Recent from talks
Nothing was collected or created yet.
Diethyl ether
View on Wikipedia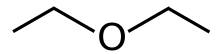 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethoxyethane | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1696894 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.425 |
| EC Number |
|
| 25444 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1155 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O | |
| Molar mass | 74.123 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Dry, rum-like, sweetish odor[1] |
| Density | 0.7134 g/cm3, liquid |
| Melting point | −116.3 °C (−177.3 °F; 156.8 K) |
| Boiling point | 34.6 °C (94.3 °F; 307.8 K)[4] |
| 6.05 g/(100 mL)[2] | |
| log P | 0.98[3] |
| Vapor pressure | 440 mmHg (58.66 kPa) at 20 °C[1] |
| −55.1·10−6 cm3/mol | |
Refractive index (nD)
|
1.353 (20 °C) |
| Viscosity | 0.224 cP (25 °C) |
| Structure | |
| 1.15 D (gas) | |
| Thermochemistry | |
Heat capacity (C)
|
172.5 J/(mol·K) |
Std molar
entropy (S⦵298) |
253.5 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−271.2 ± 1.9 kJ/mol |
Std enthalpy of
combustion (ΔcH⦵298) |
−2732.1 ± 1.9 kJ/mol |
| Pharmacology | |
| N01AA01 (WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Extremely flammable, harmful to skin, decomposes to explosive peroxides in air and light, may cause dizziness in a less ventilated place or if ingested.[1] |
| GHS labelling: | |
 
| |
| Danger | |
| H224, H302, H336 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+P312, P303+P361+P353, P304+P340, P312, P330, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | −45 °C (−49 °F; 228 K)[7] |
| 160 °C (320 °F; 433 K)[7] | |
| Explosive limits | 1.85–48.0%[5] |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
73,000 ppm (rat, 2 hr) 6500 ppm (mouse, 1.65 hr)[6] |
LCLo (lowest published)
|
106,000 ppm (rabbit) 76,000 ppm (dog)[6] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 400 ppm (1200 mg/m3)[1] |
REL (Recommended)
|
No established REL[1] |
IDLH (Immediate danger)
|
1900 ppm[1] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related ethers
|
|
Related compounds
|
|
| Supplementary data page | |
| Diethyl ether (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diethyl ether, or simply ether (abbreviated as eth.[8] or Et2O)[a][8] is an organic compound with the chemical formula (CH3CH2)2O, belonging to the ether class. It is a colourless, highly volatile, sweet-smelling (termed "ethereal odour"), extremely flammable liquid. It is a common solvent and was formerly used as a general anesthetic.[9]
Production
[edit]Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises:[10] Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%.[11]
- 2 CH3CH2OH → (CH3CH2)2O + H2O
Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis.[12]
Uses
[edit]The dominant use of diethyl ether is as a solvent. One particular application is in the production of cellulose plastics such as cellulose acetate.[10]
Laboratory solvent
[edit]It is a common solvent for the Grignard reaction in addition to other reactions involving organometallic reagents.[13] These uses exploit its basicity. Diethyl ether is a popular non-polar solvent in liquid-liquid extraction. As an extractant, it is immiscible with and less dense than water.
Although immiscible, it has significant solubility in water (6.05 g/(100 ml) at 25 °C[2]) and dissolves 1.5 g/(100 g) (1.0 g/(100 ml)) water at 25 °C.[14]
Fuel
[edit]Diethyl ether has a high cetane number of 85–96 and, in combination with petroleum distillates for gasoline and diesel engines,[15] is used as a starting fluid because of its high volatility and low flash point. Ether starting fluid is sold and used in countries with cold climates, as it can help with cold starting an engine at sub-zero temperatures. For the same reason it is also used as a component of the fuel mixture for carbureted compression ignition model engines.
Chemical reactions
[edit]Triethyloxonium tetrafluoroborate is prepared from boron trifluoride, diethyl ether, and epichlorohydrin:[16]
- 4 Et2O·BF3 + 2 Et2O + 3 C2H3OCH2Cl → 3 [Et3O]+[BF4]− + B(OCH(CH2Cl)CH2OEt)3
Diethyl ether is a common laboratory aprotic solvent. It is susceptible to the formation of hydroperoxides.
