Recent from talks
Nothing was collected or created yet.
Radiography
View on Wikipedia
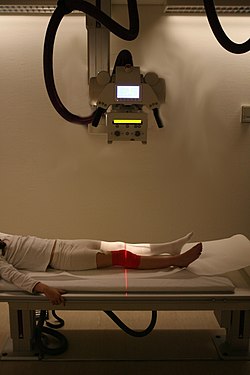 Projectional radiography of the knee in a modern X-ray machine | |
| System | Musculoskeletal |
|---|---|
| Subdivisions | Interventional, Nuclear, Therapeutic, Paediatric |
| Significant diseases | Cancer, bone fractures |
| Significant tests | screening tests, X-ray, CT, MRI, PET, bone scan, ultrasonography, mammography, fluoroscopy |
| Specialist | Radiographer |
Radiography is an imaging technique using X-rays, gamma rays, or similar ionizing radiation and non-ionizing radiation to view the internal form of an object. Applications of radiography include medical ("diagnostic" radiography and "therapeutic radiography") and industrial radiography. Similar techniques are used in airport security, (where "body scanners" generally use backscatter X-ray). To create an image in conventional radiography, a beam of X-rays is produced by an X-ray generator and it is projected towards the object. A certain amount of the X-rays or other radiation are absorbed by the object, dependent on the object's density and structural composition. The X-rays that pass through the object are captured behind the object by a detector (either photographic film or a digital detector). The generation of flat two-dimensional images by this technique is called projectional radiography. In computed tomography (CT scanning), an X-ray source and its associated detectors rotate around the subject, which itself moves through the conical X-ray beam produced. Any given point within the subject is crossed from many directions by many different beams at different times. Information regarding the attenuation of these beams is collated and subjected to computation to generate two-dimensional images on three planes (axial, coronal, and sagittal) which can be further processed to produce a three-dimensional image.

History
[edit]
Radiography's origins and fluoroscopy's origins can both be traced to 8 November 1895, when German physics professor Wilhelm Conrad Röntgen discovered the X-ray and noted that, while it could pass through human tissue, it could not pass through bone or metal.[1] Röntgen referred to the radiation as "X", to indicate that it was an unknown type of radiation. He received the first Nobel Prize in Physics for his discovery.[2]
There are conflicting accounts of his discovery because Röntgen had his lab notes burned after his death, but this is a likely reconstruction by his biographers:[3][4] Röntgen was investigating cathode rays using a fluorescent screen painted with barium platinocyanide and a Crookes tube which he had wrapped in black cardboard to shield its fluorescent glow. He noticed a faint green glow from the screen, about 1 metre away. Röntgen realized some invisible rays coming from the tube were passing through the cardboard to make the screen glow: they were passing through an opaque object to affect the film behind it.[5]

Röntgen discovered X-rays' medical use when he made a picture of his wife's hand on a photographic plate formed due to X-rays. The photograph of his wife's hand was the first ever photograph of a human body part using X-rays. When she saw the picture, she said, "I have seen my death."[5]
The first use of X-rays under clinical conditions was by John Hall-Edwards in Birmingham, England, on 11 January 1896, when he radiographed a needle stuck in the hand of an associate. On 14 February 1896, Hall-Edwards also became the first to use X-rays in a surgical operation.[6]
The United States saw its first medical X-ray obtained using a discharge tube of Ivan Pulyui's design. In January 1896, on reading of Röntgen's discovery, Frank Austin of Dartmouth College tested all of the discharge tubes in the physics laboratory and found that only the Pulyui tube produced X-rays. This was a result of Pulyui's inclusion of an oblique "target" of mica, used for holding samples of fluorescent material, within the tube. On 3 February 1896 Gilman Frost, professor of medicine at the college, and his brother Edwin Frost, professor of physics, exposed the wrist of Eddie McCarthy, whom Gilman had treated some weeks earlier for a fracture, to the X-rays and collected the resulting image of the broken bone on gelatin photographic plates obtained from Howard Langill, a local photographer also interested in Röntgen's work.[7]

X-rays were put to diagnostic use very early; for example, Alan Archibald Campbell-Swinton opened a radiographic laboratory in the United Kingdom in 1896, before the dangers of ionizing radiation were discovered. Indeed, Marie Curie pushed for radiography to be used to treat wounded soldiers in World War I. Initially, many kinds of staff conducted radiography in hospitals, including physicists, photographers, physicians, nurses, and engineers. The medical speciality of radiology grew up over many years around the new technology. When new diagnostic tests were developed, it was natural for the radiographers to be trained in and to adopt this new technology. Radiographers now perform fluoroscopy, computed tomography, mammography, ultrasound, nuclear medicine and magnetic resonance imaging as well. Although a nonspecialist dictionary might define radiography quite narrowly as "taking X-ray images", this has long been only part of the work of "X-ray departments", radiographers, and radiologists. Initially, radiographs were known as roentgenograms,[8] while skiagrapher (from the Ancient Greek words for "shadow" and "writer") was used until about 1918 to mean radiographer. The Japanese term for the radiograph, rentogen (レントゲン), shares its etymology with the original English term.
Medical uses
[edit]| Radiography | |
|---|---|
| ICD-9-CM | 87, 88.0-88.6 |
| MeSH | D011859 |
| OPS-301 code | 3–10...3–13, 3–20...3–26 |
Since the body is made up of various substances with differing densities, ionising and non-ionising radiation can be used to reveal the internal structure of the body on an image receptor by highlighting these differences using attenuation, or in the case of ionising radiation, the absorption of X-ray photons by the denser substances (like calcium-rich bones). The discipline involving the study of anatomy through the use of radiographic images is known as radiographic anatomy. Medical radiography acquisition is generally carried out by radiographers, while image analysis is generally done by radiologists. Some radiographers also specialise in image interpretation. Medical radiography includes a range of modalities producing many different types of image, each of which has a different clinical application.
Projectional radiography
[edit]
The creation of images by exposing an object to X-rays or other high-energy forms of electromagnetic radiation and capturing the resulting remnant beam (or "shadow") as a latent image is known as "projection radiography". The "shadow" may be converted to light using a fluorescent screen, which is then captured on photographic film, it may be captured by a phosphor screen to be "read" later by a laser (CR), or it may directly activate a matrix of solid-state detectors (DR—similar to a very large version of a CCD in a digital camera). Bone and some organs (such as lungs) especially lend themselves to projection radiography. It is a relatively low-cost investigation with a high diagnostic yield. The difference between soft and hard body parts stems mostly from the fact that carbon has a very low X-ray cross section compared to calcium.
Computed tomography
[edit]
Computed tomography or CT scan (previously known as CAT scan, the "A" standing for "axial") uses ionizing radiation (x-ray radiation) in conjunction with a computer to create images of both soft and hard tissues. These images look as though the patient was sliced like bread (thus, "tomography" – "tomo" means "slice"). Though CT uses a higher amount of ionizing x-radiation than diagnostic x-rays (both utilising X-ray radiation), with advances in technology, levels of CT radiation dose and scan times have reduced.[9] CT exams are generally short, most lasting only as long as a breath-hold, Contrast agents are also often used, depending on the tissues needing to be seen. Radiographers perform these examinations, sometimes in conjunction with a radiologist (for instance, when a radiologist performs a CT-guided biopsy).
Dual energy X-ray absorptiometry
[edit]DEXA, or bone densitometry, is used primarily for osteoporosis tests. It is not projection radiography, as the X-rays are emitted in two narrow beams that are scanned across the patient, 90 degrees from each other. Usually the hip (head of the femur), lower back (lumbar spine), or heel (calcaneum) are imaged, and the bone density (amount of calcium) is determined and given a number (a T-score). It is not used for bone imaging, as the image quality is not good enough to make an accurate diagnostic image for fractures, inflammation, etc. It can also be used to measure total body fat, though this is not common. The radiation dose received from DEXA scans is very low, much lower than projection radiography examinations.[10]
Fluoroscopy
[edit]Fluoroscopy is a term invented by Thomas Edison during his early X-ray studies. The name refers to the fluorescence he saw while looking at a glowing plate bombarded with X-rays.[11]
The technique provides moving projection radiographs. Fluoroscopy is mainly performed to view movement (of tissue or a contrast agent), or to guide a medical intervention, such as angioplasty, pacemaker insertion, or joint repair/replacement. The last can often be carried out in the operating theatre, using a portable fluoroscopy machine called a C-arm.[12] It can move around the surgery table and make digital images for the surgeon. Biplanar Fluoroscopy works the same as single plane fluoroscopy except displaying two planes at the same time. The ability to work in two planes is important for orthopedic and spinal surgery and can reduce operating times by eliminating re-positioning.[13]

Angiography is the use of fluoroscopy to view the cardiovascular system. An iodine-based contrast is injected into the bloodstream and watched as it travels around. Since liquid blood and the vessels are not very dense, a contrast with high density (like the large iodine atoms) is used to view the vessels under X-ray. Angiography is used to find aneurysms, leaks, blockages (thromboses), new vessel growth, and placement of catheters and stents. Balloon angioplasty is often done with angiography.
Contrast radiography
[edit]Contrast radiography uses a radiocontrast agent, a type of contrast medium, to make the structures of interest stand out visually from their background. Contrast agents are required in conventional angiography, and can be used in both projectional radiography and computed tomography (called contrast CT).[14][15]
Other medical imaging
[edit]Although not technically radiographic techniques due to not using X-rays, imaging modalities such as PET and MRI are sometimes grouped in radiography because the radiology department of hospitals handle all forms of imaging. Treatment using radiation is known as radiotherapy.
