Cochrane Database of Systematic Reviews. 4 (4) CD011190. doi:10.1002/14651858.CD011190.pub2. PMC 6478245. PMID 28398697.
- ^ Subramaniam, P; Ho, JJ; Davis, PG (18 October 2021). "Prophylactic or very early initiation of continuous positive airway pressure (CPAP) for preterm infants". The Cochrane Database of Systematic Reviews. 2021 (10) CD001243. doi:10.1002/14651858.CD001243.pub4. PMC 8521644. PMID 34661278.
- ^ Lai NM, Foong SC, Foong WC, Tan K (April 2016). "Co-bedding in neonatal nursery for promoting growth and neurodevelopment in stable preterm twins". The Cochrane Database of Systematic Reviews. 4 (4) CD008313. doi:10.1002/14651858.CD008313.pub3. PMC 6464533. PMID 27075527.
- ^ a b c d e Abiramalatha T, Thomas N, Thanigainathan S (March 2021). "High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants". The Cochrane Database of Systematic Reviews. 2021 (3) CD012413. doi:10.1002/14651858.CD012413.pub3. PMC 8092452. PMID 33733486.
- ^ a b c d e f g h Walsh V, Brown JV, Copperthwaite BR, Oddie SJ, McGuire W (December 2020). "Early full enteral feeding for preterm or low birth weight infants". The Cochrane Database of Systematic Reviews. 2020 (12) CD013542. doi:10.1002/14651858.CD013542.pub2. PMC 8094920. PMID 33368149.
- ^ a b c d e Sadrudin Premji S, Chessell L, Stewart F (June 2021). "Continuous nasogastric milk feeding versus intermittent bolus milk feeding for preterm infants less than 1500 grams". The Cochrane Database of Systematic Reviews. 2021 (6) CD001819. doi:10.1002/14651858.CD001819.pub3. PMC 8223964. PMID 34165778.
- ^ Eidelman, Arthur I.; Schanler, Richard J.; Johnston, Margreete; Landers, Susan; Noble, Larry; Szucs, Kinga; Viehmann, Laura (March 2012). "Breastfeeding and the use of human milk". Pediatrics. 129 (3): e827 – e841. doi:10.1542/peds.2011-3552. PMID 22371471.
Meta-analyses of 4 randomized clinical trials performed over the period 1983 to 2005 support the conclusion that feeding preterm infants human milk is associated with a significant reduction (58%) in the incidence of NEC.
- ^ Brown JV, Walsh V, McGuire W (August 2019). "Formula versus maternal breast milk for feeding preterm or low birth weight infants". The Cochrane Database of Systematic Reviews. 8 (8) CD002972. doi:10.1002/14651858.CD002972.pub3. PMC 6710607. PMID 31452191.
- ^ a b Brown JV, Embleton ND, Harding JE, McGuire W (May 2016). "Multi-nutrient fortification of human milk for preterm infants". The Cochrane Database of Systematic Reviews (5) CD000343. doi:10.1002/14651858.CD000343.pub3. hdl:2292/57382. PMID 27155888.
- ^ Amissah EA, Brown J, Harding JE (September 2020). "Protein supplementation of human milk for promoting growth in preterm infants". The Cochrane Database of Systematic Reviews. 2020 (9) CD000433. doi:10.1002/14651858.CD000433.pub3. PMC 8094919. PMID 32964431.
- ^ Gao C, Miller J, Collins CT, Rumbold AR (November 2020). "Comparison of different protein concentrations of human milk fortifier for promoting growth and neurological development in preterm infants". The Cochrane Database of Systematic Reviews. 2020 (11) CD007090. doi:10.1002/14651858.CD007090.pub2. PMC 8092673. PMID 33215474.
- ^ Fenton TR, Al-Wassia H, Premji SS, Sauve RS (June 2020). "Higher versus lower protein intake in formula-fed low birth weight infants". The Cochrane Database of Systematic Reviews. 6 (7) CD003959. doi:10.1002/14651858.CD003959.pub4. PMC 7387284. PMID 32573771.
- ^ Amissah EA, Brown J, Harding JE (September 2020). "Carbohydrate supplementation of human milk to promote growth in preterm infants". The Cochrane Database of Systematic Reviews. 9 (10) CD000280. doi:10.1002/14651858.CD000280.pub3. PMC 8094174. PMID 32898300.
- ^ Amissah EA, Brown J, Harding JE (August 2020). "Fat supplementation of human milk for promoting growth in preterm infants". The Cochrane Database of Systematic Reviews. 8 (10) CD000341. doi:10.1002/14651858.CD000341.pub3. PMC 8236752. PMID 32842164.
- ^ Perretta L, Ouldibbat L, Hagadorn JI, Brumberg HL (February 2021). "High versus low medium chain triglyceride content of formula for promoting short-term growth of preterm infants". The Cochrane Database of Systematic Reviews. 2021 (2) CD002777. doi:10.1002/14651858.CD002777.pub2. PMC 8094384. PMID 33620090.
- ^ Amari S, Shahrook S, Namba F, Ota E, Mori R (October 2020). "Branched-chain amino acid supplementation for improving growth and development in term and preterm neonates". The Cochrane Database of Systematic Reviews. 2020 (10) CD012273. doi:10.1002/14651858.CD012273.pub2. PMC 8078205. PMID 33006765.
- ^ Basuki F, Hadiati DR, Turner T, McDonald S, Hakimi M (June 2019). "Dilute versus full-strength formula in exclusively formula-fed preterm or low birth weight infants". The Cochrane Database of Systematic Reviews. 6 (6) CD007263. doi:10.1002/14651858.CD007263.pub3. PMC 6596360. PMID 31246272.
- ^ a b Fabrizio V, Trzaski JM, Brownell EA, Esposito P, Lainwala S, Lussier MM, Hagadorn JI (November 2020). "Individualized versus standard diet fortification for growth and development in preterm infants receiving human milk". The Cochrane Database of Systematic Reviews. 2020 (11) CD013465. doi:10.1002/14651858.CD013465.pub2. PMC 8094236. PMID 33226632.
- ^ a b Premkumar MH, Pammi M, Suresh G (November 2019). "Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates". The Cochrane Database of Systematic Reviews. 2019 (11). doi:10.1002/14651858.CD013145.pub2. PMC 6837687. PMID 31697857.
- ^ Yeo KT, Kong JY, Sasi A, Tan K, Lai NM, Schindler T (October 2019). "Stopping enteral feeds for prevention of transfusion-associated necrotising enterocolitis in preterm infants". The Cochrane Database of Systematic Reviews. 2019 (10). doi:10.1002/14651858.CD012888.pub2. PMC 6815687. PMID 31684689.
- ^ a b Thanigainathan S, Abiramalatha T (July 2020). "Early fortification of human milk versus late fortification to promote growth in preterm infants". The Cochrane Database of Systematic Reviews. 2020 (7) CD013392. doi:10.1002/14651858.CD013392.pub2. PMC 7390609. PMID 32726863.
- ^ Young L, Embleton ND, McGuire W (December 2016). "Nutrient-enriched formula versus standard formula for preterm infants following hospital discharge". The Cochrane Database of Systematic Reviews. 2016 (12) CD004696. doi:10.1002/14651858.CD004696.pub5. PMC 6463855. PMID 27958643.
- ^ Richards R, Foster JP, Psaila K (August 2021). "Continuous versus bolus intermittent intragastric tube feeding for preterm and low birth weight infants with gastro-oesophageal reflux disease". The Cochrane Database of Systematic Reviews. 2021 (8) CD009719. doi:10.1002/14651858.CD009719.pub3. PMC 8407337. PMID 34355390.
- ^ Dawson JA, Summan R, Badawi N, Foster JP (August 2021). "Push versus gravity for intermittent bolus gavage tube feeding of preterm and low birth weight infants". The Cochrane Database of Systematic Reviews. 2021 (8) CD005249. doi:10.1002/14651858.CD005249.pub3. PMC 8407046. PMID 34346056.
- ^ Akindolire A, Talbert A, Sinha I, Embleton N, Allen S (2020). "Evidence that informs feeding practices in very low birthweight and very preterm infants in sub-Saharan Africa: an overview of systematic reviews". BMJ Paediatrics Open. 4 (1) e000724. doi:10.1136/bmjpo-2020-000724. PMC 7422638. PMID 32821859.
- ^ Moon K, Athalye-Jape GK, Rao U, Rao SC (April 2020). "Early versus late parenteral nutrition for critically ill term and late preterm infants". The Cochrane Database of Systematic Reviews. 2020 (4) CD013141. doi:10.1002/14651858.CD013141.pub2. PMC 7138920. PMID 32266712.
- ^ a b c d e f g h Abiramalatha, T; Thanigainathan, S; Ramaswamy, VV; Rajaiah, B; Ramakrishnan, S (30 June 2023). "Re-feeding versus discarding gastric residuals to improve growth in preterm infants". The Cochrane Database of Systematic Reviews. 2023 (6) CD012940. doi:10.1002/14651858.CD012940.pub3. PMC 10312053. PMID 37387544.
- ^ a b c d Diller, Natasha; Osborn, David A; Birch, Pita (12 October 2023). Cochrane Neonatal Group (ed.). "Higher versus lower sodium intake for preterm infants". Cochrane Database of Systematic Reviews. 2023 (10) CD012642. doi:10.1002/14651858.CD012642.pub2. PMC 10569379. PMID 37824273.
- ^ a b c Joint Committee on Infant Hearing (October 2007). "Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs". Pediatrics. 120 (4): 898–921. doi:10.15142/fptk-b748. PMID 17908777.
- ^ Patel RM, Rysavy MA, Bell EF, Tyson JE (June 2017). "Survival of Infants Born at Periviable Gestational Ages". Clinics in Perinatology. 44 (2): 287–303. doi:10.1016/j.clp.2017.01.009. PMC 5424630. PMID 28477661.