Metabolism
[edit]A cytochrome P450 enzyme is proposed to metabolize diethyl ether.[17]
Diethyl ether inhibits alcohol dehydrogenase, and thus slows the metabolism of ethanol.[18] It also inhibits metabolism of other drugs requiring oxidative metabolism. For example, diazepam requires hepatic oxidization whereas its oxidized metabolite oxazepam does not.[19]
Safety, stability, regulations
[edit]Diethyl ether is extremely flammable and may form explosive vapour/air mixtures.[20]
Since ether is heavier than air it can collect low to the ground and the vapour may travel considerable distances to ignition sources. Ether will ignite if exposed to an open flame, though due to its high flammability, an open flame is not required for ignition. Other possible ignition sources include – but are not limited to – hot plates, steam pipes, heaters, and electrical arcs created by switches or outlets.[20] Vapour may also be ignited by the static electricity which can build up when ether is being poured from one vessel into another. The autoignition temperature of diethyl ether is 160 °C (320 °F). The diffusion of diethyl ether in air is 9.18 × 10−6 m2/s (298 K, 101.325 kPa).[citation needed]
Ether is sensitive to light and air, tending to form explosive peroxides.[20] Ether peroxides have a higher boiling point than ether and are contact explosives when dry.[20] Commercial diethyl ether is typically supplied with trace amounts of the antioxidant butylated hydroxytoluene (BHT), which reduces the formation of peroxides. Storage over sodium hydroxide precipitates the intermediate ether hydroperoxides. Water and peroxides can be removed by either distillation from sodium and benzophenone, or by passing through a column of activated alumina.[21]
Due to its application in the manufacturing of illicit substances, it is listed in the Table II precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances as well as substances such as acetone, toluene and sulfuric acid.[22]
History
[edit]The compound may have been synthesised by either Jābir ibn Hayyān in the 8th century[23] or Ramon Llull in 1275.[23][24] It was synthesised in 1540 by Valerius Cordus, who called it "sweet oil of vitriol" (oleum dulce vitrioli) – the name reflects the fact that it is obtained by distilling a mixture of ethanol and sulfuric acid (then known as oil of vitriol) – and noted some of its medicinal properties.[23] At about the same time, Paracelsus discovered the analgesic properties of the molecule in dogs.[23] The name ether was given to the substance in 1729 by August Sigmund Frobenius.[25]
It was considered to be a sulfur compound until the idea was disproved in about 1800.[26]
The synthesis of diethyl ether by a reaction between ethanol and sulfuric acid has been known since the 13th century.[26]
Anesthesia
[edit]

William T. G. Morton participated in a public demonstration of ether anesthesia on October 16, 1846, at the Ether Dome in Boston, Massachusetts. Morton had called his ether preparation, with aromatic oils to conceal its smell, "Letheon" after the Lethe River (Λήθη, meaning "forgetfulness, oblivion").[27] However, Crawford Williamson Long is now known to have demonstrated its use privately as a general anesthetic in surgery to officials in Georgia, as early as March 30, 1842, and Long publicly demonstrated ether's use as a surgical anesthetic on six occasions before the Boston demonstration.[28][29][30] British doctors were aware of the anesthetic properties of ether as early as 1840 where it was widely prescribed in conjunction with opium.[31] Diethyl ether was preferred by some practitioners over chloroform as a general anesthetic due to ether's more favorable therapeutic index, that is, a greater difference between an effective dose and a potentially toxic dose.[32]
Diethyl ether does not depress the myocardium but rather it stimulates the sympathetic nervous system leading to hypertension and tachycardia. It is safely used in patients with shock as it preserves the baroreceptor reflex.[33] Its minimal effect on myocardial depression and respiratory drive, as well as its low cost and high therapeutic index allows it to see continued use in developing countries.[34] Diethyl ether could also be mixed with other anesthetic agents such as chloroform to make C.E. mixture, or chloroform and alcohol to make A.C.E. mixture. In the 21st century, ether is rarely used. The use of flammable ether was displaced by nonflammable fluorinated hydrocarbon anesthetics. Halothane was the first such anesthetic developed and other currently used inhaled anesthetics, such as isoflurane, desflurane, and sevoflurane, are halogenated ethers.[35] Diethyl ether was found to have undesirable side effects, such as post-anesthetic nausea and vomiting. Modern anesthetic agents reduce these side effects.[28]

