Recent from talks
Nothing was collected or created yet.
Neurosurgery
View on Wikipedia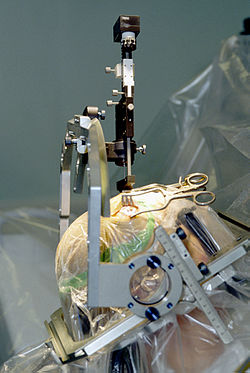 Stereotactic guided insertion of DBS electrodes in neurosurgery | |
| Occupation | |
|---|---|
Activity sectors | Surgery |
| Description | |
Education required |
or
or
|
Fields of employment | Hospitals, clinics |
Neurosurgery or/and neurological surgery, known in common parlance as brain surgery, is the medical specialty that focuses on the surgical treatment or rehabilitation of disorders which affect any portion of the nervous system including the brain, spinal cord, peripheral nervous system, and cerebrovascular system.[1] Neurosurgery as a medical specialty also includes non-surgical management of some neurological conditions.[2]
Education and context
[edit]In different countries, there are different requirements for an individual to legally practice neurosurgery, and there are varying methods through which they must be educated. In most countries, neurosurgeon training requires a minimum period of seven years after graduating from medical school.[3]
United Kingdom
[edit]In the United Kingdom, students must gain entry into medical school. The MBBS qualification (Bachelor of Medicine, Bachelor of Surgery) takes four to six years depending on the student's route. The newly qualified physician must then complete foundation training lasting two years; this is a paid training program in a hospital or clinical setting covering a range of medical specialties including surgery. Junior doctors then apply to enter the neurosurgical pathway. Unlike most other surgical specialties, it currently has its own independent training pathway which takes around eight years (ST1-8); before being able to sit for consultant exams with sufficient amounts of experience and practice behind them. Neurosurgery remains consistently amongst the most competitive medical specialties in which to obtain entry.
United States
[edit]In the United States, a neurosurgeon must generally complete four years of undergraduate education, four years of medical school, and seven years of residency (PGY-1-7).[4] Most, but not all, residency programs have some component of basic science or clinical research. Neurosurgeons may pursue additional training in the form of a fellowship after residency, or, in some cases, as a senior resident in the form of an enfolded fellowship. These fellowships include pediatric neurosurgery, trauma/neurocritical care, functional and stereotactic surgery, surgical neuro-oncology, radiosurgery, neurovascular surgery, skull-base surgery, peripheral nerve and complex spinal surgery.[5] Fellowships typically span one to two years. In the U.S., neurosurgery is a very small, highly competitive specialty, constituting only 0.5 percent of all physicians.[6]
History
[edit]Neurosurgery, or the premeditated incision into the head for pain relief, has been around for thousands of years, but notable advancements in neurosurgery have only come within the last hundred years.[7]

Ancient
[edit]During the Roman Empire, doctors and surgeons performed neurosurgery on depressed skull fractures.[8][9] The Incas appear to have practiced a procedure known as trepanation since before European colonization.[10] During the Middle Ages in Al-Andalus from 936 to 1013 AD, Al-Zahrawi performed surgical treatments of head injuries, skull fractures, spinal injuries, hydrocephalus, subdural effusions and headache.[11] Simple forms of neurosurgery were performed on King Henri II in 1559, after a jousting accident with Gabriel Montgomery fatally wounded him. Ambroise Paré and Andreas Vesalius, both experts in their field at the time, attempted their own methods, to no avail, in curing Henri.[12] In China, Hua Tuo created the first general anaesthesia called mafeisan, which he used on surgical procedures on the brain.[13]
Modern
[edit]History of tumor removal: In 1879, after locating it via neurological signs alone, Scottish surgeon William Macewen (1848–1924) performed the first successful brain tumor removal.[4] On November 25, 1884, after English physician Alexander Hughes Bennett (1848–1901) used Macewen's technique to locate it, English surgeon Rickman Godlee (1849–1925) performed the first primary brain tumor removal,[5][14] which differs from Macewen's operation in that Bennett operated on the exposed brain, whereas Macewen operated outside of the "brain proper" via trepanation.[15] On March 16, 1907, Austrian surgeon Hermann Schloffer became the first to successfully remove a pituitary tumor.[16]
Lobotomy: also known as leucotomy, was a form of psychosurgery, a neurosurgical treatment of mental disorders that involves severing connections in the brain's prefrontal cortex.[17] The originator of the procedure, Portuguese neurologist António Egas Moniz, shared the Nobel Prize for Physiology or Medicine of 1949.[18][19] Some patients improved in some ways after the operation, but complications and impairments – sometimes severe – were frequent. The procedure was controversial from its initial use, in part due to the balance between benefits and risks. It is mostly rejected as a treatment now and non-compliant with patients' rights.
History of electrodes in the brain: In 1878, Richard Caton discovered that electrical signals transmitted through an animal's brain. In 1950 Jose Delgado invented the first electrode that was implanted in an animal's brain (bull), using it to make it run and change direction.[20] In 1972 the cochlear implant, a neurological prosthetic that allowed deaf people to hear was marketed for commercial use. In 1998 researcher Philip Kennedy implanted the first Brain Computer Interface (BCI) into a human subject.[21]
A survey done in 2010 on 100 most cited works in neurosurgery shows that the works mainly cover clinical trials evaluating surgical and medical therapies, descriptions of novel techniques in neurosurgery, and descriptions of systems classifying and grading diseases.[22]
Modern surgical instruments
[edit]The main advancements in neurosurgery came about as a result of highly crafted tools. Modern neurosurgical tools, or instruments, include chisels, curettes, dissectors, distractors, elevators, forceps, hooks, impactors, probes, suction tubes, power tools, and robots.[23][24] Most of these modern tools have been in medical practice for a relatively long time. The main difference of these tools in neurosurgery, were the precision in which they were crafted. These tools are crafted with edges that are within a millimeter of desired accuracy.[25] Other tools, such as handheld power saws and robots, have only recently been commonly used inside of a neurological operating room. As an example, the University of Utah developed a device for computer-aided design / computer-aided manufacturing (CAD-CAM) which uses an image-guided system to define a cutting tool path for a robotic cranial drill.[26]
Organised neurosurgery
[edit]
The World Federation of Neurosurgical Societies (WFNS), founded in 1955 in Switzerland, as a professional, scientific, non governmental organization, is composed of 130 member societies: consisting of 5 Continental Associations (AANS, AASNS, CAANS, EANS and FLANC), 6 Affiliate Societies, and 119 National Neurosurgical Societies, representing some 50,000 neurosurgeons worldwide.[27] It has a consultative status in the United Nations. The official Journal of the Organization is World Neurosurgery.[28][29] The other global organisations being the World Academy of Neurological Surgery (WANS) and the World Federation of Skull Base Societies (WFSBS).
Main divisions
[edit]General neurosurgery involves most neurosurgical conditions including neuro-trauma and other neuro-emergencies such as intracranial hemorrhage. Most level 1 hospitals have this kind of practice.[30]
Specialized branches have developed to cater to special and difficult conditions. These specialized branches co-exist with general neurosurgery in more sophisticated hospitals. To practice advanced specialization within neurosurgery, additional higher fellowship training of one to two years is expected from the neurosurgeon. Some of these divisions of neurosurgery are:
- Vascular neurosurgery includes clipping of aneurysms and performing carotid endarterectomy (CEA).
- Stereotactic neurosurgery, functional neurosurgery, and epilepsy surgery (the latter includes partial or total corpus callosotomy – severing part or all of the corpus callosum to stop or lessen seizure spread and activity, and the surgical removal of functional, physiological and/or anatomical pieces or divisions of the brain, called epileptic foci, that are operable and that are causing seizures, and also the more radical and rare partial or total lobectomy, or even hemispherectomy – the removal of part or all of one of the lobes, or one of the cerebral hemispheres of the brain; those two procedures, when possible, are also very, very rarely used in oncological neurosurgery or to treat very severe neurological trauma, such as stab or gunshot wounds to the brain)
- Oncological neurosurgery also called neurosurgical oncology; includes pediatric oncological neurosurgery; treatment of benign and malignant central and peripheral nervous system cancers and pre-cancerous lesions in adults and children (including, among others, glioblastoma multiforme and other gliomas, brain stem cancer, astrocytoma, pontine glioma, medulloblastoma, spinal cancer, tumors of the meninges and intracranial spaces, secondary metastases to the brain, spine, and nerves, and peripheral nervous system tumors)
- Skull base surgery
- Spinal neurosurgery
- Peripheral nerve surgery
- Pediatric neurosurgery (for cancer, seizures, bleeding, stroke, cognitive disorders or congenital neurological disorders)
Commonly performed surgeries
[edit]According to an analysis by the American College of Surgeons National Surgical Quality Improvement Program (NSQIP), the most common surgeries performed by neurosurgeons in between 2006 and 2014 were the following:[31]
- Anterior cervical discectomy and fusion (ACDF)
- Craniotomy for brain tumor (CBT)
- Discectomy
- Laminectomy
- Posterolateral lumbar fusion (PLF)
Neuropathology
[edit]
Neuropathology is a specialty within the study of pathology focused on the disease of the brain, spinal cord, and neural tissue.[32] This includes the central nervous system and the peripheral nervous system. Tissue analysis comes from either surgical biopsies or post mortem autopsies. Common tissue samples include muscle fibers and nervous tissue.[33] Common applications of neuropathology include studying samples of tissue in patients who have Parkinson's disease, Alzheimer's disease, dementia, Huntington's disease, amyotrophic lateral sclerosis, mitochondria disease, and any disorder that has neural deterioration in the brain or spinal cord.[34][35]
History
[edit]While pathology has been studied for millennia only within the last few hundred years has medicine focused on a tissue- and organ-based approach to tissue disease. In 1810, Thomas Hodgkin started to look at the damaged tissue for the cause. This was conjoined with the emergence of microscopy and started the current understanding of how the tissue of the human body is studied.[36]
Neuroanesthesia
[edit]Neuroanesthesia is a field of anesthesiology which focuses on neurosurgery. Anesthesia is not used during the middle of an "awake" brain surgery. Awake brain surgery is where the patient is conscious for the middle of the procedure and sedated for the beginning and end. This procedure is used when the tumor does not have clear boundaries and the surgeon wants to know if they are invading on critical regions of the brain which involve functions like talking, cognition, vision, and hearing. It will also be conducted for procedures which the surgeon is trying to combat epileptic seizures.[37]
History
[edit]The physician Hippocrates (460–370 BCE) made accounts of using different wines to sedate patients while trepanning. In 60 CE, Dioscorides, a physician, pharmacologist, and botanist, detailed how mandrake, henbane, opium, and alcohol were used to put patients to sleep during trepanning. In 972 CE, two brother surgeons in Paramara, now India, used "samohine" to sedate a patient while removing a small tumor, and awoke the patient by pouring onion and vinegar in the patient's mouth. The combination of carbon dioxide, hydrogen, and nitrogen, was a form of neuroanesthesia adopted in the 18th century and introduced by Humphry Davy.[38]
Neurosurgery methods
[edit]| Neurosurgery | |
|---|---|
| ICD-10-PCS | 00-01 |
| ICD-9-CM | 01–05 |
| MeSH | D019635 |
| OPS-301 code | 5-01...5-05 |
Various Imaging methods are used in modern neurosurgery diagnosis and treatment. They include computer assisted imaging computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), magnetoencephalography (MEG), and stereotactic radiosurgery. Some neurosurgery procedures involve the use of intra-operative MRI and functional MRI.[39]
In conventional neurosurgery the neurosurgeon opens the skull, creating a large opening to access the brain. Techniques involving smaller openings with the aid of microscopes and endoscopes are now being used as well. Methods that utilize small craniotomies in conjunction with high-clarity microscopic visualization of neural tissue offer excellent results. However, the open methods are still traditionally used in trauma or emergency situations.[16][23]
Microsurgery is utilized in many aspects of neurological surgery. Microvascular techniques are used in EC-IC bypass surgery and in restoration carotid endarterectomy. The clipping of an aneurysm is performed under microscopic vision. Minimally-invasive spine surgery utilizes microscopes or endoscopes. Procedures such as microdiscectomy, laminectomy, and artificial disc replacement rely on microsurgery.[24]
Using stereotaxy neurosurgeons can approach a minute target in the brain through a minimal opening. This is used in functional neurosurgery where electrodes are implanted or gene therapy is instituted with high level of accuracy as in the case of Parkinson's disease or Alzheimer's disease. Using the combination method of open and stereotactic surgery, intraventricular hemorrhages can potentially be evacuated successfully.[25] Conventional surgery using image guidance technologies is also becoming common and is referred to as surgical navigation, computer-assisted surgery, navigated surgery, stereotactic navigation. Similar to a car or mobile Global Positioning System (GPS), image-guided surgery systems, like Curve Image Guided Surgery and StealthStation, use cameras or electromagnetic fields to capture and relay the patient's anatomy and the surgeon's precise movements in relation to the patient, to computer monitors in the operating room. These sophisticated computerized systems are used before and during surgery to help orient the surgeon with three-dimensional images of the patient's anatomy including the tumor.[40] Real-time functional brain mapping has been employed to identify specific functional regions using electrocorticography (ECoG)[41]
Minimally invasive endoscopic surgery is commonly utilized by neurosurgeons when appropriate. Techniques such as endoscopic endonasal surgery are used in pituitary tumors, craniopharyngiomas, chordomas, and the repair of cerebrospinal fluid leaks. Ventricular endoscopy is used in the treatment of intraventricular bleeds, hydrocephalus, colloid cyst and neurocysticercosis. Endonasal endoscopy is at times carried out with neurosurgeons and ENT surgeons working together as a team.[citation needed]
Repair of craniofacial disorders and disturbance of cerebrospinal fluid circulation is done by neurosurgeons who also occasionally team up with maxillofacial and plastic surgeons. Cranioplasty for craniosynostosis is performed by pediatric neurosurgeons with or without plastic surgeons.[42]
Neurosurgeons are involved in stereotactic radiosurgery along with radiation oncologists in tumor and AVM treatment. Radiosurgical methods such as Gamma knife, Cyberknife and Novalis Radiosurgery are used as well.[43]
Endovascular neurosurgery utilize endovascular image guided procedures for the treatment of aneurysms, AVMs, carotid stenosis, strokes, and spinal malformations, and vasospasms. Techniques such as angioplasty, stenting, clot retrieval, embolization, and diagnostic angiography are endovascular procedures.[44]
A common procedure performed in neurosurgery is the placement of ventriculo-peritoneal shunt (VP shunt). In pediatric practice this is often implemented in cases of congenital hydrocephalus. The most common indication for this procedure in adults is normal pressure hydrocephalus (NPH).[45]
Neurosurgery of the spine covers the cervical, thoracic and lumbar spine. Some indications for spine surgery include spinal cord compression resulting from trauma, arthritis of the spinal discs, or spondylosis. In cervical cord compression, patients may have difficulty with gait, balance issues, and/or numbness and tingling in the hands or feet. Spondylosis is the condition of spinal disc degeneration and arthritis that may compress the spinal canal. This condition can often result in bone-spurring and disc herniation. Power drills and special instruments are often used to correct any compression problems of the spinal canal. Disc herniations of spinal vertebral discs are removed with special rongeurs. This procedure is known as a discectomy. Generally once a disc is removed it is replaced by an implant which will create a bony fusion between vertebral bodies above and below. Instead, a mobile disc could be implanted into the disc space to maintain mobility. This is commonly used in cervical disc surgery. At times instead of disc removal a Laser discectomy could be used to decompress a nerve root. This method is mainly used for lumbar discs. Laminectomy is the removal of the lamina of the vertebrae of the spine in order to make room for the compressed nerve tissue.[46]
Surgery for chronic pain is a sub-branch of functional neurosurgery. Some of the techniques include implantation of deep brain stimulators, spinal cord stimulators, peripheral stimulators and pain pumps.[47]
Surgery of the peripheral nervous system is also possible, and includes the very common procedures of carpal tunnel decompression and peripheral nerve transposition. Numerous other types of nerve entrapment conditions and other problems with the peripheral nervous system are treated as well.[48]
Conditions
[edit]Conditions treated by neurosurgeons include, but are not limited to:[49]
- Meningitis and other central nervous system infections including abscesses
- Spinal disc herniation
- Cervical spinal stenosis and Lumbar spinal stenosis
- Hydrocephalus
- Head trauma (brain hemorrhages, skull fractures, etc.)