- ^ Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES (December 2012). "Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies)". BMJ. 345 e7976. doi:10.1136/bmj.e7976. PMC 3514472. PMID 23212881.
- ^ Fellman V, Hellström-Westas L, Norman M, Westgren M, Källén K, Lagercrantz H, et al. (June 2009). "One-year survival of extremely preterm infants after active perinatal care in Sweden". JAMA. 301 (21): 2225–2233. doi:10.1001/jama.2009.771. PMID 19491184.
- ^ Ancel PY, Goffinet F, Kuhn P, Langer B, Matis J, Hernandorena X, et al. (EPIPAGE-2 Writing Group) (March 2015). "Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE-2 cohort study". JAMA Pediatrics. 169 (3): 230–238. doi:10.1001/jamapediatrics.2014.3351. PMID 25621457.
- ^ Boland RA, Davis PG, Dawson JA, Doyle LW (March 2017). "Outcomes of infants born at 22-27 weeks' gestation in Victoria according to outborn/inborn birth status". Archives of Disease in Childhood. Fetal and Neonatal Edition. 102 (2): F153 – F161. doi:10.1136/archdischild-2015-310313. PMID 27531224. S2CID 7958596.
- ^ Chen F, Bajwa NM, Rimensberger PC, Posfay-Barbe KM, Pfister RE (September 2016). "Thirteen-year mortality and morbidity in preterm infants in Switzerland". Archives of Disease in Childhood. Fetal and Neonatal Edition. 101 (5): F377 – F383. doi:10.1136/archdischild-2015-308579. PMID 27059074. S2CID 20567764.
- ^ a b c d Saigal S, Doyle LW (January 2008). "An overview of mortality and sequelae of preterm birth from infancy to adulthood". Lancet. 371 (9608): 261–269. doi:10.1016/S0140-6736(08)60136-1. PMID 18207020. S2CID 17256481.
- ^ Phillips C, Velji Z, Hanly C, Metcalfe A (July 2017). "Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis". BMJ Open. 7 (6) e015402. doi:10.1136/bmjopen-2016-015402. PMC 5734267. PMID 28679674.
- ^ Mathew TJ, MacDorman MF (2006). "Infant Mortality Statistics from the 2003 Period Linked Birth/Infant Death Data Set". National Vital Statistics Reports. 54 (16).
- ^ Kaempf JW, Tomlinson M, Arduza C, Anderson S, Campbell B, Ferguson LA, et al. (January 2006). "Medical staff guidelines for periviability pregnancy counseling and medical treatment of extremely premature infants". Pediatrics. 117 (1): 22–29. doi:10.1542/peds.2004-2547. PMID 16396856. S2CID 20495326. Archived from the original on 18 March 2008. — in particular see TABLE 1 Survival and Neurologic Disability Rates Among Extremely Premature Infants Archived 12 June 2008 at the Wayback Machine
- ^ Morgan MA, Goldenberg RL, Schulkin J (February 2008). "Obstetrician-gynecologists' practices regarding preterm birth at the limit of viability". The Journal of Maternal-Fetal & Neonatal Medicine. 21 (2): 115–121. doi:10.1080/14767050701866971. PMID 18240080. S2CID 27735824.
- ^ Arzuaga BH, Lee BH (December 2011). "Limits of human viability in the United States: a medicolegal review". Pediatrics. 128 (6): 1047–1052. doi:10.1542/peds.2011-1689. PMID 22065266. S2CID 31065615.
- ^ Lambert SR, Lyons CJ (31 October 2016). Taylor and Hoyt's pediatric ophthalmology and strabismus (Fifth ed.). Edinburgh: Elsevier Health Sciences. ISBN 978-0-7020-6616-0. OCLC 960162637.
- ^ a b March of Dimes --> Neonatal Death Archived 24 October 2014 at the Wayback Machine Retrieved on 11 November 2014
- ^ Berbel P, Navarro D, Ausó E, Varea E, Rodríguez AE, Ballesta JJ, et al. (June 2010). "Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity". Cerebral Cortex. 20 (6): 1462–1475. doi:10.1093/cercor/bhp212. PMC 2871377. PMID 19812240.
- ^ Cloherty and Stark's Manual of Neonatal Care (9th ed.). Lippincott Williams & Wilkins. 2022. p. 162. ISBN 978-1-9751-5955-9.
- ^ a b "New BAPM Framework on Extreme Preterm Birth Published | British Association of Perinatal Medicine". www.bapm.org. Archived from the original on 4 December 2020. Retrieved 25 October 2019.
- ^ a b "World Health Organization". November 2015. Archived from the original on 18 July 2016.
- ^ a b "Extreme preemies face long-term disabilities". NBC News. 6 January 2005. Archived from the original on 12 August 2020.
- ^ "Why Do So Many Preemies Have Hearing Loss?". Audiology. 19 June 2017. Retrieved 1 April 2020.
- ^ a b Moster D, Lie RT, Markestad T (July 2008). "Long-term medical and social consequences of preterm birth". The New England Journal of Medicine. 359 (3): 262–273. doi:10.1056/NEJMoa0706475. PMID 18635431. S2CID 25921193.
- ^ "Depression linked to premature birth". The Age. Melbourne. 4 May 2004. Archived from the original on 8 April 2009. Retrieved 16 December 2008.
- ^
Böhm B, Katz-Salamon M, Institute K, Smedler AC, Lagercrantz H, Forssberg H (August 2002). "Developmental risks and protective factors for influencing cognitive outcome at 5 1/2 years of age in very-low-birthweight children". Developmental Medicine and Child Neurology. 44 (8): 508–516. doi:10.1017/S001216220100247X (inactive 26 August 2025). PMID 12206615.
{{cite journal}}: CS1 maint: DOI inactive as of August 2025 (link) - ^ Spencer MD, Moorhead TW, Gibson RJ, McIntosh AM, Sussmann JE, Owens DG, et al. (January 2008). "Low birthweight and preterm birth in young people with special educational needs: a magnetic resonance imaging analysis". BMC Medicine. 6 (1) 1. doi:10.1186/1741-7015-6-1. PMC 2241838. PMID 18234075.
- ^ Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B (October 2014). "Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis". Obesity Reviews. 15 (10): 804–811. doi:10.1111/obr.12214. PMID 25073871. S2CID 22685359.
- ^ Hack M (October 2009). "Adult outcomes of preterm children". Journal of Developmental and Behavioral Pediatrics. 30 (5): 460–470. doi:10.1097/dbp.0b013e3181ba0fba. PMID 19823140. S2CID 205574669.
- ^ Saigal S (April 2013). "Quality of life of former premature infants during adolescence and beyond". Early Human Development. 89 (4): 209–213. doi:10.1016/j.earlhumdev.2013.01.012. PMID 23462550.
- ^ Carmody JB, Charlton JR (June 2013). "Short-term gestation, long-term risk: prematurity and chronic kidney disease". Pediatrics. 131 (6): 1168–1179. doi:10.1542/peds.2013-0009. PMID 23669525. S2CID 8389988.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 11 November 2009. Retrieved 11 November 2009.
- ^ Delnord M, Blondel B, Zeitlin J (April 2015). "What contributes to disparities in the preterm birth rate in European countries?". Current Opinion in Obstetrics & Gynecology. 27 (2): 133–142. doi:10.1097/GCO.0000000000000156. PMC 4352070. PMID 25692506.
- ^ "Vital Statistics Rapid Release, Number 28 (June 2023)" (PDF). cdc.gov.
- ^ "Data and statistics". World Health Organization. Archived from the original on 16 February 2007.
- ^ Subramanian, KNS (18 June 2009). "Extremely Low Birth Weight Infant". eMedicine. Archived from the original on 21 November 2008. Retrieved 26 August 2009.
- ^ Gilbert WM, Nesbitt TS, Danielsen B (September 2003). "The cost of prematurity: quantification by gestational age and birth weight". Obstetrics and Gynecology. 102 (3): 488–492. doi:10.1016/S0029-7844(03)00617-3. PMID 12962929. S2CID 9995272.
- ^ Richard E. Behrman, Adrienne Stith Butler, Editors, Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention Archived 5 June 2011 at the Wayback Machine. Institute of Medicine. The National Academies Press, 2007. Retrieved 2010-1-14.
- ^ Spencer E. Ante. Million-Dollar Babies Archived 31 May 2009 at the Wayback Machine. BusinessWeek. 12 June 2008. Retrieved 2010-1-24.
- ^ Service, Canwest News. "Miracle child". Archived from the original on 9 December 2007. Retrieved 28 November 2007.
- ^ Guinness World Records 2004. Bantam Books. 2004. ISBN 978-0-553-58712-8.
- ^ "'Miracle baby': Born at 21 weeks, she may be the most premature surviving infant". TODAY. Retrieved 2 January 2019.
- ^ a b c "Most-premature baby allowed home". BBC News. 21 February 2007. Archived from the original on 23 March 2007. Retrieved 5 May 2007.
- ^ "trithuc.thanhnienkhcn.org.vn". Archived from the original on 24 January 2008. Retrieved 28 November 2007.
- ^ "A little miracle called Madeline". The Hindu. Chennai, India. 26 August 2004. Archived from the original on 2 December 2007. Retrieved 28 November 2007.
- ^ "World's Smallest Baby Goes Home, Cellphone-Sized Baby Is Discharged From Hospital". CBS News. 8 February 2005. Archived from the original on 1 January 2008. Retrieved 28 November 2007.
- ^ Rochman B. "Incredibly, World's Tiniest Preterm Babies Are Doing Just Fine". Time. ISSN 0040-781X. Retrieved 30 May 2019.
- ^ "World's Smallest Baby Goes Home". CBS News. 8 February 2005. Archived from the original on 1 January 2008.
- ^ Allyn B (29 May 2019). "Saybie, Born at 8.6 Ounces in San Diego, Is Now The World's Tiniest Surviving Baby". NPR. Retrieved 30 May 2019.
