Recent from talks
Nothing was collected or created yet.
Fuel cell
View on WikipediaThis article needs to be updated. (February 2021) |

A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen)[1] into electricity through a pair of redox reactions.[2] Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery.[3] Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.
The first fuel cells were invented by Sir William Grove in 1838. The first commercial use of fuel cells came almost a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for satellites and space capsules. Since then, fuel cells have been used in many other applications. Fuel cells are used for primary and backup power for commercial, industrial and residential buildings and in remote or inaccessible areas. They are also used to power fuel cell vehicles, including forklifts, automobiles, buses,[4] trains, boats, motorcycles, and submarines.
There are many types of fuel cells, but they all consist of an anode, a cathode, and an electrolyte that allows ions, often positively charged hydrogen ions (protons), to move between the two sides of the fuel cell. At the anode, a catalyst causes the fuel to undergo oxidation reactions that generate ions (often positively charged hydrogen ions) and electrons. The ions move from the anode to the cathode through the electrolyte. At the same time, electrons flow from the anode to the cathode through an external circuit, producing direct current electricity. At the cathode, another catalyst causes ions, electrons, and oxygen to react, forming water and possibly other products. Fuel cells are classified by the type of electrolyte they use and by the difference in start-up time ranging from 1 second for proton-exchange membrane fuel cells (PEM fuel cells, or PEMFC) to 10 minutes for solid oxide fuel cells (SOFC). A related technology is flow batteries, in which the fuel can be regenerated by recharging. Individual fuel cells produce relatively small electrical potentials, about 0.7 volts, so cells are "stacked", or placed in series, to create sufficient voltage to meet an application's requirements.[5] In addition to electricity, fuel cells produce water vapor, heat and, depending on the fuel source, very small amounts of nitrogen dioxide and other emissions. PEMFC cells generally produce fewer nitrogen oxides than SOFC cells: they operate at lower temperatures, use hydrogen as fuel, and limit the diffusion of nitrogen into the anode via the proton exchange membrane, which forms NOx. The energy efficiency of a fuel cell is generally between 40 and 60%; however, if waste heat is captured in a cogeneration scheme, efficiencies of up to 85% can be obtained.[6]
History
[edit]

The first references to hydrogen fuel cells appeared in 1838. In a letter dated October 1838 but published in the December 1838 edition of The London and Edinburgh Philosophical Magazine and Journal of Science, Welsh physicist and barrister Sir William Grove wrote about the development of his first crude fuel cells. He used a combination of sheet iron, copper, and porcelain plates, and a solution of sulphate of copper and dilute acid.[7][8] In a letter to the same publication written in December 1838 but published in June 1839, German physicist Christian Friedrich Schönbein discussed the first crude fuel cell that he had invented. His letter discussed the current generated from hydrogen and oxygen dissolved in water.[9] Grove later sketched his design, in 1842, in the same journal. The fuel cell he made used similar materials to today's phosphoric acid fuel cell.[10][11]
In 1932, English engineer Francis Thomas Bacon successfully developed a 5 kW stationary fuel cell.[12] NASA used the alkaline fuel cell (AFC), also known as the Bacon fuel cell after its inventor, from the mid-1960s.[12][13]
In 1955, W. Thomas Grubb, a chemist working for the General Electric Company (GE), further modified the original fuel cell design by using a sulphonated polystyrene ion-exchange membrane as the electrolyte. Three years later another GE chemist, Leonard Niedrach, devised a way of depositing platinum onto the membrane, which served as a catalyst for the necessary hydrogen oxidation and oxygen reduction reactions. This became known as the "Grubb-Niedrach fuel cell".[14][15] GE went on to develop this technology with NASA and McDonnell Aircraft, leading to its use during Project Gemini. This was the first commercial use of a fuel cell. In 1959, a team led by Harry Ihrig built a 15 kW fuel cell tractor for Allis-Chalmers, which was demonstrated across the U.S. at state fairs. This system used potassium hydroxide as the electrolyte and compressed hydrogen and oxygen as the reactants. Later in 1959, Bacon and his colleagues demonstrated a practical five-kilowatt unit capable of powering a welding machine. In the 1960s, Pratt & Whitney licensed Bacon's U.S. patents for use in the U.S. space program to supply electricity and drinking water (hydrogen and oxygen being readily available from the spacecraft tanks).
UTC Power was the first company to manufacture and commercialize a large, stationary fuel cell system for use as a cogeneration power plant in hospitals, universities and large office buildings.[16]
In recognition of the fuel cell industry and America's role in fuel cell development, the United States Senate recognized October 8, 2015 as National Hydrogen and Fuel Cell Day, passing S. RES 217. The date was chosen in recognition of the atomic weight of hydrogen (1.008).[17]
Types of fuel cells; design
[edit]Fuel cells come in many varieties; however, they all work in the same general manner. They are made up of three adjacent segments: the anode, the electrolyte, and the cathode. Two chemical reactions occur at the interfaces of the three different segments. The net result of the two reactions is that fuel is consumed, water or carbon dioxide is created, and an electric current is created, which can be used to power electrical devices, normally referred to as the load.
At the anode a catalyst ionizes the fuel, turning the fuel into a positively charged ion and a negatively charged electron. The electrolyte is a substance specifically designed so ions can pass through it, but the electrons cannot. The freed electrons travel through a wire creating an electric current. The ions travel through the electrolyte to the cathode. Once reaching the cathode, the ions are reunited with the electrons and the two react with a third chemical, usually oxygen, to create water or carbon dioxide.
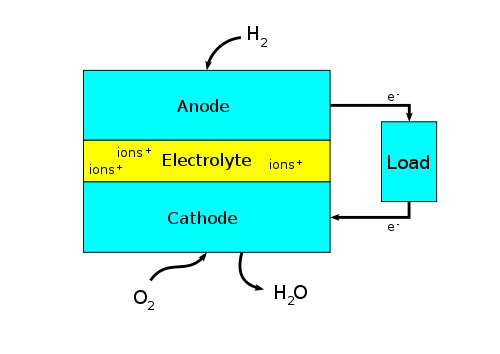
Design features in a fuel cell include:
- The electrolyte substance, which usually defines the type of fuel cell, and can be made from a number of substances like potassium hydroxide, salt carbonates, and phosphoric acid.[18]
- The most common fuel that is used is hydrogen.
- The anode catalyst, usually fine platinum powder, breaks down the fuel into electrons and ions.
- The cathode catalyst, often nickel, converts ions into waste chemicals, with water being the most common type of waste.[19]
- Gas diffusion layers that are designed to resist oxidization.[19]
A typical fuel cell produces a voltage from 0.6 to 0.7 V at a full-rated load. Voltage decreases as current increases, due to several factors:
- Activation loss
- Ohmic loss (voltage drop due to resistance of the cell components and interconnections)
- Mass transport loss (depletion of reactants at catalyst sites under high loads, causing rapid loss of voltage).[20]
To deliver the desired amount of energy, the fuel cells can be combined in series to yield higher voltage, and in parallel to allow a higher current to be supplied. Such a design is called a fuel cell stack. The cell surface area can also be increased, to allow higher current from each cell.
Proton-exchange membrane fuel cells
[edit]


In the archetypical hydrogen–oxide proton-exchange membrane fuel cell (PEMFC) design, a proton-conducting polymer membrane (typically nafion) contains the electrolyte solution that separates the anode and cathode sides.[24][25] This was called a solid polymer electrolyte fuel cell (SPEFC) in the early 1970s, before the proton-exchange mechanism was well understood. (Notice that the synonyms polymer electrolyte membrane and proton-exchange mechanism result in the same acronym.)
On the anode side, hydrogen diffuses to the anode catalyst where it later dissociates into protons and electrons. These protons often react with oxidants causing them to become what are commonly referred to as multi-facilitated proton membranes. The protons are conducted through the membrane to the cathode, but the electrons are forced to travel in an external circuit (supplying power) because the membrane is electrically insulating. On the cathode catalyst, oxygen molecules react with the electrons (which have traveled through the external circuit) and protons to form water.
In addition to this pure hydrogen type, there are hydrocarbon fuels for fuel cells, including diesel, methanol (see: direct-methanol fuel cells and indirect methanol fuel cells) and chemical hydrides. The waste products with these types of fuel are carbon dioxide and water. When hydrogen is used, the CO2 is released when methane from natural gas is combined with steam, in a process called steam methane reforming, to produce the hydrogen. This can take place in a different location to the fuel cell, potentially allowing the hydrogen fuel cell to be used indoors—for example, in forklifts.
The different components of a PEMFC are
- bipolar plates,
- electrodes,
- catalyst,
- membrane, and
- the necessary hardware such as current collectors and gaskets.[26]
The materials used for different parts of the fuel cells differ by type. The bipolar plates may be made of different types of materials, such as, metal, coated metal, graphite, flexible graphite, C–C composite, carbon–polymer composites etc.[27] The membrane electrode assembly (MEA) is referred to as the heart of the PEMFC and is usually made of a proton-exchange membrane sandwiched between two catalyst-coated carbon papers. Platinum and/or similar types of noble metals are usually used as the catalyst for PEMFC, and these can be contaminated by carbon monoxide, necessitating a relatively pure hydrogen fuel.[28] The electrolyte could be a polymer membrane.
Proton-exchange membrane fuel cell design issues
[edit]- Cost
- In 2013, the Department of Energy estimated that 80 kW automotive fuel cell system costs of US$67 per kilowatt could be achieved, assuming volume production of 100,000 automotive units per year and US$55 per kilowatt could be achieved, assuming volume production of 500,000 units per year.[29] Many companies are working on techniques to reduce cost in a variety of ways including reducing the amount of platinum needed in each individual cell. Ballard Power Systems has experimented with a catalyst enhanced with carbon silk, which allows a 30% reduction (1.0–0.7 mg/cm2) in platinum usage without reduction in performance.[30] Monash University, Melbourne uses PEDOT as a cathode.[31] A 2011-published study[32] documented the first metal-free electrocatalyst using relatively inexpensive doped carbon nanotubes, which are less than 1% the cost of platinum and are of equal or superior performance. A recently published article demonstrated how the environmental burdens change when using carbon nanotubes as carbon substrate for platinum.[33]
- Water and air management[34][35] (in PEMFCs)
- In this type of fuel cell, the membrane must be hydrated, requiring water to be evaporated at precisely the same rate that it is produced. If water is evaporated too quickly, the membrane dries, the resistance across it increases, and eventually, it will crack, creating a gas "short circuit" where hydrogen and oxygen combine directly, generating heat that will damage the fuel cell. If the water is evaporated too slowly, the electrodes will flood, preventing the reactants from reaching the catalyst and stopping the reaction. Methods to manage water in cells are being developed like electroosmotic pumps focusing on flow control. Just as in a combustion engine, a steady ratio between the reactant and oxygen is necessary to keep the fuel cell operating efficiently.
- Temperature management
- The same temperature must be maintained throughout the cell in order to prevent destruction of the cell through thermal loading. This is particularly challenging as the 2H2 + O2 → 2H2O reaction is highly exothermic, so a large quantity of heat is generated within the fuel cell.
- Durability, service life, and special requirements for some type of cells
- Stationary fuel-cell applications typically require more than 40,000 hours of reliable operation at a temperature of −35 to 40 °C (−31 to 104 °F), while automotive fuel cells require a 5,000-hour lifespan (the equivalent of 240,000 km or 150,000 miles) under extreme temperatures. Current service life is 2,500 hours (about 120,000 km or 75,000 mi).[36] Automotive engines must also be able to start reliably at −30 °C (−22 °F) and have a high power-to-volume ratio (typically 2.5 kW/L).
- Limited carbon monoxide tolerance of some (non-PEDOT) cathodes.[28]
Phosphoric acid fuel cell
[edit]Phosphoric acid fuel cells (PAFCs) were first designed and introduced in 1961 by G. V. Elmore and H. A. Tanner. In these cells, phosphoric acid is used as a non-conductive electrolyte to pass protons from the anode to the cathode and to force electrons to travel from anode to cathode through an external electrical circuit. These cells commonly work in temperatures of 150 to 200 °C. This high temperature will cause heat and energy loss if the heat is not removed and used properly. This heat can be used to produce steam for air conditioning systems or any other thermal energy-consuming system.[37] Using this heat in cogeneration can enhance the efficiency of phosphoric acid fuel cells from 40 to 50% to about 80%.[37] Since the proton production rate on the anode is small, platinum is used as a catalyst to increase this ionization rate. A key disadvantage of these cells is the use of an acidic electrolyte. This increases the corrosion or oxidation of components exposed to phosphoric acid.[38]
Solid acid fuel cell
[edit]Solid acid fuel cells (SAFCs) are characterized by the use of a solid acid material as the electrolyte. At low temperatures, solid acids have an ordered molecular structure like most salts. At warmer temperatures (between 140 and 150 °C for CsHSO4), some solid acids undergo a phase transition to become highly disordered "superprotonic" structures, which increases conductivity by several orders of magnitude. SAFC systems use cesium dihydrogen phosphate (CsH2PO4) and have demonstrated lifetimes in the thousands of hours.[39]
Alkaline fuel cell
[edit]The alkaline fuel cell (AFC) or hydrogen-oxygen fuel cell was designed and first demonstrated publicly by Francis Thomas Bacon in 1959. It was used as a primary source of electrical energy in the Apollo space program.[40] The cell consists of two porous carbon electrodes impregnated with a suitable catalyst such as Pt, Ag, CoO, etc. The space between the two electrodes is filled with a concentrated solution of KOH or NaOH which serves as an electrolyte. H2 gas and O2 gas are bubbled into the electrolyte through the porous carbon electrodes. Thus the overall reaction involves the combination of hydrogen gas and oxygen gas to form water. The cell runs continuously until the reactant's supply is exhausted. This type of cell operates efficiently in the temperature range 343–413 K (70 -140 °C) and provides a potential of about 0.9 V.[41] Alkaline anion exchange membrane fuel cell (AAEMFC) is a type of AFC which employs a solid polymer electrolyte instead of aqueous potassium hydroxide (KOH) and it is superior to aqueous AFC.
High-temperature fuel cells
[edit]Solid oxide fuel cell
[edit]Solid oxide fuel cells (SOFCs) use a solid material, most commonly a ceramic material called yttria-stabilized zirconia (YSZ), as the electrolyte. Because SOFCs are made entirely of solid materials, they are not limited to the flat plane configuration of other types of fuel cells and are often designed as rolled tubes. They require high operating temperatures (800–1000 °C) and can be run on a variety of fuels including natural gas.[6]
SOFCs are unique because negatively charged oxygen ions travel from the cathode (positive side of the fuel cell) to the anode (negative side of the fuel cell) instead of protons travelling vice versa (i.e., from the anode to the cathode), as is the case in all other types of fuel cells. Oxygen gas is fed through the cathode, where it absorbs electrons to create oxygen ions. The oxygen ions then travel through the electrolyte to react with hydrogen gas at the anode. The reaction at the anode produces electricity and water as by-products. Carbon dioxide may also be a by-product depending on the fuel, but the carbon emissions from a SOFC system are less than those from a fossil fuel combustion plant.[42] The chemical reactions for the SOFC system can be expressed as follows:[43]
- Anode reaction: 2H2 + 2O2− → 2H2O + 4e−
- Cathode reaction: O2 + 4e− → 2O2−
- Overall cell reaction: 2H2 + O2 → 2H2O
SOFC systems can run on fuels other than pure hydrogen gas. However, since hydrogen is necessary for the reactions listed above, the fuel selected must contain hydrogen atoms. For the fuel cell to operate, the fuel must be converted into pure hydrogen gas. SOFCs are capable of internally reforming light hydrocarbons such as methane (natural gas),[44] propane, and butane.[45] These fuel cells are at an early stage of development.[46]
Challenges exist in SOFC systems due to their high operating temperatures. One such challenge is the potential for carbon dust to build up on the anode, which slows down the internal reforming process. Research to address this "carbon coking" issue at the University of Pennsylvania has shown that the use of copper-based cermet (heat-resistant materials made of ceramic and metal) can reduce coking and the loss of performance.[47] Another disadvantage of SOFC systems is the long start-up, making SOFCs less useful for mobile applications. Despite these disadvantages, a high operating temperature provides an advantage by removing the need for a precious metal catalyst like platinum, thereby reducing cost. Additionally, waste heat from SOFC systems may be captured and reused, increasing the theoretical overall efficiency to as high as 80–85%.[6]
The high operating temperature is largely due to the physical properties of the YSZ electrolyte. As temperature decreases, so does the ionic conductivity of YSZ. Therefore, to obtain the optimum performance of the fuel cell, a high operating temperature is required. According to their website, Ceres Power, a UK SOFC fuel cell manufacturer, has developed a method of reducing the operating temperature of their SOFC system to 500–600 degrees Celsius. They replaced the commonly used YSZ electrolyte with a CGO (cerium gadolinium oxide) electrolyte. The lower operating temperature allows them to use stainless steel instead of ceramic as the cell substrate, which reduces cost and start-up time of the system.[48]
Molten-carbonate fuel cell
[edit]Molten carbonate fuel cells (MCFCs) require a high operating temperature, 650 °C (1,200 °F), similar to SOFCs. MCFCs use lithium potassium carbonate salt as an electrolyte, and this salt liquefies at high temperatures, allowing for the movement of charge within the cell – in this case, negative carbonate ions.[49]
Like SOFCs, MCFCs are capable of converting fossil fuel to a hydrogen-rich gas in the anode, eliminating the need to produce hydrogen externally. The reforming process creates CO2 emissions. MCFC-compatible fuels include natural gas, biogas and gas produced from coal. The hydrogen in the gas reacts with carbonate ions from the electrolyte to produce water, carbon dioxide, electrons and small amounts of other chemicals. The electrons travel through an external circuit, creating electricity, and return to the cathode. There, oxygen from the air and carbon dioxide recycled from the anode react with the electrons to form carbonate ions that replenish the electrolyte, completing the circuit.[49] The chemical reactions for an MCFC system can be expressed as follows:[50]
- Anode reaction: CO32− + H2 → H2O + CO2 + 2e−
- Cathode reaction: CO2 + ½O2 + 2e− → CO32−
- Overall cell reaction: H2 + ½O2 → H2O
As with SOFCs, MCFC disadvantages include slow start-up times because of their high operating temperature. This makes MCFC systems not suitable for mobile applications, and this technology will most likely be used for stationary fuel-cell purposes. The main challenge of MCFC technology is the cells' short life span. The high-temperature and carbonate electrolyte lead to corrosion of the anode and cathode. These factors accelerate the degradation of MCFC components, decreasing the durability and cell life. Researchers are addressing this problem by exploring corrosion-resistant materials for components as well as fuel cell designs that may increase cell life without decreasing performance.[6]
MCFCs hold several advantages over other fuel cell technologies, including their resistance to impurities. They are not prone to "carbon coking", which refers to carbon build-up on the anode that results in reduced performance by slowing down the internal fuel reforming process. Therefore, carbon-rich fuels like gases made from coal are compatible with the system. The United States Department of Energy claims that coal, itself, might even be a fuel option in the future, assuming the system can be made resistant to impurities such as sulfur and particulates that result from converting coal into hydrogen.[6] MCFCs also have relatively high efficiencies. They can reach a fuel-to-electricity efficiency of 50%, considerably higher than the 37–42% efficiency of a phosphoric acid fuel cell plant. Efficiencies can be as high as 65% when the fuel cell is paired with a turbine, and 85% if heat is captured and used in a combined heat and power (CHP) system.[49]
FuelCell Energy, a Connecticut-based fuel cell manufacturer, develops and sells MCFC fuel cells. The company says that their MCFC products range from 300 kW to 2.8 MW systems that achieve 47% electrical efficiency and can utilize CHP technology to obtain higher overall efficiencies. One product, the DFC-ERG, is combined with a gas turbine and, according to the company, it achieves an electrical efficiency of 65%.[51]
Electric storage fuel cell
[edit]The electric storage fuel cell is a conventional battery chargeable by electric power input, using the conventional electro-chemical effect. However, the battery further includes hydrogen (and oxygen) inputs for alternatively charging the battery chemically.[52]
Biofuel cell
[edit]A biofuel cell converts chemical energy from biological substances into electrical energy using biological catalysts, such as enzymes or microorganisms. The process involves the oxidation of a fuel, like glucose, at the anode, releasing electrons and protons. The electrons travel through an external circuit to generate electrical current, while at the cathode, oxygen is typically reduced to water or hydrogen peroxide, completing the circuit.[53] Applications include wastewater treatment and renewable energy production.[54] Conductive polymers may be used to improve electron transfer between enzymes and electrodes. [55]
The integration of nanomaterials, such as carbon nanotubes and metal nanoparticles, are used to enhance the performance of BFCs. These materials increase the surface area of electrodes and facilitate better electron transfer, resulting in higher power densities. Three-dimensional porous structures and graphene-based materials, have been used to improve conductivity and stability, and hybrid biofuel cells that combine BFCs with supercapacitors or secondary batteries are being developed to provide stable and continuous energy output.[56] BFCs are being explored as power sources for implantable devices like pacemakers and biosensors.to potentially eliminate the need for traditional batteries, and fiber-type EBFCs show potential in implantable applications.[57] The power density of BFCs, however, is generally lower than that of conventional energy sources, the stability of enzymes and microorganisms over extended periods is another concern, and scalability and commercial viability also pose hurdles.[58]
Comparison of fuel cell types
[edit]| Fuel cell name | Electrolyte | Qualified power (W) | Working temperature (°C) | Efficiency | Status | Cost (USD/W) | |
|---|---|---|---|---|---|---|---|
| Cell | System | ||||||
| Electro-galvanic fuel cell | Aqueous alkaline solution | < 40 | Commercial / Research | 3-7 | |||
| Direct formic acid fuel cell (DFAFC) | Polymer membrane (ionomer) | < 50 W | < 40 | Commercial / Research | 10-20 | ||
| Alkaline fuel cell | Aqueous alkaline solution | 10–200 kW | < 80 | 60–70% | 62% | Commercial / Research | 50-100 |
| Proton-exchange membrane fuel cell | Polymer membrane (ionomer) | 1 W – 500 kW | 50–100 (Nafion)[59] 120–200 (PBI)[60] |
50–70% | 30–50%[61] | Commercial / Research | 50–100 |
| Metal hydride fuel cell | Aqueous alkaline solution | > −20 (50% Ppeak @ 0 °C) |
Commercial / Research | 30-200 | |||
| Zinc–air battery | Aqueous alkaline solution | < 40 | Mass production | 150-300 | |||
| Direct carbon fuel cell | Several different | 700–850 | 80% | 70% | Commercial / Research | 18 | |
| Direct borohydride fuel cell | Aqueous alkaline solution | 70 | Commercial | 400-450 | |||
| Microbial fuel cell | Polymer membrane or humic acid | < 40 | Research | 10-50 | |||
| Upflow microbial fuel cell (UMFC) | < 40 | Research | 1-5 | ||||
| Regenerative fuel cell | Polymer membrane (ionomer) | < 50 | Commercial / Research | 200-300 | |||
| Direct methanol fuel cell | Polymer membrane (ionomer) | 100 mW – 1 kW | 90–120 | 20–30% | 10–25%[61] | Commercial / Research | 125 |
| Reformed methanol fuel cell | Polymer membrane (ionomer) | 5 W – 100 kW | 250–300 (reformer) 125–200 (PBI) |
50–60% | 25–40% | Commercial / Research | 8.50 |
| Direct-ethanol fuel cell | Polymer membrane (ionomer) | < 140 mW/cm² | > 25 ? 90–120 |
Research | 12 | ||
| Redox fuel cell[broken anchor] (RFC) | Liquid electrolytes with redox shuttle and polymer membrane (ionomer) | 1 kW – 10 MW | Research | 12.50 | |||
| Phosphoric acid fuel cell | Molten phosphoric acid (H3PO4) | < 10 MW | 150–200 | 55% | 40%[61] Co-gen: 90% |
Commercial / Research | 4.00–4.50 |
| Solid acid fuel cell | H+-conducting oxyanion salt (solid acid) | 10 W – 1 kW | 200–300 | 55–60% | 40–45% | Commercial / Research | 15 |
| Molten carbonate fuel cell | Molten alkaline carbonate | 100 MW | 600–650 | 55% | 45–55%[61] | Commercial / Research | 1000 |
| Tubular solid oxide fuel cell (TSOFC) | O2−-conducting ceramic oxide | < 100 MW | 850–1100 | 60–65% | 55–60% | Commercial / Research | 3.50 |
| Protonic ceramic fuel cell | H+-conducting ceramic oxide | 700 | Research | 80 | |||
| Planar solid oxide fuel cell | O2−-conducting ceramic oxide | < 100 MW | 500–1100 | 60–65% | 55–60%[61] | Commercial / Research | 800 |
| Enzymatic biofuel cells | Any that will not denature the enzyme | < 40 | Research | 10 | |||
| Magnesium-air fuel cell | Salt water | −20 to 55 | 90% | Commercial / Research | 15 | ||
Glossary of terms in table:
- Anode
- The electrode at which oxidation (a loss of electrons) takes place. For fuel cells and other galvanic cells, the anode is the negative terminal; for electrolytic cells (where electrolysis occurs), the anode is the positive terminal.[62]
- Aqueous solution[63]
- Of, relating to, or resembling water
- Made from, with, or by water.