Industrial radiography
[edit]Industrial radiography is a method of non-destructive testing where many types of manufactured components can be examined to verify the internal structure and integrity of the specimen. Industrial Radiography can be performed utilizing either X-rays or gamma rays. Both are forms of electromagnetic radiation. The difference between various forms of electromagnetic energy is related to the wavelength. X and gamma rays have the shortest wavelength and this property leads to the ability to penetrate, travel through, and exit various materials such as carbon steel and other metals. Specific methods include industrial computed tomography.

Image quality
[edit]Image quality will depend on resolution and density. Resolution is the ability of an image to show closely spaced structure in the object as separate entities in the image while density is the blackening power of the image. Sharpness of a radiographic image is strongly determined by the size of the X-ray source. This is determined by the area of the electron beam hitting the anode. A large photon source results in more blurring in the final image and is worsened by an increase in image formation distance. This blurring can be measured as a contribution to the modulation transfer function of the imaging system.
Radiation dose
[edit]The dosage of radiation applied in radiography varies by procedure. For example, the effective dosage of a chest x-ray is 0.1 mSv, while an abdominal CT is 10 mSv.[16] The American Association of Physicists in Medicine (AAPM) have stated that the "risks of medical imaging at patient doses below 50 mSv for single procedures or 100 mSv for multiple procedures over short time periods are too low to be detectable and may be nonexistent." Other scientific bodies sharing this conclusion include the International Organization of Medical Physicists, the UN Scientific Committee on the Effects of Atomic Radiation, and the International Commission on Radiological Protection. Nonetheless, radiological organizations, including the Radiological Society of North America (RSNA) and the American College of Radiology (ACR), as well as multiple government agencies, indicate safety standards to ensure that radiation dosage is as low as possible.[17]
Shielding
[edit]| X-rays generated by peak voltages below |
Minimum thickness of lead (depending on machine) |
|---|---|
| 75 kV | 1.0 mm |
| 100 kV | 1.5 mm |
| 125 kV | 2.0 mm |
| 150 kV | 2.5 mm |
| 175 kV | 3.0 mm |
| 200 kV | 4.0 mm |
| 225 kV | 5.0 mm |
| 300 kV | 9.0 mm |
| 400 kV | 15.0 mm |
| 500 kV | 22.0 mm |
| 600 kV | 34.0 mm |
| 900 kV | 51.0 mm |
Lead is the most common shield against X-rays because of its high density (11,340 kg/m3), stopping power, ease of installation and low cost. The maximum range of a high-energy photon such as an X-ray in matter is infinite; at every point in the matter traversed by the photon, there is a probability of interaction. Thus there is a very small probability of no interaction over very large distances. The shielding of photon beam is therefore exponential (with an attenuation length being close to the radiation length of the material); doubling the thickness of shielding will square the shielding effect.
Starting in the 1950s, personal lead shielding began to be used on directly on patients during all X-rays over the abdomen to intending to protect the gonads (reproductive organs) or a fetus if the patient was pregnant. Dental X-rays would also typically additionally use lead shielding to protect the thyroid. However, a consensus was reached between 2019[18][19][20] and 2021[21][22] that lead shielding for routine diagnostic X-rays is not necessary and may in some cases be harmful. Personal shielding for medical professionals and other people in the room is still recommended.
Rooms where X-rays are performed are lined with lead. The table in this section shows the recommended thickness of lead shielding for a room where X-rays are performed as function of X-ray energy, from the Recommendations by the Second International Congress of Radiology.[23]
Campaigns
[edit]In response to increased concern by the public over radiation doses and the ongoing progress of best practices, The Alliance for Radiation Safety in Pediatric Imaging was formed within the Society for Pediatric Radiology. In concert with the American Society of Radiologic Technologists, the American College of Radiology, and the American Association of Physicists in Medicine, the Society for Pediatric Radiology developed and launched the Image Gently campaign which is designed to maintain high quality imaging studies while using the lowest doses and best radiation safety practices available on pediatric patients.[24] This initiative has been endorsed and applied by a growing list of various professional medical organizations around the world and has received support and assistance from companies that manufacture equipment used in radiology.
Following upon the success of the Image Gently campaign, the American College of Radiology, the Radiological Society of North America, the American Association of Physicists in Medicine, and the American Society of Radiologic Technologists have launched a similar campaign to address this issue in the adult population called Image Wisely.[25] The World Health Organization and International Atomic Energy Agency (IAEA) of the United Nations have also been working in this area and have ongoing projects designed to broaden best practices and lower patient radiation dose.[26][27][28]
Provider payment
[edit]Contrary to advice that emphasises only conducting radiographs when in the patient's interest, recent evidence suggests that they are used more frequently when dentists are paid under fee-for-service.[29]
Equipment
[edit]


Sources
[edit]In medicine and dentistry, projectional radiography and computed tomography images generally use X-rays created by X-ray generators, which generate X-rays from X-ray tubes. The resultant images from the radiograph (X-ray generator/machine) or CT scanner are correctly referred to as "radiograms"/"roentgenograms" and "tomograms" respectively.
A number of other sources of X-ray photons are possible, and may be used in industrial radiography or research; these include betatrons, linear accelerators (linacs), and synchrotrons. For gamma rays, radioactive sources such as 192Ir, 60Co, or 137Cs are used.
Grid
[edit]An anti-scatter grid may be placed between the patient and the detector to reduce the quantity of scattered x-rays that reach the detector. This improves the contrast resolution of the image, but also increases radiation exposure for the patient.[30]
Detectors
[edit]Detectors can be divided into two major categories: imaging detectors (such as photographic plates and X-ray film (photographic film), now mostly replaced by various digitizing devices like image plates or flat panel detectors) and dose measurement devices (such as ionization chambers, Geiger counters, and dosimeters used to measure the local radiation exposure, dose, and/or dose rate, for example, for verifying that radiation protection equipment and procedures are effective on an ongoing basis).[31][32][33]
Side markers
[edit]A radiopaque anatomical side marker is added to each image. For example, if the patient has their right hand x-rayed, the radiographer includes a radiopaque "R" marker within the field of the x-ray beam as an indicator of which hand has been imaged. If a physical marker is not included, the radiographer may add the correct side marker later as part of digital post-processing.[34]
Image intensifiers and array detectors
[edit]As an alternative to X-ray detectors, image intensifiers are analog devices that readily convert the acquired X-ray image into one visible on a video screen. This device is made of a vacuum tube with a wide input surface coated on the inside with caesium iodide (CsI). When hit by X-rays, phosphor material causes the photocathode adjacent to it to emit electrons. These electrons are then focused using electron lenses inside the intensifier to an output screen coated with phosphorescent materials. The image from the output can then be recorded via a camera and displayed.[35]
Digital devices known as array detectors are becoming more common in fluoroscopy. These devices are made of discrete pixelated detectors known as thin-film transistors (TFT) which can either work indirectly by using photo detectors that detect light emitted from a scintillator material such as CsI, or directly by capturing the electrons produced when the X-rays hit the detector. Direct detectors do not tend to experience the blurring or spreading effect caused by phosphorescent scintillators or by film screens since the detectors are activated directly by X-ray photons.[36]
Dual-energy
[edit]Dual-energy radiography is where images are acquired using two separate tube voltages. This is the standard method for bone densitometry. It is also used in CT pulmonary angiography to decrease the required dose of iodinated contrast.[37]
See also
[edit]- Autoradiograph – Radiograph made by recording radiation emitted by samples on photographic plates
- Background radiation – Measure of ionizing radiation in the environment
- Computer-aided diagnosis – Type of diagnosis assisted by computers
- GXMO
- Imaging science – Representation or reproduction of an object's form
- List of civilian radiation accidents
- Medical imaging in pregnancy – Types of pregnancy imaging techniques
- Radiation – Waves or particles moving through space
- Digital radiography – Form of radiography
- Radiation contamination – Undesirable radioactive elements on surfaces or in gases, liquids, or solids
- Radiographer – Healthcare professional
- Thermography – Infrared imaging used to reveal temperature
References
[edit]- ^ "History of Radiography". NDT Resource Center. Iowa State University. Retrieved 27 April 2013.
- ^ Karlsson EB (9 February 2000). "The Nobel Prizes in Physics 1901–2000". Stockholm: The Nobel Foundation. Retrieved 24 November 2011.
- ^ "5 unbelievable things about X-rays you can't miss". vix.com. Archived from the original on 24 December 2020. Retrieved 23 October 2017.
- ^ Glasser O (1993). Wilhelm Conrad Röntgen and the early history of the roentgen rays. Norman Publishing. pp. 10–15. ISBN 978-0930405229.
- ^ a b Markel H (20 December 2012). "'I Have Seen My Death': How the World Discovered the X-Ray". PBS NewsHour. PBS. Archived from the original on 20 August 2020. Retrieved 27 April 2013.
- ^ "Major John Hall-Edwards". Birmingham City Council. Archived from the original on 28 September 2012. Retrieved 17 May 2012.
- ^ Spiegel PK (January 1995). "The first clinical X-ray made in America – 100 years". American Journal of Roentgenology. 164 (1). American Roentgen Ray Society: 241–3. doi:10.2214/ajr.164.1.7998549. PMID 7998549.
- ^ Ritchey B, Orban B (April 1953). "The Crests of the Interdental Alveolar Septa". The Journal of Periodontology. 24 (2): 75–87. doi:10.1902/jop.1953.24.2.75.
- ^ Jang J, Jung SE, Jeong WK, Lim YS, Choi JI, Park MY, et al. (February 2016). "Radiation Doses of Various CT Protocols: a Multicenter Longitudinal Observation Study". Journal of Korean Medical Science. 31 (Suppl 1): S24-31. doi:10.3346/jkms.2016.31.S1.S24. PMC 4756338. PMID 26908984.
- ^ "Radiation protection of patients during DXA". International Atomic Energy Agency. 27 July 2017. Retrieved 29 June 2025.