- ^ "The Tiniest Babies". University of Iowa. Archived from the original on 10 June 2010. Retrieved 22 July 2010.
- ^ "Baby Richard born at 21 weeks: One of the youngest babies to survive". Children's Minnesota. 23 December 2020. Retrieved 5 June 2021.
- ^ "Miracle Baby Thrives After Being Given 0% Chance To Live Diagnosis". 14 April 2021. Retrieved 5 June 2021.
- ^ "Most premature baby". Guinness World Records. 5 July 2020. Retrieved 15 March 2023.
- ^ Yu, Yi-Jin. "Boy sets Guinness record for world's most premature baby, celebrates 1st birthday". Good Morning America. Retrieved 25 July 2025.
- ^ Raju, TNK (1980). "Some Famous "High Risk" Newborn Babies". Historical Review and Recent Advances in Neonatal and Perinatal Medicine. Archived from the original on 11 September 2007.
- ^ Preston E (19 July 2020). "During Coronavirus Lockdowns, Some Doctors Wondered: Where Are the Preemies?". The New York Times. Retrieved 2 August 2020.
- ^ Benders MJ, Kersbergen KJ, de Vries LS (March 2014). "Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage". Clinics in Perinatology. 41 (1): 69–82. doi:10.1016/j.clp.2013.09.005. PMID 24524447.
- ^ Volpe JJ (December 2009). "The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined". Seminars in Pediatric Neurology. 16 (4): 167–178. doi:10.1016/j.spen.2009.09.005. PMC 2799246. PMID 19945651.
- ^ a b Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, et al. (January 2008). "Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome". Brain. 131 (Pt 1): 205–217. doi:10.1093/brain/awm282. PMID 18056158.
- ^ a b c Nosarti C, Nam KW, Walshe M, Murray RM, Cuddy M, Rifkin L, Allin MP (2014). "Preterm birth and structural brain alterations in early adulthood". NeuroImage. Clinical. 6: 180–191. doi:10.1016/j.nicl.2014.08.005. PMC 4215396. PMID 25379430.
- ^ Tanskanen P, Valkama M, Haapea M, Barnes A, Ridler K, Miettunen J, et al. (January 2011). "Is prematurity associated with adult cognitive outcome and brain structure?". Pediatric Neurology. 44 (1): 12–20. doi:10.1016/j.pediatrneurol.2010.07.002. PMID 21147382.
- ^ a b c Orchinik LJ, Taylor HG, Espy KA, Minich N, Klein N, Sheffield T, Hack M (November 2011). "Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten". Journal of the International Neuropsychological Society. 17 (6): 1067–1079. doi:10.1017/S135561771100107X. PMC 3282051. PMID 21923973.
- ^ a b Allin MP, Kontis D, Walshe M, Wyatt J, Barker GJ, Kanaan RA, et al. (12 October 2011). "White matter and cognition in adults who were born preterm". PLOS ONE. 6 (10) e24525. Bibcode:2011PLoSO...624525A. doi:10.1371/journal.pone.0024525. PMC 3192037. PMID 22022357. S2CID 3884637.
- ^ a b Bjuland KJ, Løhaugen GC, Martinussen M, Skranes J (June 2013). "Cortical thickness and cognition in very-low-birth-weight late teenagers". Early Human Development. 89 (6): 371–380. doi:10.1016/j.earlhumdev.2012.12.003. PMID 23273486.
- ^ a b c Cheong JL, Anderson PJ, Roberts G, Burnett AC, Lee KJ, Thompson DK, et al. (2013). "Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents". PLOS ONE. 8 (10) e77475. Bibcode:2013PLoSO...877475C. doi:10.1371/journal.pone.0077475. PMC 3793949. PMID 24130887.
- ^ a b c Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N (January 2002). "Outcomes in young adulthood for very-low-birth-weight infants". The New England Journal of Medicine. 346 (3): 149–157. doi:10.1056/NEJMoa010856. PMID 11796848.
- ^ Weisglas-Kuperus N, Hille ET, Duivenvoorden HJ, Finken MJ, Wit JM, van Buuren S, et al. (May 2009). "Intelligence of very preterm or very low birthweight infants in young adulthood". Archives of Disease in Childhood. Fetal and Neonatal Edition. 94 (3): F196 – F200. doi:10.1136/adc.2007.135095. hdl:1874/32813. PMID 18805824. S2CID 16930851.
- ^ Doyle LW, Cheong JL, Burnett A, Roberts G, Lee KJ, Anderson PJ (December 2015). "Biological and Social Influences on Outcomes of Extreme-Preterm/Low-Birth Weight Adolescents". Pediatrics. 136 (6): e1513 – e1520. doi:10.1542/peds.2015-2006. PMID 26553187. S2CID 30594886.
- ^ van Lunenburg A, van der Pal SM, van Dommelen P, van der Pal-de Bruin KM, Bennebroek Gravenhorst J, Verrips GH (March 2013). "Changes in quality of life into adulthood after very preterm birth and/or very low birth weight in the Netherlands". Health and Quality of Life Outcomes. 11 (1) 51. doi:10.1186/1477-7525-11-51. PMC 3618000. PMID 23531081.
- ^ Walther FJ, den Ouden AL, Verloove-Vanhorick SP (September 2000). "Looking back in time: outcome of a national cohort of very preterm infants born in The Netherlands in 1983". Early Human Development. 59 (3): 175–191. doi:10.1016/S0378-3782(00)00094-3. PMID 10996273.
- ^ Zwicker JG, Harris SR (February 2008). "Quality of life of formerly preterm and very low birth weight infants from preschool age to adulthood: a systematic review". Pediatrics. 121 (2): e366 – e376. doi:10.1542/peds.2007-0169. PMID 18245409. S2CID 11674158.
- ^ Martinussen M, Flanders DW, Fischl B, Busa E, Løhaugen GC, Skranes J, et al. (December 2009). "Segmental brain volumes and cognitive and perceptual correlates in 15-year-old adolescents with low birth weight". The Journal of Pediatrics. 155 (6): 848–853.e1. doi:10.1016/j.jpeds.2009.06.015. PMC 5875423. PMID 19683725.
- ^ Allin M, Henderson M, Suckling J, Nosarti C, Rushe T, Fearon P, et al. (January 2004). "Effects of very low birthweight on brain structure in adulthood". Developmental Medicine and Child Neurology. 46 (1): 46–53. doi:10.1111/j.1469-8749.2004.tb00433.x. PMID 14974647. S2CID 221649350.
- ^ Skranes J, Løhaugen GC, Martinussen M, Håberg A, Brubakk AM, Dale AM (September 2013). "Cortical surface area and IQ in very-low-birth-weight (VLBW) young adults". Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 49 (8): 2264–2271. doi:10.1016/j.cortex.2013.06.001. PMID 23845237. S2CID 32770053.
- ^ Lanaro D, Ruffini N, Manzotti A, Lista G (March 2017). "Osteopathic manipulative treatment showed reduction of length of stay and costs in preterm infants: A systematic review and meta-analysis". Medicine. 96 (12) e6408. doi:10.1097/MD.0000000000006408. PMC 5371477. PMID 28328840.
- ^ DeMarsh S, Huntzinger A, Gehred A, Stanek JR, Kemper KJ, Belsky JA (February 2021). "Pediatric Osteopathic Manipulative Medicine: A Scoping Review". Pediatrics. 147 (2) e2020016162. doi:10.1542/peds.2020-016162. PMID 33500321. S2CID 231730075.
