Recent from talks
Contribute something
Nothing was collected or created yet.
Topiramate
View on Wikipedia
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Topamax, Trokendi XR, Qudexy XR, others |
| Other names | Topiramic acid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697012 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Protein binding | 13–17%; 15–41% |
| Metabolism | Liver (20–30%) |
| Elimination half-life | 21 hours |
| Excretion | Urine (70–80%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.129.713 |
| Chemical and physical data | |
| Formula | C12H21NO8S |
| Molar mass | 339.36 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Topiramate, sold under the brand name Topamax among others, is an oral medication used to treat epilepsy and prevent migraines.[8] For epilepsy, this includes treatment for generalized or focal seizures.[9] It has also been used off-label for alcohol dependence and essential tremor.[8]
Common side effects include tingling, feeling tired, loss of appetite, abdominal pain, weight loss,[10] and decreased cognitive function such as trouble concentrating.[8][9] Serious side effects may include suicidal ideation, increased ammonia levels resulting in encephalopathy, and kidney stones.[8] Topiramate can cause birth defects, including cleft lip and palate.[11] Risks/benefits should be carefully discussed with the full treatment team. Topiramate is considered "probably compatible" with lactation and is not contraindicated for breastfeeding, though monitoring of the infant for diarrhea or poor weight gain may be considered.[12][13] Its mechanism of action is unclear.[8]
Topiramate was approved for medical use in the United States in 1996.[8] It is available as a generic medication.[9][14][15] In 2023, it was the 71st most commonly prescribed medication in the United States, with more than 9 million prescriptions.[16][17]
Medical uses
[edit]
Topiramate is used to treat epilepsy in children and adults, and it was originally used as an anticonvulsant.[18] In children, it is indicated for the treatment of Lennox-Gastaut syndrome, a disorder that causes seizures and developmental delay. It is most frequently prescribed for the prevention of migraines,[18] as it decreases the frequency of attacks.[19][20] Topiramate is used to treat medication overuse headache and is recommended by the European Federation of Neurological Societies as one of the few medications showing effectiveness for this indication.[21]
Pain
[edit]A 2018 review found topiramate of no use in chronic low back pain.[22] Topiramate has not been shown to work as a pain medicine in diabetic neuropathy, the only neuropathic condition for which it has been adequately tested.[23]
Other
[edit]One common off-label use for topiramate is in the treatment of bipolar disorder.[24][25][26] A review published in 2010 suggested a benefit of topiramate in the treatment of symptoms of borderline personality disorder; however, the authors noted that this was based only on one randomized controlled trial and requires replication.[27]
Topiramate has been used as a treatment for alcoholism.[28] The U.S. Veterans Affairs and Department of Defense 2015 guidelines on substance use disorders list topiramate as a "strong for" in its recommendations for alcohol use disorder.[29]
Other uses include treatment of obesity[30][31] and binge eating disorder,[32] and off-setting weight gain induced by taking antipsychotic medications.[33] In 2012, the combination of phentermine/topiramate was approved in the United States for weight loss.
Adverse effects
[edit]People taking topiramate should be aware of the following risks:
- Avoid activities requiring mental alertness and coordination until drug effects are realized.
- Topiramate may impair heat regulation,[34] especially in children. Use caution with activities leading to an increased core temperature, such as strenuous exercise, exposure to extreme heat, or dehydration.
- Topiramate may cause visual field defects.[35]
- Topiramate may decrease the effectiveness of oestrogen-containing oral contraceptives.
- Taking topiramate in the first trimester of pregnancy may increase the risk of cleft lip/cleft palate in infants.[36]
- As is the case for all antiepileptic drugs, it is advisable not to suddenly discontinue topiramate, as there is a theoretical risk of rebound seizures.
- Some studies have attributed loss of appetite and upper respiratory tract infection to topiramate, but studies have concluded these adverse events are not difficult to tolerate for most individuals.[37]
Frequency
[edit]Adverse effects by incidence:[38][39][40][41]
Very common (>10% incidence) adverse effects include:
- Dizziness
- Weight loss
- Paraesthesia – e.g., pins and needles
- Somnolence
- Nausea
- Diarrhea
- Fatigue
- Nasopharyngitis
- Depression
Rarely, the inhibition of carbonic anhydrase may be strong enough to cause metabolic acidosis of clinical importance.[42]
The U.S. Food and Drug Administration (FDA) has notified prescribers that topiramate can cause acute myopia and secondary angle closure glaucoma in a small subset of people who take topiramate regularly.[43] The symptoms, which typically begin in the first month of use, include blurred vision and eye pain. Discontinuation of topiramate may halt the progression of the ocular damage and may reverse the visual impairment.
Preliminary data suggests that, as with several other anti-epileptic drugs, topiramate carries an increased risk of congenital malformations.[44] This might be particularly important for women who take topiramate to prevent migraine attacks. In March 2011, the FDA notified healthcare professionals and patients of an increased risk of development of cleft lip and/or cleft palate (oral clefts) in infants born to women treated with Topamax (topiramate) during pregnancy and placed it in Pregnancy Category D.[36]
Cognitive and word-finding difficulties, which may occur in some patients, may respond to piracetam.[45][46]
Carbonation dysgeusia (distortion of the sense of taste-sensation of carbonation) may respond to and/or be prevented with zinc.[47]
Topiramate has been associated with a statistically significant increase in suicidality,[48] and "suicidal thoughts or actions" is now listed as one of the possible side effects of the drug "in a very small number of people, about 1 in 500."[34][49]
Overdose
[edit]Symptoms of acute and acute on chronic exposure to topiramate range from asymptomatic to status epilepticus, including in patients with no seizure history.[50][51] In children, overdose may also result in hallucinations.[51] Topiramate has been deemed the primary substance that led to fatal overdoses in cases that were complicated by polydrug exposure.[52] The most common signs of overdose are dilated pupils, somnolence, dizziness, psychomotor agitation, and abnormal, uncoordinated body movements.[50][51][52]
Interactions
[edit]Topiramate has many drug-drug interactions. Some of the most common are listed below:
- As topiramate inhibits carbonic anhydrase, use with other inhibitors of carbonic anhydrase (e.g. acetazolamide) increases the risk of kidney stones.[citation needed]
- Enzyme inducers (e.g. carbamazepine) can increase the elimination of topiramate, possibly necessitating dose escalations of topiramate.[citation needed]
- Topiramate may increase the plasma levels of phenytoin.