- Catalyst
- A chemical substance that increases the rate of a reaction without being consumed; after the reaction, it can potentially be recovered from the reaction mixture and is chemically unchanged. The catalyst lowers the activation energy required, allowing the reaction to proceed more quickly or at a lower temperature. In a fuel cell, the catalyst facilitates the reaction of oxygen and hydrogen. It is usually made of platinum powder very thinly coated onto carbon paper or cloth. The catalyst is rough and porous so the maximum surface area of the platinum can be exposed to the hydrogen or oxygen. The platinum-coated side of the catalyst faces the membrane in the fuel cell.[62]
- Cathode
- The electrode at which reduction (a gain of electrons) occurs. For fuel cells and other galvanic cells, the cathode is the positive terminal; for electrolytic cells (where electrolysis occurs), the cathode is the negative terminal.[62]
- Electrolyte
- A substance that conducts charged ions from one electrode to the other in a fuel cell, battery, or electrolyzer.[62]
- Fuel cell stack
- Individual fuel cells connected in a series. Fuel cells are stacked to increase voltage.[62]
- Matrix
- something within or from which something else originates, develops, or takes form.[64]
- Membrane
- The separating layer in a fuel cell that acts as electrolyte (an ion-exchanger) as well as a barrier film separating the gases in the anode and cathode compartments of the fuel cell.[62]
- Molten carbonate fuel cell (MCFC)
- A type of fuel cell that contains a molten carbonate electrolyte. Carbonate ions (CO32−) are transported from the cathode to the anode. Operating temperatures are typically near 650 °C.[62]
- Phosphoric acid fuel cell (PAFC)
- A type of fuel cell in which the electrolyte consists of concentrated phosphoric acid (H3PO4). Protons (H+) are transported from the anode to the cathode. The operating temperature range is generally 160–220 °C.[62]
- Proton-exchange membrane fuel cell (PEM)
- A fuel cell incorporating a solid polymer membrane used as its electrolyte. Protons (H+) are transported from the anode to the cathode. The operating temperature range is generally 60–100 °C for Low Temperature Proton-exchange membrane fuel cell (LT-PEMFC).[62] PEM fuel cell with operating temperature of 120-200 °C is called High Temperature Proton-exchange membrane fuel cell (HT-PEMFC).[65]
- Solid oxide fuel cell (SOFC)
- A type of fuel cell in which the electrolyte is a solid, nonporous metal oxide, typically zirconium oxide (ZrO2) treated with Y2O3, and O2− is transported from the cathode to the anode. Any CO in the reformate gas is oxidized to CO2 at the anode. Temperatures of operation are typically 800–1,000 °C.[62]
- Solution[66]
- An act or the process by which a solid, liquid, or gaseous substance is homogeneously mixed with a liquid or sometimes a gas or solid.
- A homogeneous mixture formed by this process; especially : a single-phase liquid system.
- The condition of being dissolved.
Efficiency of leading fuel cell types
[edit]Theoretical maximum efficiency
[edit]The energy efficiency of a system or device that converts energy is measured by the ratio of the amount of useful energy put out by the system ("output energy") to the total amount of energy that is put in ("input energy") or by useful output energy as a percentage of the total input energy. In the case of fuel cells, useful output energy is measured in electrical energy produced by the system. Input energy is the energy stored in the fuel. According to the U.S. Department of Energy, fuel cells are generally between 40 and 60% energy efficient.[67] This is higher than some other systems for energy generation. For example, the internal combustion engine of a car can be about 43% energy efficient.[68][69] Steam power plants usually achieve efficiencies of 30-40%[70] while combined cycle gas turbine and steam plants can achieve efficiencies above 60%.[71][72] In combined heat and power (CHP) systems, the waste heat produced by the primary power cycle - whether fuel cell, nuclear fission or combustion - is captured and put to use, increasing the efficiency of the system to up to 85–90%.[6]
The theoretical maximum efficiency of any type of power generation system is never reached in practice, and it does not consider other steps in power generation, such as production, transportation and storage of fuel and conversion of the electricity into mechanical power. However, this calculation allows the comparison of different types of power generation. The theoretical maximum efficiency of a fuel cell approaches 100%,[73] while the theoretical maximum efficiency of internal combustion engines is approximately 58%.[74]
In practice
[edit]Values are given from 40% for acidic, 50% for molten carbonate, to 60% for alkaline, solid oxide and PEM fuel cells.[75]
Fuel cells cannot store energy like a battery,[76] except as hydrogen, but in some applications, such as stand-alone power plants based on discontinuous sources such as solar or wind power, they are combined with electrolyzers and storage systems to form an energy storage system. As of 2019, 90% of hydrogen was used for oil refining, chemicals and fertilizer production (where hydrogen is required for the Haber–Bosch process),[77] and as of 2024, more than 95% hydrogen was still produced using steam methane reformation (about 95% is grey hydrogen, most of the rest is blue hydrogen, and only about 1% is green hydrogen), a process that emits carbon dioxide.[78] In addition, the overall efficiency (electricity to hydrogen and back to electricity) of such plants (known as round-trip efficiency), using pure hydrogen and pure oxygen can be "from 35 up to 50 percent", depending on gas density and other conditions.[79] The electrolyzer/fuel cell system can store indefinite quantities of hydrogen, and is therefore suited for long-term storage.
Solid-oxide fuel cells produce heat from the recombination of the oxygen and hydrogen. The ceramic can run as hot as 800 °C (1,470 °F). This heat can be captured and used to heat water in a micro combined heat and power (m-CHP) application. When the heat is captured, total efficiency can reach 80–90% at the unit, but does not consider production and distribution losses. CHP units are being developed today for the European home market.
Professor Jeremy P. Meyers, in the Electrochemical Society journal Interface in 2008, wrote, "While fuel cells are efficient relative to combustion engines, they are not as efficient as batteries, primarily due to the inefficiency of the oxygen reduction reaction (and ... the oxygen evolution reaction, should the hydrogen be formed by electrolysis of water). ... [T]hey make the most sense for operation disconnected from the grid, or when fuel can be provided continuously. For applications that require frequent and relatively rapid start-ups ... where zero emissions are a requirement, as in enclosed spaces such as warehouses, and where hydrogen is considered an acceptable reactant, a [PEM fuel cell] is becoming an increasingly attractive choice [if exchanging batteries is inconvenient]".[80] In 2013 military organizations were evaluating fuel cells to determine if they could significantly reduce the battery weight carried by soldiers.[81]
In vehicles
[edit]In a fuel cell vehicle the tank-to-wheel efficiency is greater than 45% at low loads[82] and shows average values of about 36% when a driving cycle like the NEDC (New European Driving Cycle) is used as test procedure.[83] The comparable NEDC value for a Diesel vehicle is 22%. In 2008 Honda released a demonstration fuel cell electric vehicle (the Honda FCX Clarity) with fuel stack claiming a 60% tank-to-wheel efficiency.[84]
It is also important to take losses due to fuel production, transportation, and storage into account. Fuel cell vehicles running on compressed hydrogen may have a power-plant-to-wheel efficiency of 22% if the hydrogen is stored as high-pressure gas, and 17% if it is stored as liquid hydrogen.[85]
Applications
[edit]
Power
[edit]Stationary fuel cells are used for commercial, industrial and residential primary and backup power generation. Fuel cells are very useful as power sources in remote locations, such as spacecraft, remote weather stations, large parks, communications centers, rural locations including research stations, and in certain military applications. A fuel cell system running on hydrogen can be compact and lightweight, and have no major moving parts. Because fuel cells have no moving parts and do not involve combustion, in ideal conditions they can achieve up to 99.9999% reliability.[86] This equates to less than one minute of downtime in a six-year period.[86]
Since fuel cell electrolyzer systems do not store fuel in themselves, but rather rely on external storage units, they can be successfully applied in large-scale energy storage, rural areas being one example.[87] There are many different types of stationary fuel cells so efficiencies vary, but most are between 40% and 60% energy efficient.[6] However, when the fuel cell's waste heat is used to heat a building in a cogeneration system this efficiency can increase to 85%.[6] This is significantly more efficient than traditional coal power plants, which are only about one third energy efficient.[88] Assuming production at scale, fuel cells could save 20–40% on energy costs when used in cogeneration systems.[89] Fuel cells are also much cleaner than traditional power generation; a fuel cell power plant using natural gas as a hydrogen source would create less than one ounce of pollution (other than CO2) for every 1,000 kW·h produced, compared to 25 pounds of pollutants generated by conventional combustion systems.[90] Fuel Cells also produce 97% less nitrogen oxide emissions than conventional coal-fired power plants.
One such pilot program is operating on Stuart Island in Washington State. There the Stuart Island Energy Initiative[91] has built a complete, closed-loop system: Solar panels power an electrolyzer, which makes hydrogen. The hydrogen is stored in a 500-U.S.-gallon (1,900 L) tank at 200 pounds per square inch (1,400 kPa), and runs a ReliOn fuel cell to provide full electric back-up to the off-the-grid residence. Another closed system loop was unveiled in late 2011 in Hempstead, NY.[92]
Fuel cells can be used with low-quality gas from landfills or waste-water treatment plants to generate power and lower methane emissions. A 2.8 MW fuel cell plant in California is said to be the largest of the type.[93] Small-scale (sub-5kWhr) fuel cells are being developed for use in residential off-grid deployment.[94]
Cogeneration
[edit]Combined heat and power (CHP) fuel cell systems, including micro combined heat and power (MicroCHP) systems are used to generate both electricity and heat for homes (see home fuel cell), office building and factories. The system generates constant electric power (selling excess power back to the grid when it is not consumed), and at the same time produces hot air and water from the waste heat. As the result CHP systems have the potential to save primary energy as they can make use of waste heat which is generally rejected by thermal energy conversion systems.[95] A typical capacity range of home fuel cell is 1–3 kWel, 4–8 kWth.[96][97] CHP systems linked to absorption chillers use their waste heat for refrigeration.[98]
The waste heat from fuel cells can be diverted during the summer directly into the ground providing further cooling while the waste heat during winter can be pumped directly into the building. The University of Minnesota owns the patent rights to this type of system.[99][100]
Co-generation systems can reach 85% efficiency (40–60% electric and the remainder as thermal).[6] Phosphoric-acid fuel cells (PAFC) comprise the largest segment of existing CHP products worldwide and can provide combined efficiencies close to 90%.[101][102] Molten carbonate (MCFC) and solid-oxide fuel cells (SOFC) are also used for combined heat and power generation and have electrical energy efficiencies around 60%.[103] Disadvantages of co-generation systems include slow ramping up and down rates, high cost and short lifetime.[104][105] Also their need to have a hot water storage tank to smooth out the thermal heat production was a serious disadvantage in the domestic market place where space in domestic properties is at a great premium.[106]
Delta-ee consultants stated in 2013 that with 64% of global sales the fuel cell micro-combined heat and power passed the conventional systems in sales in 2012.[81] The Japanese ENE FARM project stated that 34.213 PEMFC and 2.224 SOFC were installed in the period 2012–2014, 30,000 units on LNG and 6,000 on LPG.[107]
Fuel cell electric vehicles (FCEVs)
[edit]


Automobiles
[edit]Four fuel cell electric vehicles have been introduced for commercial lease and sale: the Honda Clarity, Toyota Mirai, Hyundai ix35 FCEV, and the Hyundai Nexo. By year-end 2019, about 18,000 FCEVs had been leased or sold worldwide.[108][109] Fuel cell electric vehicles feature an average range of 505 km (314 mi) between refuelings[110] and can be refueled in about 5 minutes.[111] The U.S. Department of Energy's Fuel Cell Technology Program states that, as of 2011, fuel cells achieved 53–59% efficiency at one-quarter power and 42–53% vehicle efficiency at full power,[112] and a durability of over 120,000 km (75,000 miles) with less than 10% degradation.[113] In a 2017 Well-to-Wheels simulation analysis that "did not address the economics and market constraints", General Motors and its partners estimated that, for an equivalent journey, a fuel cell electric vehicle running on compressed gaseous hydrogen produced from natural gas could use about 40% less energy and emit 45% less greenhouse gasses than an internal combustion vehicle.[114]
In 2015, Toyota introduced its first fuel cell vehicle, the Mirai, at a price of $57,000.[115] Hyundai introduced the limited production Hyundai ix35 FCEV under a lease agreement.[116] In 2016, Honda started leasing the Honda Clarity Fuel Cell.[117] In 2018, Hyundai introduced the Hyundai Nexo, replacing the Hyundai ix35 FCEV. In 2020, Toyota introduced the second generation of its Mirai brand, improving fuel efficiency and expanding range compared to the original Sedan 2014 model.[118]
In 2024, Mirai owners filed a class action lawsuit against Toyota in California over the lack of availability of hydrogen for fuel cell electric cars, alleging, among other things, fraudulent concealment and misrepresentation as well as violations of California's false advertising law and breaches of implied warranty.[119] The same year, Hyundai recalled all 1,600 Nexo vehicles sold in the US to that time due to a risk of fuel leaks and fire from a faulty "pressure relief device".[120]
Criticism
[edit]Some commentators believe that hydrogen fuel cell cars will never become economically competitive with other technologies[121][122][123] or that it will take decades for them to become profitable.[80][124] Elon Musk, CEO of battery-electric vehicle maker Tesla Motors, stated in 2015 that fuel cells for use in cars will never be commercially viable because of the inefficiency of producing, transporting and storing hydrogen and the flammability of the gas, among other reasons.[125] In 2012, Lux Research, Inc. issued a report that stated: "The dream of a hydrogen economy ... is no nearer". It concluded that "Capital cost ... will limit adoption to a mere 5.9 GW" by 2030, providing "a nearly insurmountable barrier to adoption, except in niche applications". The analysis concluded that, by 2030, PEM stationary market will reach $1 billion, while the vehicle market, including forklifts, will reach a total of $2 billion.[124] Other analyses cite the lack of an extensive hydrogen infrastructure in the U.S. as an ongoing challenge to Fuel Cell Electric Vehicle commercialization.[82]
In 2014, Joseph Romm, the author of The Hype About Hydrogen (2005; 2025), said that FCVs still had not overcome the high fueling cost, lack of fuel-delivery infrastructure, and pollution caused by producing hydrogen. "It would take several miracles to overcome all of those problems simultaneously in the coming decades."[126] He concluded that renewable energy cannot economically be used to make hydrogen for an FCV fleet "either now or in the future."[121] Greentech Media's analyst reached similar conclusions in 2014.[127] In 2015, CleanTechnica listed some of the disadvantages of hydrogen fuel cell vehicles.[128] So did Car Throttle.[129] A 2019 video by Real Engineering noted that, notwithstanding the introduction of vehicles that run on hydrogen, using hydrogen as a fuel for cars does not help to reduce carbon emissions from transportation. The 95% of hydrogen still produced from fossil fuels releases carbon dioxide, and producing hydrogen from water is an energy-consuming process. Storing hydrogen requires more energy either to cool it down to the liquid state or to put it into tanks under high pressure, and delivering the hydrogen to fueling stations requires more energy and may release more carbon. The hydrogen needed to move a FCV a kilometer costs approximately 8 times as much as the electricity needed to move a BEV the same distance.[130]
A 2020 assessment concluded that hydrogen vehicles are still only 38% efficient, while battery EVs are 80% efficient.[131] In 2021 CleanTechnica concluded that (a) hydrogen cars remain far less efficient than electric cars; (b) grey hydrogen – hydrogen produced with polluting processes – makes up the vast majority of available hydrogen; (c) delivering hydrogen would require building a vast and expensive new delivery and refueling infrastructure; and (d) the remaining two "advantages of fuel cell vehicles – longer range and fast fueling times – are rapidly being eroded by improving battery and charging technology."[132] A 2022 study in Nature Electronics agreed.[133] A 2023 study by the Centre for International Climate and Environmental Research (CICERO) estimated that leaked hydrogen has a global warming effect 11.6 times stronger than CO2.[134]
Buses
[edit]
As of August 2011[update], there were about 100 fuel cell buses in service around the world.[135] Most of these were manufactured by UTC Power, Toyota, Ballard, Hydrogenics, and Proton Motor. UTC buses had driven more than 970,000 km (600,000 miles) by 2011.[136] Fuel cell buses have from 39% to 141% higher fuel economy than diesel buses and natural gas buses.[114][137]
As of 2019[update], the NREL was evaluating several current and planned fuel cell bus projects in the U.S.[138]
Trains