External links
[edit]Preterm birth
View on GrokipediaDefinition and Classification
Gestational Age Categories and Subtypes
Preterm birth is defined as delivery before 37 completed weeks of gestation, with gestational age calculated from the first day of the mother's last menstrual period or more precisely via early ultrasound.[1] [2] This threshold aligns with the point at which fetal organ systems, particularly lungs and brain, are generally sufficiently mature for extrauterine survival without excessive morbidity, though risks persist even near term.[9] Subcategories of preterm birth are delineated by gestational age to reflect escalating risks of neonatal complications, such as respiratory distress, intraventricular hemorrhage, and long-term neurodevelopmental deficits, which intensify with decreasing maturity.[3] The World Health Organization (WHO) standardizes these as follows:| Category | Gestational Age Range | Key Characteristics and Risks |
|---|---|---|
| Extremely preterm | Less than 28 weeks | Highest mortality and morbidity; survival rates below 50% before 24 weeks, rising to ~80% at 27 weeks; profound immaturity of multiple organs.[1] [10] |
| Very preterm | 28 to less than 32 weeks | Improved survival (~90%+ at 30-31 weeks) but elevated risks of chronic lung disease and sepsis.[1] [11] |
| Moderate preterm | 32 to less than 34 weeks | Generally better outcomes, though jaundice, feeding issues, and readmissions common.[1] [12] |
| Late preterm | 34 to less than 37 weeks | Accounts for ~75% of preterm births; subtle risks like hypoglycemia and apnea, often underestimated.[1] [13] |
Spontaneous versus Indicated Preterm Birth
Spontaneous preterm birth refers to delivery before 37 weeks of gestation resulting from the onset of preterm labor with intact membranes or preterm premature rupture of membranes (PPROM), accounting for approximately 70 to 80 percent of all preterm births.[5] In contrast, indicated preterm birth involves medical intervention, such as labor induction or cesarean section, prompted by maternal or fetal conditions necessitating early delivery to mitigate risks, comprising the remaining 20 to 30 percent.[16] This distinction is critical for understanding underlying etiologies, as spontaneous cases often stem from intrinsic uterine or placental dysfunction, whereas indicated cases arise from extrinsic complications identifiable through antenatal monitoring.[18] Globally, preterm birth affects about 10 percent of deliveries, with spontaneous subtypes predominating; for instance, preterm labor contributes 40 to 50 percent and PPROM another 30 percent of spontaneous events.[5] In the United States, spontaneous preterm births represented roughly two-thirds of cases as of recent analyses, though exact proportions vary by region and population due to differences in healthcare access and maternal health profiles.[16] Trends indicate stability or slight declines in overall preterm rates in high-income settings from 2009 to 2020, but subtype shifts may occur with rising indicated deliveries linked to improved detection of conditions like preeclampsia.[19] Risk factors diverge markedly between subtypes. Spontaneous preterm birth is associated with prior preterm delivery, infections, cervical insufficiency, uterine overdistension (e.g., multiples), and inflammatory pathways, often unpredictable without targeted screening.[20] Indicated preterm birth correlates with maternal comorbidities such as hypertension, diabetes, heart disease, advanced age, and fetal issues like growth restriction or distress, enabling earlier intervention but reflecting chronic health burdens.[21] Pre-conception factors like tobacco use and socioeconomic status influence both but more strongly predict indicated cases.[22] Neonatal outcomes differ, with indicated preterm births sometimes showing lower rates of respiratory distress due to planned timing and antenatal corticosteroids, yet higher overall morbidity from underlying conditions; spontaneous cases carry elevated risks of infection-related complications.[23] Recurrence risks persist across subtypes, as a history of spontaneous preterm birth elevates odds for both future spontaneous and indicated events, underscoring shared vascular or inflammatory pathways.[24] Indicated preterm birth is linked to poorer maternal cardiovascular prognosis long-term compared to spontaneous.[25] Preventive strategies thus require subtype-specific approaches, such as progesterone for spontaneous risk reduction versus aggressive management of hypertensive disorders.[20]Historical Context
Early Observations and Medical Recognition
In ancient Greece, preterm births were recognized through mythological accounts, historical texts, and archeological findings, with infants termed elitomina to denote those born prematurely, often after missing months of gestation. Viability was acknowledged for births after approximately seven months, though a classical superstition held that eight-month fetuses had poorer prognoses than seven-month ones due to incomplete development. Examples include mythological figures like Dionysos, depicted as a preterm infant nurtured in a cave with incubator-like warmth by nymphs, and archeological evidence from sites in Athens and Astypalaia revealing burials of preterm infants (24-37 weeks gestation) in wells or pots, indicating awareness of their distinct fragility.[26][27][28] During the 17th and 18th centuries in Europe, particularly France, early-born or small infants were frequently classified as abortions, miscarriages, or malformed "aborted monsters" rather than viable premature beings, limiting targeted medical intervention. A shift began with François Mauriceau's 1691 description of a seven-month, eight-day infant surviving to age four or five, highlighting potential for viability with care. By 1757, Antoine Petit explicitly differentiated prematurity from abortion, asserting that post-seven-month infants could survive under proper nurturing, while Nicolas Puzos in 1759 categorized prematurity into three groups based on gestational age and weight, advancing descriptive precision.[29] Medical recognition solidified in the late 19th century as preterm infants were distinguished from other low-viability neonates around 1870, with the term "premature infant" entering English medical lexicon to denote births before full term gestation. Stéphane Tarnier's 1880 introduction of closed incubators in Paris marked a pivotal technological acknowledgment, reducing mortality from 66% in 1879 to 38% by 1882 through controlled warming. Pierre Budin's 1901 publication of the first major textbook on premature infant care further formalized the field, emphasizing gavage feeding and infection prevention, though survival remained dismal—often below two pounds birth weight was deemed the viability limit—and infants were labeled "weaklings" implying inherent debility.[30][29][31]Milestones in Survival and Care Advances
The development of infant incubators in the late 19th century marked an initial advance in preterm care, with devices introduced in European hospitals around 1880 by figures such as Alexandre Lion, who demonstrated reduced mortality for infants under 2000 grams by maintaining warmth and humidity.[32] These incubators gained public and medical attention through exhibitions, including those by Dr. Martin Couney starting in 1896, where over 6,500 premature infants were reportedly saved by 1943 through controlled environments that prevented hypothermia and supported basic oxygenation.[33] By 1922, the first permanent hospital-based premature infant unit opened in the United States, emphasizing warmth, nutrition, and infection prevention as core interventions.[31] The mid-20th century saw the establishment of specialized neonatal units, with expansions in the 1930s incorporating oxygen therapy and improved feeding techniques, though hyperoxia risks were not yet fully understood.[31] The modern neonatal intensive care unit (NICU) emerged in the 1960s, exemplified by Louis Gluck's unit at Yale in 1960, which integrated monitoring, mechanical ventilation, and parenteral nutrition to address respiratory and metabolic failures in preterm infants.[34] Miniaturized blood gas analysis and infusion pumps in the 1960s and 1970s enabled precise management of acid-base balance and fluid delivery, reducing complications from immaturity.[35] A pivotal pharmacological milestone occurred in 1972 when Sir Graham Liggins and Ross Howie demonstrated that antenatal administration of betamethasone to pregnant women at risk of preterm delivery accelerated fetal lung maturation, reducing neonatal respiratory distress syndrome (RDS) incidence by up to 50% and mortality in trials.[36] This intervention, targeting surfactant production deficiency, became standard for gestations between 24 and 34 weeks.[37] Exogenous surfactant replacement therapy, introduced clinically in the 1980s following animal studies, revolutionized RDS treatment by replenishing deficient pulmonary surfactant in preterm lungs, decreasing the need for mechanical ventilation and bronchopulmonary dysplasia rates; by the early 1990s, it was established as safe and effective, with prophylaxis in very preterm infants improving short-term survival.[38][39] Advances in less invasive techniques, such as continuous positive airway pressure (CPAP) from the 1970s onward, further minimized barotrauma risks.[40] Since the mid-1990s, integrated care protocols—including antenatal steroids, surfactant, and optimized ventilation—have substantially boosted survival rates, with infants born before 28 weeks now achieving over 80% survival in high-resource settings, compared to under 50% in earlier decades, driven by reduced RDS and intraventricular hemorrhage incidences.[41][42] Ongoing refinements in nutrition, infection control, and resuscitation have lowered overall preterm mortality, though long-term neurodevelopmental risks persist.[35]Epidemiology
Global Incidence and Trends
An estimated 13.4 million infants (95% credible interval 12.3–15.2 million) were born preterm worldwide in 2020, representing 9.9% of the approximately 135.8 million live births that year.[43] [1] Preterm birth rates vary substantially by country, ranging from 4% in some high-income nations with advanced prenatal care to 16% in regions with higher burdens of infectious diseases and limited healthcare access, such as parts of sub-Saharan Africa and South Asia.[1] These estimates derive from models integrating vital registration data, national surveys, and hospital records, though underreporting persists in low-resource settings where many births occur outside formal facilities, potentially understating true incidence in high-burden areas.[44] Global preterm birth rates have shown minimal change over the past decade, remaining stable at around 1 in 10 live births from 2010 to 2020, with the absolute number of preterm births decreasing slightly from 13.8 million to 13.4 million amid rising global birth volumes.[45] [46] The annual rate of reduction was just 0.14% during this period, insufficient to offset underlying risk factors like increasing maternal age and obesity in some populations.[44] Longitudinal analyses from 1990 to 2021 indicate an overall declining trend in crude incidence and associated disability-adjusted life years (DALYs), attributed partly to improved survival through neonatal interventions, but age-standardized incidence rates began rising after 2016, possibly reflecting better detection or shifts in obstetric practices like elective early deliveries.[4] Data limitations, including inconsistent gestational age assessment methods (e.g., last menstrual period vs. ultrasound), contribute to uncertainty in trend attribution, with calls for enhanced surveillance in low- and middle-income countries where over 60% of preterm births occur.[45]Demographic and Geographic Disparities
