Recent from talks
Nothing was collected or created yet.
Vertically transmitted infection
View on Wikipedia
| Vertically transmitted infection | |
|---|---|
 | |
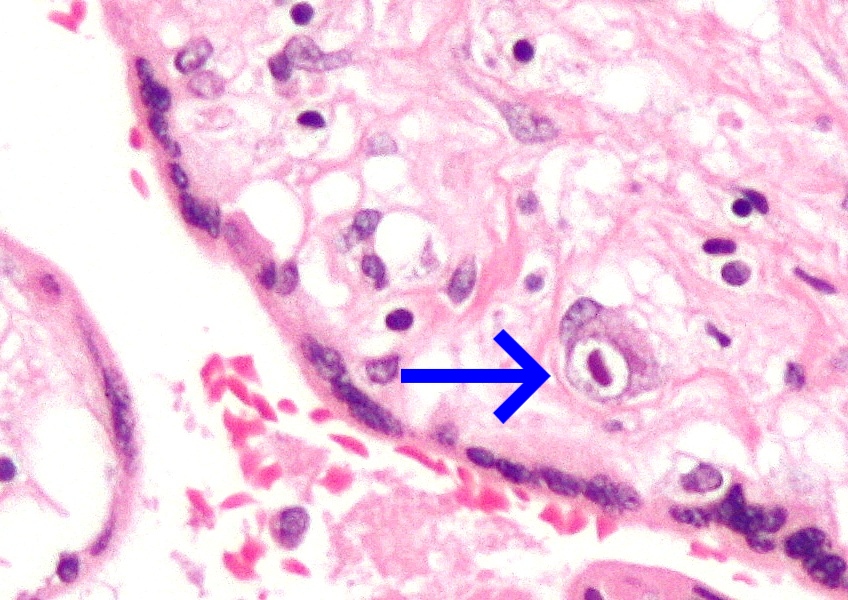
| Micrograph of cytomegalovirus (CMV) infection of the placenta (CMV placentitis), a vertically transmitted infection: The characteristic large nucleus of a CMV-infected cell is seen off-centre at the bottom right of the image, H&E stain. | |
| Specialty | Pediatrics |
A vertically transmitted infection is an infection caused by pathogenic bacteria or viruses that use mother-to-child transmission, that is, transmission directly from the mother to an embryo, fetus, or baby during pregnancy or childbirth. It can occur when the mother has a pre-existing disease or becomes infected during pregnancy. Nutritional deficiencies may exacerbate the risks of perinatal infections. Vertical transmission is important for the mathematical modelling of infectious diseases, especially for diseases of animals with large litter sizes, as it causes a wave of new infectious individuals.[1]
Types of infections
[edit]Bacteria, viruses, and other organisms are able to be passed from mother to child. Several vertically transmitted infections are included in the TORCH complex:[2]
- T – toxoplasmosis from Toxoplasma gondii
- O – other infections (see below)
- R – rubella
- C – cytomegalovirus
- H – herpes simplex virus-2 or neonatal herpes simplex
Other infections include:
- Parvovirus B19[3], small DNA virus from the family Parvoviridae. Causes disease in the pediatric population, although it also affects adults. It is the classic cause of the childhood rash called fifth disease or erythema infectiosum, or "slapped face syndrome".[4][5]
- Coxsackievirus[6], RNA virus belonging to the family Picornaviridae. It is one of the most common and important human pathogens, transmitted by the fecal–oral route.
- Chickenpox. Highly contagious caused by varicella zoster virus[7]. Causes a disease which results in a characteristic skin rash that forms small, itchy blisters, which eventually scab over.[8]
- Chlamydia[9] which is a sexually transmitted infection caused by the bacterium Chlamydia trachomatis.