- ^ Carroll QB (2014). Radiography in the Digital Age (2nd ed.). Springfield: Charles C Thomas. p. 9. ISBN 9780398080976.
- ^ Seeram E, Brennan PC (2016). Radiation Protection in Diagnostic X-Ray Imaging. Jones & Bartlett. ISBN 9781284117714.
- ^ Schueler BA (July 2000). "The AAPM/RSNA physics tutorial for residents: general overview of fluoroscopic imaging". Radiographics. 20 (4): 1115–26. doi:10.1148/radiographics.20.4.g00jl301115. PMID 10903700.
- ^ Quader MA, Sawmiller CJ, Sumpio BE (2000). "Radio Contrast Agents: History and Evolution". Textbook of Angiology. pp. 775–783. doi:10.1007/978-1-4612-1190-7_63. ISBN 978-1-4612-7039-3.
- ^ Brant WE, Helms CA (2007). "Diagnostic Imaging Methods". Fundamentals of Diagnostic Radiology (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 3. ISBN 9780781761352.
- ^ "Reducing Radiation from Medical X-rays". FDA.gov. Archived from the original on 3 June 2009. Retrieved 9 September 2018.
- ^ Goldberg J (September–October 2018). "From the Spectral to the Spectrum". Skeptical Inquirer. 42 (5).
- ^ "AAPM Position Statement on the Use of Patient Gonadal and Fetal Shielding". www.aapm.org. Retrieved 5 April 2025.
- ^ Patient Gonadal and Fetal Shielding in Diagnostic Imaging (PDF), American Association of Physicists in Medicine
- ^ Benavides, Erika; Krecioch, Joseph R.; Connolly, Roger T.; Allareddy, Trishul; Buchanan, Allison; Spelic, David; O’Brien, Kelly K.; Keels, Martha Ann; Mascarenhas, Ana Karina; Duong, Mai-Ly; Aerne-Bowe, Mickie J.; Ziegler, Kathleen M.; Lipman, Ruth D. (April 2024). "Optimizing radiation safety in dentistry". The Journal of the American Dental Association. 155 (4): 280–293.e4. doi:10.1016/j.adaj.2023.12.002. PMID 38300176.
- ^ Fujibuchi, Toshio; Matsubara, Kosuke; Hamada, Nobuyuki (1 October 2021). "NCRP statement No. 13 "NCRP recommendations for ending routine gonadal shielding during abdominal and pelvic radiography" and its accompanying documents. Underpinnings and recent developments". Hoken Butsuri (in Japanese). 56 (3): 107–115. doi:10.5453/jhps.56.107. ISSN 1884-7560.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 14 January 2021.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Alchemy Art Lead Products – Lead Shielding Sheet Lead For Shielding Applications. Retrieved 7 December 2008.
- ^ "IG new: The Alliance | image gently". Pedrad.org. Archived from the original on 9 June 2013. Retrieved 16 August 2013.
- ^ "Radiation Safety in Adult Medical Imaging". Image Wisely. Retrieved 16 August 2013.
- ^ "Optimal levels of radiation for patients – Pan American Health Organization – Organización Panamericana de la Salud". New.paho.org. 24 August 2010. Archived from the original on 25 May 2013. Retrieved 16 August 2013.
- ^ "Radiation Protection of Patients". Rpop.iaea.org. 14 March 2013. Retrieved 16 August 2013.
- ^ "Global Initiative on Radiation Safety in Healthcare Settings: Technical Meeting Report" (PDF). World Health Organization. Archived (PDF) from the original on 29 October 2013. Retrieved 16 August 2013.
- ^ Chalkley M, Listl S (March 2018). "First do no harm - The impact of financial incentives on dental X-rays". Journal of Health Economics. 58 (March 2018): 1–9. doi:10.1016/j.jhealeco.2017.12.005. hdl:2066/190628. PMID 29408150.
- ^ Bushberg JT (2002). The Essential Physics of Medical Imaging (2nd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 210. ISBN 9780683301182.
- ^ Ranger NT (1999). "Radiation detectors in nuclear medicine". Radiographics. 19 (2): 481–502. doi:10.1148/radiographics.19.2.g99mr30481. PMID 10194791.
- ^ DeWerd LA, Wagner LK (January 1999). "Characteristics of radiation detectors for diagnostic radiology". Applied Radiation and Isotopes. 50 (1): 125–36. Bibcode:1999AppRI..50..125D. doi:10.1016/S0969-8043(98)00044-X. PMID 10028632.
- ^ Anwar K (2013). "Nuclear Radiation Detectors". Particle Physics. Graduate Texts in Physics. Berlin: Springer-Verlag. pp. 1–78. doi:10.1007/978-3-642-38661-9_1. ISBN 978-3-642-38660-2.
- ^ Barry K, Kumar S, Linke R, Dawes E (September 2016). "A clinical audit of anatomical side marker use in a paediatric medical imaging department". Journal of Medical Radiation Sciences. 63 (3): 148–54. doi:10.1002/jmrs.176. PMC 5016612. PMID 27648278.
- ^ Hendee WR, Ritenour ER (2002). "Fluoroscopy". Medical Imaging Physics (4th ed.). Hoboken, NJ: John Wiley & Sons. ISBN 9780471461135.
- ^ Seibert JA (September 2006). "Flat-panel detectors: how much better are they?". Pediatric Radiology. 36 Suppl 2 (S2) 173: 173–81. doi:10.1007/s00247-006-0208-0. PMC 2663651. PMID 16862412.
- ^ Cochrane Miller J (2015). "Dual Energy CT Imaging for Suspected Pulmonary Embolism Using a Lower Dose of Contrast Agent". Radiology Rounds. 13 (7). Archived from the original on 10 May 2017. Retrieved 5 February 2018.
Further reading
[edit]- Oakley, PA; Harrison, DE (2020). X-Ray Hesitancy: Patients' Radiophobic Concerns Over Medical X-rays. Dose-Response. Specific Safety Guide No. SSG-11 (Report). Vienna: International Atomic Energy Agency. doi:10.1177/1559325820959542. PMC 7503016.
- Seliger HH (November 1995). "Wilhelm Conrad Röntgen and the Glimmer of Light". Physics Today. 48 (11): 25–31. Bibcode:1995PhT....48k..25S. doi:10.1063/1.881456. hdl:10013/epic.43596.d001.
- Shroy Jr RE (1995). "X-Ray equipment". In Bronzino JD (ed.). The Biomedical Engineering handbook. CRC Press and IEEE Press. pp. 953–960. ISBN 978-0-8493-8346-5.
- Herman GT (2009). Fundamentals of Computerized Tomography: Image Reconstruction from Projections (2nd ed.). Springer. ISBN 978-1-85233-617-2.
- Yu SB, Watson AD (September 1999). "Metal-Based X-ray Contrast Media". Chemical Reviews. 99 (9): 2353–78. doi:10.1021/cr980441p. PMID 11749484.