Prior to 2005, it was on the World Health Organization's List of Essential Medicines for use as an anesthetic.[36][37]
Medicine
[edit]Ether was once used in pharmaceutical formulations. A mixture of alcohol and ether, one part of diethyl ether and three parts of ethanol, was known as "Spirit of ether", Hoffman's Anodyne or Hoffman's Drops. In the United States this concoction was removed from the Pharmacopeia at some point prior to June 1917,[38] as a study published by William Procter, Jr. in the American Journal of Pharmacy as early as 1852 showed that there were differences in formulation to be found between commercial manufacturers, between international pharmacopoeia, and from Hoffman's original recipe.[39] It is also used to treat hiccups through instillation into the nasal cavity.[40]
Recreational use
[edit]The recreational use of ether also took place at organised parties in the 19th century called ether frolics, where guests were encouraged to inhale therapeutic amounts of diethyl ether or nitrous oxide, producing a state of excitation. Long, as well as fellow dentists Horace Wells, William Edward Clarke, and William T. G. Morton, observed that during these gatherings, people would often experience minor injuries but appear to show no reaction to them, nor memory that it had happened, demonstrating ether's anaesthetic effects.[41]
In the 19th and early 20th centuries, ether drinking was popular among Polish peasants.[42] It is a traditional and still relatively popular recreational drug among Lemkos.[43] It is usually consumed in a small quantity (kropka, or "dot") poured over milk, sugar water, or orange juice in a shot glass. As a drug, it has been known to cause psychological dependence, sometimes referred to as etheromania.[44][medical citation needed]
Ether intoxication is referenced in Hunter S. Thompson's Fear and Loathing in Las Vegas, where in one of the book's most famous quotes, protagonist Raoul Duke declares that "There is nothing in the world more helpless and irresponsible and depraved than a man in the depths of an ether binge."[45]
See also
[edit]- The Great Moment – film about William T.G. Morton and ether
- Flurothyl – fluorinated derivative
Explanatory notes
[edit]- ^ "Et" stands for monovalent ethyl group CH3CH2 (see pseudoelement symbol)
References
[edit]- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0277". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Merck Index, 10th ed., Martha Windholz, editor, Merck & Co., Inc, Rahway, NJ, 1983, p. 551
- ^ "Diethyl ether_msds".
- ^ "Diethyl ether". ChemSpider. Retrieved 19 January 2017.
- ^ Carl L. Yaws, Chemical Properties Handbook, McGraw-Hill, New York, 1999, p. 567
- ^ a b "Ethyl ether". Immediately Dangerous to Life or Health Concentrations. National Institute for Occupational Safety and Health.
- ^ a b "Ethyl Ether MSDS". J.T. Baker. Archived from the original on 2012-03-28. Retrieved 2010-06-24.
- ^ a b Logan CM, Rice MK (1987). Logan's Medical and Scientific Abbreviations (Hardbound book). J. B. Lippincott. p. 182. ISBN 0-397-54589-4.
- ^ Sakuth, Michael; Mensing, Thomas; Schuler, Joachim; Heitmann, Wilhelm; Strehlke, Günther; Mayer, Dieter (2010). "Ethers, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a10_023.pub2. ISBN 978-3-527-30385-4.
- ^ a b "Ethers, by Lawrence Karas and W. J. Piel". Kirk‑Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2004.
- ^ Ethyl Ether, Chem. Economics Handbook. Menlo Park, Calif: SRI International. 1991.
- ^ Cohen, Julius Berend (1920). A Class-book of Organic Chemistry, Volume 1. London: Macmillan and Co. p. 39.
the structure of ethyl alcohol cohen julius diethyl ether.
- ^ Moyer, W. W.; Marveltitle=Triethyl Carbinol, C. S. (1931). "Triethyl Carbinol". Organic Syntheses. 11: 98. doi:10.15227/orgsyn.011.0098.
- ^ H. H. Rowley; Wm. R. Reed (1951). "Solubility of Water in Diethyl Ether at 25 °". J. Am. Chem. Soc. 73 (6): 2960. Bibcode:1951JAChS..73.2960R. doi:10.1021/ja01150a531.
- ^ "Extra Strength Starting Fluid: How it Works". Valvovine. Archived from the original on 2007-09-27. Retrieved 2007-09-05.
- ^ H. Meerwein (1966). "Triethyloxonium fluoroborate". Organic Syntheses. 46: 113. doi:10.15227/orgsyn.046.0113.