- Topiramate itself is a weak inhibitor of CYP2C19 and induces CYP3A4; a decrease in plasma levels of estrogens and digoxin has been noted during topiramate therapy. This can reduce the effectiveness of oral contraceptives (birth control pills); use of alternative birth control methods is recommended.[53] Neither intrauterine devices (IUDs) nor Depo-Provera are affected by topiramate.[53]
- Alcohol may cause increased sedation or drowsiness, and increase the risk of having a seizure.
- As topiramate may result in acidosis, other treatments that also do so may worsen this effect.[54]
- Oligohidrosis and hyperthermia were reported in post-marketing reports about topiramate; antimuscarinic drugs (like trospium) can aggravate these disorders.[citation needed]
Pharmacology
[edit]
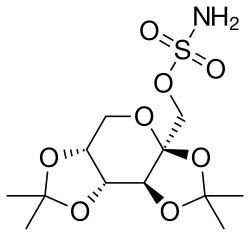
The topiramate molecule is a sulfamate modified sugar – more specifically, fructose diacetonide, an unusual chemical structure for a pharmaceutical.
Topiramate is quickly absorbed after oral use. It has a half-life of 21 hours and a steady state of the drug is reached in 4 days in patients with normal renal function.[55] Most of the drug (70%) is excreted in the urine unchanged. The remainder is extensively metabolized by hydroxylation, hydrolysis, and glucuronidation. Six metabolites have been identified in humans, none of which constitutes more than 5% of an administered dose.
Several cellular targets have been proposed to be relevant to the therapeutic activity of topiramate.[56] These include voltage-gated sodium channels, high-voltage-activated calcium channels, GABAA receptors, AMPA/kainate receptors, and carbonic anhydrase isoenzymes. There is evidence that topiramate may alter the activity of its targets by modifying their phosphorylation state instead of by direct action.[57] The effect on sodium channels could be of particular relevance for seizure protection. Although topiramate does inhibit high-voltage-activated calcium channels, its relevance to clinical activity is uncertain. Effects on specific GABAA receptor isoforms could also contribute to the anticonvulsant activity of the drug. Topiramate selectively inhibits cytosolic (type II) and membrane-associated (type IV) forms of carbonic anhydrase. Its action on carbonic anhydrase isoenzymes may contribute to the drug's side effects, including its propensity to cause metabolic acidosis and calcium phosphate kidney stones.[citation needed]
Topiramate inhibits maximal seizure activity in electroconvulsive therapy and in pentylenetetrazol-induced seizures as well as partial and secondarily generalized tonic-clonic seizures in the kindling model, findings predictive of a broad spectrum of activities clinically. Its action on mitochondrial permeability transition pores has been proposed as a mechanism.[58]
While many anticonvulsants have been associated with apoptosis in young animals, animal experiments have found that topiramate is one of the very few anticonvulsants that do not induce apoptosis in young animals at doses needed to produce an anticonvulsant effect.[59]
Detection in body fluids
[edit]Blood, serum, or plasma topiramate concentrations may be measured using immunoassay or chromatographic methods to monitor therapy, confirm a diagnosis of poisoning in hospitalized patients, or assist in a medicolegal death investigation. Plasma levels are usually less than 10 mg/L during therapeutic administration, but can range from 10 to 150 mg/L in overdose victims.[60][61][62]
History
[edit]Topiramate was discovered in 1979 by Bruce E. Maryanoff and Joseph F. Gardocki during their research work at McNeil Pharmaceuticals.[63][64] Topiramate was first sold in 1996.[65] Mylan Pharmaceuticals was granted final approval by the FDA for the sale of generic topiramate in the United States and the generic version was made available in September 2006.[66] The last patent for topiramate in the U.S. was for use in children and expired on 28 February 2009.[67]
Research
[edit]Topiramate is being studied as a potential treatment for post-traumatic stress disorder (PTSD).[68]
There is some evidence for the use of topiramate in the management of cravings related to withdrawal from dextromethorphan.[69]
A 2023 systematic review of seizure treatment for infants aged 1 to 36 months identified three studies that evaluated the use of topiramate. Though its adverse effects, including upper respiratory tract infection and loss of appetite, were rarely severe enough for the medication to be discontinued in this age group, its effectiveness in reducing seizures was inconclusive. The available research suffers from small sample sizes, inconsistent findings, and inadequate comparison groups.[70]
References
[edit]- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Trokendi XR- topiramate capsule, extended release". DailyMed. Archived from the original on 8 November 2021. Retrieved 8 November 2021.
- ^ "Topamax- topiramate tablet, coated Topamax- topiramate capsule, coated pellets". DailyMed. Archived from the original on 8 November 2021. Retrieved 8 November 2021.
- ^ "Qsymia- phentermine and topiramate capsule, extended release". DailyMed. Archived from the original on 26 October 2021. Retrieved 8 November 2021.
- ^ "Qudexy XR- topiramate capsule, extended release". DailyMed. Archived from the original on 8 November 2021. Retrieved 8 November 2021.
- ^ "Eprontia - topiramate solution". DailyMed. Archived from the original on 19 December 2021. Retrieved 19 December 2021.
- ^ "Active substance(s): topiramate" (PDF). List of nationally authorised medicinal products. European Medicines Agency. September 2022. Archived (PDF) from the original on 6 September 2022. Retrieved 6 September 2022.
- ^ a b c d e f "Topiramate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 March 2019. Retrieved 5 March 2019.
- ^ a b c British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 328. ISBN 978-0-85711-338-2.