[edit]Train operators may use hydrogen fuel cells in trains in an effort to save the costs of installing overhead electrification and to maintain the range offered by diesel trains. They have encountered expenses, however, due to fuel cells in trains lasting only three years, maintenance of the hydrogen tank and the additional need for batteries as a power buffer.[139][140] In 2018, the first fuel cell-powered trains, the Alstom Coradia iLint multiple units, began running on the Buxtehude–Bremervörde–Bremerhaven–Cuxhaven line in Germany.[141] Hydrogen trains have also been introduced in Sweden[142] and the UK.[143]
Trucks
[edit]In December 2020, Toyota and Hino Motors, together with Seven-Eleven (Japan), FamilyMart and Lawson announced that they have agreed to jointly consider introducing light-duty fuel cell electric trucks (light-duty FCETs).[144] Lawson started testing for low temperature delivery at the end of July 2021 in Tokyo, using a Hino Dutro in which the Toyota Mirai fuel cell is implemented. FamilyMart started testing in Okazaki city.[145]
In August 2021, Toyota announced their plan to make fuel cell modules at its Kentucky auto-assembly plant for use in zero-emission big rigs and heavy-duty commercial vehicles. They plan to begin assembling the electrochemical devices in 2023.[146]
In October 2021, Daimler Truck's fuel cell based truck received approval from German authorities for use on public roads.[147]
Forklifts
[edit]A fuel cell forklift (also called a fuel cell lift truck) is a fuel cell-powered industrial forklift truck used to lift and transport materials. In 2013 there were over 4,000 fuel cell forklifts used in material handling in the US,[148] of which 500 received funding from DOE (2012).[149][150] As of 2024, approximately 50,000 hydrogen forklifts are in operation worldwide (the bulk of which are in the U.S.), as compared with 1.2 million battery electric forklifts that were purchased in 2021.[151]
Most companies in Europe and the US do not use petroleum-powered forklifts, as these vehicles work indoors where emissions must be controlled and instead use electric forklifts.[152][153] Fuel cell-powered forklifts can be refueled in 3 minutes and they can be used in refrigerated warehouses, where their performance is not degraded by lower temperatures. The FC units are often designed as drop-in replacements.[154][155]
Motorcycles and bicycles
[edit]In 2005, a British manufacturer of hydrogen-powered fuel cells, Intelligent Energy (IE), produced the first working hydrogen-run motorcycle called the ENV (Emission Neutral Vehicle). The motorcycle holds enough fuel to run for four hours, and to travel 160 km (100 miles) in an urban area, at a top speed of 80 km/h (50 mph).[156] In 2004 Honda developed a fuel cell motorcycle that utilized the Honda FC Stack.[157][158]
Other examples of motorbikes[159] and bicycles[160] that use hydrogen fuel cells include the Taiwanese company APFCT's scooter[161] using the fueling system from Italy's Acta SpA[162] and the Suzuki Burgman scooter with an IE fuel cell that received EU Whole Vehicle Type Approval in 2011.[163] Suzuki Motor Corp. and IE have announced a joint venture to accelerate the commercialization of zero-emission vehicles.[164]
Airplanes
[edit]In 2003, the world's first propeller-driven airplane to be powered entirely by a fuel cell was flown. The fuel cell was a stack design that allowed the fuel cell to be integrated with the plane's aerodynamic surfaces.[165] Fuel cell-powered unmanned aerial vehicles (UAV) include a Horizon fuel cell UAV that set the record distance flown for a small UAV in 2007.[166] Boeing researchers and industry partners throughout Europe conducted experimental flight tests in February 2008 of a manned airplane powered only by a fuel cell and lightweight batteries. The fuel cell demonstrator airplane, as it was called, used a proton-exchange membrane (PEM) fuel cell/lithium-ion battery hybrid system to power an electric motor, which was coupled to a conventional propeller.[167]
In 2009, the Naval Research Laboratory's (NRL's) Ion Tiger utilized a hydrogen-powered fuel cell and flew for 23 hours and 17 minutes.[168] Fuel cells are also being tested and considered to provide auxiliary power in aircraft, replacing fossil fuel generators that were previously used to start the engines and power on board electrical needs, while reducing carbon emissions.[169][170][failed verification] In 2016 a Raptor E1 drone made a successful test flight using a fuel cell that was lighter than the lithium-ion battery it replaced. The flight lasted 10 minutes at an altitude of 80 metres (260 ft), although the fuel cell reportedly had enough fuel to fly for two hours. The fuel was contained in approximately 100 solid 1 square centimetre (0.16 sq in) pellets composed of a proprietary chemical within an unpressurized cartridge. The pellets are physically robust and operate at temperatures as warm as 50 °C (122 °F). The cell was from Arcola Energy.[171]
Lockheed Martin Skunk Works Stalker is an electric UAV powered by solid oxide fuel cell.[172]
Boats
[edit]
The Hydra, a 22-person fuel cell boat operated from 1999 to 2001 on the Rhine river near Bonn, Germany,[173] and was used as a ferry boat in Ghent, Belgium, during an electric boat conference in 2000. It was fully certified by the Germanischer Lloyd for passenger transport.[174] The Zemship, a small passenger ship, was produced in 2003 to 2013. It used a 100 kW Polymer Electrolyte Membrane Fuel Cells (PEMFC) with 7 lead gel batteries. With these systems, alongside 12 storage tanks, fuel cells provided an energy capacity of 560 V and 234 kWh.[175] Made in Hamburg, Germany, the FCS Alsterwasser, revealed in 2008, was one of the first passenger ships powered by fuel cells and could carry 100 passengers. The hybrid fuel cell technology that powered this ship was produced by Proton Motor Fuel Cell GmbH.[176]
In 2010, the MF Vågen was first produced, utilizing 12 kW fuel cells and 2- to 3-kilogram metal hydride hydrogen storage. It also utilizes 25 kWh lithium batteries and a 10 kW DC motor.[175] The Hornblower Hybrid debuted in 2012. It utilizes a diesel generator, batteries, photovoltaics, wind power, and fuel cells for energy.[175] Made in Bristol, a 12-passenger hybrid ferry, Hydrogenesis, has been in operation since 2012.[175] The SF-BREEZE is a two-motor boat that utilizes 41 × 120 kW fuel cells. With a type C storage tank, the pressurized vessel can maintain 1200 kg of LH2. These ships are still in operation today.[175] In Norway, the first ferry powered by fuel cells running on liquid hydrogen was scheduled for its first test drives in December 2022.[177][178]
The Type 212 submarines of the German and Italian navies use fuel cells to remain submerged for weeks without the need to surface.[179] The U212A is a non-nuclear submarine developed by German naval shipyard Howaldtswerke Deutsche Werft.[180] The system consists of nine PEM fuel cells, providing between 30 kW and 50 kW each. The ship is silent, giving it an advantage in the detection of other submarines.[181]
Portable power systems
[edit]Portable fuel cell systems are generally classified as weighing under 10 kg and providing power of less than 5 kW.[182] The potential market size for smaller fuel cells was estimated in 2002 at around $10 billion.[183] Within this market two groups have been identified. The first is the microfuel cell market, in the 1-50 W range for power smaller electronic devices. The second is the 1-5 kW range of generators for larger scale power generation (e.g. military outposts, remote oil fields). Microfuel cells are primarily aimed at penetrating the market for phones and laptops.[183]
Portable power systems that use fuel cells can be used in the leisure sector (i.e. RVs, cabins, marine), industry (i.e. power for remote locations including gas/oil wellsites, communication towers, security, weather stations), and the military. SFC Energy is a German manufacturer of direct methanol fuel cells for a variety of portable power systems.[184] Ensol Systems Inc. is an integrator of portable power systems, using the SFC Energy DMFC.[185] The key advantage of fuel cells in this market is the great power generation per weight. While fuel cells can be expensive, for remote locations that require dependable energy fuel cells hold great power.[182]
Other applications
[edit]- Providing power for base stations or cell sites[186][187]
- Emergency power systems are a type of fuel cell system, which may include lighting, generators and other apparatus, to provide backup resources in a crisis or when regular systems fail. They find uses in a wide variety of settings from residential homes to hospitals, scientific laboratories, data centers,[188]
- Telecommunication[189] equipment and modern naval ships.
- An uninterrupted power supply (UPS) provides emergency power and, depending on the topology, provide line regulation as well to connected equipment by supplying power from a separate source when utility power is not available. Unlike a standby generator, it can provide instant protection from a momentary power interruption.
- Smartphones, laptops and tablets for use in locations where AC charging may not be readily available.
- Portable charging docks for small electronics (e.g. a belt clip that charges a cell phone or PDA).
- Small heating appliances[190]
- Food preservation, achieved by exhausting the oxygen and automatically maintaining oxygen exhaustion in a shipping container, containing, for example, fresh fish.[191]
- Sensors, including in Breathalyzers, where the amount of voltage generated by a fuel cell is used to determine the concentration of fuel (alcohol) in the sample.[192]
Fueling stations
[edit]
According to FuelCellsWorks, an industry group, at the end of 2019, 330 hydrogen refueling stations were open to the public worldwide.[193] As of June 2020, there were 178 publicly available hydrogen stations in operation in Asia.[194] 114 of these were in Japan.[194] There were at least 177 stations in Europe, and about half of these were in Germany.[195][196] There were 44 publicly accessible stations in the US, 42 of which were located in California.[197]
A hydrogen fueling station costs between $1 million and $4 million to build.[198]
Social Implications
[edit]As of 2023, technological barriers to fuel cell adoption remain.[199] Fuel cells are primarily for material handling in warehouses, distribution centers, and manufacturing facilities.[200] They are projected to be useful and sustainable in a wider range applications.[201] But current applications do not often reach lower-income communities,[202] though some attempts at inclusivity are being made, for example in accessibility.[203]
Markets and economics
[edit]This section needs to be updated. (July 2025) |
In 2012, fuel cell industry revenues exceeded $1 billion market value worldwide, with Asian pacific countries shipping more than 3/4 of the fuel cell systems worldwide.[204] There were 140,000 fuel cell stacks shipped globally in 2010, up from 11,000 shipments in 2007, and from 2011 to 2012 worldwide fuel cell shipments had an annual growth rate of 85%.[205] Tanaka Kikinzoku expanded its manufacturing facilities in 2011.[206] Approximately 50% of fuel cell shipments in 2010 were stationary fuel cells, up from about a third in 2009, and the four dominant producers in the Fuel Cell Industry were the United States, Germany, Japan and South Korea.[207] The Department of Energy Solid State Energy Conversion Alliance found that, as of January 2011, stationary fuel cells generated power at approximately $724 to $775 per kilowatt installed.[208] In 2011, Bloom Energy, a major fuel cell supplier, said that its fuel cells generated power at 9–11 cents per kilowatt-hour, including the price of fuel, maintenance, and hardware.[209][210]
In 2016, Samsung "decided to drop fuel cell-related business projects, as the outlook of the market isn't good".[211]
Research and development
[edit]- 2013: British firm ACAL Energy developed a fuel cell that it said could run for 10,000 hours in simulated driving conditions.[212] It asserted that the cost of fuel cell construction can be reduced to $40/kW (roughly $9,000 for 300 HP).[213]
- 2014: Researchers in Imperial College London developed a new method for regeneration of hydrogen sulfide contaminated PEFCs.[214] They recovered 95–100% of the original performance of a hydrogen sulfide contaminated PEFC. They were successful in rejuvenating a SO2 contaminated PEFC too.[215] This regeneration method is applicable to multiple cell stacks.[216]
- 2019: U.S. Army Research Laboratory researchers developed a two part in-situ hydrogen generation fuel cell, one for hydrogen generation and the other for electric power generation through an internal hydrogen/air power plant.[217]
- 2022: Researchers from University of Delaware developed a hydrogen-powered fuel cell projected to function at lower costs and operate at roughly $1.4/kW. This design removes carbon dioxide from the air feed of hydroxide exchange membrane fuel cells.[218]
- 2024 KAIST researchers developed a method to create a protonic fuel cell relying on laminating slurries in a tape casting, then sintering the laminated structure. The three different slurries were created by using Resonant Acoustic Mixing (RAM) before deposition.[219]
See also
[edit]References
[edit]- ^ Saikia, Kaustav; Kakati, Biraj Kumar; Boro, Bibha; Verma, Anil (2018). "Current Advances and Applications of Fuel Cell Technologies". Recent Advancements in Biofuels and Bioenergy Utilization. Singapore: Springer. pp. 303–337. doi:10.1007/978-981-13-1307-3_13. ISBN 978-981-13-1307-3.
- ^ Khurmi, R. S. (2014). Material Science. S. Chand & Company. ISBN 978-81-219-0146-8.
- ^ Winter, Martin; Brodd, Ralph J. (28 September 2004). "What Are Batteries, Fuel Cells, and Supercapacitors?". Chemical Reviews. 104 (10): 4245–4270. doi:10.1021/cr020730k. PMID 15669155. S2CID 3091080.
- ^ "Bronx Hydrogen Fuel Cell Bus". Empire Clean Cities. Retrieved 13 April 2024.
- ^ Nice, Karim and Strickland, Jonathan. "How Fuel Cells Work: Polymer Exchange Membrane Fuel Cells". How Stuff Works, accessed 4 August 2011
- ^ a b c d e f g h i "Types of Fuel Cells" Archived 9 June 2010 at the Wayback Machine. Department of Energy EERE website, accessed 4 August 2011
- ^ Grove, W. R. (1838). "On a new voltaic combination". The London and Edinburgh Philosophical Magazine and Journal of Science. 3rd series. 13 (84): 430–431. doi:10.1080/14786443808649618. Retrieved 2 October 2013.
- ^ Grove, William Robert (1839). "On Voltaic Series and the Combination of Gases by Platinum". Philosophical Magazine and Journal of Science. 3rd series. 14 (86–87): 127–130. doi:10.1080/14786443908649684.
- ^ Schœnbein (1839). "On the voltaic polarization of certain solid and fluid substances" (PDF). The London and Edinburgh Philosophical Magazine and Journal of Science. 3rd series. 14 (85): 43–45. Archived from the original on 5 October 2013. Retrieved 2 October 2013.
- ^ Grove, William Robert (1842). "On a Gaseous Voltaic Battery". The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science. 3rd series. 21 (140): 417–420. doi:10.1080/14786444208621600.
- ^ Larminie, James; Dicks, Andrew. Fuel Cell Systems Explained (PDF).[permanent dead link]
- ^ a b "The Brits who bolstered the Moon landings". BBC. Retrieved 7 August 2019.
- ^ "Apollo 11 mission 50 years on: The Cambridge scientist who helped put man on the moon". Cambridge Independent. Retrieved 7 August 2019.
- ^ "Fuel Cell Project: PEM Fuel Cells photo #2". americanhistory.si.edu.
- ^ "Collecting the History of Proton Exchange Membrane Fuel Cells". americanhistory.si.edu.
- ^ "The PureCell Model 400 – Product Overview". UTC Power. Archived from the original on 11 December 2011. Retrieved 22 December 2011.
- ^ "S.Res.217 – A resolution designating October 8, 2015, as "National Hydrogen and Fuel Cell Day"". Congress.gov. 29 September 2015.
- ^ "Fuel Cells - EnergyGroove.net". EnergyGroove.net. Retrieved 6 February 2018.
- ^ a b "Reliable High Performance Textile Materials". Tex Tech Industries. Retrieved 6 February 2018.
- ^ Larminie, James (1 May 2003). Fuel Cell Systems Explained, Second Edition. SAE International. ISBN 978-0-7680-1259-0.
- ^ Kakati, B. K.; Deka, D. (2007). "Effect of resin matrix precursor on the properties of graphite composite bipolar plate for PEM fuel cell". Energy & Fuels. 21 (3): 1681–1687. Bibcode:2007EnFue..21.1681K. doi:10.1021/ef0603582.
- ^ "LEMTA – Our fuel cells". Perso.ensem.inpl-nancy.fr. Archived from the original on 21 June 2009. Retrieved 21 September 2009.
- ^ Yin, Xi; Lin, Ling; Chung, Hoon T; Komini Babu, Siddharth; Martinez, Ulises; Purdy, Geraldine M; Zelenay, Piotr (4 August 2017). "Effects of MEA Fabrication and Ionomer Composition on Fuel Cell Performance of PGM-Free ORR Catalyst". ECS Transactions. 77 (11): 1273–1281. Bibcode:2017ECSTr..77k1273Y. doi:10.1149/07711.1273ecst. OSTI 1463547.
- ^ Anne-Claire Dupuis, Progress in Materials Science, Volume 56, Issue 3, March 2011, pp. 289–327
- ^ "Measuring the relative efficiency of hydrogen energy technologies for implementing the hydrogen economy 2010" (PDF). Archived from the original (PDF) on 5 November 2013.
- ^ Kakati, B. K.; Mohan, V. (2008). "Development of low cost advanced composite bipolar plate for P.E.M. fuel cell". Fuel Cells. 08 (1): 45–51. doi:10.1002/fuce.200700008. S2CID 94469845.
- ^ Kakati, B. K.; Deka, D. (2007). "Differences in physico-mechanical behaviors of resol and novolac type phenolic resin based composite bipolar plate for proton exchange membrane (PEM) fuel cell". Electrochimica Acta. 52 (25): 7330–7336. doi:10.1016/j.electacta.2007.06.021.
- ^ a b Coletta, Vitor, et al. "Cu-Modified SrTiO 3 Perovskites Toward Enhanced Water-Gas Shift Catalysis: A Combined Experimental and Computational Study" , ACS Applied Energy Materials (2021), vol. 4, issue 1, pp. 452–461
- ^ Spendelow, Jacob and Jason Marcinkoski. "Fuel Cell System Cost – 2013" Archived 2 December 2013 at the Wayback Machine, DOE Fuel Cell Technologies Office, 16 October 2013 (archived version)
- ^ "Ballard Power Systems: Commercially Viable Fuel Cell Stack Technology Ready by 2010". 29 March 2005. Archived from the original on 27 September 2007. Retrieved 27 May 2007.
- ^ Online, Science (2 August 2008). "2008 – Cathodes in fuel cells". Abc.net.au. Archived from the original on 6 August 2008. Retrieved 21 September 2009.
- ^ Wang, Shuangyin (2011). "Polyelectrolyte Functionalized Carbon Nanotubes as Efficient Metal-free Electrocatalysts for Oxygen Reduction". Journal of the American Chemical Society. 133 (14): 5182–5185. Bibcode:2011JAChS.133.5182W. doi:10.1021/ja1112904. PMID 21413707. S2CID 207063759.