- Spinal cord trauma
- Traumatic injuries of peripheral nerves
- Tumors of the spine, spinal cord and peripheral nerves
- Intracerebral hemorrhage, such as subarachnoid hemorrhage, interdepartmental, and intracellular hemorrhages
- Some forms of drug-resistant epilepsy
- Some forms of movement disorders (advanced Parkinson's disease, chorea) – this involves the use of specially developed minimally invasive stereotactic techniques (functional, stereotactic neurosurgery) such as ablative surgery and deep brain stimulation surgery
- Intractable pain of cancer or trauma patients and cranial/peripheral nerve pain
- Some forms of intractable psychiatric disorders
- Vascular malformations (i.e., arteriovenous malformations, venous angiomas, cavernous angiomas, capillary telangectasias) of the brain and spinal cord
- Moyamoya disease
Recovery
[edit]Postoperative pain
[edit]Pain following brain surgery can be significant and may lengthen recovery, increase the amount of time a person stays in the hospital following surgery, and increase the risk of complications following surgery.[50] Severe acute pain following brain surgery may also increase the risk of a person developing a chronic post-craniotomy headache.[50] Approaches to treating pain in adults include treatment with nonsteroidal anti‐inflammatory drugs (NSAIDs), which have been shown to reduce pain for up to 24 hours following surgery.[50] Low-quality evidence supports the use of the medications dexmedetomidine, pregabalin or gabapentin to reduce post-operative pain.[50] Low-quality evidence also supports scalp blocks and scalp infiltration to reduce postoperative pain.[50] Gabapentin or pregabalin may also decrease vomiting and nausea following surgery, based on very low-quality medical evidence.[50]
Notable neurosurgeons
[edit]- Harvey Cushing – known as one of the founders of modern neurosurgery.
- Walter Dandy – known as one of the founders of modern neurosurgery.
- Victor Horsley – known as the first neurosurgeon.
- Sofia Ionescu-Ogrezeanu – known as the first woman neurosurgeon.[51] As a medical student at the University of Bucharest, she performed her first neurosurgical procedure in 1944, under the supervision of Dumitru Bagdasar, and saved the life of an 8-year old comatose boy with an epidural hematoma (during the WWII bombardment of Bucharest).[52]
- Hermann Schloffer invented transsphenoidal surgery in 1907.
- Wilder Penfield – known as one of the founding fathers of modern neurosurgery, and pioneer of epilepsy Neurosurgery.
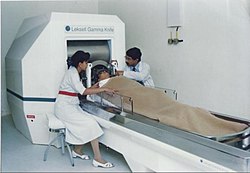
- Lars Leksell – Swedish neurosurgeon who developed the Gamma Knife.
- Wirginia Maixner – pediatric neurosurgeon at Melbourne's Royal Children's Hospital. Primarily known for separating conjoined Bangladeshi twins, Trishna and Krishna.
- Saleem Abdulrauf – developed "awake" craniotomy for complex aneurysms and vascular malformations.
- John R. Adler – Stanford University neurosurgeon who invented the Cyberknife.
- Alim-Louis Benabid – known as one of the developers of deep brain stimulation surgery for movement disorder.
- Ben Carson – retired pediatric neurosurgeon from Johns Hopkins Hospital, pioneer in hemispherectomy, and pioneer in the separation of craniopagus twins (joined at the head); former 2016 Republican Party presidential candidate, and former United States Secretary of Housing and Urban Development under the Presidency of Donald Trump.
- Henry Marsh – leading English neurosurgeon and pioneer of neurosurgical advancements in Ukraine
- Frank Henderson Mayfield – invented the Mayfield skull clamp.
- B. K. Misra – First neurosurgeon in the world to perform image-guided surgery for aneurysms, first in South Asia to perform stereotactic radiosurgery, first in India to perform awake craniotomy and laparoscopic spine surgery.[53]
- Karin Muraszko – first woman to occupy a chair of neurosurgery at an American medical school (University of Michigan).
- Hirotaro Narabayashi – a pioneer of stereotactic Neurosurgery.
- Ayub K. Ommaya – invented the Ommaya reservoir.
- Christopher Duntsch – Former neurosurgeon who killed or maimed nearly every patient he operated on before being incarcerated.
- Ludvig Puusepp – known as one of the founding fathers of modern neurosurgery, world's first professor of neurosurgery.
- Joseph Ransohoff – known for his pioneering use of medical imaging and catheterization in neurosurgery, and for founding the first neurosurgery intensive care unit.
- Majid Samii – pioneer of cerebello-pontine angle tumor surgery. World Federation of Neurosurgical Societies coined a medal of honor bearing Samii's name which would be given to outstanding neurosurgeons every two years.[54]
- Juliet Sekabunga Nalwanga – Uganda's first woman neurosurgeon.
- Robert Wheeler Rand – among the first to introduce the surgical microscope into neurosurgical procedures in 1957 and published first textbook on Microneurosurgery in 1969.
- Robert J. White – Established the Vatican's Commission on Biomedical Ethics in 1981 after his appointment to the Pontifical Academy of Sciences and was famous for his head transplants on living monkeys.[55][56]
- Gazi Yaşargil – known as the father of microneurosurgery.
- Mohana Rao Patibandla is known for performing India's first invasive brain surgery using the BrainPath system.[57][58]
Bioethics in neurosurgery
[edit]Neurosurgery is a part of practical medicine and the only specialty that involves invasive intervention in the activity of the living brain. The brain ensures the structural and functional integrity of the body and the implementation of all the main life processes of the body. Therefore, neurosurgery faces a wide range of bioethical issues and a significant selection of the latest treatment technologies.[59]
Neurosurgery has the following applied scientific and ethical problems:
- Ethical and legal aspects of clinical research;
- Αxiological deficit due to professional deformation and professional burnout;
- Limited access to expensive medical services;
- The industry-specific problem of "medical error" due to the complexity of neurosurgical pathologies and the huge number of possible technologies and tools for their treatment;
- Controversial bioethical and legal issues of surgery for the treatment of psychiatric diseases;
- Bioethical discussions regarding the instrumentation of reconstructive surgery, through the use of experimental technologies;
- Debatable bioethical issues of improving human brain activity with the help of artificial implants, for instance neurocomponents (artificial impulse quasi-neurons);
- Cyborgization in transhumanism meaning;[60]
- Ethical issue of standardization of research protocols for testing neuroengineering means of nerve tissue regeneration in order to improve the implementation of experimental research results in clinical practice.[59]
See also
[edit]- American Association of Neurological Surgeons – Scientific and educational association
- Congress of Neurological Surgeons – Professional organization
- Cranial auscultation – Medical neurological procedure to check for intracranial bruits
- Global neurosurgery – Global health subspecialty
- List of neurologists and neurosurgeons
- Polyaxial screw – Orthopedic screw
References
[edit]- ^ "Neurological Surgery Specialty Description". American Medical Association. Archived from the original on 12 October 2020. Retrieved 4 October 2020.
- ^ Greenberg, Mark S. (2020). Handbook of neurosurgery (9th ed.). New York Stuttgart Delhi: Thieme. ISBN 978-1-68420-137-2.
- ^ "Brain Surgeon: Job Description, Salary, Duties and Requirements". Science. Archived from the original on 29 December 2019. Retrieved 29 December 2019.
- ^ a b Preul, Mark C. (2005). "History of brain tumor surgery". Neurosurgical Focus. 18 (4): 1. doi:10.3171/foc.2005.18.4.1.
- ^ a b Kirkpatrick, Douglas B. (1984). "The first primary brain-tumor operation". Journal of Neurosurgery. 61 (5): 809–13. doi:10.3171/jns.1984.61.5.0809. PMID 6387062.
- ^ "Ensuring an Adequate Neurosurgical Workforce for the 21st Century" (PDF). American Association of Neurological Surgeons. Archived from the original (PDF) on 11 July 2021. Retrieved 28 May 2021.
Neurosurgery is a small specialty, constituting only 0.5 percent of all physicians.
- ^ Wickens, Andrew P. (2014-12-08). A History of the Brain: From Stone Age surgery to modern neuroscience. Psychology Press. ISBN 978-1-317-74482-5.
- ^ Desai, Tejal; Bhatia, Sangeeta N. (2007-05-26). BioMEMS and Biomedical Nanotechnology: Volume III: Therapeutic Micro/Nanotechnology. Springer Science & Business Media. p. 97. ISBN 978-0-387-25844-7.
- ^ Gillard, Arthur (2012-10-19). Traumatic Brain Injury (in Spanish). Greenhaven Publishing LLC. p. 142. ISBN 978-0-7377-7312-5.
- ^ Andrushko, Valerie A.; Verano, John W. (September 2008). "Prehistoric trepanation in the Cuzco region of Peru: A view into an ancient Andean practice". American Journal of Physical Anthropology. 137 (1): 4–13. Bibcode:2008AJPA..137....4A. doi:10.1002/ajpa.20836. PMID 18386793.
- ^ Al-Rodhan, N. R.; Fox, J. L. (1986-07-01). "Al-Zahrawi and Arabian neurosurgery, 936-1013 AD". Surgical Neurology. 26 (1): 92–95. doi:10.1016/0090-3019(86)90070-4. ISSN 0090-3019. PMID 3520907.
- ^ Kean, Sam (2014). The Tale of the Dueling Neurosurgeons: The History of the Human Brain as Revealed by True Stories of Trauma, Madness, and Recovery. New York: Little, Brown and Company. pp. 25–40.
- ^ Zhang, Yuqi (2015-03-18). "HUA Tuo: The First Neurosurgeon in the World". Translational Neuroscience and Clinics. 1: 71–72. doi:10.18679/CN11-6030_R.2015.008. S2CID 207942533. Archived from the original on 2024-01-04. Retrieved 2021-03-31.
- ^ "Alexander Hughes Bennett (1848-1901): Rickman John Godlee (1849-1925)". CA: A Cancer Journal for Clinicians. 24 (3): 169–170. 1974. doi:10.3322/canjclin.24.3.169. PMID 4210862. S2CID 45097428.
- ^ "Surgery". Archived from the original on 2021-11-13. Retrieved 2016-02-11.
- ^ a b "Cyber Museum of Neurosurgery". Archived from the original on 2017-01-06. Retrieved 2016-02-11.
- ^ "Lobotomy: Definition, Procedure & History". Live Science. Archived from the original on 2023-09-24. Retrieved 2018-06-28.
- ^ Nouri, Aria (20 October 2011). ""A brief history of lobotomy"". aaas.org. Archived from the original on 3 December 2021. Retrieved 3 December 2021.
- ^ Miguel A, Faria (5 April 2013). ""Violence, mental illness, and the brain – A brief history of psychosurgery: Part 1 – From trephination to lobotomy"". Surgical Neurology International. 4: 49. doi:10.4103/2152-7806.110146. PMC 3640229. PMID 23646259.
- ^ C.Marzullo, Timothy (Spring 2017). ""The Missing Manuscript of Dr. Jose Delgado's Radio Controlled Bulls"". Journal of Undergraduate Neuroscience Education. 15 (2): R29 – R35. PMC 5480854. PMID 28690447.
- ^ https://biomed.brown.edu/Courses/BI108/BI108_2005_Groups/03/hist.htm[full citation needed] Archived 2016-11-30 at the Wayback Machine [dead link]
- ^ Ponce FA, Lozano AM (February 2010). "Highly cited works in neurosurgery. Part I: the 100 top-cited papers in neurosurgical journals". Journal of Neurosurgery. 112 (2): 223–32. doi:10.3171/2009.12.JNS091599. PMID 20078192.
- ^ a b "Neurosurgery surgical power tool - All medical device manufacturers - Videos". Archived from the original on 2022-01-24. Retrieved 2016-02-11.
- ^ a b "Neurosurgical Instruments,Neurosurgery Instrument, Neurosurgeon, Surgical Tools". Archived from the original on 2007-05-13. Retrieved 2016-02-11.
- ^ a b "Technology increases precision, safety during neurosurgery | Penn State University". Archived from the original on 2013-09-04. Retrieved 2016-02-11.
- ^ "Robotics in Neurosurgery". Neurosurgical Focus. 42 (5). 1 May 2017. Archived from the original on 14 November 2018. Retrieved 14 November 2018.
- ^ "About the Foundation | World Federation of Neurosurgical Societies". Archived from the original on 2020-08-04. Retrieved 2020-07-10.
- ^ "Journal: World Neurosurgery". WFNS. Archived from the original on 6 June 2014. Retrieved 29 May 2014.
- ^ "World Neurosurgery, Home page". Elsevier. Archived from the original on 9 April 2016. Retrieved 29 May 2014.
- ^ Esposito, Thomas J.; Reed, R. Lawrence; Gamelli, Richard L.; Luchette, Fred A. (2005-01-01). "Neurosurgical Coverage: Essential, Desired, or Irrelevant for Good Patient Care and Trauma Center Status". Transactions of the ... Meeting of the American Surgical Association. 123 (3): 67–76. doi:10.1097/01.sla.0000179624.50455.db. ISSN 0066-0833. PMC 1357744. PMID 16135922.
- ^ M Giantini Larsen BS, Alexandra; Vishwas Karhade BE, Aditya; J Cote BS, David; R. Smith MD, Timothy (2016). Most Common Neurosurgical Procedures & Complications (Report). Cushing Neurosurgery Outcomes Center. Archived from the original on 2022-07-03. Retrieved 2022-05-17.
- ^ "Department of Pathology, Microbiology and Immunology". Archived from the original on 2021-01-25. Retrieved 2016-02-12.