- ^ "Topiramate Side Effects: Common, Severe, Long Term". Drugs.com. Archived from the original on 10 April 2021. Retrieved 3 August 2021.
- ^ "FDA Drug Safety Communication: Risk of oral clefts in children born to mothers taking Topamax (topiramate)". FDA. 18 June 2019.
- ^ "Essential Reads: Breastfeeding and Anti-Epileptic Drugs". MGH Center for Women's Mental Health. 10 August 2022. Retrieved 27 December 2023.
- ^ "Topiramate". Drugs and Lactation Database (LactMed®). Bethesda (MD): National Institute of Child Health and Human Development. 2006. PMID 30000318. Retrieved 27 December 2023.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ "First Generic Drug Approvals 2023". U.S. Food and Drug Administration (FDA). 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.
- ^ "Top 300 of 2023". ClinCalc. Archived from the original on 12 August 2025. Retrieved 12 August 2025.
- ^ "Topiramate Drug Usage Statistics, United States, 2013 - 2023". ClinCalc. Retrieved 18 August 2025.
- ^ a b "Topamax Prescribing Information" (PDF). United States Food and Drug Administration. Archived from the original (PDF) on 24 April 2016. Retrieved 11 April 2016.
- ^ Linde M, Mulleners WM, Chronicle EP, McCrory DC (June 2013). "Topiramate for the prophylaxis of episodic migraine in adults". The Cochrane Database of Systematic Reviews. 6 (6) CD010610. doi:10.1002/14651858.CD010610. PMC 7388931. PMID 23797676.
- ^ Ferrari A, Tiraferri I, Neri L, Sternieri E (September 2011). "Clinical pharmacology of topiramate in migraine prevention". Expert Opinion on Drug Metabolism & Toxicology. 7 (9): 1169–1181. doi:10.1517/17425255.2011.602067. PMID 21756204. S2CID 207491096.
- ^ Evers S, Jensen R (September 2011). "Treatment of medication overuse headache--guideline of the EFNS headache panel". European Journal of Neurology. 18 (9): 1115–1121. doi:10.1111/j.1468-1331.2011.03497.x. PMID 21834901. S2CID 2698885.
- ^ Enke O, New HA, New CH, Mathieson S, McLachlan AJ, Latimer J, et al. (July 2018). "Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis". CMAJ. 190 (26): E786 – E793. doi:10.1503/cmaj.171333. PMC 6028270. PMID 29970367.
- ^ Wiffen PJ, Derry S, Lunn MP, Moore RA (August 2013). Derry S (ed.). "Topiramate for neuropathic pain and fibromyalgia in adults". The Cochrane Database of Systematic Reviews. 2013 (8) CD008314. doi:10.1002/14651858.CD008314.pub3. PMC 8406931. PMID 23996081. Archived from the original on 31 March 2014. Retrieved 6 September 2013.
- ^ Arnone D (February 2005). "Review of the use of Topiramate for treatment of psychiatric disorders". Annals of General Psychiatry. 4 (1) 5. doi:10.1186/1744-859X-4-5. PMC 1088011. PMID 15845141.
- ^ Vasudev K, Macritchie K, Geddes J, Watson S, Young A (January 2006). Young AH (ed.). "Topiramate for acute affective episodes in bipolar disorder". The Cochrane Database of Systematic Reviews (1) CD003384. doi:10.1002/14651858.CD003384.pub2. PMID 16437453.
- ^ Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, et al. (October 2011). "Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis". Lancet. 378 (9799): 1306–1315. doi:10.1016/s0140-6736(11)60873-8. PMID 21851976. S2CID 25512763.
- ^ Lieb K, Völlm B, Rücker G, Timmer A, Stoffers JM (January 2010). "Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials". The British Journal of Psychiatry. 196 (1): 4–12. doi:10.1192/bjp.bp.108.062984. PMID 20044651.
- ^ Johnson BA, Ait-Daoud N (2010). "Topiramate in the new generation of drugs: efficacy in the treatment of alcoholic patients". Current Pharmaceutical Design. 16 (19): 2103–2112. doi:10.2174/138161210791516404. PMC 3063512. PMID 20482511.
- ^ "VA/DoD Clinical Practice Guideline for the management of substance use disorders" (PDF). healthquality.va.gov. 31 December 2015. Archived (PDF) from the original on 31 August 2017. Retrieved 30 August 2017.
- ^ Verrotti A, Scaparrotta A, Agostinelli S, Di Pillo S, Chiarelli F, Grosso S (August 2011). "Topiramate-induced weight loss: a review". Epilepsy Research. 95 (3): 189–199. doi:10.1016/j.eplepsyres.2011.05.014. PMID 21684121. S2CID 30103553.
- ^ Kramer CK, Leitão CB, Pinto LC, Canani LH, Azevedo MJ, Gross JL (May 2011). "Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials". Obesity Reviews. 12 (5): e338 – e347. doi:10.1111/j.1467-789X.2010.00846.x. PMID 21438989. S2CID 24358798.
- ^ "Topiramate for Binge Eating Disorder". wa.kaiserpermanente.org. Archived from the original on 3 August 2021. Retrieved 3 August 2021.
- ^ Mahmood S, Booker I, Huang J, Coleman CI (February 2013). "Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents". Journal of Clinical Psychopharmacology. 33 (1): 90–94. doi:10.1097/JCP.0b013e31827cb2b7. PMID 23277264. S2CID 26085987.
- ^ a b "Possible Side Effects - Topamax (topiramate)". Topamax.xom. Archived from the original on 28 January 2011. Retrieved 17 October 2014.
- ^ "Topamax (topiramate) tablets and sprinkle capsules". Fda.gov. Archived from the original on 12 January 2017. Retrieved 17 October 2014.
- ^ a b "Risk of oral clefts in children born to mothers taking Topamax (topiramate)". FDA Drug Safety Communication. Fda.gov. 6 January 2011. Archived from the original on 24 April 2019. Retrieved 11 July 2013.