- ^ Notter, Dominic A.; Kouravelou, Katerina; Karachalios, Theodoros; Daletou, Maria K.; Haberland, Nara Tudela (2015). "Life cycle assessment of PEM FC applications: electric mobility and μ-CHP". Energy Environ. Sci. 8 (7): 1969–1985. Bibcode:2015EnEnS...8.1969N. doi:10.1039/C5EE01082A.
- ^ "Water_and_Air_Management". Ika.rwth-aachen.de. Archived from the original on 14 January 2009. Retrieved 21 September 2009.
- ^ Andersson, M.; Beale, S. B.; Espinoza, M.; Wu, Z.; Lehnert, W. (15 October 2016). "A review of cell-scale multiphase flow modeling, including water management, in polymer electrolyte fuel cells". Applied Energy. 180: 757–778. Bibcode:2016ApEn..180..757A. doi:10.1016/j.apenergy.2016.08.010.
- ^ "Progress and Accomplishments in Hydrogen and Fuel Cells" (PDF). Archived from the original (PDF) on 23 November 2015. Retrieved 16 May 2015.
- ^ a b "Collecting the History of Phosphoric Acid Fuel Cells". americanhistory.si.edu.
- ^ "Phosphoric Acid Fuel Cells". scopeWe - a Virtual Engineer. Archived from the original on 10 November 2013. Retrieved 28 June 2013.
- ^ Haile, Sossina M.; Chisholm, Calum R. I.; Sasaki, Kenji; Boysen, Dane A.; Uda, Tetsuya (11 December 2006). "Solid acid proton conductors: from laboratory curiosities to fuel cell electrolytes" (PDF). Faraday Discussions. 134: 17–39. Bibcode:2007FaDi..134...17H. doi:10.1039/B604311A. ISSN 1364-5498. PMID 17326560. Archived (PDF) from the original on 15 August 2017.
- ^ Williams, K.R. (1 February 1994). "Francis Thomas Bacon. 21 December 1904 – 24 May 1992". Biographical Memoirs of Fellows of the Royal Society. 39: 2–9. doi:10.1098/rsbm.1994.0001. S2CID 71613260.
- ^ Srivastava, H. C. Nootan ISC Chemistry (12th) Edition 18, pp. 458–459, Nageen Prakashan (2014) ISBN 9789382319399
- ^ Stambouli, A. Boudghene (2002). "Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy". Renewable and Sustainable Energy Reviews. 6 (5): 433–455. Bibcode:2002RSERv...6..433S. doi:10.1016/S1364-0321(02)00014-X.
- ^ "Solid Oxide Fuel Cell (SOFC)". FCTec website', accessed 4 August 2011 Archived 8 January 2012 at the Wayback Machine
- ^ "Methane Fuel Cell Subgroup". University of Virginia. 2012. Archived from the original on 22 February 2014. Retrieved 13 February 2014.
- ^ A Kulkarni; FT Ciacchi; S Giddey; C Munnings; SPS Badwal; JA Kimpton; D Fini (2012). "Mixed ionic electronic conducting perovskite anode for direct carbon fuel cells". International Journal of Hydrogen Energy. 37 (24): 19092–19102. Bibcode:2012IJHE...3719092K. doi:10.1016/j.ijhydene.2012.09.141.
- ^ S. Giddey; S.P.S. Badwal; A. Kulkarni; C. Munnings (2012). "A comprehensive review of direct carbon fuel cell technology". Progress in Energy and Combustion Science. 38 (3): 360–399. Bibcode:2012PECS...38..360G. doi:10.1016/j.pecs.2012.01.003.
- ^ Hill, Michael. "Ceramic Energy: Material Trends in SOFC Systems" Archived 28 September 2011 at the Wayback Machine. Ceramic Industry, 1 September 2005.
- ^ "The Ceres Cell" Archived 13 December 2013 at the Wayback Machine. Ceres Power website, accessed 4 August 2011
- ^ a b c "Molten Carbonate Fuel Cell Technology". U.S. Department of Energy, accessed 9 August 2011
- ^ "Molten Carbonate Fuel Cells (MCFC)". FCTec.com, accessed 9 August 2011 Archived 3 March 2012 at the Wayback Machine
- ^ "Products". FuelCell Energy, accessed 9 August 2011 Archived 11 January 2013 at archive.today
- ^ U.S. patent 8,354,195
- ^ Huang, Wengang; Zulkifli, Muhammad Yazid Bin; Chai, Milton; Lin, Rijia; Wang, Jingjing; Chen, Yuelei; Chen, Vicki; Hou, Jingwei (August 2023). "Recent advances in enzymatic biofuel cells enabled by innovative materials and techniques". Exploration. 3 (4) 20220145. doi:10.1002/EXP.20220145. PMC 10624391. PMID 37933234.
- ^ Babanova, Sofia (2009). "Biofuel Cells – Alternative Power Sources". International Scientific Conference (FMNS2013), Blagoevgrad (Bulgaria).
- ^ Kižys, Kasparas; Zinovičius, Antanas; Jakštys, Baltramiejus; Bružaitė, Ingrida; Balčiūnas, Evaldas; Petrulevičienė, Milda; Ramanavičius, Arūnas; Morkvėnaitė-Vilkončienė, Inga (3 February 2023). "Microbial Biofuel Cells: Fundamental Principles, Development and Recent Obstacles". Biosensors. 13 (2): 221. doi:10.3390/bios13020221. PMC 9954062. PMID 36831987.
- ^ Kwon, Cheong Hoon; Ko, Yongmin; Shin, Dongyeeb; Kwon, Minseong; Park, Jinho; Bae, Wan Ki; Lee, Seung Woo; Cho, Jinhan (26 October 2018). "High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers". Nature Communications. 9 (1): 4479. Bibcode:2018NatCo...9.4479K. doi:10.1038/s41467-018-06994-5. PMC 6203850. PMID 30367069.
- ^ Cai, Jingsheng; Shen, Fei; Zhao, Jianqing; Xiao, Xinxin (February 2024). "Enzymatic biofuel cell: A potential power source for self-sustained smart textiles". iScience. 27 (2) 108998. Bibcode:2024iSci...27j8998C. doi:10.1016/j.isci.2024.108998. PMC 10850773. PMID 38333690.
- ^ Wang, Linlin; Wu, Xiaoge; Su, B. S. Qi-wen; Song, Rongbin; Zhang, Jian-Rong; Zhu, Jun-Jie (August 2021). "Enzymatic Biofuel Cell: Opportunities and Intrinsic Challenges in Futuristic Applications". Advanced Energy and Sustainability Research. 2 (8) 2100031. Bibcode:2021AdESR...200031W. doi:10.1002/aesr.202100031.
- ^ "Fuel Cell Comparison Chart" (PDF). Archived from the original (PDF) on 1 March 2013. Retrieved 10 February 2013.
- ^ E. Harikishan Reddy; Jayanti, S (15 December 2012). "Thermal management strategies for a 1 kWe stack of a high temperature proton exchange membrane fuel cell". Applied Thermal Engineering. 48: 465–475. Bibcode:2012AppTE..48..465H. doi:10.1016/j.applthermaleng.2012.04.041.
- ^ a b c d e Badwal, Sukhvinder P. S.; Giddey, Sarbjit S.; Munnings, Christopher; Bhatt, Anand I.; Hollenkamp, Anthony F. (24 September 2014). "Emerging electrochemical energy conversion and storage technologies". Frontiers in Chemistry. 2: 79. Bibcode:2014FrCh....2...79B. doi:10.3389/fchem.2014.00079. PMC 4174133. PMID 25309898.
- ^ a b c d e f g h i j "Fuel Cell Technologies Program: Glossary" Archived 23 February 2014 at the Wayback Machine. Department of Energy Energy Efficiency and Renewable Energy Fuel Cell Technologies Program. 7 July 2011. Accessed 3 August 2011.
- ^ "Aqueous Solution". Merriam-Webster Free Online Dictionary
- ^ "Matrix". Merriam-Webster Free Online Dictionary
- ^ Araya, Samuel Simon (2012). High temperature PEM fuel cells - degradation & durability: dissertation submitted to the Faculty of Engineering and Science at Aalborg University in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Aalborg: Aalborg University, Department of Energy Technology. ISBN 978-87-92846-14-3. OCLC 857436369.
- ^ "Solution". Merriam-Webster Free Online Dictionary
- ^ "Comparison of Fuel Cell Technologies" Archived 1 March 2013 at the Wayback Machine. U.S. Department of Energy, Energy Efficiency and Fuel Cell Technologies Program, February 2011, accessed 4 August 2011
- ^ "Benchmarking a 2018 Toyota Camry 2.5-Liter Atkinson Cycle Engine with Cooled-EGR" (PDF). SAE. Retrieved 2 April 2019.
- ^ Yang, Dongsheng; Lu, Guoxiang; Gong, Zewen; Qiu, An; Bouaita, Abdelhamid (2021). "Development of 43% Brake Thermal Efficiency Gasoline Engine for BYD DM-i Plug-in Hybrid". SAE Technical Paper Series. Vol. 1. SAE. doi:10.4271/2021-01-1241. Retrieved 21 September 2021.
- ^ "New Benchmarks for Steam Turbine Efficiency". August 2002. Archived from the original on 25 July 2021. Retrieved 12 March 2022.
- ^ "All Units of Nishi-Nagoya Thermal Power Station Now in Operation". Toshiba Energy Systems & Solutions Corporation. 30 March 2018.
- ^ "Chubu Electric Power's Nishi-Nagoya Thermal Power Station Unit 7-1 Recognized by Guinness World Records as World's Most Efficient Combined Cycle Power Plant: Achieved 63.08% Power Generation Efficiency". Press Release 2018. Chubu Electric. 27 March 2018.
- ^ Haseli, Y. (3 May 2018). "Maximum conversion efficiency of hydrogen fuel cells". International Journal of Hydrogen Energy. 43 (18): 9015–9021. Bibcode:2018IJHE...43.9015H. doi:10.1016/j.ijhydene.2018.03.076. ISSN 0360-3199.
- ^ "Fuel Cell Efficiency" Archived 9 February 2014 at the Wayback Machine. World Energy Council, 17 July 2007, accessed 4 August 2011
- ^ "Fuel Cells" (PDF). November 2015. Retrieved 27 December 2022.
- ^ "Batteries, Supercapacitors, and Fuel Cells: Scope". Science Reference Services. 20 August 2007. Retrieved 11 February 2009.
- ^ "Realising the hydrogen economy" Archived 5 November 2019 at the Wayback Machine,Power Technology, 11 October 2019
- ^ "Ask MIT Climate: How Clean Is Green Hydrogen?", MIT, February 27, 2024
- ^ Garcia, Christopher P.; et al. (January 2006). "Round Trip Energy Efficiency of NASA Glenn Regenerative Fuel Cell System". Preprint. p. 5. hdl:2060/20060008706.
- ^ a b Meyers, Jeremy P. "Getting Back Into Gear: Fuel Cell Development After the Hype". The Electrochemical Society Interface, Winter 2008, pp. 36–39, accessed 7 August 2011
- ^ a b "The fuel cell industry review 2013" (PDF).
- ^ a b Eberle, Ulrich and Rittmar von Helmolt. "Sustainable transportation based on electric vehicle concepts: a brief overview". Energy & Environmental Science, Royal Society of Chemistry, 14 May 2010, accessed 2 August 2011
- ^ Von Helmolt, R.; Eberle, U (20 March 2007). "Fuel Cell Vehicles:Status 2007". Journal of Power Sources. 165 (2): 833–843. Bibcode:2007JPS...165..833V. doi:10.1016/j.jpowsour.2006.12.073.
- ^ "Honda FCX Clarity – Fuel cell comparison". Honda. Archived from the original on 3 January 2009. Retrieved 2 January 2009.
- ^ "Efficiency of Hydrogen PEFC, Diesel-SOFC-Hybrid and Battery Electric Vehicles" (PDF). 15 July 2003. Archived from the original (PDF) on 21 October 2006. Retrieved 23 May 2007.
- ^ a b "Fuel Cell Basics: Benefits". Fuel Cells 2000. Archived from the original on 28 September 2007. Retrieved 27 May 2007.
- ^ "Fuel Cell Basics: Applications" Archived 15 May 2011 at the Wayback Machine. Fuel Cells 2000. Accessed 2 August 2011.
- ^ "Energy Sources: Electric Power". U.S. Department of Energy. Accessed 2 August 2011.
- ^ "2008 Fuel Cell Technologies Market Report" Archived 4 September 2012 at the Wayback Machine. Bill Vincent of the Breakthrough Technologies Institute, Jennifer Gangi, Sandra Curtin, and Elizabeth Delmont. Department of Energy Energy Efficiency and Renewable Energy. June 2010.
- ^ U.S. Fuel Cell Council Industry Overview 2010, p. 12. U.S. Fuel Cell Council. 2010.
- ^ "Stuart Island Energy Initiative". Siei.org. Archived from the original on 18 June 2013. Retrieved 21 September 2009. – gives extensive technical details
- ^ "Town's Answer to Clean Energy is Blowin' in the Wind: New Wind Turbine Powers Hydrogen Car Fuel Station". Town of Hempstead. Archived from the original on 28 January 2012. Retrieved 13 January 2012.
- ^ World's Largest Carbon Neutral Fuel Cell Power Plant Archived 28 May 2013 at the Wayback Machine, 16 October 2012
- ^ Upstart Power Announces Investment for Residential Fuel Cell Technology from Clean Tech Leaders Archived 22 January 2021 at the Wayback Machine, 16 December 2020
- ^ "Reduction of residential carbon dioxide emissions through the use of small cogeneration fuel cell systems – Combined heat and power systems". IEA Greenhouse Gas R&D Programme (IEAGHG). 11 November 2008. Archived from the original on 3 December 2013. Retrieved 1 July 2013.
- ^ "Reduction of residential carbon dioxide emissions through the use of small cogeneration fuel cell systems – Scenario calculations". IEA Greenhouse Gas R&D Programme (IEAGHG). 11 November 2008. Archived from the original on 26 October 2013. Retrieved 1 July 2013.
- ^ "cogen.org – body shop in nassau county".
- ^ "Fuel Cells and CHP" (PDF). Archived from the original (PDF) on 18 May 2012.
- ^ "Patent 7,334,406". Archived from the original on 24 February 2021. Retrieved 25 August 2011.
- ^ "Geothermal Heat, Hybrid Energy Storage System". Archived from the original on 5 March 2012. Retrieved 25 August 2011.
- ^ "Reduction of residential carbon dioxide emissions through the use of small cogeneration fuel cell systems – Commercial sector". IEA Greenhouse Gas R&D Programme (IEAGHG). 11 November 2008. Archived from the original on 5 March 2018. Retrieved 1 July 2013.
- ^ "PureCell Model 400: Overview" Archived 14 May 2011 at the Wayback Machine. UTC Power. Accessed 2 August 2011.
- ^ "Comparison of Fuel Cell Technologies" Archived 1 March 2013 at the Wayback Machine. Department of Energy Energy Efficiency and Renewable Energy Fuel Cell Technologies Program. February 2011.
- ^ Onovwiona, H.I.; Ugursal, V.I. (2006). "Residential cogeneration systems: review of the current technology". Renewable and Sustainable Energy Reviews. 10 (5): 389–431. Bibcode:2006RSERv..10..389O. doi:10.1016/j.rser.2004.07.005.
- ^ AD. Hawkes, L. Exarchakos, D. Hart, MA. Leach, D. Haeseldonckx, L. Cosijns and W. D'haeseleer. EUSUSTEL work package 3: Fuell cells, 2006.
- ^ "Reduction of residential carbon dioxide emissions through the use of small cogeneration fuel cell systems". IEA Greenhouse Gas R&D Programme (IEAGHG). 11 November 2008. Archived from the original on 4 May 2018. Retrieved 1 July 2013.
- ^ "HyER " Enfarm, enefield, eneware!". Archived from the original on 15 February 2016.
- ^ "Hydrogen Fuel Cell - Designs, reactions, FCEV, Pros and Cons". Bauaelectric. 17 July 2021. Archived from the original on 18 July 2021. Retrieved 18 July 2021.
- ^ "Global Market for Hydrogen Fuel Cell Vehicles: Forecasts for Major World Regions To 2032". 21 May 2020.
- ^ "Fuel Cell Electric Vehicles". Community Environmental Council. Archived from the original on 27 March 2018. Retrieved 26 March 2018.
- ^ Wipke, Keith, Sam Sprik, Jennifer Kurtz and Todd Ramsden. "National FCEV Learning Demonstration" Archived 19 October 2011 at the Wayback Machine. National Renewable Energy Laboratory, April 2011, accessed 2 August 2011
- ^ Garbak, John. "VIII.0 Technology Validation Sub-Program Overview" Archived 24 September 2015 at the Wayback Machine. DOE Fuel Cell Technologies Program, FY 2010 Annual Progress Report, accessed 2 August 2011
- ^ "Accomplishments and Progress" Archived 21 August 2011 at the Wayback Machine. Fuel Cell Technology Program, U.S. Dept. of Energy, 24 June 2011
- ^ a b Lathia, Rutvik Vasudev; Dobariya, Kevin S.; Patel, Ankit (10 January 2017). "Hydrogen Fuel Cells for Road Vehicles". Journal of Cleaner Production. 141: 462. Bibcode:2017JCPro.141..462L. doi:10.1016/j.jclepro.2016.09.150.
- ^ "Mirai – New and Used Car Reviews, Comparisons and News".
- ^ Korzeniewski, Jeremy (27 September 2012). "Hyundai ix35 lays claim to world's first production fuel cell vehicle title". autoblog.com. Retrieved 7 October 2012.
- ^ "Hydro Dip: 2017 Honda Clarity Fuel-Cell Leases Cheaper Than Initially Expected". Archived from the original on 27 March 2018. Retrieved 26 March 2018.
- ^ "Toyota launches second generation Mirai hydrogen fuel cell vehicle". Retrieved 21 December 2020.
- ^ Martin, Polly. "Toyota sued over lack of hydrogen availability for fuel cell cars in California", Hydrogen Insight, July 15, 2024
- ^ "Hyundai recalls hydrogen fuel cell vehicles due to fire risk and tells owners to park them outdoors", Associated Press, via Boston.com, October 18, 2024
- ^ a b Romm, Joseph. "Tesla Trumps Toyota: Why Hydrogen Cars Can't Compete With Pure Electric Cars", CleanProgress.com, 5 August 2014
- ^ "Hell and Hydrogen". Technologyreview.com. March 2007. Retrieved 31 January 2011.
- ^ Fernandez, Ray (14 April 2022). "Here's Why Hydrogen Cars Were Doomed to Fail". SlashGear. Retrieved 16 April 2022.
- ^ a b Brian Warshay, Brian. "The Great Compression: the Future of the Hydrogen Economy" Archived 15 March 2013 at the Wayback Machine, Lux Research, Inc. January 2013
- ^ "Elon Musk on why Hydrogen fuel cell is dumb (2015)", YouTube, 14 January 2015, at 10:20 of the clip
- ^ Romm, Joseph. "Tesla Trumps Toyota Part II: The Big Problem with Hydrogen Fuel Cell Vehicles", CleanProgress.com, 13 August 2014
- ^ Hunt, Tam. "Should California Reconsider Its Policy Support for Fuel-Cell Vehicles?", GreenTech Media, 10 July 2014
- ^ Brown, Nicholas. "Hydrogen Cars Lost Much of Their Support, But Why?", Clean Technica, 26 June 2015
- ^ "Engineering Explained: 5 Reasons Why Hydrogen Cars Are Stupid", Car Throttle, 8 October 2015
- ^ Ruffo, Gustavo Henrique. "This Video Compares BEVs to FCEVs and the More Efficient Is...", InsideEVs.com, 29 September 2019
- ^ Baxter, Tom. "Hydrogen cars won't overtake electric vehicles because they're hampered by the laws of science", The Conversation, 3 June 2020
- ^ Morris, Charles. "Why Are 3 Automakers Still Hyping Hydrogen Fuel Cell Vehicles?", CleanTechnica, October 14, 2021
- ^ Plötz, Patrick. "Hydrogen technology is unlikely to play a major role in sustainable road transport", Nature Electronics, vol. 5, pp. 8–10, January 31, 2022
- ^ Bjørnæs, Christian. "Global warming potential of hydrogen estimated", Centre for International Climate and Environmental Research, June 7, 2023. Retrieved June 15, 2023
- ^ "National Fuel Cell Bus Program Awards". Calstart. Accessed 12 August 2011 Archived 31 October 2012 at the Wayback Machine
- ^ "Transportation Fleet Vehicles: Overview" Archived 17 October 2011 at the Wayback Machine. UTC Power. Accessed 2 August 2011.