- HIV[10][11]. Lentivirus infecting humans, causing acquired immunodeficiency syndrome (AIDS).[12][13] Leading to a progressive failure of the immune system and allowing life-threatening opportunistic infections and cancers to thrive.[14]
- Human T-lymphotropic virus[15]
- Syphilis[16]
- Zika fever, caused by Zika virus, can cause microcephaly and other brain defects in the child.[17]
- COVID-19 in pregnancy is associated with an increased risk of stillbirth with an odds ratio of approximately 2.[18]
Hepatitis B may also be classified as a vertically transmitted infection. The hepatitis B virus is large and does not cross the placenta. Hence, it cannot infect the fetus unless breaks in the maternal-fetal barrier have occurred, but such breaks can occur in bleeding during childbirth or amniocentesis.[19]
The TORCH complex was originally considered to consist of the four conditions mentioned above,[20] with the "TO" referring to Toxoplasma. The four-term form is still used in many modern references,[21] and the capitalization "ToRCH" is sometimes used in these contexts.[22] The acronym has also been listed as TORCHES, for TOxoplasmosis, Rubella, Cytomegalovirus, HErpes simplex, and Syphilis.[citation needed]
A further expansion of this acronym, CHEAPTORCHES, was proposed by Ford-Jones and Kellner in 1995:[23]
- C – chickenpox and shingles
- H – hepatitis, C[24] (D), E
- E – enteroviruses
- A – AIDS (HIV infection)
- P – parvovirus B19 (produces hydrops fetalis secondary to aplastic anemia)
- T – toxoplasmosis
- O – other (group B streptococci, Listeria, Candida, and Lyme disease)
- R – rubella
- C – cytomegalovirus
- H – herpes simplex
- E – everything else sexually transmitted (gonorrhea, Chlamydia infection, Ureaplasma urealyticum, and human papillomavirus)
- S – Syphilis
Signs and symptoms
[edit]The signs and symptoms of a vertically transmitted infection depend on the individual pathogen. In the mother, it may cause subtle signs such as an influenza-like illness, or possibly no symptoms at all. In such cases, the effects may be seen first at birth.[citation needed]
Symptoms of a vertically transmitted infection may include fever and flu-like symptoms. The newborn is often small for gestational age. A petechial rash on the skin may be present, with small reddish or purplish spots due to bleeding from capillaries under the skin. An enlarged liver and spleen (hepatosplenomegaly) is common, as is jaundice. However, jaundice is less common in hepatitis B because a newborn's immune system is not developed well enough to mount a response against liver cells, as would normally be the cause of jaundice in an older child or adult. Hearing impairment, eye problems, mental retardation, autism, and death can be caused by vertically transmitted infections.[citation needed]
The genetic conditions of Aicardi-Goutieres syndrome are possibly present in a similar manner.[25][26]
Causal routes
[edit]The main routes of transmission of vertically transmitted infections are across the placenta (transplacental) and across the female reproductive tract during childbirth. Transmission is also possible by breaks in the maternal-fetal barrier such by amniocentesis[19] or major trauma.
Transplacental
[edit]The embryo and fetus have little or no immune function. They depend on the immune function of their mother. Several pathogens can cross the placenta and cause perinatal infection. Often, microorganisms that produce minor illness in the mother are very dangerous for the developing embryo or fetus. This can result in spontaneous abortion or major developmental disorders. For many infections, the baby is more at risk at particular stages of pregnancy. Problems related to perinatal infection are not always directly noticeable.[citation needed]
Apart from infecting the fetus, transplacental pathogens may cause placentitis (inflammation of the placenta) and/or chorioamnionitis (inflammation of the fetal membranes).[citation needed]
During childbirth
[edit]Babies can also become infected by their mothers during birth. Some infectious agents may be transmitted to the embryo or fetus in the uterus, while passing through the birth canal, or even shortly after birth. The distinction is important because when transmission is primarily during or after birth, medical intervention can help prevent infections in the infant.[citation needed]During birth, babies are exposed to maternal blood, body fluids, and to the maternal genital tract without the placental barrier intervening. Because of this, blood-borne microorganisms (hepatitis B, HIV), organisms associated with sexually transmitted infections (e.g., Neisseria gonorrhoeae and Chlamydia trachomatis), and normal fauna of the genitourinary tract (e.g., Candida albicans) are among those commonly seen in infection of newborns.[citation needed]
Pathophysiology
[edit]Virulence versus symbiosis