- ^ Love, S. (April 2004). "Post mortem sampling of the brain and other tissues in neurodegenerative disease". Histopathology. 44 (4): 309–317. doi:10.1111/j.1365-2559.2004.01794.x. ISSN 0309-0167. PMID 15049895.
- ^ "Dementia". neuropathology-web.org. Archived from the original on 2021-04-13. Retrieved 2016-02-12.
- ^ Filosto, Massimiliano; Tomelleri, Giuliano; Tonin, Paola; Scarpelli, Mauro; Vattemi, Gaetano; Rizzuto, Nicolò; Padovani, Alessandro; Simonati, Alessandro (2007). "Neuropathology of mitochondrial diseases". Bioscience Reports. 27 (1–3): 23–30. doi:10.1007/s10540-007-9034-3. PMID 17541738. S2CID 36830289.
- ^ van den Tweel, Jan G.; Taylor, Clive R. (2010). "A brief history of pathology". Virchows Archiv. 457 (1): 3–10. doi:10.1007/s00428-010-0934-4. PMC 2895866. PMID 20499087.
- ^ Kieffer, Sara (26 April 2022). "Awake Brain Surgery (Intraoperative Brain Mapping) | Imaging Services | Johns Hopkins Intraoperative Neurophysiological Monitoring Unit (IONM)". Archived from the original on 28 April 2021. Retrieved 12 February 2016.
- ^ Chivukula, Srinivas; Grandhi, Ramesh; Friedlander, Robert M. (2014). "A brief history of early neuroanesthesia". Neurosurgical Focus. 36 (4): E2. doi:10.3171/2014.2.FOCUS13578. PMID 24684332.
- ^ Castillo, Mauricio (2005). Neuroradiology Companion: Methods, Guidelines, and Imaging Fundamentals (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 1–428.
- ^ Duan, Zhaoliang; Yuan, Zhi-Yong; Liao, Xiangyun; Si, Weixin; Zhao, Jianhui (2011). "3D Tracking and Positioning of Surgical Instruments in Virtual Surgery Simulation". Journal of Multimedia. 6 (6): 502–509. doi:10.4304/jmm.6.6.502-509.
- ^ Swift, James; Coon, William; Guger, Christoph; Brunner, Peter; Bunch, M; Lynch, T; Frawley, T; Ritaccio, Anthony; Schalk, Gerwin (2018). "Passive functional mapping of receptive language areas using electrocorticographic signals". Clinical Neurophysiology. 6 (12): 2517–2524. doi:10.1016/j.clinph.2018.09.007. PMC 6414063. PMID 30342252.
- ^ Albright, L.; Pollack, I.; Adelson, D. (2015), Principles and practice of pediatric neurosurgery (3rd ed.), Thieme Medical Publishers, Inc.
- ^ "Neurosurgery". Division of Biology and Medicine, Brown University. Archived from the original on June 5, 2013. Retrieved February 11, 2016.
- ^ "Neuroradiology Patients & Families: Washington University Radiologist". Archived from the original on 2010-06-02. Retrieved 2010-06-20.
- ^ Kombogiorgas, D., The cerebrospinal fluid shunts New York: Nova Medical. 2016
- ^ "Laminectomy - Health Encyclopedia - University of Rochester Medical Center". www.urmc.rochester.edu. Retrieved 2021-05-06.
- ^ "How Neurosurgeons Treat Chronic Pain". www.aans.org. Archived from the original on 2021-04-22. Retrieved 2021-05-06.
- ^ Cutts, Steven (January 2007). "Cubital tunnel syndrome". Postgraduate Medical Journal. 83 (975): 28–31. doi:10.1136/pgmj.2006.047456. ISSN 0032-5473. PMC 2599973. PMID 17267675.
- ^ Greenberg., Mark S. (2010-01-01). Handbook of neurosurgery. Greenberg Graphics. ISBN 978-1-60406-326-4. OCLC 892183792.
- ^ a b c d e f Galvin, Imelda M.; Levy, Ron; Day, Andrew G.; Gilron, Ian (November 21, 2019). "Pharmacological interventions for the prevention of acute postoperative pain in adults following brain surgery". The Cochrane Database of Systematic Reviews. 2019 (11). doi:10.1002/14651858.CD011931.pub2. ISSN 1469-493X. PMC 6867906. PMID 31747720.
- ^ "The Professionals, by Eugenia Voda, Interview with Sofia Ionescu-Ogrezeanu". YouTube. September 2021.
- ^ https://www.eans.org/page/SofiaIonescu-Ogrezeanu-Bio.
{{cite web}}: Missing or empty|title=(help) - ^ "Past Presidents.pmd" (PDF). Neurological Society of India. Archived from the original on 18 July 2023.
- ^ "Madjid Samii Medal of Honor". www.bbraun.co.za. Retrieved 2025-03-09.
- ^ Segall, Grant; Dealer, The Plain (2010-09-16). "Dr. Robert J. White, famous neurosurgeron [sic] and ethicist, dies at 84". cleveland. Archived from the original on 2021-02-28. Retrieved 2021-05-24.
- ^ Mims, Christopher (July 2013). "First-ever human head transplant is now possible, says neuroscientist". Quartz. Archived from the original on 2013-07-01. Retrieved 2021-05-24.
- ^ "Guntur doctors perform minimally invasive surgery using BrainPath technology". The Hindu. 15 July 2024. Retrieved 29 July 2025.
- ^ "Dr. Mohana Rao Patibandla leads Dr Rao's Hospital in pioneering minimally invasive brain surgery with BrainPath system, a first in Guntur and India". ET HealthWorld. The Economic Times. 6 April 2024. Retrieved 29 July 2025.
- ^ a b Moroz, V.A. (2009). Suchasni problemy bioetyky [Modern problems of bioethics] (in Ukrainian). Akademperiodyka. ISBN 978-966-615-333-6.
- ^ Jiménez-Ponce, F.; García-Muñoz, L.; Carrillo-Ruiz, J. D. (2015-01-01). "The role of bioethics in the neurosurgical treatment of psychiatric disorders". Revista Médica del Hospital General de México. 78 (1): 47–54. doi:10.1016/j.hgmx.2015.04.001. ISSN 0185-1063. Archived from the original on 2024-06-02. Retrieved 2024-06-02.
Neurosurgery
View on GrokipediaHistory
Prehistoric and Ancient Origins
Trepanation, the surgical removal of a portion of the skull to expose the brain, represents the earliest known neurosurgical practice, with archaeological evidence dating back to the Neolithic period around 6500 BC in regions such as France, where 40 out of 120 examined skulls exhibited trepanation holes.[10] Similar findings appear globally, including in Peru from 400 BC to 200 BC, China along the Yellow River Basin from the Neolithic era onward, and Çatalhöyük in Turkey around 7000 BC, indicating widespread adoption across prehistoric cultures.[11] [12] Techniques involved scraping, drilling, or cutting with stone or flint tools, often targeting the cranial vault without damaging underlying dura mater, as evidenced by healed bone edges in many specimens.[13] Survival rates varied by region and era but demonstrate technical proficiency; for instance, approximately 62.5% of 400 trepanned skulls from ancient Peru showed healing, while Late Iron Age Swiss cases achieved 78% long-term survival, contrasting sharply with 46-56% mortality in 19th-century American Civil War cranial surgeries.[14] [15] These procedures likely addressed trauma, intracranial pressure from hematomas, or conditions like epilepsy, though ritualistic or mystical motivations cannot be ruled out based on the absence of direct textual records.[16] In ancient Egypt, the Edwin Smith Papyrus, dating to circa 1600 BC but preserving texts from around 3000 BC, documents 48 cases of head and spinal injuries, including cranial fractures and superficial wound management, marking the oldest known surgical treatise with empirical observations rather than magical incantations.[17] It describes examination, diagnosis, and prognosis for skull wounds, recognizing brain pulsations and spinal cord function, though invasive cranial procedures remained limited.[18] Greek physician Hippocrates (c. 460-355 BC) advanced trepanation for treating depressed skull fractures, contusions, and elevated intracranial pressure, advising against unnecessary intervention in linear fractures and emphasizing tools like the trepan to evacuate fluids or bone fragments.[19] His corpus detailed postoperative care to prevent infection, reflecting causal understanding of head trauma outcomes.[20] In ancient India, Sushruta (c. 700-600 BC) outlined cranial surgeries in the Sushruta Samhita, including trepanation for head injuries, sinus access, and foreign body removal, with detailed anatomy of the skull and brain meninges, alongside aseptic techniques using boiled oils and alkalis.[21] [22] Roman-era Galen (129-216 AD) built on these foundations through animal vivisections, describing neural pathways and advocating cautious trepanation for trauma, though his reliance on non-human models introduced anatomical inaccuracies regarding human ventricular functions.[19][23]19th-Century Foundations
The foundations of modern neurosurgery were laid in the 19th century through critical advances in anesthesia and antisepsis, which transformed cranial surgery from a high-mortality endeavor into a feasible practice. Prior to these innovations, surgical interventions on the brain were limited by excruciating pain and rampant postoperative infections, often resulting in near-certain fatality. The introduction of general anesthesia began with William T. G. Morton's public demonstration of diethyl ether on October 16, 1846, at Massachusetts General Hospital, enabling surgeons to perform prolonged operations without patient movement.[24] Chloroform followed in 1847, further expanding operative possibilities. Concurrently, Joseph Lister's adoption of antiseptic techniques in 1867, inspired by Louis Pasteur's germ theory, involved spraying carbolic acid during procedures to curb bacterial contamination, drastically reducing sepsis rates in surgical wounds.[25] These developments collectively lowered perioperative mortality, allowing tentative explorations of intracranial pathology.[4] Progress in cerebral localization provided a theoretical basis for targeted interventions, shifting from empirical trephination to anatomically informed surgery. Pierre Broca's 1861 identification of the left inferior frontal gyrus as a speech center, following postmortem examination of patient "Tan," demonstrated functional specificity in the brain.[25] Eduard Hitzig and Gustav Fritsch's 1870 experiments electrically stimulating the motor cortex in dogs confirmed localized excitability, laying groundwork for intraoperative mapping.[25] These findings encouraged general surgeons to attempt excisions of intracranial lesions, often guided by clinical symptoms and rudimentary diagnostics like percussion or auscultation. Pioneering operations marked the field's emergence, with William Macewen performing the first documented successful brain tumor resection on July 27, 1879, in Glasgow, removing a glioma from a nine-year-old boy based on focal seizures and hemiparesis; the patient survived seven years without recurrence.[26] In 1884, Rickman Godlee in London conducted the first intentional adult brain tumor removal, excising a meningioma verified postmortem, though the patient succumbed to infection shortly after.[27] Victor Horsley, appointed in 1886 as the first dedicated brain surgeon at London's National Hospital for the Paralysed and Epileptic, advanced the specialty through over 50 cranial procedures by the 1890s, including spinal cord tumor resections and epilepsy surgeries using cortical stimulation for localization.[28] Horsley's systematic approach, emphasizing hemostasis and asepsis, yielded survival rates up to 80% in select cases, establishing neurosurgery as a distinct pursuit amid ongoing challenges like hemorrhage and localization errors.[29] These efforts, though limited by absent imaging and antibiotics, demonstrated that intracranial surgery could yield functional recoveries, setting precedents for 20th-century refinements.[27]20th-Century Maturation
Harvey Cushing, often regarded as the father of modern neurosurgery, established the specialty in the early 20th century through systematic approaches to brain tumor surgery at Johns Hopkins Hospital, where he performed thousands of operations and reduced operative mortality rates significantly by emphasizing meticulous hemostasis and tissue handling.[5] His innovations included the use of silver clips for controlling bleeding, development of electrosurgical tools, and detailed classifications of intracranial tumors, such as meningiomas and gliomas, which informed precise surgical resections.[30] [31] Cushing's training of international fellows further disseminated these techniques globally, fostering neurosurgery's recognition as a distinct discipline by the 1920s.[32] Walter Dandy, Cushing's trainee, advanced diagnostic and therapeutic methods in the 1910s and 1920s, inventing pneumoencephalography in 1918 to visualize ventricular structures via air injection, which replaced cerebrospinal fluid and enabled X-ray imaging of brain pathologies like tumors and hydrocephalus.[33] Dandy pioneered selective ventricular approaches for hydrocephalus shunting and refined surgeries for acoustic neuromas and posterior fossa tumors, achieving high success rates through innovative exposure techniques, such as the combined supra- and infratentorial approach.[34] His work on arteriovenous malformations and hemispherectomy for intractable epilepsy laid groundwork for functional neurosurgery, though his aggressive style contrasted with Cushing's caution, highlighting evolving paradigms in risk assessment.[35] Mid-century progress included the refinement of stereotactic techniques, originating from Horsley and Clarke's 1908 apparatus for animal experiments but adapted for humans by Ernst Spiegel and Henry Wycis in 1947, who performed the first stereotactic thalamotomy for psychiatric disorders using a human-adapted frame and ventriculographic targeting.[36] [37] This enabled precise lesioning of deep brain structures for movement disorders like Parkinson's disease, reducing invasiveness compared to open craniotomy; by the 1950s, Lars Leksell introduced stereotactic radiosurgery with the gamma knife prototype in 1951, delivering focused radiation to targets like arteriovenous malformations.[38] The 1960s and 1970s saw the advent of microneurosurgery, propelled by M. Gazi Yasargil's integration of the operating microscope—initially adapted from otolaryngology—for aneurysm clipping and tumor resection, allowing visualization of vessels under 10 micrometers and improving outcomes in cerebrovascular surgery.[39] Yasargil's multi-volume treatise, published starting in 1984, codified these methods, emphasizing anatomical precision and clip application, which lowered morbidity in subarachnoid hemorrhage cases from over 50% to below 20% in specialized centers.[40] Concurrently, the computed tomography (CT) scanner, invented by Godfrey Hounsfield in 1971 and clinically deployed by 1973, transformed preoperative planning by providing non-invasive cross-sectional imaging, reducing reliance on invasive diagnostics like angiography and enabling safer interventions.[41] By the late 20th century, these cumulative advances—bolstered by antibiotics, improved anesthesia, and the 1931 founding of the American Association of Neurological Surgeons—matured neurosurgery into a high-precision field, with operative survival for gliomas exceeding 70% in select cases and endovascular techniques emerging for aneurysms by the 1990s.[6] World War II experiences further accelerated trauma management, standardizing decompressive craniectomies and infection control.[42]Post-2000 Milestones
![Parkinson's surgery showing deep brain stimulation procedure][float-right] Advancements in deep brain stimulation (DBS) post-2000 expanded its therapeutic scope for movement disorders, with refinements in electrode placement and programming enhancing outcomes for Parkinson's disease and essential tremor. DBS, initially approved by the FDA in 1997 for essential tremor and Parkinson's, saw further validation through large-scale trials demonstrating sustained motor improvements, such as the 2006 VAIT study showing 5-year benefits in advanced Parkinson's patients.[44] In 2003, FDA approval extended DBS to dystonia, addressing severe involuntary contractions via targeted thalamic or globus pallidus stimulation.[44] Intraoperative imaging technologies, particularly MRI-compatible systems, marked a pivotal shift toward precision neurosurgery. The introduction of the neuroArm in 2008 represented the first fully MRI-compatible robotic system, enabling telesurgery with haptic feedback and real-time imaging to minimize invasiveness during tumor resections and DBS placements.[45] Concurrently, endoscopic endonasal approaches gained prominence for skull base tumors, allowing transnasal access to pituitary and clival lesions without traditional craniotomy, as evidenced by increased adoption reported in series from the early 2000s onward.[46] Ablative techniques advanced with the FDA approval of magnetic resonance-guided focused ultrasound (MRgFUS) in 2016 for unilateral thalamotomy in medication-refractory essential tremor, offering a non-invasive alternative to lesioning via thermal coagulation without incisions.[47] This was expanded in 2018 to tremor-dominant Parkinson's disease, with staged bilateral treatments approved in 2025, reducing symptoms in up to 60% of patients per pivotal trials.[48] Laser interstitial thermal therapy (LITT), leveraging MRI thermography for real-time monitoring, emerged as a minimally invasive option for recurrent brain tumors and epilepsy foci in the 2010s, with systems like Visualase facilitating stereotactic laser ablation through small burr holes. Clinical series post-2010 reported reduced morbidity compared to open resection for deep-seated lesions, such as glioblastomas, achieving ablation volumes with survival extensions in select cases.[49] These innovations collectively reduced operative risks, shortened recovery times, and broadened eligibility for complex interventions, driven by interdisciplinary integration of engineering and neuroimaging.[46]Definition and Scope
Core Principles and Objectives