Preterm birth rates vary substantially by geography, with a global average of 9.9% in 2020, equating to 13.4 million preterm live births. 00878-4/fulltext) Rates range from 4% in select high-income countries to 16% or higher in low-resource settings, reflecting differences in healthcare infrastructure, nutrition, and infectious disease burden. [1] Sub-Saharan Africa and southern Asia bear the heaviest burden, with preterm births comprising about 13% of deliveries and accounting for over 65% of global cases in 2020; these regions reported 38.8 million and 36.1 million live births respectively, amid high rates of maternal infections, malaria, and poverty-related stressors. [44] 00878-4/fulltext) In contrast, European nations exhibit lower incidences, such as 5.94% in Denmark and 5.88% in Iceland, attributable to advanced prenatal care and lower exposure to environmental risks. [47] Demographic disparities are evident across racial, ethnic, and socioeconomic lines. In the United States, non-Hispanic Black women faced a preterm birth rate of 14.6% in 2022—approximately 55% higher than non-Hispanic White women at 9.4% and Hispanic women at 10.1%—a pattern persisting into 2023 with rates of 14.65%, 9.44%, and 10.14% respectively. [2] [48] These racial differences endure after controlling for socioeconomic status and prenatal care utilization, pointing to multifactorial contributors including potential genetic vulnerabilities and chronic physiological stress, though definitive causal pathways require further empirical scrutiny beyond access-related explanations. [49] [50] Socioeconomic gradients amplify risks, with preterm birth incidence rising in lower-income groups and deprived neighborhoods; for instance, non-Hispanic Black women in high-deprivation U.S. areas experience rates up to 16%, compared to under 10% for non-Hispanic White women in affluent locales. [51] [52] Maternal nativity influences outcomes, as U.S. immigrants exhibit lower preterm rates (9%) than U.S.-born women (9.7%), possibly due to healthier baseline profiles or cultural protective factors prior to acculturation. [53]| Maternal Race/Ethnicity (U.S., 2022) | Preterm Birth Rate |
|---|---|
| Non-Hispanic Black | 14.6% [2] |
| Non-Hispanic White | 9.4% [2] |
| Hispanic | 10.1% [2] |
Etiology and Pathophysiology
Core Mechanisms and Pathways
Preterm birth arises from the premature activation of the physiologic labor cascade, which normally occurs at term through coordinated hormonal, inflammatory, and mechanical signals at the maternal-fetal interface.[54] This cascade involves cervical remodeling (softening and dilation via collagen degradation and hyaluronic acid accumulation), rupture of fetal membranes (through matrix metalloproteinase activity), and myometrial contractions (driven by increased gap junctions, oxytocin receptors, and prostaglandin synthesis).[55] In spontaneous preterm cases, these processes are triggered pathologically, often converging on a final common pathway of unchecked inflammation that overrides progesterone-mediated quiescence, leading to functional progesterone withdrawal and labor initiation.[56] Infection and sterile inflammation represent a primary pathway, accounting for up to 40% of spontaneous preterm births, particularly those with preterm premature rupture of membranes (PPROM).[57] Microbial invasion of the amniotic cavity or lower genital tract elicits cytokine release (e.g., IL-1β, IL-6, TNF-α) from decidual cells, trophoblasts, and immune cells, activating the NLRP3 inflammasome and Toll-like receptors.[58] [55] This inflammatory cascade upregulates prostaglandins (PGDH inhibition fails), chemokines, and proteases, promoting neutrophil influx, membrane weakening, and uterine contractions; even non-infectious damage-associated molecular patterns (DAMPs) like cell-free fetal DNA or heat shock proteins can mimic this via alarmin signaling.[59] Systemic infections or periodontal disease amplify this through hematogenous spread or oral microbiome translocation.[60] Vascular and decidual hemorrhage pathways contribute in 15-20% of cases, often linked to placental abruption or ischemia from uterine-placental vascular malperfusion.[57] Hypoxia-reoxygenation injury releases fetal stress signals (e.g., S100B protein), triggering decidual hemorrhage and thrombin generation, which activates protease-activated receptors (PARs) to induce myometrial inflammation and prostaglandin production independently of infection.[54] This pathway intersects with preeclampsia or fetal growth restriction, where endothelial dysfunction and oxidative stress exacerbate inflammatory mediator release from the placenta.[61] Uterine overdistension and cervical insufficiency pathways mechanically initiate labor in multifetal gestations or polyhydramnios, stretching myometrium and decidua to release stretch-sensitive cytokines (e.g., IL-8) and activate stretch-activated ion channels, mimicking term signals prematurely.[57] Fetal stress from anomalies or hypoxia can signal via CRH surges or surfactant proteins, amplifying maternal pathways.[54] These heterogeneous triggers underscore multifactorial causality, with genetic-epigenetic modifiers influencing susceptibility across pathways.[61]Genetic and Heritable Components
Heritability estimates for preterm birth derived from twin and family studies range from 17% to 40%, indicating a moderate genetic contribution amid multifactorial etiology.[62][63] A Swedish registry-based study of over 244,000 individuals using an extended twin-sibling design attributed 13.1% of genetic variation to fetal factors, with maternal effects also prominent.[64] These figures underscore that while environmental and obstetric factors predominate, inherited susceptibility plays a substantive role, particularly in spontaneous preterm birth subtypes.[65] Familial recurrence patterns further evidence heritable components, with women whose mothers or sisters experienced preterm delivery facing elevated risks independent of personal obstetric history.[66] For instance, maternal history of preterm birth correlates with increased odds across daughters' pregnancies, even among those born at term, suggesting transgenerational genetic transmission rather than solely intrauterine programming.[67] Sibling studies confirm that preterm-born individuals or those with preterm siblings exhibit heightened recurrence risks, with adjusted odds ratios approximating 1.5 to 2.0 in population cohorts.[68][69] This pattern holds across diverse ancestries, though absolute risks vary with baseline incidence.[70] Genome-wide association studies (GWAS) have identified candidate loci influencing gestational duration and spontaneous preterm birth risk, often implicating pathways in immune regulation, prostaglandin synthesis, and uterine contractility. A 2017 meta-analysis of European-ancestry cohorts pinpointed variants near genes such as EBF1 and AGO3, explaining a fraction of variance in birth timing akin to twin-derived heritability estimates of 30-40%.[62] Subsequent analyses in multi-ancestry samples, including East Asians, highlight alleles in WNT4 and ADCY5, with effect sizes modest but consistent for extreme preterm events.[71] Rare variants in protein-coding regions, detected via exome sequencing, contribute marginally but may amplify risk in compound heterozygotes, particularly for inflammatory-mediated preterm birth.[64] Polygenic risk scores (PRS) aggregating common variants show promise for stratification but remain weakly predictive for preterm birth, capturing less than 5% of liability in validation sets due to polygenicity and gene-environment interplay.[64] Efforts to refine PRS by integrating maternal, fetal, and placental genotypes aim to enhance utility, though clinical translation lags pending larger, diverse genomic datasets.[72] Overall, genetic influences manifest heterogeneously, with stronger signals in familial clusters than sporadic cases, emphasizing the need for causal variant prioritization over candidate gene approaches historically prone to false positives.[73]Inflammatory, Infectious, and Vascular Factors
Intra-amniotic inflammation represents a central pathway in the pathophysiology of spontaneous preterm birth, often triggered by microbial invasion or sterile insults leading to cytokine release and uterine contractility. Dysregulated inflammatory responses, including elevated levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), activate proteases that degrade extracellular matrix in fetal membranes, promoting rupture and labor initiation.[74] [59] This process can occur independently of infection, as in sterile inflammation driven by innate immune activation via damage-associated molecular patterns (DAMPs) from placental or decidual injury.[75] Infectious factors predominantly involve ascending polymicrobial colonization from the lower genital tract, culminating in chorioamnionitis, an acute inflammation of the choriodecidual space affecting up to 40% of preterm deliveries before 32 weeks gestation. Common pathogens include Ureaplasma species, group B Streptococcus, and Escherichia coli, which breach intact or ruptured membranes to elicit Toll-like receptor-mediated responses, releasing endotoxins that amplify prostaglandin synthesis and fetal inflammatory syndrome.[76] [55] Chorioamnionitis correlates with histological evidence in 10-20% of term births but rises sharply in preterm cases, contributing to neonatal sepsis and cerebral palsy risks through fetal systemic inflammation.[77] [78] Vascular factors center on uteroplacental ischemia, where impaired spiral artery remodeling or maternal vascular maladaptation reduces placental perfusion, releasing anti-angiogenic factors like soluble fms-like tyrosine kinase-1 (sFlt-1) and triggering decidual necrosis. This ischemia-hypoxia cascade promotes thrombin generation and hemorrhage, linking to 15-20% of spontaneous preterm births via pathways overlapping with preeclampsia etiology.[79] [80] Maternal conditions such as chronic hypertension or thrombophilias exacerbate vascular insufficiency, evidenced by Doppler ultrasound showing elevated uterine artery pulsatility indices in early gestation predicting preterm delivery.[81] [82] These factors interconnect causally: infections often induce vascular compromise through endothelial damage, while ischemia fosters a pro-inflammatory milieu amplifying infection susceptibility, underscoring preterm birth as a syndrome of multifactorial disruption rather than isolated triggers.[83] Empirical data from placental histopathology studies confirm inflammation in 50% of cases, infection in 25%, and vascular lesions in 20%, with overlaps exceeding additive risks.[56]Risk Factors
Maternal Health and Lifestyle Contributors