[edit]In the spectrum of optimal virulence, vertical transmission tends to evolve benign symbiosis, so is a critical concept for evolutionary medicine. Because a pathogen's ability to pass from mother to child depends significantly on the hosts' ability to reproduce, pathogens' transmissibility tends to be inversely related to their virulence. In other words, as pathogens become more harmful to, and thus decrease the reproduction rate of, their host organism, they are less likely to be passed on to the hosts' offspring since they will have fewer offspring.[27]
Although HIV is sometimes transmitted through perinatal transmission, its virulence can be accounted for because its primary mode of transmission is not vertical. Moreover, medicine has further decreased the frequency of vertical transmission of HIV. The incidence of perinatal HIV cases in the United States has declined as a result of the implementation of recommendations on HIV counselling and voluntary testing practices and the use of zidovudine therapy by providers to reduce perinatal HIV transmission.[28]
The price paid in the evolution of symbiosis is, however, great: for many generations, almost all cases of vertical transmission continue to be pathological—in particular if any other routes of transmission exist. Many generations of random mutation and selection are needed to evolve symbiosis. During this time, the vast majority of vertical transmission cases exhibit the initial virulence.[citation needed]
In dual inheritance theory, vertical transmission refers to the passing of cultural traits from parents to children.[29]
Diagnosis
[edit]
When physical examination of the newborn shows signs of a vertically transmitted infection, the examiner may test blood, urine, and spinal fluid for evidence of the infections listed above. Diagnosis can be confirmed by culture of one of the specific pathogens or by increased levels of IgM against the pathogen.[citation needed]
Classification
[edit]A vertically transmitted infection can be called a perinatal infection if it is transmitted in the perinatal period, which starts at gestational ages between 22[30] and 28 weeks[31] (with regional variations in the definition) and ending seven completed days after birth.[30]
The term congenital infection can be used if the vertically transmitted infection persists after childbirth.[citation needed]
Treatment
[edit]
Some vertically transmitted infections, such as toxoplasmosis and syphilis, can be effectively treated with antibiotics if the mother is diagnosed early in her pregnancy. Many viral vertically transmitted infections have no effective treatment, but some, notably rubella and varicella-zoster, can be prevented by vaccinating the mother prior to pregnancy.[citation needed]
Pregnant women living in malaria-endemic areas are candidates for malaria prophylaxis. It clinically improves the anemia and parasitemia of the pregnant women, and birthweight in their infants.[32]
If the mother has active herpes simplex (as may be suggested by a pap test), delivery by Caesarean section can prevent the newborn from contact, and consequent infection, with this virus.[citation needed]
IgG2 antibody may play a crucial role in prevention of intrauterine infections and extensive research is going on for developing IgG2-based therapies for treatment and vaccination.[33]
Prognosis
[edit]Each type of vertically transmitted infection has a different prognosis. The stage of the pregnancy at the time of infection also can change the effect on the newborn.[citation needed]
See also
[edit]References
[edit]- ^ von Csefalvay, Chris (2023), "Simple compartmental models", Computational Modeling of Infectious Disease, Elsevier, pp. 19–91, doi:10.1016/b978-0-32-395389-4.00011-6, ISBN 978-0-323-95389-4, retrieved 5 March 2023
- ^ Jaan A, Rajnik M (2021). "TORCH Complex". National Center for Biotechnology Information, U.S. National Library of Medicine. PMID 32809363. Retrieved 27 August 2021.
- ^ "Parvovirus B19". The Lecturio Medical Concept Library. Retrieved 27 August 2021.
- ^ Vafaie, Janet; Schwartz, Robert A. (October 2004). "Parvovirus B19 infections". International Journal of Dermatology. 43 (10): 747–749. doi:10.1111/j.1365-4632.2004.02413.x. ISSN 0011-9059. PMID 15485533.
- ^ Sabella, C.; Goldfarb, J. (1 October 1999). "Parvovirus B19 infections". American Family Physician. 60 (5): 1455–1460. ISSN 0002-838X. PMID 10524489.
- ^ "Coxsackievirus". The Lecturio Medical Concept Library. Retrieved 27 August 2021.
- ^ "Varicella-Zoster Virus/Chickenpox". The Lecturio Medical Concept Library. Retrieved 27 August 2021.
- ^ Arvin, A.M. (1999), Chickenpox (Varicella), Contributions to Microbiology, vol. 3, Basel: KARGER, pp. 96–110, doi:10.1159/000060317, ISBN 3-8055-6884-3, PMID 10599524, retrieved 16 October 2025
- ^ Yu, Jialin; Wu, Shixiao; Li, Fang; Hu, Linyan (2009). "Vertical Transmission of Chlamydia trachomatis in Chongqing China". Current Microbiology. 58 (4): 315–320. doi:10.1007/s00284-008-9331-5. ISSN 0343-8651. PMID 19123031. S2CID 2758055.