Neurosurgery encompasses the surgical management of disorders affecting the central, peripheral, and autonomic nervous systems, including their supporting structures and vascular supply, with the primary objectives of preventing neurological deterioration, restoring or preserving function, and rehabilitating patients to optimize quality of life. These goals are pursued through precise interventions that address congenital anomalies, trauma, tumors, vascular malformations, infections, and degenerative conditions, prioritizing outcomes that mitigate morbidity while achieving therapeutic efficacy.[50] Fundamental to this practice is the preservation of eloquent brain areas and neural pathways, guided by intraoperative monitoring and advanced imaging to minimize deficits, as evidenced by reduced complication rates in procedures incorporating neuromonitoring, where functional preservation rates exceed 90% in select tumor resections.[51] Neurosurgeons often pursue elective subspecialties focusing on specific domains, including pediatric neurosurgery; complex spine and deformity surgery; cerebrovascular and endovascular neurosurgery; skull base surgery; neuro-oncology for brain and spine tumors; functional neurosurgery for epilepsy and movement disorders such as Parkinson's disease; neurocritical care; peripheral nerve surgery; and neurotrauma.[52] A core principle is adherence to the Monro-Kellie doctrine, which describes the cranium as a fixed-volume compartment containing brain tissue, blood, and cerebrospinal fluid; any volume expansion in one component necessitates compensatory reduction in others to avoid elevated intracranial pressure, informing urgent interventions like decompressive craniectomy in traumatic brain injury, where timely application has been associated with 20-30% improved survival in severe cases.[53] Surgical decision-making emphasizes rigorous preoperative evaluation, including neuroimaging and electrophysiological studies, to select patients where benefits outweigh risks, such as in elective tumor resections where gross total removal correlates with prolonged progression-free survival in low-grade gliomas (median 7-10 years versus 3-5 years for subtotal).[54] Intraoperative techniques prioritize hemostasis, minimal tissue disruption, and anatomical fidelity, often employing microsurgical dissection to navigate critical structures, reducing ischemia-related deficits.[55] Objectives extend beyond acute correction to long-term rehabilitation, integrating multidisciplinary care to manage sequelae like motor impairments or cognitive deficits post-resection, with evidence from cohort studies showing that combined surgical and rehabilitative approaches yield functional independence in 70-80% of surviving aneurysm patients at one year.[56] Ethical imperatives underscore informed consent and proportionality, ensuring interventions align with patient-specific prognoses derived from evidence-based risk stratification, such as the Spetzler-Martin grading for arteriovenous malformations, which predicts morbidity risks from 2% in low-grade lesions to over 30% in high-grade ones.[57] Overall, neurosurgical practice balances curative intent with harm avoidance, grounded in empirical outcomes from randomized trials and registries demonstrating superior survival and neurological preservation compared to non-surgical alternatives in operable pathologies.[58]Distinctions from Neurology and Other Surgical Fields
Neurosurgery differs fundamentally from neurology in its emphasis on surgical intervention for disorders of the central, peripheral, and autonomic nervous systems, whereas neurology is a non-surgical medical specialty dedicated to the diagnosis and non-operative management of these conditions through medications, lifestyle modifications, and therapies. Neurologists, trained via internal medicine pathways, focus on electrophysiological testing, pharmacological treatments, and conservative care for ailments such as epilepsy, multiple sclerosis, and migraines, referring patients requiring operative procedures to neurosurgeons. In contrast, neurosurgeons undergo extensive surgical residency—typically 7 years post-medical school—encompassing both diagnostic acumen akin to neurology and advanced operative skills for pathologies like tumors, vascular malformations, and trauma affecting the brain, spinal cord, and nerves. This dual competency allows neurosurgeons to manage perioperative care comprehensively, including preoperative optimization and postoperative rehabilitation, distinguishing their role from neurologists who collaborate but do not perform surgery.[59][60] Relative to other surgical disciplines, neurosurgery is uniquely delimited to the nervous system, precluding routine involvement in non-neural structures that dominate fields like general surgery, which addresses gastrointestinal, endocrine, and vascular issues outside the cranium or spine. General surgeons may handle peripheral trauma or basic wound care but lack the specialized training for intracranial or intraspinal procedures, such as aneurysm clipping or tumor resection, which demand precise neural preservation to avert deficits in cognition, motor function, or sensation. Neurosurgery's scope thus excludes broad abdominal or thoracic interventions, focusing instead on conditions where neural integrity is paramount, often integrating neurology-derived principles like neurophysiology to guide operative risks.[3][61] A notable overlap exists with orthopedic surgery in spinal procedures, yet distinctions arise from foundational training and procedural emphasis: orthopedic surgeons, rooted in musculoskeletal expertise, prioritize bony decompression, fusion, and instrumentation for degenerative disc disease or fractures, whereas neurosurgeons emphasize neural decompression and preservation of spinal cord or cauda equina integrity in cases involving compression or instability. Neurosurgical residency includes mandatory rotations in neurology and neuropathology, yielding greater exposure to intracranial pathologies and adult spinal case volumes—averaging over 300 spinal surgeries by graduation—compared to orthopedic counterparts, who may subspecialize via fellowships but derive from a non-neural base. This leads to divergent approaches in complex cases, such as intradural tumors or syringomyelia, where neurosurgeons' neural focus predominates, though both achieve comparable outcomes in routine lumbar fusions when fellowship-trained. Evidence from residency data indicates neurosurgeons log higher procedural hours in neural-specific spine work, underscoring the specialty's causal priority on mitigating neurological deficits over skeletal alignment alone.[62][63][64]Education and Training
Prerequisites and Core Curriculum
Aspiring neurosurgeons must first complete a bachelor's degree, typically in a science-related field, followed by four years of medical school to earn an MD or DO degree from an accredited institution.[65] [66] Admission to medical school requires competitive scores on the Medical College Admission Test (MCAT), which assesses knowledge in biological and physical sciences, critical analysis, and reasoning skills.[66] Successful completion of medical school culminates in passing the United States Medical Licensing Examination (USMLE) Steps 1 and 2, which evaluate foundational medical knowledge and clinical skills, respectively; these scores, along with clinical grades, research experience, and letters of recommendation, form the basis for competitive applications to neurosurgery residency programs via the National Resident Matching Program (NRMP).[67] [66] Entry into an Accreditation Council for Graduate Medical Education (ACGME)-accredited neurosurgery residency demands graduation from a Liaison Committee on Medical Education (LCME)- or Commission on Osteopathic College Accreditation (COCA)-approved medical school, with applicants ranked based on academic performance, surgical aptitude demonstrated during sub-internships, and peer-reviewed publications, as neurosurgery residencies accept fewer than 250 candidates annually in the United States despite high applicant volumes.[68] [69] Residents must also pass the American Board of Neurological Surgery (ABNS) Neuroanatomy Examination early in training and the Primary Examination prior to chief residency for certification eligibility.[70] The core curriculum of neurosurgery residency spans seven years (84 months), comprising 54 months of dedicated clinical neurosurgery training, 12 months as chief resident in postgraduate years (PGY) 6 or 7, and 30 months of electives in areas such as neuropathology, neuroradiology, research, or additional subspecialties.[69] [70] This structure emphasizes progressive responsibility, starting with foundational rotations in neurosurgical intensive care, ward management, and basic operative techniques, advancing to complex cranial, spinal, and pediatric cases under supervision.[71] Programs must ensure residents log minimum defined cases, including at least 205 adult cranial procedures, 95 adult spinal, 30 pediatric, and 60 critical care cases, totaling over 400 major operations, with primary sites supporting at least 500 major procedures annually per resident.[72] [73] Educational components integrate didactic sessions, grand rounds, morbidity and mortality conferences, and simulation-based training in microsurgery and endovascular techniques, aligned with ACGME core competencies in patient care, medical knowledge, practice-based learning, and systems improvement.[73] [74] Research is mandated, often yielding peer-reviewed outputs, to foster evidence-based practice and innovation, with programs like those accredited by the Society of Neurological Surgeons (SNS) emphasizing preparation for ABNS oral examinations through structured curricula in neuro-oncology, vascular neurosurgery, and functional disorders.[69] [70] Post-residency fellowships, though optional, are common for subspecialization, extending training by 1-2 years in fields like spine or endovascular surgery.[65]Variations by Region
In North America, neurosurgical training typically follows a standardized 7-year residency program after completion of medical school, encompassing 84 months of postgraduate education as mandated by the American Board of Neurological Surgery (ABNS) since 2014. This structure includes dedicated rotations in neurosurgery, neurology, neuropathology, neuroradiology, and critical care, with progressive operative responsibility and requirements for research productivity, often culminating in board certification examinations. In Canada, programs align closely with U.S. models, typically lasting 6-7 years under the Royal College of Physicians and Surgeons, emphasizing similar competencies but with variations in provincial funding and case volumes.[75] European neurosurgical training durations generally range from 4 to 6 years post-medical school, though national variations persist despite guidelines from the European Association of Neurosurgical Societies (EANS), with programs in countries like Germany or France often extending to 6 years including subspecialty tracks, while others such as the UK may integrate a 6-year specialty training phase after foundational years. Content differs significantly across borders; for instance, some nations require mandatory military or general surgery service, and certification relies on country-specific boards rather than a unified EU standard, leading to inconsistencies in operative exposure and assessment criteria. A survey of European programs reported a mean residency length of 5.7 years with a standard deviation of 1.12, highlighting decentralized structures that prioritize national accreditation over harmonization.[76][76][77] In Asia, training models diverge markedly; Japan's programs, often 6 years after a 6-year medical curriculum, emphasize intracranial procedures with neurosurgeons performing fewer spinal surgeries compared to Western counterparts, reflecting a cultural and systemic focus on brain pathology amid high workforce density exceeding 1000 residents nationwide. India's 3-year MS or 6-year MCh pathways post-MBBS incorporate broad surgical exposure but face critiques for insufficient duration to match global case complexities, prompting proposals for extension to 6 years with integrated general surgery rotations. Southeast Asian nations like Cambodia historically required 3 years of general surgery followed by overseas fellowships until recent local reforms, while higher-density regions such as South Korea mirror Japan's intensity with rigorous national exams.[78][79][80] Latin America and Africa exhibit greater heterogeneity, with programs in Brazil or Argentina spanning 4-5 years under national societies aligned loosely with WFNS standards, often constrained by resource limitations and reliance on international fellowships for advanced techniques. In contrast to high-income regions, many low-resource settings prioritize shorter apprenticeships or 3-year residencies, as seen in parts of Uzbekistan, to address urgent workforce shortages despite reduced formal standardization.[81][82]Global Disparities and Reforms
Global disparities in neurosurgical education and training are pronounced, particularly between high-income countries (HICs) and low- and middle-income countries (LMICs), where limited residency programs, inadequate infrastructure, and financial barriers restrict access to specialized training. In LMICs, few opportunities exist for neurosurgical residency due to shortages of faculty, simulation facilities, and funding, exacerbating workforce shortages that hinder local capacity building.[83][84] For instance, in 26.2% of countries surveyed, trainees must pay for neurosurgery training, with costs often exceeding annual national incomes in LMICs, deterring candidates from pursuing the specialty.[85] These gaps contribute to uneven adoption of advanced training methods like simulation, which remains more prevalent in HICs despite its potential to bridge skill deficits in resource-limited settings.[86] Workforce density serves as a proxy for training output disparities, with a global average of 0.93 neurosurgeons per 100,000 population as of 2024, but stark regional variations persist. The WHO African and Southeast Asia regions exhibit the lowest densities, often below 0.5 per 100,000, while the Western Pacific region leads with higher figures driven by established programs in countries like Japan and South Korea.[87][88] Trainee densities mirror this, with WHO European (0.36 per 100,000) and Americas (0.27 per 100,000) regions far outpacing Southeast Asia (0.04 per 100,000), reflecting fewer training slots and higher attrition in under-resourced areas.[89] Urban-rural divides within countries further compound issues, as training concentrates in metropolitan centers, leaving peripheral regions underserved.[90] Reforms aim to address these imbalances through sustainable, scalable interventions tailored to LMIC contexts, emphasizing virtual platforms, international partnerships, and policy advocacy. Organizations like the World Federation of Neurosurgical Societies (WFNS) promote global mapping and minimum density targets of 1 neurosurgeon per 200,000 population to guide resource allocation and training expansion.[91][92] Initiatives include cadaver-free courses using virtual and hands-on simulations to reduce costs and logistical barriers, as demonstrated in non-profit programs targeting LMIC trainees.[93] Early curricular reforms, such as structured lecture exposure and global health electives in HIC programs, foster bidirectional knowledge exchange, while advocacy pushes for subsidized training and infrastructure investment to mitigate brain drain.[94][95] These efforts prioritize empirical workforce data over anecdotal reforms, though challenges like funding sustainability and accreditation harmonization remain.[96]Surgical Techniques
Fundamental Approaches and Methods