Prior preterm birth represents the strongest predictor of recurrence, with risks increasing 2- to 7-fold depending on prior gestational age and whether the delivery was spontaneous or indicated; meta-analyses confirm adjusted odds ratios of 2.5 for any prior preterm and up to 6 for very early prior events.[84] Extremes of maternal age, particularly under 18 or over 35 years, elevate preterm birth risk through mechanisms including cervical immaturity or comorbidities, with cohort data showing 20-50% higher incidence in these groups after adjustment.[84] A short cervical length, typically <25 mm measured by transvaginal ultrasound in the second trimester, independently predicts spontaneous preterm birth with sensitivity around 30-50% and positive predictive value up to 50% for delivery before 35 weeks, often warranting interventions like cerclage.[20] Maternal chronic hypertension is a well-established risk factor for preterm birth, as it can impair placental blood flow and lead to preeclampsia or intrauterine growth restriction, necessitating early delivery. A study of traditional cardiovascular risk factors found that pre-pregnancy hypertension doubled the odds of preterm birth compared to normotensive women. Similarly, pre-existing or gestational diabetes mellitus elevates risk through mechanisms like macrosomia, polyhydramnios, or vascular complications, with meta-analyses indicating a 20-30% increased odds ratio for spontaneous preterm delivery in affected pregnancies.[85][18][86] Obesity, defined as pre-pregnancy BMI ≥30 kg/m², independently heightens preterm birth risk via inflammatory pathways and endothelial dysfunction, with systematic reviews reporting a J-shaped association where both obesity and underweight (BMI <18.5 kg/m²) confer elevated odds, though obesity shows stronger links to indicated preterm deliveries. Maternal infections, particularly genitourinary or periodontal, contribute through ascending inflammation or systemic cytokine release, accounting for up to 40% of cases in some cohorts; robust evidence from umbrella reviews confirms associations with bacterial vaginosis and chorioamnionitis.[87][85][88] Among lifestyle factors, cigarette smoking during pregnancy exhibits a dose-dependent relationship with preterm birth, increasing risk by 20-50% via nicotine-induced vasoconstriction and carbon monoxide hypoxia; meta-analyses of cohort studies affirm this for both active and passive exposure, with quitting before 15 weeks mitigating much of the hazard. Illicit drug use, including amphetamines and cocaine, robustly elevates odds (up to threefold for amphetamines per umbrella reviews), through uteroplacental insufficiency and abruption. Alcohol consumption, even moderate, correlates with higher preterm rates in dose-response patterns, though confounding by socioeconomic factors tempers causal inference.[89][90][84] Black race is associated with elevated preterm birth rates, approximately 50% higher than White women in U.S. data, persisting after adjustment for socioeconomic and clinical factors in multivariate analyses.[84] Inadequate prenatal care exacerbates these risks by delaying detection of complications, with women receiving late or no care facing 1.5-2 times higher preterm incidence per national surveillance data. Psychological stressors, including depression or intimate partner violence, show associative links (odds ratios 1.2-1.5) potentially via cortisol-mediated pathways, though prospective studies emphasize multifactorial interplay over direct causation.[84][90]Fetal and Placental Anomalies
Multiple gestation, such as twins or higher-order multiples, substantially increases preterm birth risk to 50-60% due to uterine overdistension, placental insufficiency, and heightened inflammatory responses, independent of maternal factors.[84] Fetal congenital anomalies, particularly major structural malformations, confer a substantially elevated risk of preterm birth through mechanisms including polyhydramnios-induced uterine distension, fetal distress prompting iatrogenic delivery, and shared pathophysiological pathways such as vascular insufficiency or genetic disruptions. In a large U.S. cohort analysis of singleton live births from 1995–2000, the prevalence of major birth defects among preterm infants (24–36 weeks gestation) was approximately 8%, yielding a prevalence ratio (PR) of 2.65 (95% CI: 2.62–2.68) compared to term births.[91] For very preterm births (24–31 weeks), the defect prevalence rose to 16%, with a PR of 5.25 (95% CI: 5.15–5.35).[91] Risk varies by anomaly type and multiplicity; central nervous system defects exhibited the strongest association, with a PR of 16.23 (95% CI: 15.49–17.00) for very preterm birth, while cardiovascular defects followed at PR 9.29 (95% CI: 9.03–9.56).[91] Pregnancies involving multiple major anomalies demonstrated the highest vulnerability, with an adjusted odds ratio (aOR) of 8.0 (95% CI: 4.6–14.1) for preterm delivery.[92] Overall, neonates with any major congenital anomaly face roughly twofold higher odds of preterm birth (aOR: 2.0, 95% CI: 1.3–2.9).[93] Placental anomalies disrupt maternal-fetal nutrient and oxygen exchange or provoke hemorrhage, often necessitating emergent or planned preterm intervention to avert fetal hypoxia or maternal instability. Placenta previa, characterized by low-lying placental implantation over the cervical os, correlates with preterm delivery rates of 43.5% in affected singleton gestations, driven by antepartum bleeding and cesarean requirements.[94] Placental abruption, involving premature separation, accounts for approximately 10% of all preterm births and yields an adjusted relative risk of 3.9 (95% CI: 3.5–4.4) for delivery before 37 weeks, reflecting acute vascular rupture and coagulopathy.[95][96] Placenta accreta spectrum disorders, where trophoblast invades beyond the decidua, are linked to preterm birth in up to 74.7% of suspected cases, primarily via scheduled cesarean hysterectomies around 34–36 weeks to mitigate hemorrhage risks.[97] Low-lying or marginal placentas confer intermediate risks, with preterm birth before 37 weeks occurring in about 27–30% of cases.[94][98] These associations underscore placental pathology's causal role in spontaneous and indicated preterm events, independent of confounding maternal factors in multivariate analyses.Iatrogenic Factors from Medical Interventions
Iatrogenic preterm birth refers to the intentional initiation of delivery before 37 weeks' gestation through medical interventions such as labor induction or cesarean section, typically to address maternal or fetal compromise including preeclampsia, eclampsia, severe fetal growth restriction, or placental insufficiency. These interventions account for 30% to 40% of all preterm births. Common underlying conditions prompting such decisions include hypertensive disorders (present in 72.8% of iatrogenic cases) and small-for-gestational-age fetuses (21.7% prevalence).[99][99][100] Assisted reproductive technologies contribute indirectly via multiple embryo transfers, which elevate the risk of twin or higher-order gestations; twin pregnancies exhibit a 60% preterm birth rate, often requiring iatrogenic delivery due to associated complications like growth discordance or preterm labor threats. Globally, iatrogenic preterm birth represents up to 50% of cases in certain regions, with modifiable factors including promotion of single embryo transfer to reduce multiples.[100][100] Invasive prenatal diagnostic procedures, such as amniocentesis or chorionic villus sampling, carry a low risk of iatrogenic preterm premature rupture of membranes (PPROM), reported in less than 1% to 2% of amniocentesis cases, which may precipitate preterm labor or necessitate early delivery; however, large studies find no overall increase in preterm delivery rates from these tests. Fetoscopic interventions pose higher risks, with PPROM rates of 3% to 5%.[101][102][101] Non-medically indicated interventions, including elective cesarean sections or inductions at 34 to 36 weeks, constitute avoidable iatrogenic factors, linked to sevenfold higher neonatal morbidity risks such as respiratory distress compared to term deliveries; rising global cesarean rates (from 6.7% in 1990 to 19.1% in 2014) amplify this when performed preterm without clear necessity. Guidelines recommend accurate gestational dating via first-trimester ultrasound and evidence-based timing to minimize such occurrences.[100][100][100]Diagnosis and Risk Stratification
Clinical Signs and Symptomatic Evaluation
Preterm labor, defined as regular uterine contractions leading to cervical changes prior to 37 weeks of gestation, presents with symptoms including regular contractions occurring every 10 minutes or more frequently (such as ≥6 contractions per 30 minutes), often accompanied by low back pain, pelvic pressure, or menstrual-like cramps.[103][104] Additional indicators include vaginal bleeding, increased vaginal discharge, or leakage of amniotic fluid, which may signal membrane rupture.[104] Patients reporting more than six contractions per hour, particularly with persistent pain, warrant immediate assessment to distinguish true labor from false alarms, as up to 30% of symptomatic women between 24 and 34 weeks do not deliver preterm.[105][104] Initial evaluation begins with a detailed history to confirm gestational age, typically verified against early ultrasound or last menstrual period, and to identify risk factors such as prior preterm birth or multiple gestation.[106] Contractions are assessed via external tocodynamometry or manual palpation, aiming for documentation of at least four contractions in 20 minutes or eight in 60 minutes over two hours, or ≥6 in 30 minutes.[103][104] A sterile speculum examination follows to evaluate for cervical discharge, bleeding, or pooling of amniotic fluid suggestive of preterm premature rupture of membranes (PPROM), tested via nitrazine or ferning if indicated; digital cervical examination is deferred in suspected PPROM to minimize infection risk.[106][104] Cervical status is then appraised digitally if safe, diagnosing preterm labor by dilation of at least 2 cm with 80% effacement or progressive change, per American College of Obstetricians and Gynecologists criteria for gestations from 20 weeks to 36 weeks 6 days.[103][104] Transvaginal ultrasound measures cervical length, with lengths under 25-30 mm indicating heightened risk, though not diagnostic alone; it also assesses fetal presentation and placental position.[106][107] Adjunctive tests like fetal fibronectin sampling from cervicovaginal secretions may aid in ruling out imminent delivery if negative (negative predictive value >95% for delivery within 7-14 days), but positive results (e.g., ≥50 ng/mL) require correlation with clinical findings due to lower specificity.[104][106] Laboratory evaluation includes urinalysis and screens for urinary tract infection, sexually transmitted infections, group B Streptococcus, or inflammatory markers if infection is suspected, guiding targeted management.[104] This multifaceted approach balances sensitivity for intervention with avoidance of unnecessary tocolysis, as overtreatment risks maternal side effects without altering outcomes in low-risk cases.[103]Predictive Biomarkers and Imaging Modalities