- ^ "Seizures and Epilepsy in Children". www.hopkinsmedicine.org. 8 August 2021. Retrieved 19 July 2023.
- ^ "Topamax Tablets and Sprinkle Capsules PRODUCT INFORMATION" (PDF). TGA eBusiness Services. JANSSEN-CILAG Pty Ltd. 30 May 2013. Archived from the original on 29 March 2019. Retrieved 18 November 2013.
- ^ "topiramate (Rx) - Topamax, Trokendi XR". Medscape Reference. WebMD. Archived from the original on 19 May 2019. Retrieved 18 November 2013.
- ^ "Topiramate 100 mg film-coated Tablets". electronic Medicines Compendium. Sandoz Limited. 6 March 2013. Archived from the original on 21 May 2014. Retrieved 18 November 2013.
- ^ "TOPIRAMATE ( topiramate ) tablet TOPIRAMATE ( topiramate ) tablet [Torrent Pharmaceuticals Limited]". DailyMed. Torrent Pharmaceuticals Limited. August 2011. Archived from the original on 20 May 2014. Retrieved 18 November 2013.
- ^ Mirza N, Marson AG, Pirmohamed M (November 2009). "Effect of topiramate on acid-base balance: extent, mechanism and effects". British Journal of Clinical Pharmacology. 68 (5): 655–661. doi:10.1111/j.1365-2125.2009.03521.x. PMC 2791971. PMID 19916989.
- ^ Hulihan J (2001). "IMPORTANT DRUG WARNING" (PDF). FDA MedWatch. Ortho-McNeil Pharmaceutical. Archived from the original (PDF) on 13 January 2017. Retrieved 11 June 2018.
- ^ Hunt S, Russell A, Smithson WH, Parsons L, Robertson I, Waddell R, et al. (July 2008). "Topiramate in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register". Neurology. 71 (4): 272–276. doi:10.1212/01.wnl.0000318293.28278.33. PMID 18645165. S2CID 13562052.
- ^ Berthier, M.L. and Dávila, G., 2023. Pharmacotherapy for post-stroke aphasia: what are the options?. Expert Opinion on Pharmacotherapy, (just-accepted).
- ^ Cumbo, E. and Ligori, L.D., 2010. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer's disease. Epilepsy & Behavior, 17(4), pp.461-466.
- ^ Charbonneau, M., Doyle-Campbell, C., Laskey, C. and Capoccia, K., 2020. Carbonation dysgeusia associated with topiramate. American Journal of Health-System Pharmacy, 77(14), pp.1113-1116.
- ^ "Suicidality and Antiepileptic Drugs". Food and Drug Administration. Archived from the original (PDF) on 10 May 2017. Retrieved 11 July 2013.
- ^ "Topiramate". PubMed Health. National Center for Biotechnology Information, U.S. National Library of Medicine. Archived from the original on 5 August 2010.
- ^ a b Wiśniewski M, Łukasik-Głebocka M, Anand JS (April 2009). "Acute topiramate overdose--clinical manifestations". Clinical Toxicology. 47 (4): 317–320. doi:10.1080/15563650601117954. PMID 19514879. S2CID 205901501.
- ^ a b c Wills B, Reynolds P, Chu E, Murphy C, Cumpston K, Stromberg P, et al. (September 2014). "Clinical outcomes in newer anticonvulsant overdose: a poison center observational study". Journal of Medical Toxicology. 10 (3): 254–260. doi:10.1007/s13181-014-0384-5. PMC 4141920. PMID 24515527.
- ^ a b Lofton AL, Klein-Schwartz W (November 2005). "Evaluation of toxicity of topiramate exposures reported to poison centers". Human & Experimental Toxicology. 24 (11): 591–595. Bibcode:2005HETox..24..591L. doi:10.1191/0960327105ht561oa. PMID 16323576. S2CID 37784043.
- ^ a b Sweetman SC, ed. (2009). "Sex hormones and their modulators". Martindale: The complete drug reference (36th ed.). London: Pharmaceutical Press. p. 2068. ISBN 978-0-85369-840-1.
- ^ "TOPAMAX (topiramate) Tablets Approved Labeling Text" (PDF). Ortho-McNeil Pharmaceutical. U.S. Food and Drug Administration. 29 June 2005. p. 14. Archived from the original (PDF) on 5 February 2007.
- ^ "FDA Data on Topamax" (PDF). Archived from the original (PDF) on 6 August 2021. Retrieved 6 August 2021.
- ^ Porter RJ, Dhir A, Macdonald RL, Rogawski MA (2012). "Mechanisms of action of antiseizure drugs". Handb Clin Neurol. Handbook of Clinical Neurology. Vol. 108. pp. 663–681. doi:10.1016/B978-0-444-52899-5.00021-6. ISBN 978-0-444-52899-5. PMID 22939059. Archived from the original on 22 June 2017. Retrieved 22 May 2014.
- ^ Meldrum BS, Rogawski MA (January 2007). "Molecular targets for antiepileptic drug development". Neurotherapeutics. 4 (1): 18–61. doi:10.1016/j.nurt.2006.11.010. PMC 1852436. PMID 17199015.
- ^ Kudin AP, Debska-Vielhaber G, Vielhaber S, Elger CE, Kunz WS (December 2004). "The mechanism of neuroprotection by topiramate in an animal model of epilepsy". Epilepsia. 45 (12): 1478–1487. doi:10.1111/j.0013-9580.2004.13504.x. PMID 15571505. S2CID 7067509.
- ^ Czuczwar K, Czuczwar M, Cieszczyk J, Gawlik P, Luszczki JJ, Borowicz KK, et al. (2004). "[Neuroprotective activity of antiepileptic drugs]". Przeglad Lekarski. 61 (11): 1268–1271. PMID 15727029.
- ^ Goswami D, Kumar A, Khuroo AH, Monif T, Rab S (November 2009). "Bioanalytical LC-MS/MS method validation for plasma determination of topiramate in healthy Indian volunteers". Biomedical Chromatography. 23 (11): 1227–41. doi:10.1002/bmc.1273. PMID 19593736.