Craniotomy serves as the primary open approach for accessing intracranial structures, involving the temporary removal of a portion of the skull known as a bone flap to expose the brain for procedures such as tumor resection or hematoma evacuation. The technique begins with scalp incision and subperiosteal dissection, followed by placement of burr holes using a drill and connection of these holes with a craniotome to elevate the flap, which is secured with plates or wires for later replacement.[97] This method, performed under general anesthesia with neuronavigation in modern practice, prioritizes preservation of dural integrity and venous sinuses to mitigate risks like epidural hematoma or infection, with complication rates reported at 5-10% in large series.[97] For spinal disorders, laminectomy provides decompression of the spinal cord or nerve roots by excising the lamina—the posterior bony arch of the vertebra—thereby enlarging the spinal canal. The procedure entails a midline incision, ligamentous dissection, and partial or total lamina removal using rongeurs or high-speed drills, often supplemented by foraminotomy for lateral recess stenosis.[98] Introduced in the early 20th century and refined through biomechanical studies, laminectomy addresses conditions like spondylotic myelopathy, with success in symptom relief exceeding 70% in degenerative cases, though it carries risks of instability necessitating fusion in up to 20% of multilevel applications.[98] Stereotactic surgery applies three-dimensional coordinate systems derived from preoperative imaging, such as CT or MRI, to guide precise instrumentation to deep-seated targets, facilitating biopsies, ablations, or electrode placements with minimal tissue disruption. Frame-based systems fixate a stereotactic frame to the skull for coordinate mapping, while frameless optical or electromagnetic navigation has become standard since the 1990s, reducing error to submillimeter accuracy.[99] Core principles emphasize target verification via intraoperative imaging and trajectory planning to avoid eloquent areas, underpinning functional neurosurgery for conditions like Parkinson's disease, where deep brain stimulation outcomes show sustained motor improvement in 50-70% of patients at five years.[100][99] Endoscopic techniques enable minimally invasive access to ventricular, skull base, or spinal pathologies via small incisions or natural orifices, using rigid or flexible endoscopes with integrated cameras and working channels for visualization and intervention. Approaches include transnasal for pituitary tumors or intraventricular for hydrocephalus, where fenestration of the septum pellucidum or third ventriculostomy diverts cerebrospinal fluid with success rates of 70-90% in obstructive cases.[101] These methods reduce operative time, blood loss, and hospital stay compared to open surgery—e.g., endoscopic third ventriculostomy averages 30-60 minutes versus hours for shunting—while leveraging irrigation for hemostasis and angled optics for blind-spot access, though limited by trajectory constraints and steeper learning curves.[102] Across these approaches, patient positioning—supine, prone, or lateral—optimizes surgical corridors and venous drainage while minimizing retraction pressure on neural tissues, a principle informed by cadaveric and imaging studies to lower ischemia risks.[103] Microsurgical enhancement via operative microscopes, standard since the 1970s, amplifies visualization to 10-40x magnification for vascular and neural preservation, integral to all open and stereotactic methods.[103] Intraoperative electrophysiological monitoring, including somatosensory evoked potentials, further refines these techniques by detecting real-time functional compromise, with evidence from randomized trials showing reduced deficits in monitored spinal cases.[103]Instrumentation and Intraoperative Tools
Neurosurgical instrumentation encompasses specialized hand-held tools engineered for precision in manipulating delicate neural tissues, including microforceps, needle holders, and dissectors with tip diameters as fine as 0.1 mm to avoid inadvertent damage.[104] Power-driven devices such as pneumatic or electric drills equipped with perforators and craniotomes facilitate cranial bone removal, with cutting speeds exceeding 80,000 rpm to ensure clean osteotomies while minimizing thermal injury to adjacent dura.[105] These instruments prioritize minimal tissue disruption, as neural structures tolerate even minor trauma poorly, often leading to deficits if vascular or axonal integrity is compromised.[106] The operating microscope provides stereoscopic magnification ranging from 4x to 40x, coaxial illumination, and depth perception essential for microvascular anastomosis and tumor resection, having been adopted in neurosurgery since 1957 at the University of Southern California.[107] Modern iterations incorporate xenon or LED light sources for enhanced color fidelity and red-free filters to delineate vascular patterns.[108] Endoscopes, typically rigid with 0° to 30° angled lenses and diameters of 1.8-6 mm, enable minimally invasive access to ventricular or skull base regions, reducing morbidity compared to open approaches by limiting brain retraction.[102] Tissue resection tools like the ultrasonic aspirator employ cavitation and vibration at 20-36 kHz to fragment soft tumors while aspirating debris, selectively sparing elastic vessels due to differential mechanical properties.[104] The Cavitron Ultrasonic Surgical Aspirator (CUSA), refined since its 1978 introduction, achieves resection rates of up to 10 cm³ per minute in gliomas, with tip irrigation preventing clogging and overheating.[109] Brain retractors, including fixed-frame systems like Leyla bars or malleable blades integrated with force sensors, apply pressures below 25 mmHg to mitigate retraction-induced ischemia, as higher forces correlate with cortical injury in animal models.[106] Intraoperative neurophysiological monitoring utilizes needle or surface electrodes to record somatosensory evoked potentials (SSEPs), motor evoked potentials (MEPs), and electromyography (EMG), alerting surgeons to signal amplitude drops exceeding 50% that indicate potential neural compromise.[110] Triggered EMG thresholds below 10 mA during pedicle testing confirm safe screw placement in spine surgery.[111] Robotic assistants, such as the ROSA system, provide sub-millimeter accuracy in stereotactic biopsy or deep brain stimulation lead placement via haptic feedback and tremor filtration, building on early PUMA 560 applications from the 1980s.[112] These tools collectively enhance surgical safety and efficacy, with adoption rates surpassing 90% in high-volume centers for complex cranial procedures by 2020.[105]
Integration of Imaging and Navigation
Neuronavigation systems integrate preoperative imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) with intraoperative tracking to provide real-time spatial orientation during neurosurgical procedures.[113] These systems enable precise localization of intracranial targets, compensating for anatomical distortions like brain shift caused by tumor resection or cerebrospinal fluid drainage.[114] Accuracy typically ranges from 1 to 2 mm, though application errors can reach 1.5 to 5.4 mm depending on registration methods and imaging fusion.[115][116] Frame-based stereotaxy, originating with the Horsley-Clarke apparatus in 1908, laid the foundation by using rigid head frames for three-dimensional targeting based on anatomical atlases and ventriculography.[36] Frameless neuronavigation emerged in 1986 with the development of optical tracking systems, eliminating the need for invasive frames and allowing flexible instrument guidance via infrared cameras or electromagnetic fields.[117] Modern systems often fuse multiple imaging datasets, incorporating diffusion tensor imaging for white matter tractography or functional MRI for eloquent area mapping, to enhance trajectory planning and avoid critical structures.[118] Intraoperative imaging updates navigation to address dynamic changes; intraoperative MRI (iMRI), introduced clinically in the 1990s, allows rescanning during surgery to verify resection extent and adjust for shifts up to several millimeters.[119] Studies demonstrate iMRI increases gross total resection rates in gliomas from approximately 20-30% with conventional methods to 50-70%, correlating with prolonged progression-free survival without elevating neurological deficits.[120][121] Intraoperative ultrasound (iUS) and CT provide complementary real-time fusion, with iUS offering cost-effective deformation modeling for soft-tissue navigation.[122][123] Clinical applications span tumor resections, deep brain stimulation electrode placements, and vascular procedures, where navigation reduces operative time and morbidity compared to freehand techniques.[124] For supratentorial gliomas, neuronavigation-assisted surgery achieves higher rates of complete resection and fewer deficits than non-navigated approaches.[125] Limitations include registration inaccuracies from patient movement or edema, and dependency on high-quality preoperative scans, underscoring the need for hybrid systems integrating robotics for sub-millimeter precision in select cases.[113][126]Conditions Treated
Intracranial Pathologies
Neurosurgery addresses a variety of intracranial pathologies that involve structural abnormalities, masses, or disruptions within the brain parenchyma, meninges, or ventricular system, often requiring surgical access via craniotomy, stereotactic techniques, or shunts to alleviate mass effect, restore cerebrospinal fluid dynamics, or prevent hemorrhage. These conditions include primary and metastatic brain tumors, vascular anomalies such as aneurysms and arteriovenous malformations (AVMs), hemorrhagic lesions, hydrocephalus, infectious processes like abscesses, and sequelae of traumatic brain injury (TBI). Surgical intervention is indicated when conservative management fails to control symptoms like elevated intracranial pressure (ICP), neurological deficits, or risk of rupture, with outcomes influenced by lesion location, size, and patient comorbidities.[127][128] Brain tumors constitute a primary neurosurgical domain, with gliomas, meningiomas, pituitary adenomas, and metastases comprising the most frequent targets for resection. Craniotomy enables maximal safe tumor removal, which correlates with prolonged progression-free survival in high-grade gliomas, as gross total resection reduces recurrence risk compared to biopsy alone. For metastatic lesions, surgical excision is prioritized in patients with good performance status and limited systemic disease, often followed by adjuvant radiotherapy or chemotherapy. Meningiomas, typically benign, are resected for symptomatic compression, with Simpson grade 1 removal (including dura) achieving over 90% long-term control rates.[129][130] Vascular pathologies, including saccular aneurysms and AVMs, demand prompt neurosurgical management to mitigate rupture risks, which for unruptured aneurysms exceed 1% annually depending on size and location. Microsurgical clipping secures the aneurysm neck, preserving parent vessel patency, and remains preferred for complex wide-necked lesions unsuitable for endovascular coiling. AVMs, prone to hemorrhage with annual rates of 2-4%, undergo staged treatment involving preoperative embolization to reduce nidus size, followed by microsurgical resection for definitive cure, particularly in Spetzler-Martin grade I-III lesions where obliteration rates approach 95%.[131][132][133] Hemorrhagic conditions, such as intracerebral hemorrhage (ICH) or subdural/epidural hematomas from trauma or rupture, prompt evacuation when volumes exceed thresholds like 30 cm³ for epidural hematomas or when accompanied by midline shift greater than 5 mm, as these correlate with poor outcomes without intervention. Decompressive craniectomy serves as a salvage procedure for refractory ICP in severe TBI, reducing mortality by allowing brain expansion, though it carries risks of hydrocephalus and syndrome of the trephined.[134][97] Hydrocephalus, arising from obstruction or overproduction of cerebrospinal fluid, is surgically managed via ventriculoperitoneal shunt implantation, which diverts fluid to the peritoneal cavity and resolves symptoms in over 80% of cases initially, despite complication rates of 10-20% including infection and malfunction requiring revision. Endoscopic third ventriculostomy offers a shunt-independent alternative for obstructive etiologies like aqueductal stenosis.[135][136] Intracranial infections, notably brain abscesses, necessitate drainage for lesions exceeding 2 cm in diameter or those multiloculated, via stereotactic aspiration yielding diagnostic yield and symptom relief in 70-90% of supratentorial cases, supplemented by antibiotics; craniotomy with excision is reserved for refractory or posterior fossa abscesses to prevent rupture or brainstem compression.[137][138]Spinal and Peripheral Disorders
Neurosurgeons manage spinal disorders involving the vertebral column, intervertebral discs, spinal cord, and nerve roots, often intervening surgically when conservative measures fail. Common degenerative conditions include lumbar disc herniation, which carries a lifetime risk of approximately 30% and symptomatic incidence of 1-3%, typically presenting with radiculopathy due to nerve root compression.[139] Spinal stenosis, characterized by narrowing of the spinal canal and resultant neural compression, affects older adults and may necessitate decompression procedures to alleviate pain, weakness, or numbness.[140] These pathologies contribute to 5-15% of low back pain cases requiring evaluation.[141] Traumatic spinal injuries, such as fractures or dislocations from high-impact events, demand urgent stabilization to prevent cord damage; neurosurgeons perform instrumentation and fusion to restore alignment and protect neurological function.[142] Neoplastic conditions, including spinal tumors like meningiomas or metastases, are addressed through resection or decompression, with minimally invasive techniques reducing morbidity in select cases.[143] Infections such as discitis or epidural abscesses require debridement and antibiotics, guided by intraoperative imaging to minimize recurrence.[144] Vascular anomalies, including arteriovenous malformations, pose risks of hemorrhage and are treated via embolization or microsurgery to preserve spinal cord perfusion.[145] Peripheral nerve disorders treated by neurosurgeons encompass entrapments, injuries, and tumors affecting nerves beyond the spinal cord. Carpal tunnel syndrome, involving median nerve compression at the wrist, is alleviated by release surgery in refractory cases, restoring sensory and motor function.[146] Brachial plexus injuries from trauma often require nerve transfers or grafts to reconstruct pathways, with outcomes depending on injury chronicity and patient factors.[147] Nerve sheath tumors, such as schwannomas, are excised microsurgically to prevent progression while sparing healthy tissue.[148] These interventions prioritize nerve decompression and repair, contrasting with non-surgical management for milder neuropathies.[149]Vascular and Functional Conditions
Vascular conditions treated by neurosurgeons include cerebral aneurysms, arteriovenous malformations (AVMs), and cavernous malformations, which pose risks of subarachnoid hemorrhage or neurological deficits due to rupture or mass effect.[150][151][152] Cerebral aneurysms, saccular dilations of arterial walls, are managed via microsurgical clipping to isolate the aneurysm sac or endovascular coiling to promote thrombosis within it, with both approaches aiming to prevent rupture; coiling has become a front-line therapy, particularly for certain anatomies, showing comparable outcomes to clipping in select randomized trials.[150][153] AVMs, tangled vascular nidus prone to bleeding, are addressed through microsurgical resection, endovascular embolization, or stereotactic radiosurgery, often in multimodal fashion to reduce hemorrhage risk, with complete obliteration conferring definitive cure.[151] Cavernous malformations, low-flow lesions with popcorn-like appearance on imaging, are excised microsurgically when symptomatic from hemorrhage or seizures, guided by eloquence of surrounding brain tissue to minimize deficits.[152] Stroke-related vascular interventions fall under neurosurgical purview, including carotid endarterectomy for symptomatic atherosclerotic stenosis, which removes plaque to restore lumen patency and prevent ischemic events; this procedure, performed via cervical incision, demonstrates reduced stroke risk in patients with 70-99% stenosis per North American Symptomatic Carotid Endarterectomy Trial data.[154] For acute hemorrhagic stroke, evacuation of intracerebral hematomas may be undertaken in select cases with large volumes or deterioration, though evidence from randomized trials like STICH indicates limited overall benefit outside specific cerebellar or superficial supratentorial locations.[154] Functional neurosurgery targets aberrant neural circuitry in disorders such as Parkinson's disease (PD), essential tremor, dystonia, and refractory epilepsy, employing techniques like deep brain stimulation (DBS) or lesioning to modulate activity. In PD, DBS of the subthalamic nucleus or globus pallidus interna yields 50-60% improvement in motor Unified Parkinson's Disease Rating Scale scores off medications, with sustained benefits up to 15 years in reducing dyskinesias and medication requirements, though it does not halt underlying neurodegeneration.[155][156][157] Efficacy in essential tremor and dystonia similarly involves tremor suppression and functional gains, with hardware-related complications like infection occurring in 5-10% of cases.[158] Epilepsy surgery, a cornerstone of functional intervention, includes temporal lobectomy for mesial temporal sclerosis, achieving Engel Class I seizure freedom in 60-70% of patients at long-term follow-up, with higher rates in lesional cases identifiable on MRI; anterior temporal resection outcomes remain favorable even beyond 10 years, underscoring the procedure's durability for drug-resistant focal epilepsy.[159][160][161] Emerging indications extend DBS to obsessive-compulsive disorder, cluster headache, and depression, though evidence levels vary, with psychiatric applications requiring rigorous patient selection to balance risks like hardware failure against potential symptom relief.[158] Overall, functional procedures prioritize precise targeting via stereotaxy, with outcomes influenced by disease etiology, preoperative testing concordance, and multidisciplinary evaluation.[162]Common Procedures
Tumor Resections and Decompressions