Fetal fibronectin (fFN), detected via cervicovaginal swab between 22 and 35 weeks gestation, serves as a biomarker for disrupted maternal-fetal interface, with a negative predictive value exceeding 95% for spontaneous preterm birth (sPTB) before 34 weeks in symptomatic women.[108] In asymptomatic high-risk women, fFN negativity predicts low risk of delivery within 7-14 days, though positive predictive value remains modest at 20-30%, limiting its utility for confirming imminent birth.[109] Quantitative fFN thresholds (e.g., ≥50 ng/mL) improve positive predictive value to 32-61% for sPTB <34 weeks, but recent commercial withdrawal of certain fFN assays has prompted exploration of alternatives.[110][109] Phosphorylated insulin-like growth factor-binding protein-1 (phIGFBP-1), measured in cervicovaginal fluid during mid-trimester, identifies membrane rupture risk and predicts sPTB with sensitivity around 70-80% in asymptomatic women, particularly when combined with clinical history.[111] Emerging maternal serum biomarkers, such as biglycan and decorin, show elevated levels in sPTB cases, with area under the curve (AUC) values of 0.75-0.85 for prediction before 37 weeks, though validation in diverse cohorts is ongoing.[112] Metabolomic profiles from maternal plasma or urine, analyzed via mass spectrometry, reveal altered lipid and amino acid patterns predictive of sPTB as early as first trimester, with some panels achieving AUC >0.80, but reproducibility across populations remains a challenge due to confounding factors like ethnicity and diet.[113] Transvaginal ultrasound (TVUS) measurement of cervical length (CL) between 16-24 weeks is the primary imaging modality for sPTB risk stratification, with CL <25 mm indicating 20-50% risk of delivery <34 weeks in singleton pregnancies, outperforming digital exams.[114][115] Serial TVUS in high-risk women (e.g., prior preterm birth) detects progressive shortening, guiding interventions like progesterone; guidelines recommend screening at 16-20 weeks for those with history, with interobserver variability minimized via standardized protocols.[116] Transabdominal or transperineal ultrasound offers less invasive alternatives but yields higher variability and lower accuracy compared to TVUS.[117] Magnetic resonance imaging (MRI) of the cervix provides superior visualization of internal os funneling and tissue integrity, predicting sPTB with sensitivity up to 90% in selected cohorts, though its higher cost and limited availability restrict routine use over TVUS.[118][114] Advanced ultrasound techniques, including automated texture analysis of cervical images, enhance predictive AUC to 0.85-0.90 for mid-trimester sPTB by quantifying echogenicity patterns linked to remodeling.[119] Integrating biomarkers with imaging via machine learning models, as in recent cohorts from 2019-2022, achieves prediction accuracies of 80-90% for sPTB in low-risk women under 35, but prospective validation is needed to address overfitting and generalizability.[120] Despite advances, no single modality or biomarker universally predicts all preterm birth subtypes, with ongoing research emphasizing multi-omic panels to overcome limitations in positive predictive power.[121]Prevention Approaches
Preconception and Lifestyle Optimization
Preconception optimization involves addressing modifiable risk factors prior to conception to mitigate the likelihood of preterm birth, defined as delivery before 37 weeks of gestation. Evidence from systematic reviews indicates that preconception interventions, including lifestyle modifications, can reduce preterm birth rates by improving maternal health status and minimizing inflammatory or metabolic stressors that contribute to early labor.[122] For instance, achieving optimal preconception health through targeted counseling has been linked to lower incidences of adverse perinatal outcomes, including preterm delivery, particularly in high-risk groups such as women with diabetes.[123] Folic acid supplementation before conception plays a key role in risk reduction. Periconceptional folic acid use is associated with a 14% overall decrease in preterm birth risk, with longer-term supplementation (one year or more) yielding 50-70% reductions in early spontaneous preterm births.[124] [125] Higher dietary folate intake during the preconception period further supports this protective effect against preterm delivery.[126] Maintaining a healthy preconception body mass index (BMI) of 18.5-24.9 kg/m² is crucial, as both underweight (BMI <18.5 kg/m²) and obesity (BMI ≥30 kg/m²) elevate preterm birth risks through mechanisms like impaired placentation or chronic inflammation.[87] [127] Preconception weight management, including diet and exercise, can normalize these risks, with adherence to health-conscious dietary patterns—rich in vegetables, fruits, protein sources, and whole grains—correlating with lower preterm birth incidence independent of BMI.[123] [128] Cessation of tobacco, alcohol, and illicit drug use prior to conception substantially lowers preterm birth probability. Women who quit smoking before pregnancy exhibit preterm birth risks comparable to never-smokers, with cessation yielding up to a 26% risk reduction in subsequent pregnancies.[129] [130] Similarly, abstaining from alcohol and drugs mitigates associated vascular and neurotoxic effects that precipitate preterm labor, as substance use during the preconception window heightens overall adverse outcome risks.[131] [132] Physical activity and management of chronic conditions, such as optimizing glycemic control in diabetes, further enhance preconception resilience against preterm birth triggers.[133] [123] Comprehensive preconception counseling integrating these elements—nutrition, weight control, substance avoidance, and exercise—offers a multifaceted approach grounded in causal pathways like reduced oxidative stress and improved endothelial function.[134]Antenatal Screening and Prophylactic Measures
Transvaginal ultrasound measurement of cervical length between 16 and 24 weeks gestation serves as a key antenatal screening tool for identifying women at risk of spontaneous preterm birth, particularly those with a prior history of preterm delivery. A cervical length below 25 mm is associated with a significantly elevated risk of preterm birth before 34 weeks, with evidence from randomized trials showing that targeted interventions in this group can mitigate outcomes.[135][117] Routine universal cervical length screening is not recommended for low-risk asymptomatic women due to lack of proven benefit in reducing overall preterm birth rates, though selective screening in high-risk populations is supported by professional guidelines.[136][137] Fetal fibronectin testing, performed via cervicovaginal swab between 22 and 35 weeks in women with symptoms of preterm labor such as contractions or cervical changes, aids in risk stratification by detecting the protein's presence, which indicates potential placental detachment and labor onset. A negative test result rules out delivery within 7-14 days with high negative predictive value (approximately 95-99%), allowing avoidance of unnecessary hospitalizations or tocolysis, though it has lower positive predictive value and is not endorsed for routine asymptomatic screening.[138][139] Prophylactic vaginal progesterone supplementation, initiated from 16 weeks gestation in women with a singleton pregnancy and either a prior spontaneous preterm birth or a short cervix (<25 mm) on ultrasound, reduces the relative risk of preterm birth before 35 weeks by 30-40% based on meta-analyses of randomized controlled trials. Guidelines from the American College of Obstetricians and Gynecologists recommend 200 mg daily vaginal progesterone for these indications, with intramuscular 17-alpha-hydroxyprogesterone caproate as an alternative for history-indicated cases, though vaginal administration shows superior efficacy in short cervix subgroups without increasing adverse neonatal outcomes.[140][141][142] Cervical cerclage, a surgical stitch placed around the cervix, is indicated prophylactically in select high-risk cases: history-indicated for women with prior second-trimester losses due to cervical insufficiency, ultrasound-indicated for shortening cervix (<25 mm before 24 weeks) with preterm birth history, or emergency for advanced dilation. Meta-analyses indicate cerclage reduces preterm birth before 34 weeks by about 26% in ultrasound-indicated cases, with stronger benefits when combined with vaginal progesterone, though prophylactic use in low-risk or multifetal pregnancies lacks robust evidence and may increase intervention risks without net gain.[143][144][145] Other measures, such as serial monitoring in specialist preterm birth clinics for high-risk women, incorporate these screenings and interventions, with observational data suggesting reduced preterm birth rates through multidisciplinary care, though randomized evidence remains limited.[146] No broad population-based prophylactic strategies beyond targeted use have demonstrated consistent efficacy in preventing preterm birth across diverse risk groups.[147]Interventions for High-Risk Pregnancies
For women with a history of spontaneous preterm birth, vaginal progesterone supplementation, administered daily from 16-20 weeks gestation until 36 weeks or delivery, reduces the risk of recurrent preterm birth before 34 weeks by approximately 30-40% in singleton pregnancies, based on meta-analyses of randomized trials.[148][149] This approach is recommended by guidelines for those without contraindications, as intramuscular 17-alpha hydroxyprogesterone caproate showed no benefit in large trials like the 2020 PROLONG study and was discontinued by the FDA in 2023.[140] Vaginal progesterone is particularly effective when combined with cervical length screening, showing greater absolute risk reduction in women with a short cervix (<25 mm) detected via transvaginal ultrasound before 24 weeks.[148] Cervical cerclage, a surgical procedure to reinforce the cervix, is indicated for high-risk cases such as prior preterm birth before 34 weeks or cervical insufficiency, with history-indicated placement typically at 12-14 weeks. Ultrasound-indicated cerclage, performed when cervical length shortens to <25 mm in women with prior spontaneous preterm birth, lowers preterm birth rates by 30-50% compared to expectant management, per randomized controlled trials and FIGO guidelines.[150][151] Shirodkar or McDonald techniques are used, with removal planned at 36-37 weeks or earlier if labor ensues; however, cerclage does not benefit twin gestations or those without prior preterm history, as evidenced by trials like OPPTIMUM and CERNET.[144] Complications include infection or membrane rupture, occurring in <5% of cases.[150] In multiple gestations, a high-risk category comprising 3-5% of pregnancies but 20-30% of preterm births, interventions are limited; vaginal progesterone may modestly delay delivery in select singletons but lacks consistent efficacy in twins, and routine cerclage or pessaries are not recommended due to neutral or adverse outcomes in meta-analyses.[144][152] For women with asymptomatic bacteriuria or smoking, targeted treatments like antibiotics or cessation programs reduce preterm risk by 20-50%, though these are adjunctive rather than primary interventions.[153] Bed rest remains unsupported by evidence and may increase thrombosis risk without preventing preterm birth.[154] Ongoing trials explore combined therapies, such as progesterone plus cerclage, which preliminary data suggest further lowers rates of birth before 32 weeks in ultra-high-risk cases.[145] Overall, personalized risk stratification via history and ultrasound guides intervention selection, prioritizing those with proven reductions in neonatal morbidity.[151]Clinical Management
Labor Suppression and Delay Tactics