- ^ Brandt C, Elsner H, Füratsch N, Hoppe M, Nieder E, Rambeck B, et al. (June 2010). "Topiramate overdose: a case report of a patient with extremely high topiramate serum concentrations and nonconvulsive status epilepticus". Epilepsia. 51 (6): 1090–1093. doi:10.1111/j.1528-1167.2009.02395.x. PMID 19889015. S2CID 35752877.
- ^ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 1567–1569.
- ^ Maryanoff BE, Nortey SO, Gardocki JF, Shank RP, Dodgson SP (May 1987). "Anticonvulsant O-alkyl sulfamates. 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate and related compounds". Journal of Medicinal Chemistry. 30 (5): 880–887. doi:10.1021/jm00388a023. PMID 3572976.
- ^ Maryanoff BE, Costanzo MJ, Nortey SO, Greco MN, Shank RP, Schupsky JJ, et al. (April 1998). "Structure-activity studies on anticonvulsant sugar sulfamates related to topiramate. Enhanced potency with cyclic sulfate derivatives". Journal of Medicinal Chemistry. 41 (8): 1315–1343. doi:10.1021/jm970790w. PMID 9548821.
- ^ Pitkänen A, Schwartzkroin PA, Moshé SL (2005). Models of Seizures and Epilepsy. Burlington: Elsevier. p. 539. ISBN 978-0-08-045702-4. Archived from the original on 8 September 2017. Retrieved 17 September 2017.
- ^ Waknine Y (22 September 2006). "First-Time Generic Approvals: Seasonale, Imodium Advanced, and Topamax". Medscape.com. Archived from the original on 20 May 2013. Retrieved 11 July 2013.
- ^ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". Accessdata.fda.gov. Archived from the original on 25 April 2016. Retrieved 17 October 2014.
- ^ Andrus MR, Gilbert E (November 2010). "Treatment of civilian and combat-related posttraumatic stress disorder with topiramate". The Annals of Pharmacotherapy. 44 (11): 1810–1816. doi:10.1345/aph.1P163. PMID 20923947. S2CID 12137726.
- ^ Roy AK, Hsieh C, Crapanzano K (2015). "Dextromethorphan Addiction Mediated Through the NMDA System: Common Pathways With Alcohol?". Journal of Addiction Medicine. 9 (6): 499–501. doi:10.1097/ADM.0000000000000152. PMID 26441400.
- ^ Treadwell JR, Wu M, Tsou AY (25 October 2022). Management of Infantile Epilepsies (Report). Agency for Healthcare Research and Quality. doi:10.23970/ahrqepccer252.
External links
[edit]![]() Media related to Topiramate at Wikimedia Commons
Media related to Topiramate at Wikimedia Commons
Topiramate
View on GrokipediaMedical Uses
Epilepsy
Topiramate is approved for use as initial monotherapy in patients aged 2 years and older with partial-onset seizures or primary generalized tonic-clonic seizures, as well as adjunctive therapy for partial-onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome in the same age group. These indications are supported by clinical trials demonstrating its broad-spectrum antiepileptic activity through mechanisms including sodium channel blockade, enhancement of GABA activity, and antagonism of glutamate receptors. Dosing typically begins at 25-50 mg per day for adults, titrated weekly by 25-50 mg increments to a maintenance dose of 200-400 mg per day, divided into two doses, depending on seizure type and response. In children aged 2-16 years, initial dosing is weight-based at 5-9 mg/kg per day, similarly titrated to a maintenance of 5-9 mg/kg per day, with adjustments to avoid exceeding adult-equivalent exposures. Pivotal randomized controlled trials (RCTs) established efficacy, showing median seizure reductions of 27-48% for partial-onset seizures in adults (versus 12% for placebo) and 33% in children (versus 11% for placebo), with responder rates (≥50% reduction) of 24-46% in adults and 39% in children.[5][6] For primary generalized tonic-clonic seizures, RCTs reported a 57% median reduction (versus 9% placebo),[7] and for Lennox-Gastaut syndrome, a 15% reduction in drop attacks (versus a 5% increase for placebo).[8] Long-term efficacy data from open-label extensions of these RCTs indicate sustained seizure control over 1-2 years, with over 50% of patients achieving at least 50% reduction and approximately 10-11% becoming seizure-free for periods exceeding 6 months.[9] In pediatric populations, topiramate demonstrates a comparable safety profile to adults when dosed appropriately, with common adverse effects including somnolence and anorexia, though cognitive and behavioral effects require monitoring; RCTs confirm tolerability as adjunctive therapy, with 47% responder rates in refractory cases.[6]Migraine Prevention
Topiramate is approved by the U.S. Food and Drug Administration (FDA) for the preventive treatment of migraines in adults, with a recommended maintenance dose of 100 mg per day administered as 50 mg twice daily.[10] Treatment typically begins with a titration schedule starting at 25 mg once daily for the first week, increasing to 25 mg twice daily in the second week, 50 mg twice daily in the third week, and reaching the target dose of 100 mg per day (50 mg twice daily) by the fourth week to minimize side effects.[11] This dosing regimen allows for gradual adjustment while achieving therapeutic levels for migraine prophylaxis. Clinical evidence from randomized controlled trials (RCTs) supports topiramate's efficacy in reducing migraine frequency compared to placebo. In a pivotal 2004 double-blind RCT involving 483 adults with episodic migraine, topiramate at 100 mg per day resulted in a 50% or greater reduction in monthly migraine frequency (responder rate) in approximately 50% of patients, compared to 26% in the placebo group, alongside a mean reduction of 2.1 monthly migraine attacks from baseline.[12] These findings were consistent across secondary outcomes, including reductions in migraine days and severity, establishing topiramate as an effective option for migraine prevention in adults. For adolescents aged 12 to 17 years, topiramate is also FDA-approved for migraine prevention, typically at lower doses of 50 to 100 mg per day following a similar titration approach adjusted for body weight. A 2009 randomized, double-blind, placebo-controlled study in 157 pediatric patients demonstrated that topiramate at 100 mg per day significantly reduced the mean monthly migraine attack rate by 2.4 attacks compared to placebo (p=0.038), though the 50 mg per day dose did not show statistical significance.[13] This supports its use in this population for prophylactic therapy. A 2025 phase 3 head-to-head trial (TEMPLE study) compared topiramate to the newer calcitonin gene-related peptide receptor antagonist atogepant in adults with episodic or chronic migraine, revealing higher discontinuation rates with topiramate due to adverse events. Over 24 weeks, 29.6% of topiramate-treated patients discontinued treatment because of adverse events, compared to 12.1% in the atogepant group, highlighting tolerability challenges that may impact long-term adherence in migraine prevention.[14]Weight Management