Tumor resections in neurosurgery involve the surgical removal of intracranial or spinal neoplasms to achieve maximal cytoreduction while preserving neurological function, often combined with decompressions to alleviate mass effect on surrounding structures such as the brain parenchyma or spinal cord.[163] The primary objective is gross total resection (GTR), defined as complete removal of contrast-enhancing tumor volume, which correlates with improved progression-free and overall survival compared to subtotal resection (STR) or biopsy alone, particularly in gliomas.[164] For high-grade gliomas like glioblastoma, GTR can extend median overall survival to 20-25 months versus 12-15 months with STR, though outcomes vary by tumor grade, location, and molecular markers such as IDH mutation status.[165] Decompressions, such as suboccipital craniectomy for posterior fossa tumors or laminectomy for spinal lesions, prioritize rapid relief of intracranial hypertension or myelopathy, reducing symptoms like headache, ataxia, or paraparesis.[166] Intracranial procedures typically employ microsurgical techniques via craniotomy, with en bloc resection preferred for metastases to minimize seeding and recurrence risk, achieving local control rates exceeding 80% in up to three lesions.[167] Intraoperative adjuncts enhance precision: 5-aminolevulinic acid (5-ALA) fluorescence guides resection of non-enhancing margins, while neuronavigation and intraoperative MRI (iMRI) increase GTR rates from 20-30% in conventional surgery to 50-70% in gliomas by detecting residual tumor in real-time.[168] [120] iMRI specifically outperforms standard methods in complete resection of enhancing glioma components, extending progression-free survival without elevating neurological deficits.[169] For low-grade gliomas in pediatric patients, GTR rates average 74-78%, independently associating with superior survival over STR.[170] Benign tumors, such as meningiomas, yield 90-95% success in full removal, with low morbidity in non-eloquent regions.[171] Spinal tumor resections target intradural or extradural lesions, with microsurgical excision effective for ependymomas and hemangioblastomas, often achieving total removal and neurological stabilization.[172] Decompressive laminectomy combined with tumor debulking in metastatic cases significantly reduces pain and restores motor function in acute cord compression, though stabilization via instrumentation is required to prevent instability.[173] For benign intradural tumors, resection rapidly improves quality-of-life metrics, including pain interference scores, with minimal recurrence.[174] High-volume centers report lower 1-year mortality post-craniotomy or spondylectomy, emphasizing surgeon experience in complex cases.[175] Risks include new deficits (5-15% transient cranial neuropathies or hemiparesis), infection (1-3%), and cerebrospinal fluid leaks, mitigated by awake mapping in eloquent areas and postoperative protocols.[176] Overall 30-day morbidity approximates 12% for brain tumor surgery, with mortality under 2%, favoring GTR's survival benefits despite added operative time.[177] Adjuvant therapies follow, but resection extent remains a causal determinant of prognosis, independent of confounding biases in observational data.[178]Spine and Disc Interventions
Spine and disc interventions in neurosurgery primarily target degenerative conditions such as herniated discs, spinal stenosis, and instability, which compress neural elements and cause radiculopathy or myelopathy. These procedures aim to decompress neural structures, stabilize the spine, or restore disc function while minimizing tissue disruption through minimally invasive techniques where feasible. Common approaches include discectomy for focal disc herniations and laminectomy for central canal narrowing, often combined with fusion for cases involving spondylolisthesis or recurrent instability.[179][180] Microdiscectomy, a standard procedure for lumbar disc herniation, involves a small incision and microscopic visualization to excise the extruded disc fragment impinging on the nerve root, preserving the majority of the disc and annulus. Performed under general anesthesia, the surgeon retracts paraspinal muscles, removes a portion of the lamina if needed, and uses a nerve root retractor to access and extract the herniated material, typically resolving sciatica in 80-90% of patients within weeks. This technique reduces blood loss and postoperative pain compared to open discectomy, with hospital stays often limited to one day.[181][179][181] Laminectomy addresses lumbar spinal stenosis by excising the lamina and hypertrophic ligaments to enlarge the spinal canal and alleviate nerve compression from facet joint overgrowth or ligamentum flavum thickening. In decompressive laminectomy, the procedure decompresses multiple levels if multilevel stenosis exists, with bilateral facetectomies preserving at least 50% of facets to avoid instability; fusion is added if preoperative instability exceeds 3 mm of translation on flexion-extension radiographs. Long-term studies indicate sustained pain relief and improved function in 60-70% of patients at five years, though reoperation rates for adjacent segment disease reach 10-15%.[182][180][183] Spinal fusion techniques stabilize the spine by promoting bony union between vertebrae, indicated for disc collapse with instability or post-decompression deformity. Posterior lumbar interbody fusion (PLIF) or transforaminal lumbar interbody fusion (TLIF) involve disc removal, graft placement (autograft or allograft), and pedicle screw instrumentation to immobilize segments, achieving fusion rates of 85-95% with modern biologics like bone morphogenetic protein. Minimally invasive variants use tubular retractors to limit muscle stripping, reducing operative time to 2-3 hours per level and lowering infection risk to under 2%.[184][185][186] Artificial disc replacement offers a motion-preserving alternative to fusion for isolated degenerative disc disease at one or two levels, replacing the nucleus and annulus with a metal-polyethylene or metal-on-metal prosthesis via anterior or lateral access. FDA-approved devices like the Charité or ProDisc-L demonstrate equivalent pain relief to fusion at two years but with faster return to work (average 26 days versus 71 days), though facet joint degeneration may necessitate later fusions in 5-10% of cases. Suitability requires intact facet joints and no significant osteoporosis, as assessed by preoperative DEXA scans.[187][188][189]Vascular and Endovascular Repairs
Vascular repairs in neurosurgery target cerebrovascular pathologies, including intracranial aneurysms, arteriovenous malformations (AVMs), and stenotic lesions, to mitigate risks of hemorrhage, ischemia, or infarction. These interventions encompass both open microsurgical techniques, which involve direct vessel exposure via craniotomy, and endovascular approaches, utilizing catheter-based access through femoral arteries to deploy devices within the vasculature. Selection between modalities depends on aneurysm morphology, patient comorbidities, and rupture status, with endovascular methods often favored for their minimally invasive nature and reduced perioperative morbidity in suitable candidates.[190][191] For ruptured intracranial aneurysms, the International Subarachnoid Aneurysm Trial (ISAT), involving 2143 patients randomized between 1994 and 2002, demonstrated that endovascular coiling reduced the absolute risk of death or dependency at one year by 7% (23.7% vs. 30.6%; relative risk reduction 24%) compared to surgical clipping, particularly in anterior circulation aneurysms amenable to both techniques.[192][193] Long-term follow-up from ISAT confirmed sustained benefits in functional outcomes, though coiling carries a higher rebleeding risk from recanalization (2.6% vs. 1.0% for clipping).[194] Surgical clipping, employing temporary vessel occlusion and aneurysm neck isolation with titanium clips, remains preferred for wide-necked or posterior circulation aneurysms where coil stability is compromised.[190] Endovascular adjuncts like balloon remodeling or stent-assisted coiling enhance occlusion rates, achieving complete aneurysm filling in over 80% of remnant cases post-clipping.[195] Arteriovenous malformations, characterized by tangled vessel nests causing high-flow shunts, often require multimodal therapy including endovascular embolization to devascularize feeding arteries with liquid agents like n-butyl cyanoacrylate, reducing intraoperative blood loss by up to 50% in subsequent resections.[196] Embolization alone suffices for small, superficial AVMs but typically precedes microsurgery or stereotactic radiosurgery for larger lesions, with complete obliteration rates of 70-90% in combined approaches.[197] In acute ischemic stroke from large-vessel occlusion, endovascular thrombectomy involves mechanical clot retrieval using stentrievers or aspiration catheters, extending treatment windows to 24 hours in select patients with salvageable tissue.[198] Trials such as TENSION (2023) affirmed efficacy, showing improved 90-day functional independence (modified Rankin Scale 0-3) versus medical management alone in large-core infarcts, with mortality reductions up to 15%.[199][200] Extracranial interventions, such as for carotid artery stenosis exceeding 70%, compare surgical endarterectomy—plaque excision via arteriotomy—with angioplasty and stenting. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), randomizing 2502 patients from 2000 to 2008, found equivalent long-term stroke prevention (7.2% vs. 6.8% at 10 years), though periprocedural stroke risk was higher with stenting (4.1% vs. 2.3%) and myocardial infarction with endarterectomy (2.3% vs. 1.1%).[201][202] Stenting predominates in high-surgical-risk anatomies, like hostile necks, while endarterectomy offers durability in younger, low-risk patients.[203] Flow diversion devices, such as pipeline stents, represent advanced endovascular options for unruptured aneurysms, promoting aneurysm thrombosis via endoluminal reconstruction while preserving parent vessel patency; complete occlusion occurs in 76% at one year with low morbidity (5%).[204] Overall, endovascular adoption has surged, comprising over 70% of aneurysm treatments in specialized centers by 2020, driven by technological refinements and trial evidence favoring reduced invasiveness without compromising efficacy in appropriately selected cases.[190]Ancillary Disciplines
Neuroanesthesia Practices
Neuroanesthesia practices center on safeguarding cerebral physiology during procedures that risk disrupting autoregulation, oxygenation, or intracranial dynamics, with empirical emphasis on preventing secondary insults like ischemia or edema. Core objectives involve constraining intracranial pressure (ICP) to under 22 mmHg in cases of elevated risk, such as traumatic brain injury, and sustaining cerebral perfusion pressure (CPP)—defined as mean arterial pressure minus ICP—at a minimum of 60 mmHg to ensure adequate cerebral blood flow without exceeding autoregulatory limits.[205][206] Intraoperative monitoring integrates invasive arterial blood pressure for beat-to-beat assessment, central venous pressure, and advanced neuromodality tools including somatosensory evoked potentials (SSEPs), motor evoked potentials (MEPs), raw electroencephalography (EEG), and electromyography (EMG) to identify intraoperative neural compromise promptly.[207][208] Multimodal brain monitoring, such as tissue oxygenation probes or microdialysis, may supplement in high-stakes scenarios like severe intracranial hypertension.[205] Anesthetic induction prioritizes agents that attenuate sympathetic responses to laryngoscopy, typically propofol (1.5-2.5 mg/kg) combined with opioids like fentanyl or remifentanil, while avoiding hypercapnia or volatile agents that provoke cerebral vasodilation and ICP elevation.[209] Maintenance anesthesia predominantly employs total intravenous techniques with propofol infusions (target 1-2 μg/mL via target-controlled infusion) and remifentanil (0.1-0.2 μg/kg/min), outperforming volatile anesthetics in providing brain relaxation, hemodynamic stability, and ICP suppression, as volatiles increase cerebral blood volume despite metabolic depression.[210][211] Cerebral protection strategies leverage dose-dependent metabolic suppression from anesthetics to widen the ischemia tolerance window, alongside maneuvers like mild hyperventilation (PaCO2 30-35 mmHg) for ICP reduction or hypertonic saline boluses (3% solution at 2-5 mL/kg over 10-20 minutes) for refractory edema.[205] In vascular neurosurgery, such as aneurysm clipping, temporary clip application induces burst suppression (via propofol boluses to EEG flattening) coupled with deliberate hypertension (MAP 20-30% above baseline) to bolster collateral perfusion during occlusion.[205] For resections near eloquent areas, awake craniotomy protocols follow an asleep-awake-asleep sequence, initiating with dexmedetomidine (0.5-1 μg/kg bolus over 10 minutes, then 0.2-0.7 μg/kg/h) and propofol for tolerance, transitioning to wakefulness for cortical mapping via bispectral index-guided titration and high-flow nasal oxygenation.[205] Adjunctive regional blocks, such as scalp infiltration or cervical plexus anesthesia, mitigate systemic opioid needs and enhance recovery profiles.[212] Postoperatively, rapid emergence facilitates neurological assessment, with vigilance for delayed complications like cerebral hyperperfusion syndrome.[205]Neuropathological Assessment
Neuropathological assessment in neurosurgery involves the microscopic examination of neural tissue specimens obtained during surgical procedures to provide rapid diagnostic information that informs intraoperative decision-making.[213] This process primarily occurs through intraoperative consultations, where pathologists evaluate biopsies to confirm the presence of lesional tissue, distinguish neoplasms from non-neoplastic conditions, and guide the extent of resection.[214] The primary goals include verifying adequate sampling and avoiding unnecessary removal of healthy tissue, particularly in eloquent brain areas.[215] Intraoperative techniques emphasize speed and feasibility under surgical constraints, with frozen sections and cytologic smears being the most common methods. Frozen sections involve rapid freezing, sectioning, and staining of tissue for hematoxylin and eosin evaluation, allowing assessment of cellular architecture and tumor margins within minutes.[216] Cytologic touch preparations or smears provide quicker alternatives by crushing tissue onto slides for immediate microscopic review, offering high diagnostic yield for distinguishing tumors from inflammation or necrosis, though they may underperform in low-grade gliomas.[217] Diagnostic concordance between intraoperative and permanent sections reaches 85-95% for common entities like high-grade gliomas, but discrepancies arise in heterogeneous tumors or sampling errors, necessitating postoperative confirmation with immunohistochemistry and molecular testing.[218] In stereotactic biopsies, neuropathological assessment enhances safety by confirming representative tissue acquisition, with frozen sections yielding non-diagnostic rates below 5% in frameless systems when integrated.[219] Pathologists also evaluate gross features like tumor consistency and vascularity to advise on risks such as hemorrhage.[220] Emerging adjuncts, including stimulated Raman histology for label-free imaging, aim to reduce artifacts from freezing and enable real-time molecular insights, particularly in pediatric cases.[221] Overall, this assessment bridges surgical pathology and neurosurgery, optimizing outcomes while accounting for limitations like tissue distortion in frozen artifacts.[222]Professional Organization
Governing Bodies and Societies
The World Federation of Neurosurgical Societies (WFNS), founded in 1955 in Switzerland, functions as the principal international coordinating entity for neurosurgery, uniting over 130 national and regional member societies to advance global standards, education, and research in the field.[223] It organizes world congresses every four years, supports specialized committees on topics such as spine surgery and neuro-oncology, and facilitates resource distribution to low-resource regions, thereby addressing disparities in neurosurgical access and training worldwide.[224] In the United States, the American Association of Neurological Surgeons (AANS), established in 1931 as the Harvey Cushing Society, promotes the art and science of neurosurgery through advocacy, guideline development, and continuing medical education programs for its members, who include practicing neurosurgeons, residents, and researchers.[6] The Congress of Neurological Surgeons (CNS), formed in 1951, complements this by prioritizing clinical practice advancement, evidence-based guidelines, and annual scientific meetings that disseminate procedural innovations and outcomes data.[225] Together, these organizations collaborate on policy matters, such as residency accreditation influences via joint Washington Committee efforts since 1975, while maintaining distinct emphases on research versus practical application.[226] The Society of Neurological Surgeons (SNS), the oldest such body dating to 1920, shapes foundational aspects of neurosurgical training and leadership selection, including oversight of residency program standards through its role in the Residency Review Committee.[227] Domestically, the Council of State Neurosurgical Societies (CSNS) addresses socioeconomic challenges, providing a platform for state-level advocacy on reimbursement, liability, and regulatory issues affecting clinical practice.[228] Regionally, the European Association of Neurosurgical Societies (EANS) integrates national European societies to enhance knowledge exchange, training fellowships, and representation in policy, with a focus on harmonizing subspecialty standards across diverse healthcare systems.[229] Globally, over 189 neurosurgical associations exist across 131 countries, reflecting the specialty's decentralized governance, though many nations lack dedicated societies, underscoring WFNS's role in bridging gaps.[224] These bodies collectively influence ethical guidelines, technological adoption, and professional certification pathways without direct regulatory authority, relying instead on voluntary adherence to promote evidence-based care.[230]Certification, Accreditation, and Practice Standards