- ^ "FY 2010 annual progress report: VIII.0 Technology Validation Sub-Program Overview" Archived 24 September 2015 at the Wayback Machine, John Garbak. Department of Energy Hydrogen Program.
- ^ "Fuel Cell Electric Bus Evaluations", U.S. Dept. of Energy, accessed 10 September 2019
- ^ Hajek, Stefan (17 August 2023). "Wasserstoff- und Batterie-Züge: "Die Batterie setzt sich fast immer gegen den Wasserstoff durch". www.wiwo.de (in German). Retrieved 15 July 2024.
- ^ Parkes, Rachel (22 August 2023). "Hydrogen will 'almost always' lose out to battery-electric in German rail transport: train manufacturer". hydrogeninsight.com. Retrieved 15 July 2024.
- ^ "Fuel cell powered trains". Alstom Coradia iLint.
- ^ "Alstom's Coradia iLint hydrogen train runs for the first time in Sweden". Alstom.com.
- ^ "Hydrogen trains in U.K." HydroFlex.
- ^ "Toyota and Hino Launch Initiative with Seven-Eleven, FamilyMart, and Lawson to Introduce Light-Duty Fuel Cell Electric Trucks". Toyota. 8 December 2020. Retrieved 25 November 2021.
- ^ "ローソンとファミマが燃料電池トラック導入、トヨタいすゞ日野が車両開発" [Lawson and FamilyMart introduced fuell cell trucks developed by Toyota and Hino]. IT media, Japan. 11 August 2021. Retrieved 25 November 2021.
- ^ Ohnsman, Alan (25 August 2021). "Toyota to Make Fuel Cell Modules for Hydrogen Big Rigs At Kentucky Plant". Forbes. Retrieved 25 November 2021.
- ^ "Daimler Truck's hydrogen-based fuel-cell truck receives license for road use" (Press release). Daimler Truck. 25 October 2021. Retrieved 4 April 2022.
- ^ "再生医療専門クリニック リペアセルクリニック 東京院" (PDF). 21 August 2013. Archived from the original (PDF) on 21 August 2013.
- ^ "Fuel cell technologies program overview" (PDF). Archived from the original (PDF) on 3 December 2013.
- ^ "Economic Impact of Fuel Cell Deployment in Forklifts and for Backup Power under the American Recovery and Reinvestment Act" (PDF). Archived from the original (PDF) on 3 December 2013.
- ^ Barnard, Michael. "On Hydrogen Forklifts, Bitcoin Mining and Green Fertilizer", CleanTechnica, January 2, 2024
- ^ "Global and Chinese Forklift Industry Report, 2014-2016", Research and Markets, 6 November 2014
- ^ "Full Fuel-Cycle Comparison of Forklift Propulsion Systems" (PDF). Archived from the original (PDF) on 17 February 2013.
- ^ "Fuel cell technology". Archived from the original on 3 December 2013. Retrieved 24 November 2013.
- ^ "Creating Innovative Graphite Solutions for Over 125 Years". GrafTech International. Archived from the original on 6 December 2010.
- ^ "The ENV Bike". Intelligent Energy. Archived from the original on 6 March 2008. Retrieved 27 May 2007.
- ^ "Honda Develops Fuel Cell Scooter Equipped with Honda FC Stack". Honda Motor Co. 24 August 2004. Archived from the original on 2 April 2007. Retrieved 27 May 2007.
- ^ Bryant, Eric (21 July 2005). "Honda to offer fuel-cell motorcycle". autoblog.com. Archived from the original on 16 July 2012. Retrieved 27 May 2007.
- ^ 15. Dezember 2007 (15 December 2007). "Hydrogen Fuel Cell electric bike". Youtube.com. Archived from the original on 30 October 2021. Retrieved 21 September 2009.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ "Horizon fuel cell vehicles: Transportation: Light Mobility" Archived 22 July 2011 at the Wayback Machine. Horizon Fuel Cell Technologies. 2010. Accessed 2 August 2011.
- ^ "Asia Pacific Fuel Cell Technologies, Ltd. --fuel cell systems and fuel cell powered vehicles". Archived from the original on 1 January 2013.
- ^ "The fuel cell industry review 2012" (PDF).
- ^ Burgman_Fuel-Cell_Scooter; "Products History 2000s". Global Suzuki. Suzuki Motor Corporation. Archived from the original on 24 October 2013. Retrieved 25 October 2013.
- ^ "Eco energy firm in Suzuki deal". Leicester Mercury. 6 February 2012. Archived from the original on 29 October 2013. Retrieved 26 October 2013.; "Suzuki and IE to commercialize FC cars and bikes". Gizmag. 8 February 2012. Retrieved 26 October 2013.
- ^ "First Fuel Cell Microaircraft". Archived from the original on 6 January 2010.
- ^ "Horizon Fuel Cell Powers New World Record in UAV Flight" Archived 14 October 2011 at the Wayback Machine. Horizon Fuel Cell Technologies. 1 November 2007.
- ^ "Boeing Successfully Flies Fuel Cell-Powered Airplane". Archived from the original on 9 May 2013.. Boeing. 3 April 2008. Accessed 2 August 2011.
- ^ "Fuel Cell Powered UAV Completes 23-hour Flight". Alternative Energy: News. 22 October 2009. Accessed 2 August 2011.
- ^ CNBC.com, Anmar Frangoul | Special to (2 February 2016). "Hydrogen fuel cells… on a plane?". CNBC. Retrieved 6 February 2018.
- ^ "Hydrogen-powered unmanned aircraft completes set of tests" Archived 15 October 2015 at the Wayback Machine.www.theengineer.co.uk. 20 June 2011. Accessed 2 August 2011.
- ^ Coxworth, Ben (8 February 2016). "Drone flight powered by lightweight hydrogen-producing pellets". www.gizmag.com. Retrieved 9 February 2016.
- ^ Eshel, Tamir (19 August 2011). "Stalker EX Mini-UAV Set for Eight Hour Endurance Missions".
- ^ FC Applications
- ^ "GL- Rules for classification and construction" (PDF). Archived from the original (PDF) on 3 December 2013. Retrieved 27 November 2023.
- ^ a b c d e Sürer, Meryem Gizem; Arat, Hüseyin Turan (26 May 2022). "Advancements and current technologies on hydrogen fuel cell applications for marine vehicles". International Journal of Hydrogen Energy. The Fifth International Hydrogen Technologies Congress. 47 (45): 19865–19875. Bibcode:2022IJHE...4719865S. doi:10.1016/j.ijhydene.2021.12.251. ISSN 0360-3199. S2CID 246104205.
- ^ "First fuel cell passenger ship unveiled in Hamburg". Fuel Cells Bulletin. 2008 (10): 4–5. 1 October 2008. Bibcode:2008FCBu.2008SV..4.. doi:10.1016/S1464-2859(08)70372-9. ISSN 1464-2859.
- ^ "First Liquid Hydrogen Ferry equipped with Fuel Cells". 28 November 2022. Retrieved 28 November 2022.
- ^ "Fuel cells installed onboard the world's first liquid hydrogen-powered ferry". 18 November 2022. Archived from the original on 28 November 2022. Retrieved 28 November 2022.
- ^ "A Revolution in Submarine Propulsion". U.S. Naval Institute. 1 October 2020. Retrieved 3 December 2024.
- ^ "Super-stealth sub powered by fuel cell" Archived 4 August 2011 at the Wayback Machine. Frederik Pleitgen. CNN Tech: Nuclear Weapons. 22 February 2011. Accessed 2 August 2011.
- ^ "U212 / U214 Attack Submarines, Germany". Naval-Technology.com. Accessed 2 August 2011. Archived 3 October 2012 at the Wayback Machine
- ^ a b Agnolucci, Paolo (December 2007). "Economics and market prospects of portable fuel cells". International Journal of Hydrogen Energy. 32 (17): 4319–4328. Bibcode:2007IJHE...32.4319A. doi:10.1016/j.ijhydene.2007.03.042. S2CID 98471675.
- ^ a b Dyer, C.K> (April 2002). "Fuel cells for portable applications". Journal of Power Sources. 106 (1–2): 31–34. Bibcode:2002JPS...106...31D. doi:10.1016/S0378-7753(01)01069-2.
- ^ "SFC Energy AG - Clean energy everywhere". SFC Energy.
- ^ systems, ensol. "ensol systems". Ensol Systems.
- ^ "Ballard fuel cells to power telecom backup power units for motorola" Archived 6 July 2011 at the Wayback Machine. Association Canadienne de l'hydrogene et des piles a combustible. 13 July 2009. Accessed 2 August 2011.
- ^ "India telecoms to get fuel cell power". Archived from the original on 26 November 2010.
- ^ "Cottbus receives new local data center" Archived 30 September 2011 at the Wayback Machine. T Systems. 21 March 2011.
- ^ "Fuel Cell Applications" Archived 15 May 2011 at the Wayback Machine. Fuel Cells 2000. Accessed 2 August 2011
- ^ DVGW VP 119 Brennstoffzellen-Gasgeräte bis 70 kW Archived 26 February 2021 at the Wayback Machine. DVGW. (German)
- ^ Laine Welch (18 May 2013). "Laine Welch: Fuel cell technology boosts long-distance fish shipping". Anchorage Daily News. Archived from the original on 9 June 2013. Retrieved 19 May 2013.
- ^ "Fuel Cell Technology Applied to Alcohol Breath Testing". Intoximeters, Inc. Retrieved 24 October 2013.
- ^ "In 2019: 83 New Hydrogen Refuelling Stations Worldwide - FuelCellsWorks". 19 February 2020.
- ^ a b "In 2019, 83 new hydrogen refuelling stations worldwide/". 19 February 2020. Retrieved 10 June 2020.
- ^ "Filling up with H2". 10 June 2020. Retrieved 10 June 2020.
- ^ "About | Hydrogen Mobility Europe". h2me.eu. 19 November 2015. Retrieved 24 March 2020.
- ^ Alternative Fueling Station Counts by State, Alternative Fuels Data Center, accessed 31 August 2020
- ^ Kurtz, Jennifer; Sprik, Sam; Bradley, Thomas H. (2019). "Review of Transportation Hydrogen Infrastructure Performance and Reliability". International Journal of Hydrogen Energy. 44 (23). National Renewable Energy Laboratory: 12010–12023. Bibcode:2019IJHE...4412010K. doi:10.1016/j.ijhydene.2019.03.027. OSTI 1506613. S2CID 132085841. Retrieved 7 October 2020.
- ^ "Fuel Cells: Current Status and Future Challenges". NAE Website. Retrieved 2 December 2023.
- ^ "Fuel Cell Applications 101: Where Are Fuel Cells Used Today? - Plug Power". www.plugpower.com. 19 January 2023. Retrieved 2 December 2023.[permanent dead link]
- ^ İnci, Mustafa (1 October 2022). "Future vision of hydrogen fuel cells: A statistical review and research on applications, socio-economic impacts and forecasting prospects". Sustainable Energy Technologies and Assessments. 53 102739. Bibcode:2022SETA...5302739I. doi:10.1016/j.seta.2022.102739. ISSN 2213-1388. S2CID 252235918.
- ^ Jessel, Sonal; Sawyer, Samantha; Hernández, Diana (12 December 2019). "Energy, Poverty, and Health in Climate Change: A Comprehensive Review of an Emerging Literature". Frontiers in Public Health. 7 357. Bibcode:2019FrPH....7..357J. doi:10.3389/fpubh.2019.00357. ISSN 2296-2565. PMC 6920209. PMID 31921733.
- ^ "Hydrogen Fuel Cell: Bus Technologies". www.zemo.org.uk. Retrieved 2 December 2023.
- ^ "Navigant: fuel cell industry passed $1-billion revenue mark in 2012", Green Car Congress, 12 August 2013
- ^ "Fuel cell report highlights continued growth in material handling applications". 20 November 2013.
- ^ "Tanaka precious metals constructs dedicated plant for the development and manufacture of fuel cell catalysts", FuelCellToday.com, 26 February 2013, accessed 16 November 2013
- ^ Adamson, Karry-Ann and Clint Wheelock. "Fuel Cell Annual Report 2011" Archived 17 October 2011 at the Wayback Machine. 2Q 2011, Pike Research, accessed 1 August 2011
- ^ "Solid State Energy Conversion Alliance SECA Cost Reduction". U.S. Dept. of Energy, 31 January 2011, accessed 1 August 2011
- ^ "Lower & Lock-In Energy Costs" Archived 3 August 2011 at the Wayback Machine, Bloom Energy, accessed 3 August 2011
- ^ Wesoff, Eric. "Bloom Energy Plays the Subsidy Game Like a Pro", 13 April 2011, accessed 1 August 2011 Archived 11 April 2012 at the Wayback Machine
- ^ Yoo-chul, Kim. "Samsung to drop fuel cell business", Korea Times, 12 April 2016
- ^ "Hydrogen Fuel Cell That's As Durable As A Conventional Engine". Archived from the original on 16 October 2013.
- ^ "ACAL poster on Fuel Cell costs and efficiency" (PDF). Archived from the original (PDF) on 16 October 2013.
- ^ Kakati, Biraj Kumar; Kucernak, Anthony RJ (15 March 2014). "Gas phase recovery of hydrogen sulfide contaminated polymer electrolyte membrane fuel cells". Journal of Power Sources. 252: 317–326. Bibcode:2014JPS...252..317K. doi:10.1016/j.jpowsour.2013.11.077.
- ^ Kakati, Biraj Kumar; Unnikrishnan, Anusree; Rajalakshmi, Natarajan; Jafri, RI; Dhathathreyan, KS (2016). "Kucernak". Anthony RJ. 41 (12): 5598–5604. doi:10.1016/j.ijhydene.2016.01.077. hdl:10044/1/28872.
- ^ Kakati, BK. "In-situ O3 rejuvenation of SO2 contaminated Polymer Electrolyte Fuel Cell: Electrochemistry, single cell and 5-cells stack studies" (PDF). 5th European PEFC & H2 Forum. Archived from the original (PDF) on 14 July 2015. Retrieved 14 July 2015.
- ^ "In Situ Hydrogen Generation Fuel Cell for Future Soldier Power Systems – HDIAC". Hdiac. Retrieved 7 February 2023.
- ^ Shi, Lin; Zhao, Yun; Matz, Stephanie; Gottesfeld, Shimshon; Setzler, Brian P.; Yan, Yushan (March 2022). "A shorted membrane electrochemical cell powered by hydrogen to remove CO2 from the air feed of hydroxide exchange membrane fuel cells". Nature Energy. 7 (3): 238–247. Bibcode:2022NatEn...7..238S. doi:10.1038/s41560-021-00969-5. ISSN 2058-7546. S2CID 246585109.
- ^ "Method for manufacturing protonic ceramic fuel cell, and protonic ceramic fuel cell manufactured thereby".
Further reading
[edit]- Vielstich, W.; et al., eds. (2009). Handbook of fuel cells: advances in electrocatalysis, materials, diagnostics and durability. Hoboken: John Wiley and Sons.
- Gregor Hoogers (2003). Fuel Cell Technology – Handbook. CRC Press.
- James Larminie; Andrew Dicks (2003). Fuel Cell Systems Explained (Second ed.). Hoboken: John Wiley and Sons.
- Subash C. Singhal; Kevin Kendall (2003). High Temperature Solid Oxide Fuel Cells-Fundamentals, Design and Applications. Elsevier Academic Press.
- Frano Barbir (2005). PEM Fuel Cells-Theory and Practice. Elsevier Academic Press.
- EG&G Technical Services, Inc. (2004). Fuel Cell Technology-Handbook, 7th Edition. U.S. Department of Energy.
- Matthew M. Mench (2008). Fuel Cell Engines. Hoboken: John Wiley & Sons, Inc.
- Noriko Hikosaka Behling (2012). Fuel Cells: Current Technology Challenges and Future Research Needs (First ed.). Elsevier Academic Press.