External links
[edit]- MedPix Medical Image Database
- Video on X-ray inspection and industrial computed tomography, Karlsruhe University of Applied Sciences
- NIST's XAAMDI: X-Ray Attenuation and Absorption for Materials of Dosimetric Interest Database
- NIST's XCOM: Photon Cross Sections Database
- NIST's FAST: Attenuation and Scattering Tables
- A lost industrial radiography source event
- RadiologyInfo - The radiology information resource for patients: Radiography (X-rays)
Radiography
View on GrokipediaFundamentals
Principles of X-ray Imaging
Radiography is an imaging modality that employs X-rays to visualize the internal structures of objects, such as the human body, by exploiting the differential absorption of these rays by various tissues or materials. This technique relies on the fact that denser materials, like bone, absorb more X-rays than softer tissues, such as muscle, resulting in varying intensities of transmitted radiation that form the basis of the image.[2] The fundamental principle governing image formation is X-ray attenuation, which describes the reduction in intensity of the X-ray beam as it passes through matter. This process follows the Beer-Lambert law, expressed as , where is the transmitted intensity, is the initial intensity, is the linear attenuation coefficient (dependent on the material's atomic number, density, and X-ray energy), and is the thickness of the material. Attenuation occurs primarily through interactions that remove or redirect photons, enabling the differentiation of structures based on their absorption properties.[2][4] In projectional radiography, the most basic form of X-ray imaging, a two-dimensional shadowgram is produced by projecting the three-dimensional object onto a detector plane, where overlapping structures create a composite image. Radiographic density refers to the overall blackness or whiteness of the image, determined by the total transmitted radiation reaching the detector, while contrast describes the differences in density between adjacent areas, highlighting structural boundaries. This projection inherently leads to superposition of features, limiting spatial resolution but providing a rapid overview of internal anatomy.[5] The primary mechanisms of X-ray interaction with matter that contribute to attenuation and image formation are the photoelectric effect, Compton scattering, and pair production. In the photoelectric effect, the incident photon is completely absorbed by an inner-shell electron, ejecting it and leading to high attenuation in high atomic number materials, which enhances contrast for structures like bone. Compton scattering involves partial energy transfer from the photon to an outer-shell electron, scattering the photon at an angle and contributing to image fog by reducing primary beam intensity. Pair production, relevant at higher energies above 1.02 MeV, occurs when a photon interacts with the nuclear field to create an electron-positron pair, resulting in total photon annihilation but playing a minor role in diagnostic imaging due to typical low-energy X-rays.[6][7] Image contrast arises from two main sources: subject contrast and detector contrast. Subject contrast is inherent to the object being imaged and stems from differences in X-ray attenuation due to variations in atomic number (Z) and physical density; for example, bone (high Z and density) attenuates more than soft tissue, producing darker areas on the radiograph. Detector contrast, on the other hand, refers to the ability of the imaging system (film or digital detector) to differentiate between varying radiation intensities, amplifying or preserving the subject contrast in the final image. Optimal imaging requires balancing these to maximize visibility of anatomical details without excessive noise.[5][8]Physics of Ionizing Radiation
X-rays are a form of ionizing electromagnetic radiation characterized by wavelengths ranging from 0.01 to 10 nm, corresponding to photon energies between approximately 0.12 keV and 120 keV in diagnostic applications.[2] These photons exhibit wave-particle duality, behaving as both electromagnetic waves and discrete particles capable of interacting with matter through absorption, scattering, or transmission.[2] X-rays are classified into soft and hard categories based on energy: soft X-rays have lower energies (typically below 5–10 keV) and shorter penetration depths, while hard X-rays possess higher energies (above 5–10 keV) and greater penetrating power.[2] In radiography, X-rays are primarily produced in vacuum tubes where high-speed electrons are accelerated from a negatively charged cathode filament toward a positively charged anode target, usually made of tungsten due to its high atomic number and melting point.[9] The tube voltage, measured in kilovolt peak (kVp), determines the maximum electron kinetic energy and thus the highest possible X-ray photon energy, while the tube current (in milliamperes, mA) controls the rate of electron emission and the intensity of the X-ray output.[9] Higher kVp shifts the spectrum toward higher energies and increases the total number of photons (proportional to kVp squared), producing a polyenergetic beam with a continuous distribution of energies up to the peak voltage.[9] In contrast, increasing mA boosts photon quantity without altering the energy spectrum.[9] X-ray production occurs via two main mechanisms: bremsstrahlung and characteristic radiation. Bremsstrahlung, or "braking radiation," arises when decelerating electrons interact with the electric field of atomic nuclei in the anode, converting kinetic energy into a continuous spectrum of X-ray photons with energies from near zero up to the incident electron energy.[9] Characteristic radiation, on the other hand, is emitted when incoming electrons eject inner-shell (e.g., K-shell) electrons from anode atoms, and higher-shell electrons cascade down to fill the vacancy, releasing photons at discrete energies corresponding to the binding energy differences (e.g., K-alpha or K-beta lines for tungsten at around 59 keV and 67 keV).[9] This results in sharp peaks superimposed on the bremsstrahlung continuum, with the overall beam remaining polyenergetic due to the dominance of the continuous component.[10] Upon propagation through materials, X-ray penetration depends on photon energy and the atomic number and density of the medium; higher-energy photons interact less frequently via photoelectric absorption or Compton scattering, allowing deeper traversal, while lower-energy photons are more readily attenuated.[2] As ionizing radiation, X-rays possess sufficient energy to eject orbital electrons from atoms, creating ion pairs along their tracks.[11] This ionization can lead to direct action, where photons or secondary electrons directly break molecular bonds, or indirect action, where radiolysis of surrounding water molecules produces reactive species like hydroxyl radicals that diffuse and cause damage.[11] The linear energy transfer (LET), defined as energy deposited per unit track length (typically in keV/μm), quantifies this; low-LET radiation like diagnostic X-rays (around 2–3 keV/μm) produces sparse ionizations, whereas higher LET increases clustering of damage sites.[11]Historical Development
Early Discoveries
In 1895, German physicist Wilhelm Conrad Röntgen discovered X-rays while experimenting with cathode-ray tubes at the University of Würzburg. On November 8, during a late-night session, Röntgen observed that a barium platinocyanide screen fluoresced when placed near his vacuum tube, even though the rays could not be explained by known cathode ray properties; he termed these unknown rays "X-rays" due to their mysterious nature.[12] Röntgen's subsequent investigations revealed that X-rays could penetrate soft tissues but were absorbed by denser materials like bone, producing shadow images on photographic plates.[13] Röntgen captured the first medical X-ray image on December 22, 1895, exposing his wife Anna Bertha Ludwig's hand for 15 minutes, which clearly outlined her bones and wedding ring.[14] This breakthrough image demonstrated the potential for non-invasive visualization of internal structures, sparking immediate worldwide interest. By 1896, the discovery's impact led to the rapid establishment of dedicated X-ray facilities; for instance, Glasgow Royal Infirmary opened the world's first hospital X-ray department in March 1896, followed by similar units in major medical centers across Europe and North America.[15] Early applications focused on bone fractures and foreign bodies, with dental radiography emerging shortly after: German dentist Otto Walkhoff produced the first intraoral dental X-ray in January 1896, enabling visualization of tooth roots and jaw structures.[16] Key contributors advanced practical implementation in the late 1890s. American inventor Thomas Edison developed the first practical fluoroscope in 1896, a device using a calcium tungstate screen to provide real-time X-ray visualization, which he patented and commercialized for medical examinations.[17] French physician Antoine Béclère, recognizing the need for systematic medical use, established the world's first radiology teaching laboratory at Tenon Hospital in Paris in 1897 and advocated for physician-led standardization of techniques to ensure diagnostic reliability.[18] However, early adoption occurred without awareness of radiation hazards, resulting in severe injuries among pioneers, known as "X-ray martyrs." Operators like Edison's assistant Clarence Dally suffered burns, hair loss, and cancers from prolonged unprotected exposure; Dally died in 1904 from metastatic squamous cell carcinoma linked to chronic X-ray exposure.[19] By the early 1900s, these incidents prompted basic precautions, though widespread safety measures were absent. Radiography transitioned to more efficient film-based systems in 1918, with George Eastman introducing flexible celluloid film coated in photographic emulsion, replacing cumbersome glass plates and enabling portable, higher-quality imaging.[20]Technological Evolution
The early 20th century marked significant advancements in radiography equipment, driven by the need to reduce exposure times and improve image quality during clinical and wartime applications. Intensifying screens, which used fluorescent materials like calcium tungstate to amplify X-ray signals and shorten exposure durations by factors of 10 to 50 compared to direct film exposure, saw key refinements in the 1910s and 1920s, enabling safer and more efficient imaging.[21] In 1913, German radiologist Gustav Bucky introduced the Bucky grid, a device that absorbed scattered radiation to enhance contrast in radiographic images, fundamentally improving diagnostic clarity and remaining a standard component in modern systems.[22] World War I accelerated portability innovations, with the British Army deploying at least 10 mobile X-ray units to France by 1915, allowing battlefield imaging in vans equipped with generators and screens for rapid wound assessment.[23] From the 1950s to the 1970s, radiography transitioned toward higher energy sources and real-time capabilities, addressing limitations in penetration and visualization. The adoption of high-voltage X-ray tubes, operating at 100-150 kVp or higher, became widespread in the 1950s, producing harder X-rays for better tissue penetration and reduced patient dose in thicker body regions.[24] Image intensifiers for fluoroscopy, first commercialized by Westinghouse in 1953, electronically amplified X-ray images up to 1,000 times brighter, enabling low-dose dynamic procedures like gastrointestinal studies without darkroom adaptation.[25] A pivotal milestone occurred in 1971 when British engineer Godfrey Hounsfield developed the first clinical computed tomography (CT) scanner at EMI Laboratories, reconstructing cross-sectional images from multiple projections and revolutionizing volumetric diagnostics.[26] The 1980s and 1990s ushered in the digital era, shifting radiography from analog film to electronic capture and storage. Computed radiography (CR) systems, introduced by Fuji in 1983, used photostimulable phosphor plates to capture latent images that were scanned into digital format, offering wider dynamic range and post-processing flexibility over traditional screens.[27] Direct radiography (DR) emerged in the mid-1990s with flat-panel detectors that converted X-rays directly to electrical signals via amorphous selenium or silicon, eliminating intermediate plates and enabling near-instantaneous image acquisition with resolutions up to 5 line pairs per millimeter.[28] Concurrently, picture archiving and communication systems (PACS), conceptualized in the late 1970s and implemented widely by the 1990s, digitized and networked radiographic images for remote access and storage, reducing film costs by up to 90% in large hospitals.[29] Since the 2010s, radiography has integrated advanced computing and detector technologies to enhance precision and automation. Photon-counting detectors, which directly measure individual X-ray photon energies using cadmium telluride semiconductors, entered clinical trials in the early 2010s and gained FDA approval for CT systems by 2021, with additional approvals for systems like Siemens Healthineers' expansions in March 2025 and Canon's in June 2025, improving spatial resolution to sub-millimeter levels and enabling material-specific imaging with reduced noise.[30][31][32] Artificial intelligence, particularly deep learning algorithms, has been increasingly applied for image analysis since the mid-2010s, automating tasks like lesion detection in chest radiographs with sensitivities exceeding 90% in validated studies, thus aiding radiologists in workflow efficiency.[33] These developments build on foundational milestones, including Wilhelm Röntgen's 1901 Nobel Prize in Physics for discovering X-rays and the 1979 Nobel Prize in Physiology or Medicine shared by Hounsfield and Allan Cormack for CT principles, underscoring radiography's evolution from empirical tool to sophisticated diagnostic modality.[34][35]Medical Applications