Tocolysis involves the administration of pharmacological agents to inhibit uterine contractions and temporarily delay preterm labor, primarily to allow time for antenatal corticosteroids to enhance fetal lung maturity or for maternal transfer to a tertiary care facility. According to American College of Obstetricians and Gynecologists (ACOG) guidelines, tocolysis is recommended for gestations between 24 and 33 weeks 6 days when preterm labor is diagnosed and there are no contraindications, aiming for a delay of at least 48 hours rather than indefinite prolongation, as evidence does not support reduced rates of preterm birth or improved neonatal outcomes beyond this window.[103][155] Common tocolytic classes include beta-2 adrenergic agonists (e.g., ritodrine or terbutaline), which relax uterine smooth muscle via cyclic AMP elevation but are associated with maternal side effects such as tachycardia, pulmonary edema, and hyperglycemia; calcium channel blockers like nifedipine, which inhibit calcium influx to reduce contractility and demonstrate superior efficacy over beta-agonists and magnesium sulfate in delaying delivery by 48 hours or more with fewer adverse effects; cyclooxygenase inhibitors such as indomethacin, effective short-term (up to 48 hours) by blocking prostaglandin synthesis but limited by fetal risks including ductal-dependent closure after 32 weeks; and magnesium sulfate, used intravenously for its neuromuscular blocking effects, though it provides minimal prolongation compared to alternatives and carries risks of maternal respiratory depression and hypotension.[156][157][158] Systematic reviews indicate that while individual agents like nifedipine and prostaglandin inhibitors offer the highest probability of short-term delay and improved maternal tolerance, no tocolytic class consistently reduces perinatal mortality, respiratory distress syndrome, or long-term neurodevelopmental impairment, with benefits largely confined to facilitating corticosteroid administration rather than altering overall preterm birth incidence.[159][160] Combination therapies, such as ritodrine with nifedipine, show promise in select trials for extended delay beyond seven days, but broader adoption lacks robust endorsement due to insufficient large-scale data on safety and synergy.[161] Atosiban, an oxytocin receptor antagonist, has not demonstrated improvements in neonatal outcomes when initiated between 30 and 33 weeks.[162] Non-pharmacological tactics include activity restriction or bed rest, which lack empirical support for suppressing labor or preventing preterm birth and may increase risks of venous thromboembolism, muscle atrophy, and gestational weight loss without prolonging gestation.[163][164] Cervical cerclage, a surgical stitch to reinforce the cervix, is not a general tactic for active preterm labor but serves as a delay strategy in cases of cervical insufficiency or short cervix (<25 mm before 24 weeks), reducing preterm birth risk before 35 weeks by approximately 30-40% in high-risk singleton pregnancies when placed prophylactically or emergently, though it carries complications like infection or membrane rupture in 1-2% of procedures.[165][150] Contraindications for all tactics encompass advanced labor (cervical dilation >4 cm), infection, abruption, or fetal demise, prioritizing maternal-fetal safety over prolongation.[155]Antenatal Corticosteroids and Maternal Therapies
Antenatal corticosteroids, typically betamethasone or dexamethasone, are administered intramuscularly to pregnant women at risk of preterm delivery between 24 and 34 weeks of gestation to accelerate fetal lung maturation and organ development. A standard single course consists of two 12 mg doses of betamethasone given 24 hours apart or four 6 mg doses of dexamethasone every 12 hours. This intervention reduces the incidence of respiratory distress syndrome by approximately 34%, intraventricular hemorrhage by 46%, and neonatal mortality by 31%, based on meta-analyses of randomized controlled trials involving over 3,900 participants. The foundational evidence derives from the 1972 Liggins and Howie trial, with subsequent Cochrane reviews confirming these benefits for gestations under 34 weeks.[166][167] For late preterm births (34 to 36+6 weeks), the 2016 ALPS trial demonstrated that betamethasone reduces neonatal respiratory complications from 11.6% to 8.1% in women with planned delivery in this window, prompting updated guidelines to extend use selectively when delivery is anticipated within 7 days and no contraindications exist. However, benefits diminish if delivery occurs more than 7 days after administration, and evidence indicates potential harms in non-delivering cases, including transient neonatal hypoglycemia and possible long-term neurodevelopmental risks, though large cohort studies show no overall increase in impairment up to age 6 years. Repeat courses are reserved for persistent threat after 7 days from initial treatment, as the MACS trial found marginal additional respiratory benefits outweighed by reduced fetal growth. Dexamethasone and betamethasone exhibit comparable efficacy, with some observational data suggesting dexamethasone may confer a slight edge in reducing perinatal death (relative risk 0.88).[168][169][170] Maternal magnesium sulfate therapy, administered intravenously prior to preterm delivery before 32 weeks, provides fetal neuroprotection by mitigating excitotoxic brain injury, reducing the risk of cerebral palsy by 32% and gross motor dysfunction by 30%, per the 2009 Magpie and 2010 PREMAG trials meta-analyzed in Cochrane reviews. A loading dose of 4-6 g followed by 1-2 g/hour maintenance for 24 hours or until delivery is standard, with monitoring for maternal side effects like hypotension and respiratory depression. Unlike corticosteroids, magnesium does not promote lung maturity but complements it in imminent preterm scenarios. For preterm premature rupture of membranes (PPROM), maternal broad-spectrum antibiotics (e.g., erythromycin or ampicillin plus azithromycin) for 48 hours or 7 days extend latency by 7 days and reduce chorioamnionitis, though they do not alter overall perinatal mortality. These therapies prioritize fetal benefit over maternal comfort, with decisions guided by gestational age, fetal viability, and maternal infection status to avoid overuse amid variable prediction accuracy of delivery timing.[37][167]Delivery and Immediate Neonatal Support
The mode of delivery for preterm births is determined by gestational age, fetal presentation, maternal condition, and fetal well-being, with vaginal delivery preferred for cephalic presentations absent contraindications to optimize outcomes.[171] For breech presentations at or below 32 weeks' gestation, cesarean delivery is associated with lower perinatal mortality compared to vaginal delivery, based on meta-analyses of observational data showing reduced risks of neonatal death and severe morbidity.00683-5/fulltext) Cesarean section may also decrease the incidence of intraventricular hemorrhage in uncomplicated deliveries before 32 weeks, though overall evidence for routine cesarean in preterm cephalic births remains insufficient to establish superiority over vaginal delivery.[172] Following delivery, delayed umbilical cord clamping for at least 30 seconds is recommended for preterm infants to enhance placental transfusion, increasing neonatal hemoglobin levels and reducing risks of intraventricular hemorrhage and necrotizing enterocolitis without increasing polycythemia or jaundice requiring therapy.[173] Immediate neonatal support begins with a multidisciplinary team prepared for resuscitation according to Neonatal Resuscitation Program (NRP) guidelines, initiating with warming, drying, and stimulation while assessing heart rate, respirations, and color.[174] For preterm infants, particularly those below 32 weeks' gestation, thermoregulation is critical; placement in polyethylene occlusive bags or wraps prevents heat loss, as hypothermia correlates with increased mortality.[175] Respiratory support escalates as needed: positive pressure ventilation with 21-30% oxygen for preterm infants not breathing adequately, titrated to target saturations, followed by continuous positive airway pressure (CPAP) or intubation for persistent apnea or bradycardia.[176] Chest compressions and epinephrine are employed if heart rate remains below 60 beats per minute post-ventilation.[177] Stable preterm newborns benefit from immediate skin-to-skin contact (kangaroo mother care) to stabilize temperature, cardiorespiratory function, and promote bonding, as endorsed by WHO guidelines reducing mortality in low-birth-weight infants.[178] Transfer to a neonatal intensive care unit follows for ongoing monitoring and specialized interventions such as surfactant administration or mechanical ventilation.[179]Outcomes and Long-Term Prognosis
Short-Term Morbidity and Mortality Rates
Short-term mortality for preterm infants, defined as death within the first 28 days of life, varies inversely with gestational age (GA), with extremely preterm infants (born before 28 weeks) exhibiting the highest rates. Globally, complications from preterm birth accounted for approximately 900,000 neonatal deaths in 2019, representing the leading cause of under-5 mortality.[1] In high-resource settings like the United States, the preterm-specific infant mortality rate rose slightly from 33.59 to 34.78 per 1,000 live births between 2021 and 2022, reflecting persistent vulnerabilities despite advances in neonatal care.[180] Survival to discharge from neonatal intensive care units (NICUs) for infants born at 22-25 weeks' GA reached 24.9% overall in recent U.S. cohorts, with cumulative mortality peaking at 41.7% in the first three months for extremely preterm cases.[181][182]| Gestational Age | Approximate Survival Rate to NICU Discharge |
|---|---|
| 22 weeks | 7-25% |
| 24 weeks | 30% |
| 25 weeks | 68% |
| 31 weeks | 94% |
Neurodevelopmental and Health Sequelae
Preterm infants face substantially elevated risks of neurodevelopmental impairments, with prevalence inversely proportional to gestational age at birth. In very preterm infants (born before 32 weeks), rates of cerebral palsy range from 7% to 19%, depending on the subgroup; for example, among extremely preterm infants (22-27 weeks), cerebral palsy affects up to 18.8% of survivors.[193][194] Cognitive deficits are also common, including lower IQ scores and executive function impairments; meta-analyses indicate that preterm birth, particularly below 32 weeks, is associated with standardized mean differences in cognitive scores of -0.5 to -1.0 standard deviations compared to term-born peers, persisting into adolescence and adulthood.[195][196] Behavioral issues, such as attention-deficit/hyperactivity disorder and autism spectrum traits, occur at 1.5- to 2-fold higher rates in preterm cohorts, often linked to early brain injuries like intraventricular hemorrhage or white matter damage.[197] Beyond neurodevelopment, preterm birth confers lifelong health risks across multiple systems. Respiratory sequelae predominate, with bronchopulmonary dysplasia evolving into chronic obstructive patterns; adults born preterm exhibit reduced lung function, including lower forced expiratory volume, predisposing to early chronic lung disease.[198] Cardiovascular abnormalities persist, including smaller cardiac structures and hypertension, with preterm survivors showing 1.5- to 3-fold increased odds of ischemic heart disease in adulthood.[199] Metabolic and renal issues are elevated, encompassing higher incidences of diabetes, obesity, and chronic kidney disease, attributed to disrupted organ maturation and programming effects from neonatal stressors.[200] Overall mortality remains higher into adulthood, with preterm birth linked to 1.5- to 2-fold excess risk of death from cardiorespiratory and other causes.[201] These outcomes vary by gestational age and neonatal interventions, with extreme preterm infants bearing the highest burden despite advances in survival.[202]Prognostic Modifiers and Survival Data
Survival rates for preterm infants are strongly correlated with gestational age at birth, with rates increasing markedly from below 50% for deliveries before 24 weeks to over 90% at 28 weeks or later. In a global pooled analysis of studies up to 2025, survival to discharge for infants born at 22 weeks gestation averaged 27.6% (95% CI: 19.77–35.43). For those at 24 weeks, survival approximates 60–70%, rising to 70–80% at 25 weeks, 80% at 26 weeks, and 85–90% at 27–28 weeks. Infants born between 32 and 36 weeks exhibit survival exceeding 95%, approaching term levels. In the United States, overall preterm infant mortality declined across all gestational age strata from 1995 to 2020, reflecting advancements in neonatal intensive care.[185][203][204]| Gestational Age | Approximate Survival to Discharge |
|---|---|
| 22 weeks | 20–30% |
| 23–24 weeks | 50–70% |
| 25–26 weeks | 70–80% |
| 27–28 weeks | 85–90% |
| 29–32 weeks | >95% |