Topiramate is approved by the U.S. Food and Drug Administration (FDA) in combination with phentermine as Qsymia for chronic weight management in adults with an initial body mass index (BMI) of ≥30 kg/m² or ≥27 kg/m² in the presence of at least one weight-related comorbidity, such as hypertension, type 2 diabetes mellitus, or dyslipidemia, as an adjunct to a reduced-calorie diet and increased physical activity.[15] This approval was granted on July 17, 2012, based on pivotal phase 3 trials demonstrating clinically meaningful weight reduction.[16] In these studies, patients on the recommended maintenance dose of 7.5 mg phentermine/46 mg topiramate extended-release once daily achieved an average weight loss of approximately 9-10% of baseline body weight over 56 weeks, compared to 1-2% with placebo, with 62-70% of patients achieving at least 5% weight loss.[15] The dosing regimen typically begins at 3.75 mg phentermine/23 mg topiramate extended-release once daily for 14 days, titrating to 7.5 mg/46 mg, and potentially escalating to 15 mg/92 mg if additional loss is needed and tolerated.[15] As monotherapy, topiramate is used off-label for weight loss, particularly in patients with obesity or overweight with comorbidities, though it lacks formal FDA approval for this indication. Clinical trials have shown that topiramate monotherapy leads to an average body weight reduction of 5-10% over 6-12 months, with dose-ranging studies reporting mean losses of 5-6.3% at 24 weeks on doses of 96-192 mg daily.[17] Sustained weight loss has been observed in longer-term studies, with benefits maintained through 12 months in responsive patients when combined with lifestyle interventions.[18] Topiramate contributes to weight loss primarily through appetite suppression and metabolic effects, including modulation of neurotransmitters such as gamma-aminobutyric acid (GABA) and glutamate to enhance satiety and reduce food cravings, as well as potential alterations in taste perception that decrease caloric intake.[18] It also promotes metabolic improvements, such as reduced fat accumulation and lowered triglyceride levels, supporting sustained weight reduction in 6-12 month trials.[19] These mechanisms have led to brief exploration in off-label contexts like binge eating disorder, where weight management is a secondary outcome.[18]Off-Label Uses
Topiramate has been investigated for the off-label treatment of alcohol use disorder, where randomized controlled trials (RCTs) demonstrate its efficacy in reducing heavy drinking. In a multicenter, double-blind RCT involving 371 alcohol-dependent individuals, topiramate titrated to 300 mg/day over 6 weeks significantly reduced the percentage of heavy drinking days by 16.2% (95% CI, 10.8%-21.6%; P < .001) compared to placebo, alongside decreases in drinks per drinking day and improvements in plasma γ-glutamyltransferase levels as a biomarker of alcohol consumption.[20] A meta-analysis of seven RCTs further supports moderate efficacy (Hedges' g = 0.406, P < .01) for reducing heavy drinking and promoting abstinence, with typical dosing ranging from 100-300 mg/day.[21] Emerging 2025 research also suggests potential benefits in alcohol use modulated by GRIK1 genotype.[22] Preliminary evidence from 2025 indicates topiramate's potential in managing cocaine use-related disorders, with reviews highlighting its role in substance use treatment alongside mood and anxiety benefits.[23] As an adjunct in bipolar disorder, topiramate shows limited evidence for mood stabilization from small-scale trials, though it is not considered first-line therapy. A review of controlled studies indicates potential benefits in prophylaxis of mood episodes when added to standard treatments, but results are inconsistent across larger evaluations, with no robust support for acute mania or depression management.[24] Dosing in these adjunctive contexts typically starts at 25-50 mg/day and titrates to 100-200 mg/day based on tolerability. For essential tremor, studies from the 2010s provide limited but positive efficacy data, positioning topiramate as a second-line option after propranolol or primidone. A 2015 meta-analysis of RCTs found topiramate superior to placebo in reducing total tremor scores (mean difference -8.58, 95% CI -15.46 to -1.70; P < .05), with common dosing of 50-400 mg/day divided twice daily.[25] Similarly, for neuropathic pain, 2010s investigations reveal limited efficacy, particularly in diabetic neuropathy, where a Cochrane review of available trials concluded no clear benefit over placebo despite some symptom reduction in small cohorts.[26] In binge eating disorder, phase 3-level multicenter RCTs support topiramate's role in reducing binge episodes, often at doses of 75-200 mg/day. A double-blind, placebo-controlled trial with 61 obese participants with binge eating disorder showed significant decreases in binge frequency (from 4.7 to 1.3 episodes/week vs. 4.5 to 3.6 with placebo; P < .001) and body weight after 16 weeks of treatment up to 200 mg/day.[27] Ongoing research explores topiramate's potential in post-traumatic stress disorder, with preliminary trials suggesting possible reductions in symptoms when used adjunctively.[28]Contraindications and Precautions
Pregnancy and Reproductive Risks