- ^ 109. Aspergillus flavus mutant strain 241, blocked in aflatoxin biosynthesis, does not accumulate aflR transcript. Archived 2017-09-17 at the Wayback Machine Matthew P. Brown and Gary A. Payne, North Carolina State University, Raleigh, NC, fgsc.net
- ^ P. T. Normann; A. Ripel; J. Morland (1987). "Diethyl Ether Inhibits Ethanol Metabolism in Vivo by Interaction with Alcohol Dehydrogenase". Alcoholism: Clinical and Experimental Research. 11 (2): 163–166. doi:10.1111/j.1530-0277.1987.tb01282.x. PMID 3296835.
- ^ Larry K. Keefer; William A. Garland; Neil F. Oldfield; James E. Swagzdis; Bruce A. Mico (1985). "Inhibition of N-Nitrosodimethylamine Metabolism in Rats by Ether Anesthesia" (PDF). Cancer Research. 45 (11 Pt 1): 5457–5460. PMID 4053020.
- ^ a b c d "Archived copy" (PDF). Archived from the original (PDF) on 2014-11-13. Retrieved 2014-02-15.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ W. L. F. Armarego; C. L. L. Chai (2003). Purification of laboratory chemicals. Boston: Butterworth-Heinemann. ISBN 978-0-7506-7571-0.
- ^ Microsoft Word – RedListE2007.doc Archived February 27, 2008, at the Wayback Machine
- ^ a b c d Toski, Judith A; Bacon, Douglas R; Calverley, Rod K (2001). The history of Anesthesiology. In: Barash, Paul G; Cullen, Bruce F; Stoelting, Robert K. Clinical Anesthesia (4th ed.). Lippincott Williams & Wilkins. p. 3. ISBN 978-0-7817-2268-1.
- ^ Hademenos, George J.; Murphree, Shaun; Zahler, Kathy; Warner, Jennifer M. (2008). McGraw-Hill's PCAT. McGraw-Hill. p. 39. ISBN 978-0-07-160045-3. Retrieved 2011-05-25.
- ^ "VIII. An account of a spiritus vini æthereus, together with several experiments tried therewith". Philosophical Transactions of the Royal Society of London. 36 (413): 283–289. 1730. doi:10.1098/rstl.1729.0045. S2CID 186207852.
- ^ a b Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 9 (11th ed.). Cambridge University Press. p. 806.
- ^ Cavendish, Marshall (2008). Inventors and Inventions, Volume 4. Marshall Cavendish. p. 1129. ISBN 978-0-7614-7767-9.
- ^ a b Hill, John W. and Kolb, Doris K. Chemistry for Changing Times: 10th Edition. p. 257. Pearson: Prentice Hall. Upper Saddle River, New Jersey. 2004.
- ^ Madden, M. Leslie (May 14, 2004). "Crawford Long (1815–1878)". New Georgia Encyclopedia. University of Georgia Press. Retrieved February 13, 2015.
- ^ "Crawford W. Long". Doctors' Day. Southern Medical Association. Archived from the original on February 13, 2015. Retrieved February 13, 2015.
- ^ Gratian, N. (1840-11-07). "Dr. Grattan on the Treatment of Uterine Haemorrhage". BMJ. s1-1 (6): 107. doi:10.1136/bmj.s1-1.6.107. ISSN 0959-8138. PMC 2488546. PMID 21372908.
- ^ Calderone, F.A. (1935). "Studies on Ether Dosage After Pre-Anesthetic Medication with Narcotics (Barbiturates, Magnesium Sulphate and Morphine)" (PDF). Journal of Pharmacology and Experimental Therapeutics. 55 (1): 24–39.
- ^ "Ether effects". 31 October 2010.
- ^ "Ether and its effects in Anesthesia". Anesthesia General. 2010-10-31.
- ^ Morgan, G. Edward, Jr. et al. (2002). Clinical Anesthesiology 3rd Ed. New York: McGraw-Hill. p. 3.
- ^ "Essential Medicines WHO Model List (revised April 2003)" (PDF). apps.who.int (13th ed.). Geneva, Switzerland: World Health Organization. April 2003. Retrieved 6 September 2017.
- ^ "Essential Medicines WHO Model List (revised March 2005)" (PDF). apps.who.int (14th ed.). Geneva, Switzerland: World Health Organization. March 2005. Archived from the original (PDF) on 5 August 2005. Retrieved 6 September 2017.