Projectional Radiography
Projectional radiography, also known as plain film radiography, is a fundamental imaging technique in medical diagnostics that produces two-dimensional images by projecting X-rays through the body onto a detector, capturing the differential attenuation of tissues to visualize internal structures. This method relies on the varying absorption of X-rays by different anatomical densities, such as bone, soft tissue, and air, to create contrast in the resulting image. It serves as the first-line imaging modality for a wide range of conditions due to its simplicity and effectiveness in routine evaluations.[36] The procedure for projectional radiography begins with careful patient positioning to ensure accurate projection and minimize distortion. For example, in a posteroanterior (PA) chest view, the patient stands erect facing the image receptor with the chin raised and shoulders rotated forward to displace the scapulae laterally, allowing optimal visualization of the lungs and heart. Collimation is essential to restrict the X-ray beam to the area of interest, reducing scatter radiation that can degrade image quality and unnecessary patient exposure; typically, the beam is collimated superiorly 5 cm above the shoulders, inferiorly to the 12th rib, and laterally to the acromioclavicular joints for chest imaging. Exposure factors, including kilovoltage peak (kVp) and milliampere-seconds (mAs), are selected based on body part thickness and desired contrast—higher kVp (e.g., 100-110 kVp for chest) penetrates denser tissues while lower mAs (e.g., 4-8 mAs) controls dose for optimal optical density without overexposure. These parameters are adjusted to balance image quality and radiation safety, often using automatic exposure control when available.[37][38][39] Common views in projectional radiography are standardized to target specific anatomical regions and pathologies. The anteroposterior (AP) or PA chest view is routinely used to detect pneumonia, cardiomegaly, or pleural effusions by projecting the thoracic structures onto a single plane. For skull assessment, a lateral view involves positioning the head parallel to the receptor with the interpupillary line perpendicular to it, aiding in the identification of fractures or shifts in intracranial structures. Extremity imaging for fractures often employs AP and lateral projections; for instance, in the wrist, the AP view requires the hand pronated with fingers extended, while the lateral view aligns the forearm and hand in a true lateral position to reveal bone alignment and potential breaks. These orthogonal views help mitigate superimposition and provide comprehensive diagnostic information.[36][40][36] Projectional radiography offers key advantages including rapid acquisition and interpretation, typically within minutes, making it ideal for emergency settings; low cost compared to advanced modalities, with estimates showing it as one of the most economical imaging options; and widespread availability in nearly all healthcare facilities worldwide. It excels in detecting conditions like pneumonia through lung opacity patterns and bone fractures via discontinuity in cortical lines, providing essential initial diagnostic insights without requiring specialized equipment.[41][42][36] Despite its utility, projectional radiography has limitations stemming from its two-dimensional nature, including the overlap of anatomical structures that can obscure pathologies, such as vessels projecting over lung fields in chest images. This superimposition leads to projection artifacts, where depth information is lost, potentially complicating the differentiation of overlapping tissues and requiring additional views for clarification.[43][43] The field has shifted from traditional film-based systems to digital methods, particularly computed radiography (CR), which uses photostimulable phosphor plates to capture and digitize images, eliminating chemical processing and reducing overall time from exposure to availability—often from 20-30 minutes with film to near-instant review. This transition enhances workflow efficiency, enables post-processing adjustments for contrast and brightness, and integrates seamlessly with picture archiving and communication systems (PACS) for storage and sharing, while maintaining comparable diagnostic accuracy to film for most applications.[44][44]Computed Tomography
Computed tomography (CT), also known as computed axial tomography (CAT), is a radiographic imaging technique that utilizes multiple X-ray projections acquired from various angles around the body to reconstruct cross-sectional images, providing detailed three-dimensional views of internal structures. Unlike traditional projectional radiography, which produces two-dimensional shadow images, CT employs a rotating gantry with an X-ray source and detectors to capture attenuation data, enabling the differentiation of tissues based on their density and atomic number. This method was pioneered in the early 1970s and has become essential for volumetric imaging in medical diagnostics.[45][46] The core mechanics of CT involve a motorized table that moves the patient through the gantry while the X-ray tube and detector array rotate synchronously, typically completing a full 360-degree rotation in less than a second in modern systems. Data acquisition occurs in a fan-beam or cone-beam configuration, with projections collected at hundreds of angles per rotation to form a sinogram, a dataset representing the line integrals of X-ray attenuation. Helical (or spiral) scanning enhances efficiency by continuously rotating the gantry while the table advances linearly, creating a corkscrew path of the X-ray beam relative to the patient; this allows for faster coverage of large volumes, such as the entire chest or abdomen, in a single breath-hold and reduces motion artifacts. Image reconstruction primarily relies on filtered back-projection (FBP) algorithms, which correct for the blurring inherent in simple back-projection by applying a ramp filter in the frequency domain to sharpen edges and restore high-frequency details, transforming the projection data into a tomographic image. The resulting images are quantified using the Hounsfield unit (HU) scale, a standardized measure of radiodensity where air is -1000 HU, water is 0 HU, and dense bone approaches +3000 HU, facilitating precise tissue characterization.[46][47][48][49] CT systems have evolved through several generations, each improving speed, resolution, and dose efficiency. First-generation scanners (1970s) used a translate-rotate mechanism with a single pencil-beam X-ray and dual detectors, requiring up to 5 minutes per slice and limited to head imaging. Second-generation systems introduced multiple detectors (up to 10) in a fan-beam setup with linear translation, reducing scan times to 20 seconds per slice. Third-generation scanners, dominant since the 1980s, employ a rotating fan-beam with a curved detector array, achieving sub-5-second rotations and enabling body imaging. Fourth-generation designs use a fixed ring of detectors with a rotating X-ray source, though less common today. Modern multi-slice (or multi-detector row) CT (MSCT), representing fifth-generation advancements since the late 1990s, features 64 or more detector rows, supporting cone-beam geometries for isotropic voxel resolution below 1 mm and simultaneous acquisition of multiple slices per rotation, with 256- or 320-slice systems now allowing whole-organ coverage in one rotation.[26] Clinically, CT excels in evaluating acute conditions like head trauma, where non-contrast scans rapidly detect intracranial hemorrhage, fractures, or edema with high sensitivity. In oncology, it aids cancer staging by delineating tumor extent, lymph node involvement, and distant metastases across the body. Vascular imaging, often enhanced with iodinated contrast, visualizes arterial and venous structures for detecting aneurysms, stenoses, or pulmonary emboli, guiding interventions like stent placement. These applications leverage CT's superior contrast resolution for soft tissues and bones compared to projectional methods.[50][51][52] CT delivers higher radiation doses than projectional radiography—typically 100-800 times that of a single chest X-ray for a full-body scan—due to the multiple projections required for reconstruction, raising concerns for cumulative exposure in repeated exams. To mitigate risks, the ALARA (As Low As Reasonably Achievable) principle guides protocol optimization, incorporating techniques like automatic exposure control, iterative reconstruction to reduce noise at lower doses, and limiting scan ranges to essential anatomy, thereby balancing diagnostic quality with patient safety.[53][54]Fluoroscopy and Real-Time Imaging
Fluoroscopy enables real-time visualization of dynamic anatomical structures by continuously projecting X-rays through the body onto an image receptor, producing a series of low-dose radiographic images akin to a motion picture.[55] This technique is essential for interventional procedures where immediate feedback on motion and positioning is required, differing from static imaging by prioritizing temporal resolution over high spatial detail.[56] In traditional fluoroscopic systems, the image intensifier chain serves as the core component for amplifying the faint X-ray signal into a visible image. Incoming X-rays strike the input phosphor, typically cesium iodide, which converts them into light photons; these photons then interact with the photocathode to release electrons via the photoelectric effect. The electrons are accelerated and focused by an electrostatic lens onto the output phosphor, where they produce a brightened light image that is optically coupled to a television camera for display. To mitigate patient radiation exposure from continuous X-ray beams, modern systems employ pulsed fluoroscopy, where the X-ray tube emits short pulses synchronized with image capture, reducing the overall dose by up to 80% compared to continuous modes while maintaining adequate temporal fidelity.[57] Common clinical applications include guiding catheterizations for vascular interventions, such as angiography and stent placement, where real-time imaging ensures precise navigation through blood vessels.[58] Fluoroscopy also facilitates barium swallow studies to assess swallowing dynamics and esophageal motility by tracking the radiopaque contrast as it moves through the upper gastrointestinal tract.[59] In orthopedics, it supports reductions of fractures or dislocations, allowing surgeons to verify alignment intraoperatively with minimal disruption.[60] Frame rates in fluoroscopy typically range from 7.5 to 30 frames per second (fps), balancing the need for smooth motion depiction against radiation dose and image quality. Higher rates, such as 30 fps, minimize motion blur in fast-moving structures but increase quantum noise and cumulative dose; conversely, lower rates like 7.5 fps reduce dose by limiting exposures while potentially introducing blur or temporal aliasing in dynamic scenes.[61] These trade-offs are managed through automatic brightness control, which adjusts exposure parameters dynamically.[62] Digital flat-panel detectors (FPDs) have largely supplanted traditional image intensifiers in contemporary fluoroscopic systems, offering superior spatial resolution up to 3-5 line pairs per millimeter and reduced geometric distortion due to their rigid, distortion-free structure. Unlike the curved intensifier tube, FPDs use a scintillator layer coupled to a thin-film transistor array for direct digital readout, enabling faster image acquisition and lower electronic noise, which enhances low-contrast detectability in real-time imaging.[63] Hybrid systems integrate fluoroscopy with cone-beam computed tomography (CBCT) to provide intraoperative 3D imaging, where rotational scans from the C-arm generate volumetric reconstructions overlaid on live 2D fluoroscopy for enhanced guidance.[64] This fusion supports precise interventions, such as spinal screw placements, by combining real-time 2D navigation with 3D anatomical context without transferring the patient to a separate CT suite.[65]Contrast-Enhanced Techniques