Topiramate is contraindicated during pregnancy due to an increased risk of congenital malformations in the offspring, particularly oral clefts. Data from the U.S. Food and Drug Administration (FDA) in 2011 indicated that exposure to topiramate early in pregnancy is associated with a 2- to 5-fold increased risk of oral clefts compared to the general population.[29] Specifically, the prevalence of oral clefts was reported as 1.4% in infants exposed to topiramate monotherapy, versus 0.38% in unexposed infants.[30] Overall, the risk of major congenital malformations with first-trimester exposure ranges from 1.4% to 2.5%, exceeding the baseline population risk of approximately 0.7%.[31] In the United States, the FDA recommends that women of childbearing potential use effective contraception during topiramate treatment and that the benefits be weighed against fetal risks, but does not mandate a formal Pregnancy Prevention Programme.[4] To mitigate these teratogenic risks, regulatory authorities in other regions have implemented strict preventive measures for women of childbearing potential. In 2024, the European Medicines Agency (EMA) and the UK's Medicines and Healthcare products Regulatory Agency (MHRA) introduced a Pregnancy Prevention Programme (PPP) for topiramate, making it contraindicated in pregnancy and in women who could become pregnant unless specific conditions are met.[32][33] Under the PPP, prescribers must confirm a negative pregnancy test prior to initiation, ensure the use of highly effective contraception throughout treatment, and conduct monthly pregnancy monitoring.[34] Patients receive education on the risks, and treatment discontinuation is required if pregnancy occurs.[35] Topiramate may also impact fertility and reproductive health in women. It has been associated with menstrual irregularities, including irregular periods and breakthrough bleeding, potentially due to its effects on hormonal balance.[36] Additionally, at doses greater than 200 mg/day, topiramate may reduce the efficacy of hormonal contraceptives, such as combined oral contraceptives, by accelerating their metabolism, potentially increasing the risk of unintended pregnancy; however, at lower doses used for migraine prevention, studies indicate no increased risk of unintended pregnancies.[37][38][39] Women using topiramate are advised to employ alternative or additional non-hormonal contraception methods as part of risk management.[40] In 2025, Medsafe in New Zealand issued updates reinforcing these precautions, particularly for migraine prophylaxis. Topiramate is now explicitly contraindicated for migraine prevention in women of childbearing potential unless no suitable alternatives exist and the PPP conditions are strictly followed, emphasizing the need to avoid exposure in fertile women to prevent fetal harm such as low birth weight and other malformations.[41][42]Other Contraindications
Topiramate is contraindicated in patients with known hypersensitivity to the drug itself. Although topiramate is a sulfamate-substituted derivative structurally related to sulfonamides, cross-reactivity with sulfonamide allergies is not established as an absolute contraindication, but caution is advised in such cases due to potential hypersensitivity reactions.[1][43] Topiramate carries a risk of inducing acute myopia and secondary angle-closure glaucoma through mechanisms involving choroidal effusion and ciliary body edema, typically occurring within the first month of therapy; patients with a history of acute angle-closure glaucoma should be monitored closely or alternative therapy considered. Discontinuation is recommended if symptoms such as ocular pain or blurred vision develop.[44][4] In cases of severe hepatic impairment, topiramate requires caution despite no routine dose adjustment, as reduced hepatic function may increase the risk of hyperammonemia and other metabolic disturbances; ammonia levels should be monitored. For renal impairment (creatinine clearance <70 mL/min/1.73 m²), the recommended starting dose is half of the usual dose, with slower titration to maintenance levels based on tolerability.[1] Topiramate is contraindicated or requires extreme caution in patients predisposed to metabolic acidosis, such as those with chronic kidney disease, severe respiratory disorders, or conditions involving renal bicarbonate loss, due to the drug's inhibition of carbonic anhydrase isoenzymes leading to hyperchloremic non-anion gap acidosis. Baseline and periodic serum bicarbonate monitoring is essential, with dose reduction or discontinuation considered if acidosis persists below 20 mEq/L.[44] Elderly patients warrant special precautions with topiramate due to age-related declines in renal function, resulting in approximately 20% lower clearance, a 13% longer half-life, and 25% higher systemic exposure compared to younger adults. Dose adjustments are recommended if creatinine clearance is below 70 mL/min/1.73 m², and the risk of falls increases due to potential adverse effects like dizziness and somnolence.[1]Adverse Effects
Common Adverse Effects
Topiramate is commonly associated with central nervous system and metabolic adverse effects, which occur in a significant proportion of patients during treatment for epilepsy or migraine prophylaxis. These effects are typically mild to moderate, dose-dependent, and often diminish with gradual dose titration or time. Clinical trials indicate that over 10% of patients experience paresthesia, fatigue, dizziness, somnolence, anorexia, and weight loss, with incidences varying by indication, dose, and patient population.[4] Paresthesia, characterized by tingling or numbness in the extremities or perioral region, is the most frequently reported adverse effect, affecting 40% to 51% of adults in monotherapy epilepsy trials (at 400 mg/day) and migraine prophylaxis trials (at 100 mg/day), compared to 6% to 12% with placebo. In pediatric populations, the incidence is lower, ranging from 4% to 19%. This sensory disturbance is often benign and dose-related, typically resolving upon dose reduction or discontinuation. Weight loss is another prevalent effect, observed in 9% to 17% of patients across trials, with mean reductions of 2% to 6% of body weight; notably, 6% of adults in migraine trials experienced at least 10% body weight loss versus 1% on placebo. This can be beneficial for weight management but requires monitoring in underweight individuals or children to prevent growth impacts.[4] Fatigue, dizziness, and somnolence are reported in 6% to 29% of patients, with higher rates (15% to 29%) in adjunctive epilepsy therapy at 200 to 400 mg/day. Cognitive slowing, including word-finding difficulties, psychomotor slowing, and concentration issues, occurs in 6% to 13% of adults, manifesting as "brain fog" or slowed verbal fluency; up to 10% of patients may report such symptoms overall, particularly at doses above 200 mg/day. Anorexia and nausea, which contribute to weight loss, affect 10% to 24% and 8% to 13% of patients, respectively, and are more pronounced during rapid titration but frequently resolve with slower dose escalation (e.g., weekly increments of 25 to 50 mg). Post-marketing surveillance confirms these effects occur in more than 10% of users, emphasizing the need for individualized dosing to improve tolerability.[4] At higher doses, these common effects may intensify and transition toward more serious manifestations, necessitating close clinical monitoring. Management strategies include starting at low doses (25 mg/day) and titrating gradually over 4 to 8 weeks, alongside patient education on symptom recognition and lifestyle adjustments like hydration to mitigate paresthesia.Serious Adverse Effects