- ^ The National Druggist, Volume 47, June 1917, pp. 220
- ^ Procter, William Jr. (1852). "On Hoffman's Anodyne Liquor". American Journal of Pharmacy. 28.
- ^ ncbi, Treatment of hiccups with instillation of ether into the nasal cavity.
- ^ "How Ether Went from a Recreational 'Frolic' Drug to the First Surgery Anesthetic". Smithsonian Magazine. Retrieved 2020-10-11.
- ^ Zandberg, Adrian (2010). "Short Article "Villages … Reek of Ether Vapours": Ether Drinking in Silesia before 1939". Medical History. 54 (3): 387–396. doi:10.1017/s002572730000466x. PMC 2890321. PMID 20592886.
- ^ Kaszycki, Nestor (2006-08-30). "Łemkowska Watra w Żdyni 2006 – pilnowanie ognia pamięci". Histmag.org – historia od podszewki (in Polish). Kraków, Poland: i-Press. Retrieved 2009-11-25.
Dawniej eteru używało się w lecznictwie do narkozy, ponieważ ma właściwości halucynogenne, a już kilka kropel inhalacji wystarczyło do silnego znieczulenia pacjenta. Jednak eter, jak każda ciecz, może teoretycznie być napojem. Łemkowie tę teorię praktykują. Mimo to, nazywanie skroplonego eteru – "kropki" – ich "napojem narodowym" byłoby przesadą. Chociaż stanowi to pewną część mitu "bycia Łemkiem".
- ^ Krenz, Sonia; Zimmermann, Grégoire; Kolly, Stéphane; Zullino, Daniele Fabio (August 2003). "Ether: a forgotten addiction". Addiction. 98 (8): 1167–1168. doi:10.1046/j.1360-0443.2003.00439.x. PMID 12873252.
- ^ "Hunter S. Thompson Quotes (Author of Fear and Loathing in las Vegas)".
External links
[edit]Diethyl ether
View on GrokipediaMolecular structure and properties
Chemical structure
Diethyl ether has the molecular formula C₄H₁₀O, commonly represented as (CH₃CH₂)₂O. Its IUPAC name is ethoxyethane, though it is more widely known by its common name, diethyl ether.[1] The molecular structure features a central oxygen atom bonded to two identical ethyl groups (–CH₂CH₃) via two sigma bonds, resulting in a bent C–O–C arrangement characteristic of ethers. This configuration classifies diethyl ether as a symmetrical dialkyl ether, where the alkyl substituents on either side of the oxygen are the same. The C–O–C bond angle is approximately 110°, influenced by the sp³ hybridization of the oxygen atom and lone pair repulsions./18%3A_Ethers_and_Epoxides_Thiols_and_Sulfides/18.01%3A_Names_and_Properties_of_Ethers)[5] For structural context, diethyl ether can be compared to related ethers such as dimethyl ether ((CH₃)₂O), which has shorter methyl groups and a slightly larger C–O–C angle of about 112°, or ethyl methyl ether (CH₃CH₂OCH₃), an unsymmetrical dialkyl ether with differing alkyl chains on each side of the oxygen./Ethers/Properties_of_Ethers/Physical_Properties_of_Ether) Spectroscopic techniques confirm this structure. In infrared (IR) spectroscopy, the C–O stretching vibration appears as a strong absorption band at approximately 1100 cm⁻¹. Proton nuclear magnetic resonance (¹H NMR) spectroscopy shows the methyl (CH₃) protons as a triplet at around 1.2 ppm and the methylene (CH₂) protons adjacent to oxygen as a quartet at about 3.5 ppm, reflecting their distinct chemical environments due to the ether linkage./18%3A_Ethers_and_Epoxides_Thiols_and_Sulfides/18.08%3A_Spectroscopy_of_Ethers)[1]Physical properties
Diethyl ether is a colorless, volatile liquid with a characteristic sweet, pungent odor.[1] The low boiling point of diethyl ether arises from weak intermolecular forces, as the ether oxygen cannot participate in hydrogen bonding to the same extent as in alcohols.[6] Key physical constants for diethyl ether under standard conditions are summarized in the following table:| Property | Value | Conditions |
|---|---|---|
| Molar mass | 74.12 g/mol | - |
| Melting point | -116.3 °C | - |
| Boiling point | 34.6 °C | 760 mmHg |
| Density | 0.7134 g/cm³ | 20 °C |
| Refractive index | 1.3526 | 20 °C (D line) |