External links
[edit]Fuel cell
View on GrokipediaFundamental Principles
Operating Mechanism
A fuel cell is an electrochemical device that converts the chemical energy of a fuel, typically hydrogen, and an oxidant, usually oxygen, directly into electrical energy through redox reactions, producing water and heat as byproducts without combustion.[2] This process enables higher theoretical efficiency compared to heat engines, as it bypasses thermodynamic limitations like the Carnot cycle by directly harnessing Gibbs free energy changes.[10] The fundamental operating mechanism involves three primary components: an anode where oxidation occurs, a cathode where reduction takes place, and an electrolyte that conducts ions but not electrons between them. At the anode, hydrogen gas is supplied and, in the presence of a catalyst such as platinum, dissociates into protons and electrons via the half-reaction: H₂ → 2H⁺ + 2e⁻. The electrons flow through an external circuit to the cathode, generating direct current electricity, while protons pass through the electrolyte.[1][11] At the cathode, oxygen from air reacts with the incoming protons and electrons: ½O₂ + 2H⁺ + 2e⁻ → H₂O, forming water. The net cell reaction is thus H₂ + ½O₂ → H₂O, with the electrical potential arising from the spatial separation of the oxidation and reduction processes. Operating voltages for individual cells typically range from 0.6 to 0.7 volts under load, necessitating stacking multiple cells in series to achieve practical power levels.[11][10] Fuel cells require continuous supply of reactants to sustain operation, distinguishing them from batteries which store finite energy. Catalysts accelerate reaction kinetics, particularly the oxygen reduction reaction, which is inherently sluggish and determines much of the performance limitations. Electrolyte choice dictates ion type (protons, hydroxide ions, or oxygen ions) and operating temperature, influencing efficiency, durability, and fuel flexibility, though the core charge separation mechanism remains invariant.[12][10]Electrochemical Reactions
In fuel cells, electrochemical reactions occur at the anode and cathode, separated by an electrolyte, to convert chemical energy directly into electrical energy without combustion. At the anode, fuel undergoes oxidation, releasing electrons that flow through an external circuit to generate current, while ions migrate through the electrolyte to the cathode. At the cathode, the oxidant is reduced by combining with the ions and electrons, producing water or other byproducts depending on the fuel cell type.[2][13] For hydrogen-oxygen fuel cells, the primary example in proton exchange membrane fuel cells (PEMFCs), the anode reaction involves the oxidation of hydrogen: H₂ → 2H⁺ + 2e⁻, facilitated by a catalyst such as platinum to split the hydrogen molecule into protons and electrons. The electrons travel via the external circuit to power the load, while protons pass through the proton-conducting electrolyte membrane.[2][14][13] At the cathode, oxygen reduction occurs: ½O₂ + 2H⁺ + 2e⁻ → H₂O, where atmospheric oxygen or pure oxygen combines with protons and electrons to form water, releasing heat as a byproduct. The overall reaction is H₂ + ½O₂ → H₂O, with a standard cell potential of approximately 1.23 V under standard conditions, though practical voltages are lower due to overpotentials and losses.[2][14][13] These reactions are reversible in principle, enabling fuel cells to operate in electrolysis mode for hydrogen production, but inefficiencies arise from kinetic barriers, particularly the sluggish oxygen reduction reaction at the cathode, which necessitates high catalyst loadings. In other fuel types, such as direct methanol fuel cells, the anode reaction shifts to CH₃OH + H₂O → CO₂ + 6H⁺ + 6e⁻, but hydrogen remains the most efficient fuel due to its high electrochemical reactivity.[2][13]Key Components and Materials
A fuel cell's core structure comprises two electrodes—the anode and cathode—separated by an electrolyte that permits ion conduction while preventing electron flow through it. The anode facilitates fuel oxidation, releasing electrons and ions, while the cathode enables oxidant reduction, consuming electrons and ions to form products such as water. These electrodes are typically porous to allow reactant access and product removal.[10][12] Catalysts coat the electrodes to accelerate sluggish reaction kinetics; platinum or platinum alloys supported on carbon black are standard for low-temperature cells like proton exchange membrane fuel cells (PEMFCs), with loadings often reduced to 0.1-0.5 mg/cm² to mitigate costs associated with platinum's scarcity. High-temperature cells, such as solid oxide fuel cells (SOFCs), employ nickel-based catalysts due to internal reforming capabilities and thermal stability.[10][12] The electrolyte's material varies by fuel cell type: perfluorosulfonic acid polymers like Nafion in PEMFCs for proton conduction at 60-80°C; concentrated phosphoric acid in phosphoric acid fuel cells (PAFCs) for operation near 200°C; or yttria-stabilized zirconia ceramics in SOFCs for oxide ion transport above 700°C. Electrodes often consist of carbon composites for electrical conductivity and porosity in low-temperature systems, or metallic alloys like nickel-chromium in molten carbonate or SOFCs to withstand corrosive, high-temperature environments.[10] In practical assemblies, gas diffusion layers (GDLs)—typically carbon fiber paper or cloth treated with polytetrafluoroethylene (PTFE)—adjunct the electrodes to distribute gases, manage water, and aid electron conduction. Bipolar plates, constructed from graphite, carbon-polymer composites, or stainless steel, form flow channels for reactants, provide mechanical support, and enable electrical series connection in stacks. Gaskets of elastomeric polymers seal components to prevent leaks. Material durability remains critical, as degradation from impurities like sulfur (tolerances below 0.5-50 ppm depending on type) or mechanical stress limits longevity.[12][10]History
Early Conceptualization and Inventions
The foundational concept of the fuel cell emerged from early 19th-century experiments in electrolysis and the reversal of chemical decomposition processes. In 1800, William Nicholson and Anthony Carlisle demonstrated the electrolytic decomposition of water into hydrogen and oxygen using electric current from a voltaic pile, laying groundwork for understanding reversible electrochemical reactions.[15] This principle suggested the potential for recombining the gases to generate electricity without combustion's thermal inefficiencies, though practical implementation required further innovation. In January 1839, German chemist Christian Friedrich Schönbein observed the fuel cell effect using platinum electrodes exposed to hydrogen and oxygen gases, noting electrical current generation from gas recombination.[16] Independently, British scientist Sir William Robert Grove constructed the first functional fuel cell later that year, termed a "gas voltaic battery." Grove's device featured two platinum foil electrodes: one bubbled with hydrogen in dilute sulfuric acid and the other with oxygen in nitric acid, separated by a porous ceramic pot, yielding approximately 1 volt and continuous current through the electrochemical oxidation of hydrogen and reduction of oxygen to form water.[17][18] Grove detailed his invention in a 1842 letter to Michael Faraday and subsequent publications, emphasizing its efficiency over heat engines by directly converting chemical energy to electrical energy.[18] The apparatus, while rudimentary, demonstrated sustained power output, with multiple cells stacked to increase voltage, though high costs of platinum electrodes limited scalability.[17] These early inventions highlighted the viability of fuel cells but remained experimental curiosities due to material constraints and incomplete understanding of electrode catalysis.20th Century Developments and Space Applications
In the 1930s, British engineer Francis Thomas Bacon revived practical fuel cell research by developing an alkaline electrolyte design using potassium hydroxide (KOH), operating at elevated temperatures around 200–240°C and pressures up to 45 atm to mitigate electrode flooding issues inherent in earlier low-temperature attempts. This "Bacon cell" employed porous nickel electrodes and successfully demonstrated continuous operation with hydrogen and oxygen, achieving power densities sufficient for a 5 kW stack by 1959, which was showcased to NASA representatives.[19] Bacon's innovations addressed durability challenges through high-temperature operation that facilitated water management and catalyst performance without precious metals like platinum.[20] Concurrently in the United States, General Electric (GE) advanced proton exchange membrane (PEM) fuel cells in the late 1950s, pioneered by Thomas Grubb and Leonard Niedrach, incorporating ion-exchange membranes like sulfonated polystyrene for efficient proton conduction at near-ambient temperatures.[21] These efforts aligned with military and space interests, culminating in NASA's adoption for manned missions; the Gemini V spacecraft in 1965 marked the first in-space use of PEM fuel cells, delivering 1 kW of power while generating potable water as a byproduct from the electrochemical reaction of liquid hydrogen and oxygen.[22] The system's reliability stemmed from compact design and high efficiency, exceeding 50% in converting reactants to electricity, though initial missions revealed issues like membrane degradation under microgravity, prompting iterative improvements.[23] For the Apollo program, NASA shifted to alkaline fuel cells (AFCs) derived from Bacon's technology, licensed through Pratt & Whitney, with each of the three 1.5 kW modules in the service module providing primary electrical power, lighting, and life support for missions including the 1969 Moon landing.[24] These AFCs operated at around 200°C with circulating KOH electrolyte, achieving efficiencies near 70% and producing up to 1.42 kg of water per kilowatt-hour, critical for crew hydration in extended lunar operations.[25] The space program's demands accelerated material advancements, such as asbestos-based matrices for electrolyte retention and static water removal via gas separators, enabling over 2,500 hours of operation per cell stack during Apollo 11. This era established fuel cells as viable for high-reliability, closed-loop power systems, influencing subsequent Shuttle orbiter deployments through 2011.[25]Post-2000 Commercialization Attempts and Setbacks
In the early 2000s, the U.S. Department of Energy launched the FreedomCAR initiative in 2002, allocating $150 million to advance hydrogen fuel cell technologies for vehicles, aiming to reduce costs and improve performance through partnerships with automakers.[26] Despite such efforts, commercialization faced persistent barriers including high manufacturing costs, limited durability, and inadequate hydrogen infrastructure.[27] By 2014, Toyota introduced the Mirai, the first mass-produced hydrogen fuel cell vehicle, but global sales remained negligible, totaling fewer than 2,000 units in 2024 amid declining demand outside Japan.[28] Companies like Ballard Power Systems, a key developer of PEM fuel cells since the 1980s, invested over $1 billion in R&D but reported cumulative losses exceeding $1.3 billion from 2000 to 2023 without achieving profitability, leading to the sale of its automotive division to Daimler and Ford in 2007.[29] Similarly, FuelCell Energy underwent restructuring in response to ongoing financial losses and slow market uptake in stationary applications.[30] Efforts in heavy-duty sectors, such as fuel cell buses, saw Ballard secure orders for about 1,600 engines (130 MW total) in 2024, yet broader adoption stalled due to high system costs—still roughly twice the threshold for sustainable markets—and reliability issues.[31][32] Key setbacks included the dependence on platinum catalysts, driving up costs and supply risks, alongside membrane degradation reducing lifespan below commercial viability targets of 5,000–10,000 hours for vehicles.[33] Hydrogen refueling infrastructure remained sparse, with U.S. stations numbering under 100 by 2025, exacerbating range anxiety and limiting sales, as evidenced by an 80% drop in Toyota Mirai registrations in California during late 2023.[34] Global hydrogen fuel cell vehicle sales fell 27% in the first half of 2025, reflecting competition from battery electric vehicles, which benefited from lower operational costs and denser charging networks.[35] Despite optimistic projections for market growth to billions by 2030–2034, empirical data through 2025 indicates minimal penetration, with annual U.S. deployments in the low thousands at best, underscoring fundamental challenges in scaling production and achieving cost parity without sustained subsidies.[36][37]Types of Fuel Cells
Proton Exchange Membrane Fuel Cells (PEMFCs)
Proton exchange membrane fuel cells (PEMFCs) operate using a solid polymer electrolyte membrane that conducts protons while preventing the passage of electrons or gases, typically functioning at temperatures between 60°C and 80°C. Hydrogen gas is supplied to the anode where it dissociates into protons and electrons via a platinum-based catalyst; protons migrate through the hydrated membrane to the cathode, while electrons travel externally to generate electricity. At the cathode, protons, electrons, and oxygen combine to form water, the primary byproduct.[5][38] Key components include the proton exchange membrane (e.g., Nafion, a perfluorosulfonic acid polymer), catalyst layers with platinum nanoparticles on carbon supports, gas diffusion layers for reactant distribution and water removal, and bipolar plates for current collection and flow field management. The membrane electrode assembly (MEA) integrates the membrane with catalyst layers, enabling compact stack designs with high power densities up to 2 W/cm² under practical conditions. Platinum loading has been reduced to around 0.1-0.4 mg/cm² through advanced nanostructured catalysts, though costs remain high at approximately $50-100/kW due to precious metal dependency.[39][40] PEMFCs achieve electrical efficiencies of 40-60% based on higher heating value, benefiting from rapid startup times under 1 minute and dynamic load response suitable for automotive applications. Advantages encompass low operating temperatures facilitating quick thermal cycling, minimal emissions limited to water vapor, and high volumetric power density enabling lightweight systems. However, challenges include catalyst poisoning by trace carbon monoxide (requiring >99.99% pure hydrogen), membrane degradation reducing lifespan to 5,000-10,000 hours in vehicles, and flood-prone water management necessitating precise humidity control.[5][41] Commercial applications focus on transportation, with Toyota's Mirai sedan achieving over 300,000 units sold globally by 2023 using a 114 kW stack, and hydrogen buses deployed in cities like London since 2011. Stationary uses include backup power, while portable variants power electronics. Recent advancements (2023-2025) emphasize platinum-group-metal-free catalysts and anion-exchange membranes to cut costs below $30/kW and extend durability, alongside high-temperature PEMFCs operating at 120°C for improved tolerance to impurities.[42][43][44]Alkaline Fuel Cells (AFCs)
Alkaline fuel cells (AFCs) utilize an aqueous solution of potassium hydroxide (KOH) as the electrolyte, typically at concentrations of 30-50% by weight, operating at temperatures between 60°C and 120°C.[19] The electrolyte is immobilized in a porous matrix, such as asbestos or other separators, to prevent flooding and ensure ionic conductivity.[45] AFCs employ non-precious metal catalysts like nickel, silver, or metal oxides for both anode and cathode, reducing material costs compared to platinum-dependent systems.[19] The electrochemical reactions in AFCs involve hydrogen oxidation at the anode and oxygen reduction at the cathode in an alkaline medium. At the anode: H₂ + 2OH⁻ → 2H₂O + 2e⁻; at the cathode: ½O₂ + H₂O + 2e⁻ → 2OH⁻; yielding the overall reaction H₂ + ½O₂ → H₂O, producing electricity, heat, and water.[19] These cells achieve electrical efficiencies of 60-70% in practical applications, with NASA systems in space missions demonstrating up to 70% efficiency under controlled conditions.[20] AFCs were among the earliest fuel cell types to reach practical deployment, with foundational work by Francis Thomas Bacon in the 1930s-1940s leading to a functional prototype by 1959.[46] NASA adopted AFC technology for the Gemini program in 1965, followed by Apollo missions from 1968-1972 and Space Shuttle flights through 2011, where stacks provided 1-12 kW of power and generated potable water as a byproduct.[19][22] A primary limitation of traditional AFCs is their sensitivity to carbon dioxide (CO₂), which reacts with KOH to form potassium carbonate (K₂CO₃), increasing electrolyte viscosity, reducing conductivity, and precipitating solids that clog pores.[47] This necessitates pure oxygen feeds, excluding ambient air and limiting terrestrial applications without CO₂ scrubbing.[5] Additional challenges include corrosion from the alkaline environment and electrolyte management issues like evaporation or leakage in liquid systems.[48] Recent advancements focus on anion exchange membrane fuel cells (AEMFCs), a variant of AFCs using solid polymer membranes to replace liquid electrolytes, mitigating CO₂ sensitivity and enabling air operation with ongoing catalyst and membrane durability improvements.[5] Commercial interest persists, with companies like AFC Energy developing systems for backup power and material handling, projecting market growth from USD 0.38 billion in 2025 at a CAGR of 28.77% through 2030, driven by hydrogen economy initiatives.[49][50]Phosphoric Acid Fuel Cells (PAFCs)
Phosphoric acid fuel cells (PAFCs) employ a liquid phosphoric acid electrolyte immobilized in a porous matrix, typically composed of silicon carbide particles bonded with polytetrafluoroethylene (PTFE), which separates the anode and cathode compartments while facilitating proton conduction.[5] The cells operate at temperatures between 150°C and 220°C, enabling the use of reformed hydrocarbon fuels such as natural gas, as the elevated temperature promotes tolerance to carbon monoxide impurities up to 1–2% in the fuel stream without significant catalyst poisoning.[5] [10] Platinum catalysts supported on porous carbon electrodes drive the electrochemical reactions: at the anode, hydrogen oxidizes to protons and electrons (2H₂ → 4H⁺ + 4e⁻), while at the cathode, oxygen reduces to water (O₂ + 4H⁺ + 4e⁻ → 2H₂O), yielding a theoretical cell voltage of approximately 0.7 V under load.[10] PAFCs achieve electrical efficiencies of 40–50% on a lower heating value basis, with overall system efficiencies exceeding 80–90% when configured for cogeneration, recovering waste heat for steam or hot water production.[5] This performance stems from the acid's low vapor pressure at operating temperatures, minimizing electrolyte loss, and the matrix's ability to retain the concentrated (around 100%) phosphoric acid despite gradual dilution by water product.[10] Power densities typically range from 100–200 mW/cm², constrained by the need for thick electrodes to manage acid distribution and corrosion resistance.[10] The technology's primary advantages include proven durability, with stacks demonstrating over 40,000 hours of operation in commercial units, and compatibility with impure fuels derived from fossil sources, reducing preprocessing requirements compared to proton exchange membrane fuel cells.[51] Over 500 PAFC systems, ranging from 200 kW to 11 MW, have been deployed globally since the 1970s, primarily for stationary applications such as hospitals, utilities, and wastewater treatment facilities, where reliable baseload power and heat recovery justify the capital costs.[51] United Technologies Corporation (UTC), through its Power subsidiary, led commercialization efforts, installing units in Japan and the United States by the early 1990s, supported by U.S. Department of Energy programs initiated amid the 1970s energy crises.[52] Challenges include corrosion of cell components by the acidic electrolyte, necessitating specialized materials like graphite bipolar plates and leading to gradual performance degradation; platinum loading of 0.5–1 mg/cm² adds to costs, estimated at $3,000–$4,000/kW for early systems.[53] [10] Sensitivity to sulfur contaminants above 50 ppm requires fuel desulfurization, and the high operating temperature precludes rapid startup, limiting suitability for transportation or intermittent use.[10] Despite these limitations, PAFCs represent the most mature fuel cell variant for distributed stationary power, with ongoing research focusing on advanced catalysts to reduce platinum dependency and improve tolerance to impurities.[54]Solid Oxide Fuel Cells (SOFCs)
Solid oxide fuel cells (SOFCs) operate at high temperatures, typically between 600°C and 1000°C, utilizing a solid ceramic electrolyte that conducts oxygen ions from the cathode to the anode.[5] This elevated temperature enables direct internal reforming of hydrocarbon fuels such as natural gas or biogas at the anode, eliminating the need for external preprocessing and enhancing fuel flexibility compared to lower-temperature fuel cells.[55] The core electrochemical reaction involves the reduction of oxygen at the cathode to form O²⁻ ions, which migrate through the electrolyte to react with fuel at the anode, producing water, carbon dioxide (if hydrocarbons are used), and electrons that generate electricity via an external circuit.[56] SOFCs achieve electrical efficiencies of around 60% in practical systems, with potential for higher combined heat and power efficiencies exceeding 80% due to recoverable waste heat.[5][55] The electrolyte is typically composed of yttria-stabilized zirconia (YSZ), a dense, non-porous ceramic that provides high ionic conductivity at operating temperatures while maintaining mechanical stability and chemical inertness.[5][57] YSZ, doped with 8-10 mol% yttria, exhibits oxygen ion vacancy-mediated conduction, with conductivity increasing exponentially with temperature to enable efficient ion transport.[58] The anode consists of a porous nickel-YSZ cermet, where nickel serves as the electrocatalyst for fuel oxidation and provides electronic conductivity, while YSZ ensures compatibility and prevents nickel agglomeration under reducing conditions. Cathodes are generally perovskite-structured materials like lanthanum strontium manganite (LSM) infiltrated or composited with YSZ, optimizing oxygen reduction reaction kinetics and minimizing polarization losses at high temperatures.[59] Interconnects, often lanthanum chromite-based ceramics, provide structural support, gas separation, and current collection, though metallic alternatives like ferritic stainless steels are explored for cost reduction in intermediate-temperature variants.[60] SOFCs' all-solid-state construction avoids corrosion and electrolyte management issues inherent in liquid-based cells, enabling long-term durability in stationary applications such as distributed power generation and auxiliary power units.[61] Their tolerance for impurities like sulfur in fuels stems from the high operating temperature, which kinetically favors reforming over poisoning.[55] However, challenges include thermal expansion mismatch between components, leading to cracking during startup, shutdown, or thermal cycling; material degradation via sintering, phase instability, or chromium poisoning from interconnects; and slow response times due to high thermal mass, limiting dynamic load following.[59] Efforts to lower operating temperatures to 500-700°C via thin-film electrolytes or alternative materials like scandia-stabilized zirconia aim to mitigate these issues and expand material choices, though they often trade off conductivity and stability.[58] As of 2024, SOFC systems have demonstrated over 40,000 hours of operation in field tests, with ongoing research focusing on stack scalability and reversible operation for electrolysis integration.[62]Molten Carbonate Fuel Cells (MCFCs)