Topiramate carries a boxed warning for the risk of suicidal ideation and behavior, implemented by the FDA in 2008 following a meta-analysis of antiepileptic drugs showing approximately a twofold increase in risk compared to placebo (0.43% incidence in treated patients versus 0.24% in placebo).[4] This class effect applies across indications, including epilepsy and migraine prevention, and patients should be monitored closely for emerging or worsening depression, unusual changes in mood or behavior, or suicidal thoughts, with immediate medical intervention recommended if observed.[4] Metabolic acidosis is another serious adverse effect associated with topiramate, resulting from its inhibition of carbonic anhydrase in the renal tubules, leading to a non-anion gap hyperchloremic acidosis. Subclinical reductions in serum bicarbonate occur in 30-40% of patients, while symptomatic cases, defined by markedly low levels (e.g., <17 mEq/L), affect 1-3% and may manifest as fatigue, dyspnea, or cardiac arrhythmias.[4] Monitoring of baseline and periodic serum bicarbonate levels is essential, with dose reduction or discontinuation advised if persistent acidosis develops to prevent complications such as osteomalacia or growth retardation in children.[4] Topiramate increases the risk of nephrolithiasis (kidney stones) to 1-2% in adults, with a higher incidence of up to 7% in pediatric patients aged 1-24 months, due to urinary citrate reduction and acidic urine pH changes. A 2025 retrospective cohort study reported a 3-year cumulative incidence of symptomatic kidney stones of 2.9% in topiramate users versus 1.2% in non-users.[45] Concomitant use with other carbonic anhydrase inhibitors or ketogenic diets exacerbates this risk, and preventive measures include maintaining high fluid intake (at least 8 glasses of water daily). Additionally, topiramate can cause oligohydrosis (reduced sweating) and hyperthermia, reported primarily in children and during hot weather or exercise, potentially leading to heatstroke if unrecognized.[4] Patients should be advised to monitor for decreased perspiration and elevated body temperature, avoiding overheating environments. Acute visual disturbances, including secondary angle-closure glaucoma and permanent visual field defects, represent rare but vision-threatening effects of topiramate, often linked to idiosyncratic ciliochoroidal effusion and myopic shifts.[4] These typically occur within the first month of therapy and require immediate discontinuation to prevent irreversible damage, with symptoms such as blurred vision, eye pain, or halos around lights warranting urgent ophthalmologic evaluation.[4]Overdose
Symptoms of topiramate overdose can include seizures, drowsiness or severe somnolence, speech difficulties, blurred or double vision, cognitive impairment or trouble thinking, agitation, ataxia or loss of coordination, abdominal pain, dizziness, depression, loss of appetite, irregular heartbeat, fast or shallow breathing, and metabolic acidosis. In severe cases, it may lead to loss of consciousness or coma.[3][1] There is no specific antidote for topiramate overdose. Management involves supportive care, such as monitoring and stabilizing vital signs, treating symptoms (e.g., anticonvulsants for seizures), and providing general emergency medical support. Hemodialysis is recommended for severe or refractory cases to enhance drug elimination, as topiramate is dialyzable. Patients or caregivers should immediately contact poison control (e.g., 1-800-222-1222 in the US) or emergency services if overdose is suspected.[3][1]Drug Interactions
Topiramate interacts with various medications, primarily through effects on cytochrome P450 enzymes, renal excretion, and carbonic anhydrase inhibition, which can alter drug levels, efficacy, or increase adverse effects. Clinicians should monitor patients and adjust doses as needed.[4]Antiepileptic Drugs
Concomitant use with other antiepileptic drugs (AEDs) requires caution:- Carbamazepine and phenytoin decrease topiramate plasma concentrations by approximately 40% and 48%, respectively, due to CYP3A4 induction; dosage adjustment of topiramate may be necessary.[4]
- Valproic acid increases the risk of hyperammonemia (with or without encephalopathy) and hypothermia; monitor ammonia levels and body temperature.[4] No significant interactions occur with lamotrigine or phenobarbital at typical doses.[4]
Hormonal Contraceptives
Topiramate reduces the efficacy of estrogen-containing oral contraceptives, particularly at doses greater than 200 mg/day, by increasing ethinyl estradiol clearance (18-30% decrease in levels), leading to breakthrough bleeding and risk of unintended pregnancy. Use alternative non-hormonal contraception or higher-dose formulations.[4]Carbonic Anhydrase Inhibitors and Metformin
- Other carbonic anhydrase inhibitors (e.g., acetazolamide, zonisamide) increase the risk of metabolic acidosis and nephrolithiasis; avoid concomitant use if possible and monitor acid-base balance.[4]
- Metformin exposure increases (AUC by 25%), and topiramate's risk of causing metabolic acidosis may contraindicate metformin use in affected patients; assess clinical significance and monitor for lactic acidosis.[4][1]
Central Nervous System Depressants
Topiramate may enhance CNS depression when used with alcohol, benzodiazepines, or other sedatives, increasing risks of drowsiness and impaired coordination; advise caution.[4]Other Significant Interactions
- Lithium: Increases lithium systemic exposure (AUC by 26%) at topiramate doses up to 600 mg/day; monitor lithium levels.[4]
- Hydrochlorothiazide: Increases topiramate exposure (AUC by 29%) and may cause hypokalemia; dose reduction of topiramate may be required.[4]
- Pioglitazone: Decreases pioglitazone exposure; monitor glycemic control in diabetic patients.[4]
- Amitriptyline: May substantially increase amitriptyline concentrations; titrate dose based on clinical response.[4] Minor interactions include a 12% decrease in digoxin AUC; no significant changes with propranolol, diltiazem, or venlafaxine.[4]