- ^ Ugen, K E; Goedert, J J; Boyer, J; et al. (June 1992). "Vertical transmission of human immunodeficiency virus (HIV) infection. Reactivity of maternal sera with glycoprotein 120 and 41 peptides from HIV type 1". Journal of Clinical Investigation. 89 (6): 1923–1930. doi:10.1172/JCI115798. ISSN 0021-9738. PMC 295892. PMID 1601999.
- ^ Fawzi, Wafaie W.; Msamanga, Gernard; Hunter, David; et al. (2000). "Randomized Trial of Vitamin Supplements in Relation to Vertical Transmission of HIV-1 in Tanzania". Journal of Acquired Immune Deficiency Syndromes. 23 (3): 246–254. doi:10.1097/00042560-200003010-00006. ISSN 1525-4135. PMID 10839660. S2CID 35936352.
- ^ Weiss, Robin A. (28 May 1993). "How Does HIV Cause AIDS?". Science. 260 (5112): 1273–1279. Bibcode:1993Sci...260.1273W. doi:10.1126/science.8493571. ISSN 0036-8075. PMID 8493571.
- ^ Douek, Daniel C.; Roederer, Mario; Koup, Richard A. (1 February 2009). "Emerging Concepts in the Immunopathogenesis of AIDS". Annual Review of Medicine. 60 (1): 471–484. doi:10.1146/annurev.med.60.041807.123549. ISSN 0066-4219. PMC 2716400. PMID 18947296.
- ^ Powell, Marta K.; Benková, Kamila; Selinger, Pavel; Dogoši, Marek; Kinkorová Luňáčková, Iva; Koutníková, Hana; Laštíková, Jarmila; Roubíčková, Alena; Špůrková, Zuzana; Laclová, Lucie; Eis, Václav; Šach, Josef; Heneberg, Petr (9 September 2016). Sturtevant, Joy (ed.). "Opportunistic Infections in HIV-Infected Patients Differ Strongly in Frequencies and Spectra between Patients with Low CD4+ Cell Counts Examined Postmortem and Compensated Patients Examined Antemortem Irrespective of the HAART Era". PLOS ONE. 11 (9) e0162704. Bibcode:2016PLoSO..1162704P. doi:10.1371/journal.pone.0162704. ISSN 1932-6203. PMC 5017746. PMID 27611681.
- ^ Hisada, Michie; Maloney, Elizabeth M.; Sawada, Takashi; et al. (2002). "Virus Markers Associated with Vertical Transmission of Human T Lymphotropic Virus Type 1 in Jamaica". Clinical Infectious Diseases. 34 (12): 1551–1557. doi:10.1086/340537. ISSN 1058-4838. PMID 12032888.
- ^ Lee, M.-J.; Hallmark, R.J.; Frenkel, L.M.; Del Priore, G. (1998). "Maternal syphilis and vertical perinatal transmission of human immunodeficiency virus type-1 infection". International Journal of Gynecology & Obstetrics. 63 (3): 247–252. doi:10.1016/S0020-7292(98)00165-9. ISSN 0020-7292. PMID 9989893. S2CID 22297001.
- ^ "CDC Concludes Zika Causes Microcephaly and Other Birth Defects". CDC Newsroom Releases. Centers for Disease Control and Prevention. 13 April 2016.
- ^ Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N (2021). "The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis". CMAJ. 193 (16): E540 – E548. doi:10.1503/cmaj.202604. PMC 8084555. PMID 33741725.
- ^ a b "Hepatitis B". Emergencies preparedness, response. World Health Organization. Retrieved 29 April 2016.
- ^ Kinney, JS; Kumar, ML (December 1988). "Should we expand the TORCH complex? A description of clinical and diagnostic aspects of selected old and new agents". Clinics in Perinatology. 15 (4): 727–44. doi:10.1016/S0095-5108(18)30670-5. ISSN 0095-5108. PMID 2850128.