Contrast-enhanced techniques in radiography involve the administration of exogenous agents to increase the visibility of specific anatomical structures, particularly soft tissues and vascular systems that are otherwise poorly delineated on plain X-ray images. These methods rely on the differential attenuation of X-rays by contrast materials, allowing for detailed imaging of organs such as the gastrointestinal tract, urinary system, and blood vessels. Commonly used in medical diagnostics, these techniques have evolved to minimize risks while improving diagnostic accuracy.[66] Key types of contrast agents include barium sulfate for gastrointestinal studies, iodinated compounds for intravenous and angiographic applications, and historically, air as a negative contrast medium. Barium sulfate, an insoluble suspension with high density, is ingested or administered rectally to opacify the esophagus, stomach, small bowel, and colon during procedures like upper gastrointestinal series or barium enemas. Its inert nature prevents systemic absorption, making it suitable for luminal imaging. Iodinated contrast agents, typically water-soluble organic molecules containing iodine, are injected intravenously or intra-arterially to enhance vascular and parenchymal structures; iodine's high atomic number (Z=53) provides strong X-ray attenuation. Air was used in early pneumoencephalography, where it was introduced into the cerebrospinal fluid spaces to outline brain ventricles as a negative contrast, but this method has been largely abandoned due to its invasiveness and discomfort.[67][68][66][69] Representative procedures utilizing these agents include intravenous pyelography (IVP) and hysterosalpingography. In IVP, iodinated contrast is injected intravenously to assess kidney function, ureteral patency, and bladder anatomy, with images captured as the agent is filtered and excreted into the urinary tract. Hysterosalpingography employs iodinated contrast injected through the cervix to evaluate uterine cavity shape and fallopian tube patency, aiding fertility assessments by detecting blockages or abnormalities. These procedures often combine with fluoroscopy for dynamic visualization.[70][71][72] The primary mechanism of these agents is enhanced X-ray attenuation due to their high atomic numbers and density, which promote photoelectric absorption and Compton scattering, resulting in brighter (positive contrast) or darker (negative contrast) appearances relative to surrounding tissues. For instance, barium (Z=56) and iodine strongly absorb low-energy X-rays, creating clear outlines of vessels, organs, or lumens against softer tissues. This differential absorption improves contrast resolution, enabling the detection of pathologies like tumors, strictures, or occlusions.[73] Despite their utility, contrast agents carry risks, including allergic reactions and nephrotoxicity, necessitating careful patient selection. Iodinated agents can trigger hypersensitivity responses ranging from mild urticaria to anaphylaxis, with incidence rates of approximately 0.04-0.2% for severe reactions; risk factors include prior allergies and asthma. Contrast-induced nephropathy, a form of acute kidney injury, occurs in 5-20% of at-risk patients due to renal vasoconstriction and direct tubular toxicity. Barium sulfate risks are primarily local, such as aspiration pneumonia or bowel obstruction if not cleared. Low-osmolar, non-ionic iodinated agents are preferred over high-osmolar ionic ones to reduce osmolality-related hemodynamic effects and CIN risk by up to 50% in vulnerable populations. Premedication with corticosteroids and antihistamines may mitigate allergic risks in susceptible individuals. While gadolinium-based agents serve similar enhancement roles in MRI, they are not suitable for X-ray radiography due to lower atomic number and different imaging physics.[74][75][76][77][78]Bone Densitometry and Specialized Scans
Bone densitometry techniques, such as dual-energy X-ray absorptiometry (DEXA or DXA), utilize two distinct X-ray energy levels, typically generated at voltages ranging from 40 to 100 kVp, to differentiate between bone mineral content and soft tissue attenuation, thereby enabling precise measurement of bone mineral density (BMD) in units of grams per square centimeter (g/cm²).[79] This method subtracts the lower-energy beam's absorption (more affected by soft tissue) from the higher-energy beam's (less affected) to isolate bone-specific signals, primarily assessing sites like the lumbar spine, proximal femur, and forearm.[80] DEXA remains the gold standard for BMD evaluation due to its low radiation dose (approximately 1-10 μSv per scan) and high precision, with reproducibility errors under 1-2% for repeat measurements.[81] Diagnosis of osteoporosis via DEXA relies on standardized scores derived from BMD comparisons to reference populations. The T-score, calculated as the number of standard deviations (SD) below the mean BMD of young healthy adults, identifies osteoporosis when ≤ -2.5 SD at the spine, femoral neck, or total hip, per World Health Organization (WHO) criteria established in 1994 and reaffirmed in subsequent guidelines.[82] The Z-score, comparing an individual's BMD to age- and sex-matched peers, flags potential secondary causes of bone loss if ≤ -2.0 SD, particularly in premenopausal women or men under 50.[83] These metrics integrate with tools like the Fracture Risk Assessment Tool (FRAX) to predict 10-year fracture probability, enhancing clinical decision-making beyond BMD alone.[79] Beyond central DEXA, peripheral techniques offer accessible alternatives for BMD assessment. Digital X-ray radiogrammetry (DXR) analyzes standard hand radiographs to estimate metacarpal cortical bone thickness and derive BMD (DXR-BMD) in g/cm², correlating strongly (r > 0.9) with central DEXA measurements and providing a low-cost option for longitudinal monitoring.[84] It automates measurements of bone geometry in the second through fourth metacarpals, with precision errors around 0.004 g/cm², making it suitable for pediatric and adult populations where full DEXA access is limited.[85] Peripheral quantitative computed tomography (pQCT), often at the radius or tibia, delivers true volumetric BMD (mg/cm³) by separating cortical and trabecular compartments via 3D imaging, though it involves higher radiation (10-50 μSv) than DEXA.[86] High-resolution variants (HR-pQCT) further quantify microarchitecture, such as trabecular number and cortical porosity, for research into bone quality.[87] These modalities primarily screen for osteoporosis in postmenopausal women aged 65 or older, or earlier if risk factors like glucocorticoid use are present, as recommended by the U.S. Preventive Services Task Force to prevent hip and vertebral fractures.[88] DEXA also monitors treatment efficacy, such as with bisphosphonates (e.g., alendronate), where a 3-5% BMD increase at the spine after 2-3 years indicates response, guiding decisions on therapy continuation or adjustment.[89] DXR and pQCT support similar monitoring in peripheral sites, particularly for patients unable to undergo central scans.[90] A key limitation of DEXA and DXR is their reliance on 2D projection imaging, which conflates bone depth with density and overlooks trabecular bone details, potentially underestimating fragility in conditions like hyperparathyroidism.[91] pQCT mitigates this by providing volumetric data but is confined to appendicular sites, limiting its use for axial skeleton evaluation.[86] Overall, these techniques prioritize fracture risk stratification over comprehensive structural analysis, often complemented by clinical history for holistic assessment.[92]Industrial and Non-Medical Applications
Non-Destructive Testing
Non-destructive testing (NDT) using radiography plays a crucial role in industrial manufacturing by enabling the inspection of material integrity without causing damage to components. This technique employs X-rays or gamma rays to penetrate materials and reveal internal defects such as voids, inclusions, and structural irregularities that could compromise safety or performance. In sectors like oil and gas, aerospace, and heavy engineering, radiographic NDT ensures compliance with quality standards during production and maintenance, preventing failures in critical infrastructure.[93] Key applications include weld inspection in pipelines, where radiography detects incomplete fusion, lack of penetration, and cracks that could lead to leaks or ruptures under pressure. In aerospace manufacturing, it evaluates casting porosity in turbine blades and engine components, identifying gas pockets or shrinkage defects that affect structural strength. Similarly, for aircraft maintenance, radiography assesses corrosion in fuselages and wings, measuring the extent of material degradation to guide repairs without disassembly. These inspections are vital for high-stakes environments, allowing operators to verify component reliability before deployment.[94][95][96] Techniques in radiographic NDT often utilize gamma ray sources for penetrating thick materials; iridium-192 (Ir-192) is commonly applied to steel up to 75 mm thick due to its energy range of 0.14 to 0.66 MeV, while cobalt-60 (Co-60) handles denser sections up to 200 mm with higher energies around 1.17 and 1.33 MeV. For dynamic processes, real-time radioscopy employs continuous X-ray beams and digital detectors to provide live imaging on assembly lines, facilitating rapid defect detection during automated manufacturing of automotive or electronic parts. This method supports on-line process control, reducing downtime compared to static film-based approaches.[97][98] Standards govern radiographic NDT to ensure consistent quality and sensitivity. ASTM E94 provides guidelines for radiographic examination using film, specifying requirements for image quality, exposure techniques, and processing to achieve reliable defect visibility. For weld-specific inspections, ISO 17636 outlines radiographic testing procedures for fusion-welded joints, including acceptance criteria for indications like cracks and porosity based on material thickness and joint type. Adherence to these standards is mandatory in certified operations to validate results across industries.[99] Radiographic NDT offers distinct advantages, including the creation of permanent visual records for archival review, auditing, and legal documentation of inspections. It excels at detecting volumetric flaws such as internal cracks, voids, and inclusions that surface methods might miss, providing a comprehensive assessment of material volume. These capabilities make it indispensable for ensuring the longevity and safety of manufactured goods.[100][101] The shift to digital methods, particularly computed radiography (CR), has accelerated NDT efficiency in oil and gas sectors by replacing film with reusable phosphor plates that produce high-resolution digital images in minutes rather than hours. This transition enables faster on-site inspections of pipelines and rigs, reducing processing time by up to 90% and minimizing chemical waste, while maintaining or improving defect resolution for volumetric analysis.[102][103]Security and Material Inspection