Molten carbonate fuel cells (MCFCs) operate at atmospheric pressure and temperatures of approximately 650°C, utilizing a molten alkali metal carbonate electrolyte, typically a eutectic mixture of lithium carbonate (Li₂CO₃) and potassium carbonate (K₂CO₃), which provides ionic conductivity through carbonate ions (CO₃²⁻).[5][10] The high operating temperature enables internal reforming of hydrocarbon fuels such as natural gas directly within the cell, enhancing fuel flexibility compared to lower-temperature fuel cells, and allows the use of non-precious metal catalysts like nickel for the anode and lithiated nickel oxide for the cathode.[5][63] In the electrochemical process, hydrogen or reformed syngas (H₂ and CO) oxidizes at the nickel-based anode, producing water, carbon dioxide, and electrons: H₂ + CO₃²⁻ → H₂O + CO₂ + 2e⁻ and CO + CO₃²⁻ → 2CO₂ + 2e⁻. At the cathode, oxygen from air reacts with CO₂ and electrons to regenerate carbonate ions: ½O₂ + CO₂ + 2e⁻ → CO₃²⁻. This requires a CO₂ supply to the cathode (often recycled from the anode exhaust) and separation from the anode side, enabling inherent tolerance to CO₂ and potential integration with carbon capture systems.[5][10] The cell stack incorporates a porous lithium aluminate (LiAlO₂) matrix to immobilize the electrolyte, separating anode and cathode compartments while permitting ion transport.[10] MCFCs achieve electrical efficiencies of 45-60% in simple cycle operation, rising to 85-90% in combined heat and power (CHP) configurations by recovering high-grade waste heat for steam generation or reforming.[64][65] They demonstrate robustness against fuel impurities like sulfur up to 1-25 ppm, owing to the high-temperature desulfation kinetics, and support direct use of biogas or coal syngas after minimal cleanup.[5] Primary applications target stationary power generation in the multi-megawatt range, such as distributed energy systems or grid support, with commercial deployments by FuelCell Energy exceeding 100 MW cumulative capacity as of 2023, including hybrid systems integrating gas turbines for efficiencies over 65%.[5][66] Despite these strengths, MCFCs face durability challenges from the corrosive molten electrolyte and high temperatures, leading to cathode NiO dissolution (up to 1-2 μm/year), anode Ni coarsening, and electrolyte evaporation or creep in metallic components, which limit stack lifetimes to 40,000-60,000 hours under optimized conditions.[5][67] Startup times exceed 12-24 hours due to thermal cycling stresses, restricting responsiveness, while sensitivity to trace contaminants like chlorine can accelerate degradation.[64] Ongoing research focuses on alternative cathode materials (e.g., Cu-based) and electrolyte additives to mitigate dissolution and improve long-term stability, with pilot projects demonstrating over 5 years of continuous operation in utility-scale tests.[67][63]Other Variants
Direct methanol fuel cells (DMFCs) employ liquid methanol as the anode fuel in a configuration akin to proton exchange membrane fuel cells, with a polymer electrolyte membrane facilitating proton conduction; they operate at relatively low temperatures of 60–130 °C, enabling simplified thermal management and potential portability.[2] Methanol's high volumetric energy density (4.8 kWh/L compared to 0.7 kWh/L for compressed hydrogen at 700 bar) supports compact systems suitable for consumer electronics or auxiliary power units, though methanol crossover through the membrane reduces efficiency to 20–30% and generates parasitic currents.[68] Practical power densities reach 100–200 mW/cm² under optimized conditions, with ongoing research targeting catalyst enhancements to mitigate CO poisoning of platinum anodes.[69] Anion exchange membrane fuel cells (AEMFCs) utilize hydroxide-conducting polymer membranes as electrolytes, operating in alkaline conditions (pH >13) that enable the use of non-platinum group metal (non-PGM) catalysts such as silver or nickel-based materials, potentially reducing costs by over 50% relative to PEMFCs reliant on scarce platinum.[70] These cells function at 60–80 °C with hydrogen or reformed fuels, achieving peak power densities up to 2 W/cm² in lab prototypes as of 2023, though durability remains limited to 500–1000 hours due to hydroxide-induced membrane degradation and carbonate formation from CO₂ exposure.[71] AEMFCs offer compatibility with liquid fuels like hydrazine or methanol without extensive reforming, positioning them for stationary and transportation applications where PGM avoidance is prioritized.[72] Direct carbon fuel cells (DCFCs) directly oxidize solid carbon fuels such as coal, biomass, or graphite at the anode, bypassing gasification steps and theoretically attaining efficiencies of 70–80% through the reaction C + O₂ → CO₂, with variants employing molten carbonate or solid oxide electrolytes at 650–900 °C.[73] This approach leverages abundant, low-cost carbon sources, yielding lower emissions than combustion-based systems when using high-purity carbon, but faces challenges including anode polarization from carbon deposition and sulfur impurities in raw fuels, which corrode cell components.[74] Demonstrated stack outputs exceed 1 kW with fuel utilization rates over 90%, though commercialization is hindered by material stability and fuel handling logistics.[75] Microbial fuel cells (MFCs) harness exoelectrogenic bacteria, such as Geobacter species, to catalyze organic substrate oxidation at the anode under anaerobic conditions, generating low-level electricity (power densities of 0.1–1 W/m²) while treating wastewater by breaking down compounds like acetate or glucose into CO₂, water, and electrons transferred via biofilms.[76] Operating at ambient temperatures (20–40 °C) and neutral pH, MFCs achieve chemical oxygen demand removals of 50–90% in continuous-flow systems, but electron transfer inefficiencies and slow microbial kinetics limit scalability for power generation, confining applications to integrated bioelectrochemical sensors or small-scale remediation rather than grid-level energy production.[77]Comparative Analysis of Types
Fuel cell types are distinguished by their electrolytes, which determine operating temperatures, electrochemical reactions, and performance characteristics. Low-temperature variants, such as proton exchange membrane fuel cells (PEMFCs) and alkaline fuel cells (AFCs), operate below 120°C, enabling rapid startup times of seconds to minutes and high power densities suitable for transportation and portable applications, but they demand high-purity hydrogen and exhibit sensitivity to impurities like CO and CO₂, necessitating extensive fuel processing.[10][64] In contrast, intermediate-temperature phosphoric acid fuel cells (PAFCs) at 150–200°C offer moderate impurity tolerance (e.g., up to 1–2% CO) and proven reliability in stationary cogeneration, achieving electrical efficiencies of 35–42% but requiring hours for startup.[10] High-temperature types, including molten carbonate fuel cells (MCFCs) at 600–700°C and solid oxide fuel cells (SOFCs) at 500–1,000°C, support internal reforming of hydrocarbons, tolerate impurities like H₂S (up to 3,000 ppm in some SOFC designs), and deliver electrical efficiencies of 45–60%, with cogeneration systems exceeding 80%, though slow startup (hours to days) and corrosion limit them to base-load stationary power.[10][64] These differences arise from electrolyte properties: polymer membranes in PEMFCs enable fast ion conduction but degrade under impurities, while ceramic electrolytes in SOFCs withstand high temperatures for versatile fuel use but accelerate material degradation. Practical efficiencies fall below theoretical maxima (up to 83% for hydrogen-oxygen reactions) due to losses from activation, ohmic resistance, and mass transport, with high-temperature cells benefiting from reduced kinetic barriers.[10] Fuel flexibility correlates inversely with temperature; low-temperature cells require reformed hydrogen with <10–50 ppm CO for PEMFCs, whereas SOFCs and MCFCs process natural gas or syngas internally, reducing infrastructure needs but increasing system complexity.[10]| Type | Operating Temperature (°C) | Electrical Efficiency (%) | Power Range | Startup Time | Impurity Tolerance | Primary Applications |
|---|---|---|---|---|---|---|
| PEMFC | <120 (typically 60–100) | 35–60 (H₂), 40 (reformed) | <1 kW–100 kW | Seconds–minutes | Low (CO <10–50 ppm, sensitive to S) | Transportation, backup/portable power |
| AFC | <100 (typically 65–260) | 40–60 | 1–100 kW | Minutes–hours | Low (sensitive to CO₂, CO, S) | Military/space, niche backup |
| PAFC | 150–200 | 35–42 | 5–400 kW | Hours | Moderate (CO <1–2%, H₂S <50 ppm) | Stationary cogeneration, distributed generation |
| MCFC | 600–700 | 45–57 | 300 kW–3 MW | Hours–days | High (CO tolerant, H₂S <0.5 ppm) | Utility-scale stationary, CHP |
| SOFC | 500–1,000 | 40–60 | 1 kW–2 MW | Hours–days | High (H₂S up to 3,000 ppm planar) | Stationary/hybrid power, APUs |
Efficiency and Performance Metrics
Theoretical Maximum Efficiency
The theoretical maximum efficiency of a fuel cell is determined by the ratio of the Gibbs free energy change (ΔG) to the enthalpy change (ΔH) of the electrochemical reaction, expressed as η = ΔG / ΔH. This represents the fraction of the fuel's chemical energy that can be converted to electrical work under reversible, isothermal conditions, without the Carnot limitation imposed on heat engines by temperature gradients.[78][79] For the standard hydrogen-oxygen reaction (H₂ + ½O₂ → H₂O) at 25°C and 1 atm, ΔG° = -237.2 kJ/mol and ΔH° = -285.8 kJ/mol when water is produced as liquid, yielding η ≈ 83% on a higher heating value (HHV) basis. On a lower heating value (LHV) basis, assuming gaseous water product, ΔG° = -228.6 kJ/mol and ΔH° = -241.8 kJ/mol, resulting in η ≈ 94.5%.[78][79][80] This efficiency decreases with operating temperature due to the temperature dependence of ΔG (ΔG = ΔH - TΔS), as the TΔS term reduces the available free energy; for example, at 80°C with liquid water, η drops to ≈80%, and at 1000°C, it falls to ≈62%. The choice of HHV versus LHV reflects whether latent heat of water vaporization is recoverable, with LHV more applicable to high-temperature fuel cells where water exits as steam.[78][79]Practical Efficiencies by Type
Proton exchange membrane fuel cells (PEMFCs) achieve practical electrical efficiencies of 40-60% in automotive and stationary applications, with automotive systems often reaching 50-60% under optimized conditions due to high power density and rapid startup capabilities.[64] [81] These efficiencies are limited by factors such as membrane hydration requirements, catalyst losses, and system auxiliaries like compressors, which consume 10-20% of the output.[10] Alkaline fuel cells (AFCs) demonstrate practical electrical efficiencies exceeding 60%, with some systems attaining up to 70% in controlled environments like space applications, owing to their non-noble catalysts and high ionic conductivity in alkaline electrolytes.[82] However, real-world terrestrial deployments are constrained by CO2 sensitivity, which degrades performance and reduces achievable efficiencies to around 50-60% without pure hydrogen feeds.[48] Phosphoric acid fuel cells (PAFCs) operate at practical electrical efficiencies of 37-42% in commercial stationary units, such as the UTC PC25 systems, benefiting from tolerance to impurities and stable operation at 150-200°C but hindered by corrosion and phosphoric acid evaporation.[6] [64] Molten carbonate fuel cells (MCFCs) yield practical electrical efficiencies of 45-50% in simple cycle configurations, with potential for 50-60% when integrated with gas turbines, leveraging high-temperature operation (600-700°C) for internal reforming and reduced activation losses.[83] [84] Solid oxide fuel cells (SOFCs) exhibit the highest practical electrical efficiencies among common types, ranging from 50-60% in standalone systems and up to 65% in hybrid configurations, enabled by ceramic electrolytes operating at 500-1000°C that allow fuel flexibility and minimal losses from ohmic resistance.[64] [85] These values are achieved in pilot-scale demonstrations, though material degradation at high temperatures can reduce long-term performance.[10]| Fuel Cell Type | Practical Electrical Efficiency (%) | Basis | Key Limitations |
|---|---|---|---|
| PEMFC | 40-60 | LHV | Auxiliaries, membrane losses[64] |
| AFC | 50-70 | LHV | CO2 poisoning[82] |
| PAFC | 37-42 | LHV | Corrosion, acid management[6] |
| MCFC | 45-60 | LHV | Electrolyte stability[83] |
| SOFC | 50-65 | LHV | Thermal cycling durability[64] |
Well-to-Wheel Efficiency Considerations
Well-to-wheel (WTW) efficiency measures the overall energy conversion from primary resource extraction to vehicle propulsion, accounting for losses across production, delivery, and utilization stages.[86] For hydrogen fuel cell vehicles (FCVs), this includes well-to-tank (WTT) hydrogen supply chain losses and tank-to-wheel (TTW) conversion in the fuel cell and drivetrain.[87] TTW efficiencies for proton exchange membrane fuel cells (PEMFCs) typically range from 50% to 62%, outperforming gasoline internal combustion engines (20-30%) due to electrochemical conversion avoiding thermal inefficiencies.[88] [89] WTT efficiency dominates variability, depending on hydrogen production pathways. Steam methane reforming (SMR) of natural gas achieves WTT efficiencies of 65-75%, yielding WTW values of 25-35% for central production with pipeline delivery, though this relies on fossil feedstocks with associated upstream extraction losses.[90] [91] Electrolysis pathways, using grid or renewable electricity, exhibit lower WTT (50-70%) due to 65-80% electrolyzer efficiency and electricity generation/transmission losses (5-10%), resulting in WTW efficiencies of 15-30% even with dedicated renewables; liquefaction for transport adds 25-30% losses if cryogenic storage is employed.[92] [93] Compression to 350-700 bar for onboard storage incurs additional 10-15% energy penalties across pathways.[90]| Hydrogen Pathway | Approximate WTW Efficiency (%) | Key Losses |
|---|---|---|
| Central SMR with pipeline | 28-32 | Feedstock reforming (20-30%), delivery (minimal)[92] |
| Distributed NG SMR | 25-30 | On-site reforming (higher compression needs)[90] |
| Central electrolysis (renewable grid) | 20-25 | Electricity-to-H2 (25-35%), transport (5-10%)[93] [92] |
| Biomass gasification | 18-25 | Feedstock preprocessing (high variability), gasification (30-40%)[92] |
Advantages and Technical Challenges
Primary Advantages
Fuel cells offer higher electrical efficiency than conventional combustion-based systems by directly converting chemical energy from fuel into electricity via electrochemical reactions, bypassing the thermal inefficiencies of combustion and mechanical generation. Polymer electrolyte membrane (PEM) fuel cells typically achieve 40-60% efficiency, compared to 20-30% for internal combustion engines, while high-temperature variants like molten carbonate fuel cells (MCFCs) can exceed 60% when capturing waste heat for cogeneration.[1][96] This direct conversion minimizes energy losses associated with Carnot cycle limitations in heat engines, enabling better utilization of fuel input.[97] Emissions from fuel cells are significantly lower than those from fossil fuel combustion technologies, with hydrogen-oxygen fuel cells producing only water vapor and heat as byproducts, eliminating nitrogen oxides (NOx), sulfur oxides (SOx), and particulate matter at the point of use. Even when using hydrocarbon fuels in reformed systems, fuel cells generate fewer pollutants due to the absence of combustion flames, addressing air quality concerns in urban stationary and mobile applications.[1][98] Lifecycle emissions depend on fuel production pathways, but direct operation avoids the diffuse pollutant dispersion typical of internal combustion processes.[99] Fuel cells exhibit high reliability and low maintenance requirements owing to their solid-state components and lack of moving parts, unlike reciprocating engines prone to mechanical wear; operational lifetimes in stationary applications often exceed 40,000 hours with minimal downtime. Their modular design allows scalable deployment by stacking units, providing redundancy—if one module fails, others maintain output—facilitating applications from kilowatt-scale backups to megawatt power plants without efficiency penalties at partial loads.[100][97] Quiet operation, typically below 60 decibels, further suits noise-sensitive environments like hospitals or residential areas.[98] Certain fuel cell types demonstrate fuel flexibility, operating on hydrogen, natural gas, biogas, or syngas through internal reforming, reducing dependence on single feedstocks and enabling integration with diverse energy sources. Combined heat and power (CHP) configurations recapture waste heat for thermal needs, boosting overall system efficiency to 85-90% in cogeneration setups, surpassing standalone electrical generation.[101][102] These attributes position fuel cells as resilient alternatives for distributed generation, where grid instability or remote locations demand self-sufficient power.[103]Material Durability and Degradation Issues
Material degradation in fuel cells arises from chemical, mechanical, thermal, and electrochemical stresses that erode performance over operational lifetimes. In polymer electrolyte membrane fuel cells (PEMFCs), catalyst layers suffer platinum dissolution, particularly during potential cycling above 0.8 V versus the reversible hydrogen electrode, where Pt oxidizes and solubilizes, migrating into the ionomer or membrane and forming bands that reduce electrochemical active surface area by 20-40% in accelerated stress tests equivalent to 5000-8000 hours.[104][105] Ostwald ripening further coarsens Pt nanoparticles, exacerbating activity loss at rates of 1-5 μg Pt per hour under dynamic loads.[106] Membrane degradation involves peroxide radical attacks on perfluorosulfonic acid chains, causing unzipping and fluoride emission, with thinning rates up to 10 μm per 1000 hours in harsh conditions, compromising gas crossover resistance.[107][108] High-temperature fuel cells face intensified thermal and microstructural challenges. Solid oxide fuel cells (SOFCs) exhibit anode Ni coarsening via sintering at 600-800°C, reducing triple-phase boundary density and increasing ohmic and polarization resistances by 0.5-2% per 1000 hours under isothermal operation, with rapid thermal cycling amplifying delamination and cracking due to thermal expansion mismatches exceeding 10 ppm/K between components.[109][110] Cathode materials like lanthanum strontium manganite degrade through Sr segregation and phase instability, contributing to 10-20% power density loss over 10,000 hours.[111] Molten carbonate fuel cells (MCFCs) endure nickel cathode dissolution in alkaline carbonate melts, leading to NiO precipitation in the gas channel and electrolyte loss, with degradation rates of 0.5-1% per 1000 hours limiting stack lifetimes to 40,000 hours in optimized systems, though corrosive environments accelerate electrolyte creep and matrix plugging.[5][112] These mechanisms collectively hinder commercial viability, as U.S. Department of Energy targets for automotive PEMFCs demand under 10 μV/hour voltage decay for 8000 hours at 0.7 V, yet real-world testing often exceeds 50 μV/hour due to impurity poisoning and load fluctuations. Mitigation efforts, such as Pt alloying with Co or Ir for reduced dissolution or Ni-YSZ cermet stabilization in SOFCs, extend durability but introduce trade-offs in cost and initial performance. Empirical data from stack tests underscore that multi-mechanism interactions, like coupled catalyst-membrane degradation in PEMFCs, amplify losses beyond isolated effects, necessitating integrated modeling for prediction.[113][114]Scalability and Operational Limitations
Fuel cells encounter substantial scalability barriers when transitioning to commercial volumes, primarily due to manufacturing inconsistencies that compromise durability and reliability. In proton exchange membrane fuel cells (PEMFCs), scaling production introduces variations in catalyst layers and membrane electrode assemblies, leading to reduced stack performance and lifespan below targets like 8,000 hours under automotive conditions. [115] [116] High costs of platinum-group metals, which constitute up to 40% of stack expenses, hinder cost reductions without breakthroughs in catalyst loading below 0.125 g/kW or alternatives like non-precious metals, though these remain unproven at scale. [117] U.S. Department of Energy analyses indicate that while megawatt-scale PEM systems could lower costs to $50/kW through modular stacking, current fabrication yields below 90% limit economic viability for heavy-duty applications. [118] Operational constraints further impede widespread adoption, particularly in dynamic environments. High-temperature variants like solid oxide fuel cells (SOFCs) require 30 minutes to several hours for thermal ramp-up to 600–1000°C, restricting them to baseload power rather than intermittent or backup roles. [119] PEMFCs enable sub-minute startups at ambient conditions but exhibit performance drops during cold starts below 0°C, where ice formation in the membrane increases resistance by up to 50% until thawing. [120] Load-following demands accelerate degradation via potential cycling and water management imbalances, with dynamic operation raising hydrogen consumption by 10–20% compared to steady-state and shortening lifespan by factors of 2–3 in vehicle simulations. [121] [122] Partial-load efficiency declines notably across types, dropping 10–15% below peak at 50% utilization due to increased parasitic losses from pumps and humidifiers. [123] Reverse current and flooding during shutdowns exacerbate anode degradation, necessitating purge strategies that waste 1–5% of fuel per cycle. [124] These limitations, rooted in electrochemical kinetics and thermal management, demand auxiliary systems that reduce net system efficiency to 40–50% in real-world cycling, far from theoretical maxima. [9]Fuel Supply and Infrastructure
Hydrogen Production Pathways