- ^ Abdel-Fattah, Sherif A.; Bhat, Abha; Illanes, Sebastian; et al. (November 2005). "TORCH test for fetal medicine indications: only CMV is necessary in the United Kingdom". Prenatal Diagnosis. 25 (11): 1028–1031. doi:10.1002/pd.1242. ISSN 0197-3851. PMID 16231309. S2CID 25481658.
- ^ Li, Ding; Yang, Hao; Zhang, Wen-Hong; et al. (2006). "A Simple Parallel Analytical Method of Prenatal Screening". Gynecologic and Obstetric Investigation. 62 (4): 220–225. doi:10.1159/000094092. ISSN 1423-002X. PMID 16791006. S2CID 41493830.
- ^ Ford-Jones, E. L.; Kellner, J. D. (1995). ""Cheap torches": An acronym for congenital and perinatal infections". The Pediatric Infectious Disease Journal. 14 (7): 638–640. doi:10.1097/00006454-199507000-00028. PMID 7567307.
- ^ Tosone, G.; Maraolo, A.E.; Mascolo, S.; et al. (2014). "Vertical hepatitis C virus transmission: Main questions and answers". World Journal of Hepatology. 6 (8): 538–548. doi:10.4254/wjh.v6.i8.538. PMC 4163737. PMID 25232447.
- ^ Knoblauch, Hans; Tennstedt, Cornelia; Brueck, Wolfgang; Hammer, Hannes; Vulliamy, Tom; Dokal, Inderjeet; Lehmann, Rüdiger; Hanefeld, Folker; Tinschert, Sigrid (2003). "Two brothers with findings resembling congenital intrauterine infection-like syndrome (pseudo-TORCH syndrome)". American Journal of Medical Genetics. 120A (2): 261–265. doi:10.1002/ajmg.a.20138. ISSN 0148-7299. PMID 12833411. S2CID 25402036.
- ^ Vivarelli, Rossella; Grosso, Salvatore; Cioni, Maddalena; Galluzzi, Paolo; Monti, Lucia; Morgese, Guido; Balestri, Paolo (March 2001). "Pseudo-TORCH syndrome or Baraitser–Reardon syndrome: diagnostic criteria". Brain and Development. 23 (1): 18–23. doi:10.1016/S0387-7604(00)00188-1. ISSN 0387-7604. PMID 11226724. S2CID 21209676.
- ^ Stewart, Andrew D.; Logsdon, John M.; Kelley, Steven E. (April 2005). "An empirical study of the evolution of virulence under both horizontal and vertical transmission". Evolution. 59 (4): 730–739. doi:10.1554/03-330. ISSN 0014-3820. PMID 15926685. S2CID 198155952.
- ^ Joo, Esther; Carmack, Anne; Garcia-Buñuel, Elizabeth; Kelly, Chester J. (February 2000). "Implementation of guidelines for HIV counseling and voluntary HIV testing of pregnant women". American Journal of Public Health. 90 (2): 273–276. doi:10.2105/AJPH.90.2.273. ISSN 0090-0036. PMC 1446152. PMID 10667191.
- ^ Cavalli-Sforza, Luigi Luca; Feldman, Marcus W. (1981). Cultural Transmission and Evolution: A Quantitative Approach. Monographs in Population Biology. Vol. 16. Princeton University Press. pp. 1–388. ISBN 0-691-08283-9. PMID 7300842. Retrieved 30 April 2016.
- ^ a b "Definitions and Indicators in Family Planning. Maternal & Child Health and Reproductive Health" (PDF). Archived from the original (PDF) on 25 January 2012. By European Regional Office, World Health Organization. Revised March 1999 & January 2001. In turn citing: WHO Geneva, WHA20.19, WHA43.27, Article 23
- ^ Singh, Meharban (2010). Care of the Newborn. p. 7. Edition 7. ISBN 9788170820536
- ^ Radeva-Petrova, D; Kayentao, K; ter Kuile, FO; Sinclair, D; Garner, P (10 October 2014). "Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment". The Cochrane Database of Systematic Reviews. 2014 (10) CD000169. doi:10.1002/14651858.CD000169.pub3. PMC 4498495. PMID 25300703.
- ^ Syal K and Karande AA. IgG2 Subclass Isotype Antibody and Intrauterine Infections. Current Science Vol. 102, No. 11, 10 June 2012.