In the United States, certification in neurosurgery is overseen by the American Board of Neurological Surgery (ABNS), which requires completion of an Accreditation Council for Graduate Medical Education (ACGME)-accredited residency program consisting of 84 months of postgraduate training, including at least 54 months of core neurosurgical experience, 12 months as chief resident in the final two years, and three months in basic neuroscience disciplines such as neurology or neuropathology.[70] Candidates must pass a primary written examination assessing foundational knowledge in neurosurgery, submit a case log demonstrating surgical competency, and undergo an oral examination evaluating clinical judgment, typically scheduled within four years of residency completion.[231] [232] Initial certification hinges on ABNS approval of training credentials, examination performance, and professional practice review, with ongoing maintenance of certification involving periodic reassessments to ensure sustained competence.[233] Accreditation of neurosurgical residency programs is managed by the ACGME's Review Committee for Neurological Surgery, which enforces program requirements emphasizing core competencies in patient care, medical knowledge, systems-based practice, and procedural proficiency for both operative and non-operative management of neurological disorders.[234] [68] These standards mandate structured curricula, faculty supervision, case volume minimums (e.g., defined categories for craniotomies and spinal procedures), and rotations to foster comprehensive training, with programs undergoing periodic site visits and data reviews for continued accreditation status.[235] The Society of Neurological Surgeons complements ACGME oversight by defining essential training milestones, ensuring alignment with evolving clinical demands.[227] Practice standards in neurosurgery are primarily established through evidence-based guidelines developed by professional organizations such as the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), which systematically review clinical literature to recommend protocols for conditions like tumors, vascular malformations, and spinal disorders.[236] These guidelines prioritize high-quality data abstraction and grading of evidence to guide decision-making, addressing variations in care while promoting patient outcomes, though adherence remains voluntary and influenced by institutional protocols.[237] Joint AANS/CNS sections on subspecialties, such as tumors or pediatrics, further refine standards through targeted position statements and consensus processes.[238] Internationally, certification processes diverge, with entities like the European Association of Neurosurgical Societies (EANS) and the Union Européenne des Médecins Spécialistes (UEMS) accrediting training centers based on competency frameworks, though lacking a unified global standard comparable to ABNS.[239] Variability in requirements—ranging from exam-based assessments to portfolio evaluations—reflects regional differences in training duration and oversight, underscoring the U.S. model's emphasis on rigorous, centralized validation.[240]Outcomes and Risks
Recovery Protocols
Recovery protocols in neurosurgery emphasize multimodal, evidence-based strategies to minimize complications, promote early functional restoration, and expedite discharge while mitigating risks such as cerebral edema, infection, and thromboembolism. Enhanced Recovery After Surgery (ERAS) protocols, adapted for cranial and spinal procedures, integrate preoperative optimization with postoperative elements like early mobilization and standardized monitoring, reducing hospital length of stay by 1-2 days in elective craniotomies without elevating readmission rates.[241] [242] For brain tumor resections, protocols typically involve 24-48 hours of intensive care unit (ICU) or post-anesthesia care unit (PACU) surveillance for low-risk elective cases, focusing on hourly neurological assessments to detect deficits from hematoma, ischemia, or swelling, with invasive monitoring like intracranial pressure devices reserved for high-risk patients.[243] [244] Pain management prioritizes non-opioid multimodal analgesia, including acetaminophen, NSAIDs, and gabapentinoids, to avoid respiratory depression and delirium, which occur in up to 20% of postoperative neurosurgical patients; scheduled anticonvulsants like levetiracetam are administered prophylactically for 7 days post-craniotomy in tumor cases to prevent seizures, supported by randomized trials showing reduced incidence from 25% to under 10%.[245] [246] Fluid and electrolyte balance is maintained with goal-directed therapy to prevent hyponatremia or hypervolemia exacerbating brain edema, while venous thromboembolism prophylaxis via intermittent pneumatic compression and low-molecular-weight heparin begins within 24 hours unless contraindicated by hemorrhage risk.[247] Rehabilitation commences intraoperatively or on postoperative day 0 (POD0) with bedside physical and occupational therapy, progressing to 1-2 hours daily of targeted exercises for mobility, strength, and cognition; in glioma resection patients, early mobilization protocols achieve ambulation within 24 hours in 80% of cases, correlating with shorter stays and preserved long-term outcomes without increased adverse events.[248] [249] For spinal interventions, protocols include log-rolling techniques to avoid strain, brace usage for 4-6 weeks post-fusion, and graded return to activity, with evidence from cohort studies indicating reduced pseudarthrosis rates through supervised therapy.[250] Discharge criteria mandate stable neurology, pain control on oral agents, and independent ambulation, typically by POD1-3 for uncomplicated cranial procedures under ERAS, followed by outpatient imaging and clinic visits at 2 weeks, 1 month, and 3 months to assess fusion or recurrence.[251] [252]Complication Rates and Management
Complication rates in neurosurgery vary by procedure type, patient factors, and institutional protocols, with overall perioperative adverse event rates reported between 8% and 18% in large cohorts. In a national database analysis of over 94,000 cases, the 30-day complication rate was 8.2%, with lower figures (5.6%) for elective procedures. A single-center study of 2,872 patients identified 18.5% adverse events, predominantly non-neurosurgical (8.1%), such as medical issues rather than direct surgical errors. Vascular neurosurgery exhibits the highest rates at 36.5%, followed by spine and peripheral nerve procedures at 21.7%.[253][254][255] Infections occur in 1-5% of cases, with surgical site infections post-craniotomy ranging from 1-3% despite prophylactic antibiotics. Hemorrhagic complications, including intraoperative bleeding or postoperative hematoma, affect 2-5% overall, higher in aneurysm repairs where adverse events impact outcomes significantly. Neurological deficits, such as transient or permanent paresis, arise in 4-11% of intracranial surgeries, with permanent rates around 4% in gamma knife procedures. Systemic issues like delirium post-intracranial surgery occur in 19%, influenced by patient age and operative duration.[256][257][258][259]| Complication Type | Approximate Rate | Common Contexts |
|---|---|---|
| Surgical site infection | 1-5% | Craniotomy, spine fusion[256] |
| Postoperative hemorrhage | 2-5% | Vascular repairs, tumor resections[257] |
| Neurological deficit (permanent) | 4% | Stereotactic procedures[258] |
| Delirium | 19% | Intracranial surgery[259] |
| Overall 30-day | 8.2% | Elective cases[253] |
Long-Term Efficacy Data
Long-term efficacy in neurosurgery varies by procedure and pathology, with durable benefits observed in functional interventions like deep brain stimulation (DBS) for Parkinson's disease, where subthalamic nucleus DBS sustains motor improvements and reduces dopaminergic medication needs beyond 15 years, though disease progression persists and 10-year survival stands at approximately 51%.[264][265] Similarly, epilepsy resection achieves sustained seizure freedom in 41-44% of adult and pediatric cases over extended follow-up, with temporal lobe surgeries yielding 70.8% freedom at 12 years, frontal lobe resections at 45%, and probabilities declining to 56% at 10 years among those initially seizure-free due to late recurrences.[266][267][268] In oncologic neurosurgery, maximal safe resection extends survival for gliomas, with aggressive removal of at least 75% of low-grade tumors correlating with improved long-term outcomes, while glioblastoma patients undergoing multiple resections achieve median survival of 10.7 months from diagnosis, with 24-month rates around 20%; however, overall 5-year survival for malignant brain tumors remains low at 22-33%, underscoring surgery's palliative role amid tumor recurrence and limited curative potential.[269][270][271] Vascular procedures demonstrate comparable long-term durability between clipping and endovascular coiling for ruptured saccular aneurysms, with no significant differences in rebleeding, mortality, or complete occlusion rates over years, despite coiling's initial advantages in short-term independence (7% absolute risk reduction at 1 year).[272][193] These outcomes reflect selection biases in observational data, where patient comorbidities and aneurysm morphology influence procedural choice, and randomized trials like ISAT highlight sustained equivalence beyond acute phases.[273] Across domains, long-term efficacy is tempered by progression of underlying conditions, hardware-related complications in neuromodulation (e.g., 31% dementia incidence by 6-9 years post-DBS), and the need for adjunct therapies, with systematic reviews emphasizing multidisciplinary follow-up to mitigate declines.[274]Controversies and Criticisms
Debates on Surgical Necessity vs. Conservatism
In conditions such as spontaneous intracerebral hemorrhage (ICH), debates persist regarding the superiority of surgical evacuation over conservative management, with randomized trials yielding conflicting results. The Surgical Trial in Intracerebral Haemorrhage (STICH I), published in 2005, found no significant benefit from early surgery compared to initial conservative treatment followed by surgery if needed, reporting a 24% absolute risk reduction in poor outcomes that failed to reach statistical significance (odds ratio 0.71, 95% CI 0.45-1.14).17736-6/fulltext) In contrast, the STICH II trial of 2013, focusing on superficial lobar ICH amenable to minimally invasive evacuation, demonstrated improved functional outcomes at six months with early surgery (adjusted OR 0.69, 95% CI 0.47-1.00), suggesting subgroup-specific benefits but highlighting selection biases and applicability limitations.60986-1/fulltext) A 2022 retrospective study on conventional craniotomy versus conservative care for hypertensive brainstem ICH reported higher favorable outcomes at 12 months with conservatism (81% vs. 44%, p<0.001), attributing advantages to avoiding surgical morbidity in eloquent regions.[275] These discrepancies underscore causal uncertainties, as surgical risks—including infection, rebleeding, and perioperative mortality (up to 20% in some cohorts)—may offset benefits in patients with comorbidities or deep-seated hemorrhages, where hematoma stabilization via blood pressure control and reversal of coagulopathy often suffices.[276] For acute subdural hematoma (ASDH) in traumatic brain injury, center-specific preferences for immediate surgery versus initial observation do not correlate with superior outcomes, per a 2024 multinational cohort analysis of over 1,000 patients, which adjusted for confounders and found no association between surgical propensity and six-month mortality or functional status (adjusted common OR 1.05, 95% CI 0.85-1.30).[277] Conservative strategies, incorporating serial imaging and medical optimization, yielded comparable survival (28.8% mortality vs. 32.7% surgical) in a 2025 randomized trial of moderate TBI cases, emphasizing that mass effect resolution without intervention occurs in up to 20% of select patients, thereby avoiding craniotomy risks like brain herniation exacerbation.[278] Proponents of conservatism argue that empirical data reveal overtreatment driven by tradition rather than causal evidence, as delayed surgery post-deterioration matches acute intervention efficacy without upfront complications, though critics note higher recurrence risks (up to 15%) in non-operated cases requiring vigilant monitoring.[279] In degenerative spine conditions like lumbar disc herniation causing sciatica, prolonged conservative management—encompassing physical therapy, analgesics, and epidural injections—matches surgical discectomy outcomes at one year, with a 2007 multicenter trial of 283 patients showing no significant difference in leg pain relief (mean improvement 53 mm vs. 52 mm on VAS scale) or disability scores, despite 39% of conservative patients eventually crossing over to surgery after 4.5 months.[280] Systematic reviews affirm that 70-90% of herniations resolve spontaneously within six months via resorption mechanisms, rendering microdiscectomy elective for refractory cases and questioning routine surgical endorsement amid risks of dural tear (5-10%) and recurrent herniation (7%).[281] For incidental neurosurgical findings, such as asymptomatic meningiomas or low-grade gliomas detected on imaging, watch-and-wait protocols mitigate overtreatment, as serial MRI surveillance reveals growth in only 20-30% over five years, with intervention reserved for symptomatic progression to preserve quality of life without iatrogenic deficits.[282] Broader critiques highlight systemic incentives for surgical intervention, including fee-for-service models and liability fears, potentially inflating procedure rates despite level I evidence favoring conservatism in select cohorts; a 2020 analysis defined overtreatment as interventions where harm probability exceeds net benefit, estimating 10-20% neurosurgical volume as potentially avoidable based on natural history data.[283] Nonetheless, first-principles evaluation—prioritizing causal pathways like mass effect reversal—supports surgery in acute deterioration or focal compression, as evidenced by reduced mortality in pyogenic spondylodiscitis with early debridement (OR 0.45 for survival).[284] Ongoing trials aim to refine patient stratification via biomarkers and AI predictive models to resolve these debates empirically.[285]Ethical Dilemmas in High-Risk Cases
In high-risk neurosurgical cases, such as those involving severe traumatic brain injury, advanced brain tumors, or complex cerebrovascular malformations, ethical dilemmas often center on the balance between patient autonomy and the principle of non-maleficence, where procedures carry substantial risks of mortality or permanent disability exceeding 20-30% in some scenarios.[286] Surgeons must weigh potential benefits against harms, particularly when evidence indicates limited long-term efficacy, as in glioblastoma resections where median survival remains under 15 months despite aggressive intervention.[287] This tension is exacerbated by variability in prognostic assessments, with studies showing inter-surgeon disagreement rates up to 40% on operative candidacy for high-risk patients.[288] A primary dilemma involves informed consent, where patients or surrogates struggle to comprehend probabilistic risks and alternatives due to the technical complexity of neurosurgical outcomes. Research indicates that in major high-risk surgeries, including craniotomies, only about 50-60% of patients demonstrate adequate understanding of disclosed risks post-consultation, often underestimating complications like aphasia or hemiparesis.[289][290] Legal precedents, such as the 1995 Wisconsin case Johnson v. Kokemoor, underscore the obligation to disclose surgeon-specific experience, as failure to reveal low personal success rates (e.g., 30% vs. literature benchmarks of 70%) for aneurysm clipping violated informed consent standards, leading to multimillion-dollar liability.[291] Shared decision-making frameworks aim to mitigate this by incorporating patient values, yet implementation falters in acute settings like penetrating brain injuries, where time constraints limit voluntariness and comprehension.[292][293] Futility judgments represent another core ethical challenge, particularly in geriatric or neurologically devastated patients, where surgery may extend physiological life without restoring meaningful function. In elderly cranial neurosurgery for tumors or trauma, futility arises when interventions fail to achieve quality-adjusted life expectancy beyond minimal thresholds, such as a Glasgow Outcome Scale score below 3, prompting debates over withholding procedures to avoid prolonging vegetative states.[286][294] Empirical data from neurocritical care cohorts reveal that aggressive surgical escalation in futile cases correlates with higher family distress and resource depletion, yet unilateral physician determinations risk overriding surrogate autonomy, as evidenced by disputes in decompressive craniectomies for malignant strokes where 6-month functional independence rates hover at 10-20%.[295][296] Ethical frameworks like utilitarianism advocate prioritizing net societal benefit in resource-scarce environments, but principlist approaches emphasize case-specific deliberation via ethics committees to resolve impasses.[297] In pediatric or incompetent patient scenarios, dilemmas intensify around substituted judgment, as guardians may favor intervention despite dismal prognoses, such as in severe hypoxic-ischemic encephalopathy requiring ventriculostomy. Guidelines from bodies like the American Association of Neurological Surgeons stress multidisciplinary review, yet surveys of neurosurgeons indicate persistent variability, with 25-30% reporting discomfort overriding family demands in perceived futile cases due to litigation fears.[298][299] These conflicts highlight the need for advanced care planning and prognostic tools, like multimodal outcome predictors, to enhance transparency and align decisions with empirical evidence rather than optimism bias.[288]Systemic Issues: Errors, Oversupply, and Liability