The predominant method for hydrogen production worldwide is steam-methane reforming (SMR), which accounts for approximately 70-75% of global output, utilizing natural gas as feedstock to react methane with steam in the presence of a catalyst at high temperatures (700-1000°C) and pressures (3-25 bar), yielding hydrogen, carbon monoxide, and carbon dioxide.[125][126] This process achieves energy efficiencies of 65-75%, but generates significant emissions, typically 9-12 kg CO₂ per kg of hydrogen produced, contributing to its classification as "grey" hydrogen without carbon capture.[127][128] In 2023, global hydrogen production totaled 97 million tonnes, with over 99% derived from fossil fuel-based routes like SMR, primarily serving refining and chemical sectors rather than fuel cell applications.[129] Coal gasification represents about 20% of production, concentrated in regions like China, where coal is reacted with oxygen and steam at high temperatures (above 700°C) to produce syngas, followed by water-gas shift to increase hydrogen yield.[126] This method incurs higher emissions, ranging from 18-26 kg CO₂ equivalent per kg of hydrogen, due to the carbon-intensive feedstock and process inefficiencies, making it less viable for low-emission fuel cell pathways without extensive mitigation.[130][131] Electrolysis, which splits water into hydrogen and oxygen using electricity, constitutes less than 1% of current production but is central to "green" hydrogen when powered by renewables.[129] Common variants include alkaline electrolysis (AWE) and proton exchange membrane (PEM), with practical efficiencies requiring 50-60 kWh per kg of hydrogen—far above the theoretical minimum of 39.4 kWh/kg at 100% efficiency—due to overpotentials and system losses.[132] Production costs in 2024 hover at $5-6 per kg, driven by electrolyzer capital expenses ($600-1200/kW) and electricity prices, with scalability hindered by intermittent renewable supply, high water demands (9-15 liters per kg), and grid integration challenges that limit capacity factors to around 20-50%.[133][134] "Blue" hydrogen variants of SMR incorporate carbon capture and storage (CCS), aiming to sequester 90-95% of CO₂ emissions, but real-world capture rates in operational plants often fall below 95%, with methane leakage from natural gas supply chains further elevating lifecycle emissions to 2-4 kg CO₂e per kg hydrogen even at high capture.[135][136] Abatement costs for CCS in SMR range from $60-110 per tonne of CO₂, increasing hydrogen prices by 50-100% compared to grey production, while unproven long-term storage reliability and infrastructure needs constrain deployment.[137] Overall, fossil-derived pathways dominate due to lower costs ($1-2 per kg for grey hydrogen) and established infrastructure, but their high emissions undermine fuel cell viability for decarbonization unless paired with effective CCS, which remains technologically and economically immature at scale.[133][138]Storage and Transportation Hurdles
Hydrogen's low volumetric energy density, approximately 8 MJ/L in liquid form compared to 32 MJ/L for gasoline, necessitates specialized storage systems to achieve practical capacities for fuel cell applications.[139] This inherent property results in larger storage volumes or high-pressure containment, complicating vehicle and stationary system design.[140] Compressed gaseous storage requires tanks rated at 350–700 bar (5,000–10,000 psi) to increase density, but compression consumes 10–15% of the hydrogen's lower heating value energy content, equivalent to about 8.17 MJ/kg for 700 bar from ambient conditions.[141] [142] High-pressure vessels add weight and cost, with material challenges including hydrogen embrittlement that degrades steel and other alloys over time.[120] Liquid hydrogen storage demands cryogenic temperatures of -253°C, with liquefaction processes requiring up to 30% of the fuel's energy due to refrigeration inefficiencies.[140] Even in advanced insulated tanks, boil-off losses occur at rates of 0.1–5% per day from heat ingress, necessitating venting or active cooling systems that further reduce net efficiency.[143] [144] Alternative methods like metal hydrides or chemical carriers offer higher densities but suffer from slow release kinetics, high regeneration energy, and material degradation, limiting scalability.[145] Transportation amplifies these issues due to the absence of dedicated infrastructure; unlike natural gas pipelines, hydrogen requires compression or liquefaction before trucking or shipping, incurring losses of 1–3% from boil-off in liquid form during transit.[146] Road transport of compressed hydrogen over 100 km costs 3–5 USD per kg, driven by specialized trailers and energy for recompression, while long-distance options like pipelines face compatibility hurdles from hydrogen's permeability and embrittlement risks.[147] [120] Overall, these logistics elevate delivered hydrogen costs by 20–50% compared to on-site production, hindering fuel cell adoption without subsidies or breakthroughs in materials.[148]Refueling and Distribution Networks
Hydrogen refueling for fuel cell electric vehicles (FCEVs) relies on a sparse global network of stations dispensing compressed hydrogen at pressures of 350 to 700 bar, enabling refueling times comparable to gasoline vehicles, typically 3 to 5 minutes for a 5 to 6 kg fill sufficient for 300 to 500 km range. By the end of 2024, over 1,000 hydrogen refueling stations operated worldwide, with 125 new openings that year, including 42 in Europe, 30 in China, 25 in South Korea, 8 in Japan, and others elsewhere.[149] Asia-Pacific hosts 62% of these, totaling 849 stations, while concentrations in California (around 50 stations) and Germany (over 100) support limited FCEV fleets but reveal geographic clustering that constrains broader adoption.[150] Station costs range from $1 to $3 million each, driven by high-pressure compressors and storage vessels, with utilization rates often below 20% in early markets due to low vehicle volumes.[151] Hydrogen distribution to refueling stations and stationary fuel cell sites occurs mainly via truck transport of compressed gas cylinders or cryogenic liquid, as dedicated pipelines remain nascent. In the United States, over 90% of hydrogen delivery uses tube trailers at 200 to 500 bar, limiting economical transport distances to under 500 km and contributing 10-15% to delivered hydrogen costs through energy-intensive compression and liquefaction.[152] Emerging pipeline development includes Germany's approval of a 9,000 km "core hydrogen grid" by 2032, with initial flows expected in 2025 connecting production hubs to industrial consumers and stations.[153] Europe plans 3,300 km of new interconnectors across Austria, Germany, and Italy by mid-decade, building on 1,600 km of existing repurposed lines, though blending hydrogen into natural gas grids is capped at 5-20% volumetrically to avoid pipeline degradation.[154] Repurposing natural gas infrastructure faces hydrogen embrittlement risks, requiring steel alloy upgrades or coatings, which elevate retrofit costs by 20-50% over new builds.[155] Key challenges include high capital and operational expenses—hydrogen delivery can exceed $5-10 per kg at stations—exacerbated by low demand volumes and regulatory fragmentation, with safety standards varying by jurisdiction and complicating cross-border networks.[156] For stationary fuel cells in power generation or backup systems, on-site production via steam methane reforming or electrolysis bypasses distribution bottlenecks but ties efficiency to local energy prices, while remote sites depend on trucked supplies prone to supply chain disruptions. Scaling requires coordinated investment, as current networks support fewer than 50,000 FCEVs globally against millions of battery electric vehicles, underscoring infrastructure as a primary barrier to fuel cell commercialization.[157]Applications
Stationary Power Generation
Stationary fuel cells serve as primary power sources, backup systems, grid stabilizers, and combined heat and power (CHP) units, particularly in settings requiring reliable, decentralized electricity such as data centers, hospitals, and remote facilities.[158] These systems convert chemical energy from fuels like hydrogen or natural gas directly into electricity via electrochemical reactions, avoiding combustion and enabling high uptime with minimal mechanical wear.[98] In CHP configurations, they capture waste heat for heating or cooling, achieving overall efficiencies up to 90% in some solid oxide fuel cell (SOFC) setups.[159] Proton exchange membrane (PEM) and SOFC technologies dominate stationary applications due to their scalability from kilowatts to megawatts. PEM fuel cells operate at lower temperatures (around 80°C), suiting quick-start backup roles, while SOFCs function at 600–1000°C, allowing internal reforming of natural gas or biogas for fuel flexibility.[98] Electrical efficiencies typically range from 40–60% for PEM systems and 50–65% for SOFCs, surpassing combined cycle gas turbines (up to 60%) when accounting for heat recovery, though net efficiency depends on fuel reforming losses.[98] [160] Systems like those from Bloom Energy, which deploy SOFC stacks fueled by natural gas, have demonstrated field efficiencies around 50–55% in commercial operations, with real-time monitoring of billions of data points confirming stable performance over thousands of hours.[161] Deployments have accelerated, driven by demand for resilient power amid grid constraints, especially for AI data centers. As of 2023, the global stationary fuel cell market reached approximately $1.2 billion, with projections to exceed $8 billion by 2035 at a 16.7% CAGR, fueled by installations in North America and Asia.[162] Bloom Energy, a market leader, powers facilities like CoreWeave's AI data center in Illinois (announced July 2024) and a major Wyoming hyperscale site (September 2025), providing megawatts of onsite generation to bypass transmission delays.[163] [164] FuelCell Energy's molten carbonate fuel cells support utility-scale CHP, such as a 60 MW plant in California operational since 2018, capturing CO2 for enhanced environmental performance.[165] Despite advantages, high capital costs ($3,000–$10,000 per kW) and degradation rates (1–3% annual efficiency loss) limit widespread adoption, necessitating subsidies for competitiveness against batteries or renewables.[165] [166] Natural gas dependence in many systems results in upstream emissions, though lower than grid averages in fossil-heavy regions; pure hydrogen variants remain cost-prohibitive without green production scales.[98] Ongoing U.S. Department of Energy efforts target durability beyond 40,000 hours and cost reductions to $1,000/kW by 2030 to enable broader grid integration.[159]Transportation Uses
Fuel cell electric vehicles (FCEVs) represent a primary transportation application, converting hydrogen and oxygen into electricity to power electric motors, emitting only water vapor. Passenger car FCEVs, such as the Toyota Mirai and Hyundai Nexo, achieve driving ranges of 300-400 miles per tank, with refueling times of 3-5 minutes, offering advantages over battery electric vehicles (BEVs) for long-distance travel where charging infrastructure limits BEV practicality.[167][168] However, global FCEV sales declined in the first half of 2025 across all markets, reflecting challenges including high costs and sparse hydrogen refueling stations.[169] In heavy-duty transport, fuel cells suit buses and trucks due to their high energy density and rapid refueling, enabling extended ranges without the weight penalties of large batteries. As of early 2025, Europe operated around 370 fuel cell buses with plans exceeding 1,200 by year-end, while U.S. deployments grew via federal funding, targeting over 1,100 buses.[170][171] Truck demonstrations include Hyundai's XCIENT models in Canada and commitments for 1,000 units in China by the late 2020s.[172][173] Evaluations by the National Renewable Energy Laboratory indicate fuel cell buses achieve operational efficiencies comparable to diesel in real-world transit, though hydrogen supply costs remain a barrier.[174] Marine applications leverage fuel cells for air-independent propulsion (AIP) in submarines, enhancing stealth by eliminating frequent surfacing for air. Polymer electrolyte membrane fuel cells (PEMFCs) in Germany's Type 212A submarines, operational since 2005, provide 240-300 kW of power, extending submerged endurance to three weeks without diesel engines.[175] Similar systems equip Singapore's Invincible-class submarines, commissioned in 2024, and fourth-generation units from thyssenkrupp enable longer underwater operations with reduced noise.[176][177] Overall, FCEVs exhibit tank-to-wheel efficiencies of 50-60%, but well-to-wheel figures drop to 22% due to hydrogen production and delivery losses, versus 70% for BEVs assuming grid electricity.[178][179] Despite projections of a $90 billion FCEV market by 2045, adoption lags behind BEVs owing to infrastructure deficits and upstream energy inefficiencies.[180]
Portable and Niche Applications
Portable fuel cells, predominantly proton exchange membrane (PEM) types, target applications demanding high energy density and refuelability over battery recharging, such as military field units and unmanned systems. The U.S. Department of Energy highlights their deployment in soldier portable power for extended missions and in UAVs for longer flight durations, where fuel cells provide 2-3 times the energy per unit weight of lithium-ion batteries under certain conditions.[181][182] However, system costs remain high at approximately $300/kW for gasoline-reformed PEM units, far exceeding DOE targets and battery equivalents around $5,000/kW normalized, limiting scalability.[183] Efforts to integrate fuel cells into consumer electronics, like laptops and cell phones, have encountered persistent barriers including compact hydrogen storage, thermal management, methanol crossover in direct methanol fuel cells (DMFCs), and safety risks from fuel handling. These issues have resulted in negligible market penetration, with analyses deeming portable fuel cells a commercial failure in this segment due to inferior power density and complexity compared to advancing battery technologies.[184][185] Recent prototypes for drones and robotics show promise, with companies delivering systems under U.S. Department of Defense contracts achieving outputs up to several kilowatts, yet broader adoption hinges on resolving fuel logistics.[186] In niche maritime applications, PEM fuel cells enable air-independent propulsion (AIP) in non-nuclear submarines, allowing silent, extended submerged patrols without battery depletion or diesel snorkeling. Germany's Type 212 class, commissioned starting in 2005, employs PEM stacks rated at 30-300 kW, using stored hydrogen and oxygen to generate electricity and potable water, thereby enhancing operational stealth and endurance to over three weeks underwater.[187][177] Similar systems are under development for other navies, including Sweden's Gotland class, demonstrating fuel cells' viability where acoustic discretion outweighs infrastructure costs.[188] Space exploration represents a longstanding niche for fuel cells, particularly alkaline variants in NASA's Apollo missions from 1965-1972, which powered spacecraft systems while producing 1.3 kg of water per kg of hydrogen consumed. The Space Shuttle program utilized similar 12-16 kW stacks from 1981-2011, achieving high reliability with over 140,000 hours of operation across missions. Contemporary efforts focus on PEM adaptations for planetary rovers and habitats, leveraging their low-temperature operation and water byproduct for life support, though challenges persist in radiation tolerance and fuel storage in microgravity.[181][189]Environmental and Lifecycle Assessment
Direct Emissions Profile
Fuel cells produce electricity via electrochemical oxidation of hydrogen (or other fuels) with oxygen, yielding water and heat as primary byproducts in direct stack emissions, without combustion-related pollutants such as carbon dioxide, nitrogen oxides, or particulate matter.[1] This profile holds for hydrogen-oxygen systems across major types, including proton exchange membrane (PEM), alkaline (AFC), phosphoric acid (PAFC), molten carbonate (MCFC), and solid oxide (SOFC) cells, where the core reaction—H₂ + ½O₂ → H₂O—generates no criteria air pollutants or greenhouse gases at the point of use.[10] In PEM fuel cells, the most common for vehicular applications, exhaust consists exclusively of water vapor (or liquid water under certain conditions) and inert air components, with no detectable tailpipe emissions of CO₂, NOx, SOx, volatile organic compounds, or particulates.[190] Fuel cell electric vehicles (FCEVs) certified under U.S. standards thus qualify as zero-emission vehicles for direct outputs, emitting only water vapor and warm air.[191] Empirical testing confirms these systems achieve near-total elimination of local air toxics compared to internal combustion engines.[192] High-temperature variants like SOFCs maintain a similarly clean profile with pure hydrogen, producing water vapor but potentially trace NOx if operating conditions exceed 800°C and involve nitrogen from ambient air, though levels remain orders of magnitude below combustion thresholds due to the absence of flame kinetics.[193] Fuel impurities or anode off-gases in reformed-fuel setups can introduce minor unreacted hydrocarbons or CO, but these are minimized to below 10 ppm in optimized stacks, with overall nitrogen and sulfur emissions negligible.[10] Stationary PEM and PAFC systems similarly exhibit ultra-low direct emissions, often under regulatory limits for non-attainment areas without aftertreatment.[1] Water vapor output, while not a pollutant, equates to roughly 9 kg per kg of hydrogen consumed, potentially influencing local humidity in enclosed applications but inconsequential for atmospheric greenhouse forcing.[194]Full Lifecycle Emissions by Production Method
The full lifecycle greenhouse gas (GHG) emissions associated with fuel cell systems are dominated by the hydrogen production phase, encompassing feedstock extraction, processing, purification, compression, liquefaction or storage, and transportation to end-use sites, while the fuel cell operation itself emits only water vapor and heat with negligible direct GHGs. Assessments typically measure emissions in kilograms of CO2-equivalent (CO2e) per kilogram of hydrogen (kg H2) produced, with well-to-wheel (WTW) analyses for applications like fuel cell electric vehicles (FCEVs) incorporating delivery and efficiency losses that can increase totals by 20-50% beyond production alone due to energy penalties in compression and distribution. Empirical data from lifecycle analyses reveal stark variations by method, with fossil-based routes emitting 10-20 times more than renewable electrolysis under optimal conditions, though grid-dependent electrolysis can inherit high upstream emissions from electricity sources.[137][195][196] Steam methane reforming (SMR) of natural gas, the dominant method producing over 70% of global hydrogen as of 2023, yields "gray" hydrogen with lifecycle emissions of 9-12 kg CO2e/kg H2 without carbon capture and storage (CCS), driven by methane feedstock processing and venting leaks that amplify the global warming potential. Adding CCS to SMR ("blue" hydrogen) reduces emissions to 1-3 kg CO2e/kg H2 by capturing 90-95% of process CO2, though residual fugitive methane and energy-intensive capture limit further cuts, with real-world pilots showing variability based on natural gas sourcing and capture efficiency. Coal gasification, less common but significant in regions like China, emits up to 18-20 kg CO2e/kg H2 unabated due to higher carbon intensity of coal, dropping to around 2-4 kg with CCS but still exceeding blue natural gas pathways owing to upstream mining emissions.[137][130][197] Electrolysis pathways offer the lowest potential emissions when powered by low-carbon electricity. Alkaline or proton exchange membrane (PEM) electrolysis using wind or solar renewables achieves 0.5-2 kg CO2e/kg H2, accounting for equipment manufacturing, water inputs, and indirect grid balancing, with wind-sourced examples as low as 0.6 kg in 2024 assessments. However, electrolysis tied to fossil-heavy grids (e.g., coal-dominated) can exceed 20 kg CO2e/kg H2, negating benefits and highlighting the causal dependence on electricity decarbonization; for instance, U.S. average grid electrolysis yields 10-15 kg, comparable to gray hydrogen. Biomass-derived methods, such as dark fermentation or gasification, range from near-zero (with CO2 uptake credits) to 5-10 kg CO2e/kg H2, but scale limitations and feedstock competition with food production constrain viability.[196][131][198]| Production Method | Lifecycle GHG Emissions (kg CO2e/kg H2) | Key Factors Influencing Emissions |
|---|---|---|
| SMR (gray, unabated) | 9-12 | Methane processing, venting leaks[137][195] |
| SMR + CCS (blue) | 1-3 | Capture efficiency, residual methane[199] |
| Coal gasification (unabated) | 18-20 | High feedstock carbon intensity[197] |
| Electrolysis (renewables) | 0.5-2 | Electricity source cleanliness, equipment lifecycle[196] |
| Electrolysis (fossil grid) | 10-20+ | Inherited grid emissions[198] |
Comparative Environmental Footprint vs. Alternatives
Fuel cell systems, when powered by hydrogen derived from fossil fuels via steam methane reforming, exhibit higher well-to-wheel greenhouse gas (GHG) emissions than battery electric vehicles (BEVs) operating on average global electricity grids, primarily due to the energy-intensive hydrogen production process yielding approximately 9-12 kg CO2e per kg H2, compared to BEVs' effective emissions of 50-150 g CO2e per km depending on grid carbon intensity.[202] In contrast, fuel cell electric vehicles (FCEVs) using renewably produced "green" hydrogen—via electrolysis powered by low-carbon sources—can achieve lifecycle GHG emissions as low as 20-40 g CO2e per km, surpassing BEVs in scenarios with high battery manufacturing impacts or fossil-heavy grids, though such green hydrogen currently constitutes less than 1% of global supply.[203] Versus internal combustion engine (ICE) vehicles, FCEVs reduce tailpipe emissions to zero (emitting only water), but total lifecycle emissions exceed those of gasoline ICEs only if hydrogen is low-carbon; gray hydrogen FCEVs emit 1.5-2 times more GHG than efficient ICEs on a well-to-wheel basis.[204]| Powertrain | Lifecycle GHG (g CO2e/km, mid-2020s average) | Key Assumptions |
|---|---|---|
| Gasoline ICE | 200-250 | Tailpipe + upstream refining |
| BEV | 50-150 | Grid mix; battery production ~15-20% of total |
| FCEV (gray H2) | 150-300 | SMR H2; fuel cell stack production |
| FCEV (green H2) | 20-60 | Renewable electrolysis; optimistic scaling |
Economic Realities
Manufacturing and Operational Costs
Manufacturing costs for fuel cell systems remain a primary barrier to widespread adoption, driven by expensive materials such as platinum-group catalysts in proton exchange membrane fuel cells (PEMFCs) and complex ceramic components in solid oxide fuel cells (SOFCs). For automotive PEMFC systems, current projections indicate costs around $55/kW in 2025, exceeding the U.S. Department of Energy (DOE) target of $40/kW, with stack costs comprising roughly 50% of the total at lower production volumes but dropping below 15% at high-volume manufacturing.[212][213] In stationary applications, PEMFC systems have achieved unit costs as low as $7,000 for small-scale units, while SOFC investment costs range from 4,000 to 8,000 euros per kW as of 2024, reflecting improvements in stack fabrication and balance-of-plant integration.[158][160] Cost reductions depend heavily on production scale; for instance, SOFC combined heat and power systems could fall from $2,650/kW at 100 units annually to $1,100/kW at 50,000 units, though real-world volumes remain far below this threshold.[214]| Fuel Cell Type | Application | Current Cost Estimate (2024-2025) | DOE/Target Cost | Key Cost Drivers |
|---|---|---|---|---|
| PEMFC | Automotive/Transport | $55/kW (system) | $35-40/kW by 2025 | Catalysts (Pt), membranes, assembly labor |
| SOFC | Stationary (CHP) | 4,000-8,000 €/kW | $900/kWe by 2030 | Ceramics, high-temperature seals, scaling |
| PEMFC | Backup Power (5-10 kW) | Stack <50% of system at low volume | N/A | Balance-of-plant, hydrogen storage |