Radiography plays a critical role in security applications, particularly for screening baggage, cargo, and vehicles at airports, borders, and checkpoints to detect concealed threats without physical intrusion.[104] These systems employ X-ray imaging to produce detailed views of contents, enabling operators to identify anomalies such as weapons or explosives.[105] In airport settings, dual-view X-ray scanners are widely used, providing two angled perspectives of luggage to enhance detection accuracy.[104] These systems often incorporate dual-energy techniques, utilizing two X-ray energy levels to differentiate organic materials (like plastics or explosives) from inorganic ones (such as metals) based on their absorption characteristics.[106] Additionally, backscatter X-ray technology complements transmission imaging by scattering X-rays off surfaces, revealing hidden items on or near the exterior of bags or packages with high resolution.[107] For cargo and border security, high-energy radiography systems rely on linear accelerators to generate X-rays in the MeV range, capable of penetrating dense materials like steel containers.[108] These accelerators accelerate electrons to 3-9 MeV, producing intense X-ray beams that allow non-intrusive scanning of full truckloads or shipping containers without unloading.[109] Such systems facilitate rapid inspection at ports and borders, visualizing dense cargo interiors to uncover smuggling or threats.[110] Threat detection in these radiographic systems is augmented by automated algorithms that analyze images for potential hazards like explosives or weapons.[111] These algorithms employ artificial intelligence and deep learning to perform real-time detection, flagging suspicious shapes or densities for human review.[112] Material discrimination is achieved through effective atomic number (Z_eff) estimation, which classifies substances by their X-ray attenuation properties, distinguishing low-Z organics (e.g., explosives) from high-Z inorganics (e.g., metals).[113] This approach improves accuracy in cluttered environments, reducing false alarms.[114] Portable radiographic units extend security capabilities to field operations, including forensics and checkpoint vehicle scans.[107] Handheld devices, often backscatter-based, allow investigators to scan suspicious packages or surfaces in real-time during forensic examinations.[115] Mobile vehicle scanners, deployable at checkpoints, use compact X-ray sources to image undercarriages or interiors of cars and vans for hidden contraband.[116] These units prioritize mobility and quick setup for tactical scenarios.[107] Privacy and safety are paramount in security radiography, with systems designed to deliver minimal radiation doses to operators and bystanders. The U.S. Food and Drug Administration (FDA) sets safety standards limiting the maximum permissible dose for general-use X-ray security systems to 0.25 μSv per screening.[117] These guidelines ensure no measurable health risks from routine operations, aligned with principles for ionizing radiation exposure.Equipment and Components
X-ray Sources
X-ray sources are essential components in radiography, responsible for generating the high-energy photon beams used to produce diagnostic and industrial images. In conventional systems, these sources primarily consist of X-ray tubes that accelerate electrons to strike a target anode, producing bremsstrahlung and characteristic radiation through electron interactions.[118] The design and operation of these tubes vary based on application demands, such as heat dissipation and beam intensity.[119] Two main types of X-ray tubes dominate radiographic applications: stationary anode and rotating anode designs. Stationary anode tubes, featuring a fixed tungsten target, are suited for low-power scenarios like dental imaging and portable units, where exposure times are short and heat loads are minimal, typically operating below 100 kV.[120] In contrast, rotating anode tubes, which spin the anode disk at speeds up to 10,000 RPM to distribute heat across a larger surface area, enable higher power outputs and prolonged exposures essential for medical computed tomography (CT) and fluoroscopy, handling loads exceeding 100 kW.[119] This rotation, often driven by an induction motor within the tube envelope, significantly extends tube lifespan in high-throughput clinical settings.[120] Key parameters of X-ray tubes influence beam characteristics and image quality. The focal spot size, defined as the area on the anode where electrons impact, typically ranges from 0.1 to 2 mm and directly affects spatial resolution; smaller spots reduce geometric unsharpness but limit power due to increased heat concentration.[121] Beam filtration, using materials like aluminum (1-3 mm thick) or copper (0.1-0.5 mm), removes low-energy photons to harden the spectrum, reducing patient dose while minimizing soft tissue contrast loss from beam hardening artifacts.[122] Aluminum is common for general radiography, while copper provides finer control in CT for deeper penetration.[123] Spectrum control is achieved primarily through kilovoltage peak (kVp) selection, which determines beam penetration and energy distribution. In medical radiography, kVp settings of 50-150 are standard, with lower values (e.g., 60-80 kVp) for extremities to enhance soft tissue contrast and higher (100-150 kVp) for thoracic imaging to ensure adequate penetration through dense structures.[124] Industrial applications often require energies above 1 MeV for inspecting thick materials like welds or cargo, achieved via specialized tubes or accelerators.[125] Alternative X-ray sources extend capabilities beyond conventional tubes. Synchrotrons, large-scale accelerators producing tunable, coherent beams from bending magnets or undulators, are used in research radiography for high-resolution imaging of biological samples, offering flux densities orders of magnitude higher than lab sources with energies from keV to MeV.[126] Linear accelerators (linacs), which accelerate electrons in a straight-line waveguide to energies of 4-10 MeV before conversion to X-rays via a tungsten target, serve industrial and security needs, such as non-destructive testing of aircraft components or container scanning, due to their high output and portability in some designs.[127] Maintenance of X-ray tubes is critical to prevent failures like arcing or pitting. Vacuum seals, typically glass-to-metal or ceramic-metal joints, must be monitored for slow leaks that degrade the high-vacuum environment (10^{-6} Torr or better) needed for electron acceleration, often checked via pressure gauges during routine servicing.[119] Cooling systems, including oil-immersed baths or water-circulating jackets for rotating anodes, dissipate up to 1 MJ of heat per exposure by maintaining temperatures below 2,000°C at the focal spot, with regular fluid replacement and flow verification to avoid thermal runaway.[128]Image Detectors and Grids
Image detectors in radiography capture the remnant X-ray beam after it passes through the patient, converting it into a visible or digital image while minimizing noise and distortion. Traditional analog systems rely on film-screen combinations, whereas modern digital detectors offer improved efficiency and flexibility. These detectors are essential for achieving diagnostic image quality, with performance evaluated through key metrics such as detective quantum efficiency (DQE) and modulation transfer function (MTF).[129] In film-screen radiography, the detector consists of a radiographic film coated with a silver halide emulsion, typically silver bromide or iodobromide crystals suspended in gelatin on a flexible polyester base. These crystals absorb X-ray photons or visible light to form a latent image through the reduction of silver ions, which is then developed chemically into a visible density pattern. To enhance sensitivity and reduce patient dose, intensifying screens made of calcium tungstate (CaWO₄) or rare-earth phosphors are paired with the film; these screens fluoresce upon X-ray absorption, emitting visible light (primarily blue or green) that exposes about 95% of the silver halide crystals, amplifying the signal by a factor of 50-100 compared to direct exposure.[130][131][130] Digital detectors have largely replaced analog systems, divided into indirect and direct conversion types. Indirect detectors use a scintillator layer, such as cesium iodide (CsI:Tl), to convert X-rays into visible light photons, which are then detected by a thin-film transistor (TFT) array of photodiodes, typically amorphous silicon, to generate electrical charge stored as digital signals. This two-step process allows high absorption efficiency (up to 70-80% for CsI) but introduces potential light scatter that can degrade resolution. Direct detectors, in contrast, employ a photoconductor like amorphous selenium (a-Se), which directly converts X-ray photons into electron-hole pairs under an applied electric field, producing charge that is collected by TFT electrodes without intermediate light conversion, thereby preserving higher spatial resolution.[132][133][134] Antiscatter grids are physical barriers placed between the patient and detector to improve image contrast by absorbing scattered X-rays that would otherwise fog the image. Parallel grids feature lead strips aligned straight and perpendicular to the detector, suitable for a wide range of source-to-image distances but prone to cutoff artifacts at beam edges due to the diverging X-ray beam. Focused grids, more commonly used, have lead strips angled to match the beam's divergence at a specific focal distance (e.g., 100-180 cm), minimizing off-focus radiation and grid lines while enhancing primary beam transmission. Grid ratios, defined as the height of lead strips to the distance between them, range from 5:1 for low-kVp applications like mammography to 16:1 for high-kVp exams, with higher ratios rejecting more scatter (up to 90%) but requiring precise alignment. The use of grids increases patient dose by the Bucky factor, typically 2-5 times, as more primary radiation is needed to compensate for absorbed scatter and lead attenuation.[135][135][135] Detector performance is quantified by DQE, which measures the fraction of incident X-ray quanta contributing useful signal relative to an ideal detector, accounting for sensitivity, noise, and resolution; values range from 0 to 1, with modern digital systems achieving 0.3-0.7 at low frequencies for better low-dose imaging. MTF assesses spatial sharpness by describing how well the detector preserves contrast at different spatial frequencies (cycles/mm), derived from the point spread function; high MTF at high frequencies (e.g., >10 lp/mm) is crucial for fine detail, with direct a-Se detectors often outperforming indirect ones due to reduced spread.[129][129] The transition from analog film-screen to digital radiography (DR) has significantly reduced retake rates, from 10-35% in screen-film systems—often due to exposure errors—to near 0-5% in DR, thanks to wider dynamic range and post-acquisition adjustments that tolerate underexposure or overexposure without loss of diagnostic utility. This shift, accelerated since the early 2000s, enhances workflow efficiency while maintaining or lowering overall radiation doses when optimized.[136][137]Ancillary Devices