Neurosurgery exhibits elevated rates of medical errors compared to many specialties, often stemming from systemic factors beyond individual technical skill. A review of departmental error logs identified technical errors in 27.8% of cases, contamination in 25.3%, equipment failures or missing items in 18.2%, and delays in 12.5%, with only about one-quarter attributable to surgical faults alone; the remainder involved coordination lapses, nursing oversights, or anesthesia issues.[300] Malpractice litigation data further highlight intraprocedural errors (37.4%), delayed diagnoses (32.1%), and failure to treat (28.8%) as predominant claim types, underscoring systemic vulnerabilities in preoperative assessment, intraoperative execution, and postoperative monitoring.[301] Cognitive biases contribute to predictable judgment errors in decision-making, such as overconfidence in risk assessment or anchoring on initial diagnoses, which can propagate through high-stakes procedures like tumor resections or aneurysm clippings. These biases, while not always leading to adverse outcomes, systematically distort clinical reasoning in complex cases involving incomplete information or time pressure. Systemic inaccuracies in outcome databases, such as the National Surgical Quality Improvement Program, also undermine research validity and quality improvement efforts by inflating or underreporting complication rates specific to neurosurgical interventions.[302][303] Concerns over oversupply center on procedure utilization rather than practitioner numbers, with evidence of overuse in elective spinal interventions; for instance, certain neurosurgeons have performed spinal fusions at rates up to ten times the peer average, prompting investigations into financial incentives under fee-for-service models. Globally, neurosurgical capacity deficits affect over 5 million treatable cases annually without intervention, yet in high-income settings like the US, regional competition among surgeons—coupled with defensive practices—may drive unnecessary imaging or minor procedures to mitigate liability exposure.[304][305] Liability pressures exacerbate these issues, with neurosurgeons facing annual malpractice claims at 19.1%—the highest among specialties—and spending over 25% of their careers defending open suits, averaging $439,000 in indemnity payouts per resolved claim.[306][307][308] Defensive medicine is widespread, with practitioners in high-litigation-risk states 50% more likely to order extra tests, consultations, or avoid high-risk patients altogether, potentially increasing costs without reducing errors and altering care patterns toward conservatism or excess.[309] Such behaviors, driven by premiums exceeding $300,000 annually for some, reflect causal links between tort environments and resource allocation, though they offer limited protection against suits.[310][311]Notable Figures
Historical Pioneers
Sir William Macewen (1848–1924), a Scottish surgeon, achieved the first documented successful resection of a brain tumor on July 27, 1879, when he removed a glioma from the arm area of the brain in a patient named Barbara Watson, who survived and regained function without recurrence.[312] This operation relied on precise localization through clinical symptoms and Macewen's adoption of antiseptic techniques, marking a shift from exploratory craniotomies to targeted interventions.[313] Macewen also pioneered surgical treatment of brain abscesses by advocating early drainage based on his analysis of 25 cases with a 32% mortality rate, emphasizing rapid intervention to prevent fatal complications.[314] Sir Victor Horsley (1857–1916), appointed as the first dedicated neurosurgeon at London's National Hospital for the Paralysed and Epileptic in 1886, performed the inaugural modern neurosurgical procedure for epilepsy that year, excising a cortical tuber in a patient with Jacksonian seizures.[315] Horsley introduced innovations such as antiseptic wax for hemostasis, the first laminectomy for spinal neoplasm in 1887, and carotid ligation for cerebral aneurysm, while conducting foundational experiments on intracranial pressure and cerebral circulation that informed safe operative tolerances.[315] His 1890 report on 44 brain surgeries established academic neurosurgery as a distinct specialty, though his high operative mortality rates—around 50% early on—reflected the era's limitations in anesthesia and infection control.[316] Harvey Cushing (1869–1939), widely regarded as the father of modern neurosurgery, systematized the field through meticulous operative techniques and patient outcomes tracking, achieving a brain tumor mortality rate drop from 50% to under 10% by the 1920s via innovations like silver clip hemostasis, electrocautery, and precise tumor localization using X-rays and patient positioning.[5] Cushing performed over 2,000 intracranial operations, authoring seminal works on pituitary tumors (1912), acoustic neuromas (1917), and glioma classification, while founding training programs that emphasized residency-based skill development over apprenticeship.[30] His collaboration with pathologist Louise Eisenhardt enabled accurate intraoperative tumor grading, reducing postoperative surprises and advancing neuropathological integration into surgery.[317] Walter Dandy (1886–1946), a protégé of Cushing who diverged into innovative diagnostics, introduced pneumoventriculography in 1918 by injecting air into cerebral ventricles to visualize obstructions on X-ray, revolutionizing hydrocephalus treatment and tumor localization when myelography proved insufficient.[318] Dandy pioneered selective posterior rhizotomy for trigeminal neuralgia in 1925, achieving pain relief in over 90% of cases through targeted nerve root sectioning guided by anatomical precision rather than crude decompression.[319] He also developed hemispherectomy for intractable epilepsy and gliomas in the 1920s–1930s, performing the procedure on children with Rasmussen's encephalitis-like syndromes, though long-term risks like hemiplegia limited its adoption until refinements.[35] Dandy's emphasis on operative anatomy over Cushing's gradualism influenced generations, with his techniques enabling safer access to posterior fossa and vascular lesions.[34]Contemporary Innovators
Robert F. Spetzler advanced cerebrovascular neurosurgery through pioneering the use of hypothermia and cardiac arrest techniques to access and treat complex brain lesions, including aneurysms and arteriovenous malformations (AVMs).[320][321] These methods allow temporary cessation of blood flow, enabling safer resection of high-risk vascular anomalies that were previously inoperable.[321] Spetzler performed over 6,000 aneurysm surgeries and contributed more than 600 peer-reviewed articles, establishing standardized grading systems like the Spetzler-Martin scale for AVM risk assessment.[322] Michael T. Lawton built on vascular neurosurgery foundations with innovations in skull base anatomy, defining operative triangles that enhance precision in aneurysm clipping and bypass procedures.[323] His clinical experience includes treating more than 4,000 brain aneurysms, 800 AVMs, and 1,000 cavernous malformations, alongside authoring textbooks and over 1,000 publications that disseminate advanced microneurosurgical techniques.[324][325] Lawton's educational efforts, including observership programs training over 100 surgeons, have propagated these methods globally.[326] In neuro-oncology, Henry Brem introduced implantable carmustine wafers (Gliadel), the first FDA-approved local chemotherapy for glioblastoma, following pivotal clinical trials he led that demonstrated extended survival.[327][328] Brem also pioneered image-guided stereotactic navigation systems, improving tumor resection accuracy while sparing eloquent brain areas.[328] His work at Johns Hopkins has shaped translational research, mentoring leaders in brain tumor therapies.[329] Mitchel S. Berger refined intraoperative brain mapping to preserve language and motor functions during glioma resections, integrating electrical stimulation with advanced imaging for maximal safe tumor removal.[330][331] As director of UCSF's Brain Tumor Center, Berger's research correlates extent of resection with outcomes, advocating aggressive yet functional-sparing surgery supported by studies showing improved survival.[332] His recruitment of interdisciplinary teams has accelerated innovations like fluorescence-guided surgery.[333]Recent Advances and Future Directions
Technological Innovations (2020s)
Robotic systems have seen significant enhancements in neurosurgery during the 2020s, enabling greater precision in minimally invasive procedures such as stereotactic biopsies and deep brain stimulation. In 2020, Duke University implemented awake robotic spine fusion using systems like the Mazor X, reducing patient discomfort and improving accuracy in pedicle screw placement compared to traditional fluoroscopy-guided methods. By 2023, the FDA approved neurosurgical robots with integrated AI for tremor filtering and obstacle avoidance, allowing for motion scaling and force sensing that minimize intraoperative errors in cranial and spinal interventions. These platforms, including evolutions of the ROSA system, have expanded to neuroendoscopy and functional neurosurgery, with studies reporting reduced blood loss and shorter recovery times in procedures like tumor resections.[334][335][112] Artificial intelligence applications have proliferated for preoperative planning, intraoperative guidance, and postoperative prediction in neurosurgical workflows. Deep learning algorithms, reviewed in systematic studies up to 2025, enhance diagnostic accuracy by analyzing MRI and CT scans to detect anomalies like aneurysms with sensitivity exceeding 90% in some models, outperforming traditional radiologist assessments in speed and consistency. Intraoperative AI integrates with navigation systems for real-time tissue segmentation and risk stratification, as seen in platforms developed for spine surgery that predict complications and optimize trajectories. These tools, while promising, require validation against human oversight to mitigate algorithmic biases from training datasets.[336][337][338] Focused ultrasound (FUS) has emerged as a non-invasive alternative for targeted ablation, particularly in movement disorders. In 2021, expanded FDA approvals enabled MRI-guided FUS thalamotomy for essential tremor, with clinical trials demonstrating tremor reduction in over 70% of patients at one-year follow-up without incisions or radiation. By September 2025, staged bilateral FUS received FDA clearance for advanced Parkinson's disease, allowing sequential treatments to the subthalamic nucleus with improved motor scores and minimal cognitive side effects in phase III trials. Ongoing research explores FUS for blood-brain barrier opening in tumor drug delivery and Alzheimer's, though long-term efficacy data remains preliminary.[339][340][341] Brain-computer interfaces (BCIs) represent a frontier in restorative neurosurgery, with implantable devices facilitating neural signal decoding for paralyzed patients. In 2024, endovascular BCIs like Synchron's Stentrode enabled thought-controlled device operation in clinical trials, achieving safe implantation via blood vessels without craniotomy and allowing quadriplegic individuals to perform tasks like texting at rates comparable to manual input. High-density electrode arrays, tested in studies at institutions like Mount Sinai, recorded unprecedented brain activity resolution for mapping epilepsy foci and restoring speech in ALS patients, with decoding accuracies reaching 97% for phonetic reconstruction. These innovations, while advancing functional recovery, face challenges in biocompatibility and signal stability over years.[342][343][344]Emerging Research Frontiers
Research in brain-computer interfaces (BCIs) represents a pivotal frontier in neurosurgery, enabling direct neural signal decoding for restoring function in patients with severe neurological impairments. In August 2025, Stanford Medicine researchers developed a BCI that detects inner speech in speech-impaired individuals, achieving decoding accuracy sufficient to reconstruct intended words from brain activity alone, offering potential for communication restoration without physical articulation.[345] Similarly, UC Davis studies in 2024 demonstrated BCIs translating brain signals into synthesized speech for ALS patients, with systems generating words at rates approaching natural conversation, though long-term implant stability remains a challenge requiring further surgical refinements.[343] Endovascular BCIs, implanted via minimally invasive vascular access, have shown feasibility in systematic reviews, reducing craniotomy risks while enabling high-resolution cortical recording for motor and sensory applications.[346] Artificial intelligence (AI) and machine learning are transforming neurosurgical precision and decision-making, with applications in preoperative planning, intraoperative guidance, and postoperative prediction. A July 2025 special issue of Neurosurgical Focus highlighted AI models achieving up to 97.5% accuracy in brain tumor detection via imaging analysis, outperforming traditional radiology in identifying subtle lesions and reducing diagnostic delays.[347] Machine learning algorithms integrated into neuronavigation systems provide real-time feedback during resections, decreasing error rates by up to 30% in complex cases like glioma surgery, as evidenced by predictive analytics for complication risks.[348] These tools leverage large datasets for personalized prognostication, such as forecasting intensive care needs post-craniotomy with natural language processing of clinical notes, though validation across diverse populations is ongoing to mitigate algorithmic biases.[349] Regenerative approaches using stem cells aim to repair neural tissue damage, particularly in spinal cord injuries and stroke. Mayo Clinic's 2024 phase I trial confirmed the safety of mesenchymal stem cell injections for subacute and chronic spinal cord injuries, with some participants showing improved sensory-motor function up to two years post-treatment, attributed to paracrine effects promoting endogenous repair rather than direct cell replacement.[350] UCSD's 2024 preclinical data indicated neural stem cell transplants fostering axonal regrowth in chronic spinal models, enhancing locomotion scores in rodents, paving the way for human trials targeting persistent deficits.[351] In stroke models, USC's 2025 study reported stem cell transplants inducing brain cell proliferation and functional recovery in mice, with glycoengineered variants boosting neural connectivity post-ischemia.[352] Non-invasive modalities like MR-guided focused ultrasound (MRgFUS) are expanding neurosurgical options for movement disorders and beyond. Approved for essential tremor thalamotomy, MRgFUS ablates deep targets without incisions, with 2024 clinical data showing sustained tremor reduction in 70-80% of patients at one year, minimizing infection risks compared to deep brain stimulation.[353] Emerging applications include blood-brain barrier opening for drug delivery in tumors and Alzheimer's, as explored in recent reviews, enabling targeted chemotherapy with reduced systemic toxicity.[354] A 2024 special issue in Neurosurgical Focus underscored MRgFUS's role in neuropsychiatric conditions, with ongoing trials assessing efficacy for obsessive-compulsive disorder via precise lesioning.[355] These advancements collectively promise reduced invasiveness and enhanced outcomes, contingent on rigorous long-term efficacy studies.[356]References
- ./assets/Parkinson_surgery.jpg