Ancillary devices in radiography encompass a range of supportive tools that optimize procedural accuracy, minimize patient exposure, and ensure image reliability without directly contributing to radiation generation or detection. These devices facilitate precise beam control, patient stabilization, anatomical identification, tissue compression, and system calibration, collectively enhancing diagnostic outcomes while adhering to safety protocols. Collimators, typically constructed from lead shutters, are essential for restricting the x-ray beam to the specific anatomical region of interest. By limiting the field size, collimators significantly reduce scatter radiation, which can degrade image contrast, and thereby lower the overall radiation dose to the patient. [138] This dose reduction is particularly vital in procedures involving sensitive areas, where improper collimation can increase unnecessary exposure without improving diagnostic utility. [139] Positioning aids, such as sponges, sandbags, and film cassettes, play a critical role in immobilizing patients to prevent motion artifacts that compromise image sharpness. Sponges and sandbags provide non-invasive support to maintain limb or body alignment during exposure, especially in pediatric or uncooperative patients, ensuring reproducible positioning across serial studies. [140] In traditional film-based systems, cassettes securely hold the radiographic film or phosphor plates in place, protecting them from light exposure while facilitating efficient image capture. [141] Lead markers are small, radiopaque identifiers embedded with letters and numbers, placed on the image during exposure to denote anatomical sides (right/left), procedure date, and technician initials. These markers prevent laterality errors in interpretation, which could lead to misdiagnosis, and provide essential metadata for record-keeping and legal documentation. [142] Their use is a standard practice in digital and analog radiography to maintain traceability and compliance with quality assurance standards. Compression bands, often implemented as adjustable paddles in mammography units, evenly distribute pressure to flatten and thin the breast tissue. This reduction in thickness—typically by 1 to 2 cm—improves x-ray penetration uniformity, enhances contrast between tissue types, and minimizes motion blur during the brief exposure time. [143] By stabilizing the breast, these devices also contribute to lower radiation doses required for adequate image quality. Quality control phantoms are standardized test objects designed to simulate human tissue properties for routine calibration of radiographic systems. These phantoms incorporate patterns to assess low-contrast detectability and high-resolution limits, enabling technicians to verify system performance metrics such as spatial resolution (often up to 4-5 line pairs per millimeter) and contrast sensitivity. [144] Regular phantom imaging ensures consistent output, detects degradation in components, and supports accreditation requirements by quantifying deviations in image quality parameters. [145]Image Formation and Quality
Factors Influencing Image Quality
Image quality in radiography is determined by a combination of geometric, subject-related, and technical factors that affect sharpness, contrast, and noise levels, ultimately influencing diagnostic accuracy. These elements must be optimized during image acquisition to ensure clear visualization of anatomical structures without introducing unwanted degradations. Geometric factors play a critical role in controlling magnification and sharpness. The source-to-image distance (SID), typically ranging from 100 cm for tabletop procedures to 180 cm for chest imaging, reduces geometric unsharpness and scatter radiation when increased, though it requires compensatory adjustments in exposure to maintain adequate photon flux.[146] The object-to-image distance (OID) directly impacts magnification, with smaller OID values minimizing enlargement and associated blurring of structures, as magnification increases with the ratio of OID to SID, approximated as 1 + (OID/SID) for small values.[147] Subject-related factors, including patient motion and tissue thickness, can degrade image clarity. Motion blur occurs when involuntary or respiratory movements exceed the exposure time, resulting in loss of edge definition, particularly in areas like the lungs or abdomen.[147] Variations in body part thickness alter x-ray attenuation, necessitating adjustments in technique factors; thicker regions require higher kilovoltage peak (kVp) to penetrate adequately while maintaining exposure latitude—the range of densities that produce a usable image. Balancing kVp and milliampere-seconds (mAs) is essential, as higher kVp increases exposure latitude by reducing subject contrast, allowing better visualization across varying tissue densities, while mAs primarily controls overall density without significantly altering latitude.[147][148] Noise sources further compromise image quality by introducing random variations that obscure subtle details. Quantum mottle, arising from insufficient photon numbers in low-mAs exposures, manifests as grainy patterns and is the primary noise in analog and digital systems, reducible by increasing mAs to enhance photon statistics.[147] In digital radiography, electronic noise from detector readout circuits and amplifiers adds a baseline hiss, particularly noticeable at low signal levels, and can be mitigated through improved detector design but remains a factor in high-resolution imaging.[149] Artifacts represent unintended image features that can mimic or obscure pathology. Grid lines appear as periodic stripes when stationary antiscatter grids are misaligned or used with incompatible digital sampling rates, reducing contrast in the affected regions.[150] Foreign bodies, such as metallic objects or dense materials within or external to the patient, produce superimposed radiopaque shadows that distort underlying anatomy.[151] Distortion types include foreshortening, where structures appear compressed due to excessive tube angulation toward the image receptor, and elongation, resulting from insufficient angulation causing apparent lengthening of objects.[3] Key metrics quantify these influences for objective evaluation. Signal-to-noise ratio (SNR) measures the strength of the useful signal relative to noise, improving with higher mAs and thus better delineating low-contrast features like soft tissues.[147] Contrast-to-noise ratio (CNR) assesses the separability of adjacent structures, incorporating both inherent contrast (affected by kVp) and noise levels, with higher values indicating superior diagnostic utility.[147]Processing and Enhancement Methods
In analog radiography, the processing of exposed film relies on wet chemistry techniques to convert the latent image into a visible radiograph. The development stage involves immersing the film in a developer solution, which reduces exposed silver halide crystals to metallic silver grains, thereby creating areas of varying optical density that form the image.[152] Following development, the fixing process uses a fixer bath containing sodium thiosulfate to dissolve and remove unexposed silver halide crystals, halting further reaction and stabilizing the image for archival purposes.[152] These steps must be performed under controlled temperature and time conditions to ensure consistent results, with automatic processors often integrating development, fixing, washing, and drying in a continuous sequence.[153] A key tool for evaluating analog film performance is the characteristic curve, or Hurter and Driffield (H&D) curve, which plots optical density against the logarithm of exposure to quantify the film's response.[154] This S-shaped curve delineates regions of low contrast (toe and shoulder), high contrast (straight-line portion), and overall latitude, enabling assessment of density range and contrast for optimizing exposure techniques in clinical settings.[154] Base-plus-fog density typically measures 0.1–0.2, while maximum density (Dmax) depends on emulsion thickness and processing efficiency.[154] Transitioning to digital radiography, post-acquisition processing employs algorithms to refine raw pixel data for enhanced interpretability without altering the underlying exposure. Window and level adjustments, implemented via grayscale value-of-interest (VOI) look-up tables, remap pixel values to control image brightness (level) and contrast range (width), allowing radiologists to highlight specific anatomical structures interactively.[155] Edge enhancement techniques, such as unsharp masking or 1/MTF filtering, amplify high-frequency components to sharpen boundaries and fine details, compensating for detector blur while applying noise suppression at higher frequencies to avoid exaggerating quantum mottle.[155] Noise reduction in digital images often utilizes spatial filters like the Gaussian kernel, which convolves neighboring pixels with a bell-shaped weighting to smooth random fluctuations, particularly Poisson-Gaussian noise inherent in low-dose acquisitions.[156] Adaptive variants, such as Bayesian wavelet coring, further preserve edges by thresholding high-pass bands, improving low-contrast visibility in areas like soft tissues.[155] For global contrast optimization, histogram equalization redistributes intensity values across the full dynamic range, stretching the histogram to enhance underexposed or overexposed regions without introducing artifacts.[157] In practice, contrast-limited adaptive histogram equalization (CLAHE) processes local blocks to prevent over-amplification of noise, yielding superior bony and soft-tissue delineation in x-ray images compared to manual adjustments.[157] Integration with Picture Archiving and Communication Systems (PACS) standardizes digital processing through the Digital Imaging and Communications in Medicine (DICOM) protocol, facilitating seamless storage, retrieval, and viewing of radiographic images.[158] DICOM enables workstation-based annotations, such as measurements and coded observations linked to image coordinates via Structured Reporting, supporting collaborative interpretation while maintaining data integrity across networks.[158] Post-2020 advancements in artificial intelligence (AI) have introduced automated tools for processing enhancement, including deep learning models for lesion detection that analyze pixel patterns to flag abnormalities like fractures or tumors with high sensitivity.[159] Dose-aware processing leverages generative adversarial networks and denoising autoencoders to reconstruct high-quality images from low-radiation acquisitions, reducing patient exposure by up to 50% while preserving diagnostic fidelity through AI-optimized noise suppression and contrast restoration.[160] These AI methods, often integrated into PACS workflows, prioritize clinically validated outputs to augment rather than replace radiologist oversight.[160]Radiation Safety and Dosimetry
Dose Quantification
Dose quantification in radiography involves measuring and expressing the amount of ionizing radiation absorbed by tissues to assess potential biological risks. The primary units used are the absorbed dose, measured in grays (Gy), which quantifies the energy deposited per unit mass of tissue (1 Gy = 1 joule per kilogram).[161] For X-rays used in radiography, the equivalent dose in sieverts (Sv) is numerically equal to the absorbed dose in Gy because the radiation weighting factor for photons is 1.[162] The effective dose, also in Sv, further accounts for varying sensitivities of different tissues by applying tissue weighting factors, providing a measure of overall stochastic risk comparable across exposures.[163] Common methods for measuring dose in radiography include personal dosimeters for occupational exposure and procedure-specific metrics for patients. Thermoluminescent dosimeters (TLDs), which use crystals that emit light upon heating proportional to absorbed dose, and film badges, which darken based on radiation exposure, are widely used to monitor personnel doses.[164] For patient doses, computed tomography dose index (CTDI), measured in mGy, assesses average dose in a scanned volume for CT scans, while dose area product (DAP), in Gy·cm², quantifies total radiation output in projectional radiography by multiplying air kerma by beam area.[165] Typical effective doses in diagnostic radiography are low compared to annual background radiation, which averages about 3 mSv globally from natural sources. A standard posteroanterior chest X-ray delivers approximately 0.1 mSv, equivalent to about 10 days of background exposure.[166] In contrast, an abdominal CT scan imparts around 10 mSv, roughly equivalent to three years of background radiation.[167] Several factors influence radiation dose in radiographic procedures. Exposure time directly affects dose, as longer durations increase photon fluence; techniques like automatic exposure control minimize this by adjusting based on patient attenuation. Distance from the source follows the inverse square law, where dose intensity decreases with the square of the distance, emphasizing the importance of positioning to reduce exposure.[164] Tissue weighting factors in effective dose calculations reflect radiosensitivity: for example, gonads have a factor of 0.08, while bone marrow is 0.12, highlighting higher risks to hematopoietic tissues.[168]| Tissue/Organ | Tissue Weighting Factor (w_T, ICRP 103) |
|---|---|
| Bone marrow (red) | 0.12 |
| Gonads | 0.08 |
| Colon | 0.12 |
| Lung | 0.12 |
| Stomach | 0.12 |

