Recent from talks
Nothing was collected or created yet.
Formaldehyde
View on Wikipedia
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Formaldehyde[1] | |||
| Systematic IUPAC name
Methanal[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1209228 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.002 | ||
| EC Number |
| ||
| E number | E240 (preservatives) | ||
| 445 | |||
| KEGG | |||
| MeSH | Formaldehyde | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2209 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[7] | |||
| CH2O | |||
| Molar mass | 30.026 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 0.8153 g/cm3 (−20 °C)[2] (liquid) | ||
| Melting point | −92 °C (−134 °F; 181 K) | ||
| Boiling point | −19 °C (−2 °F; 254 K)[2] | ||
| 400 g/L | |||
| log P | 0.350 | ||
| Vapor pressure | > 1 atm[3] | ||
| Acidity (pKa) | 13.27 (hydrate)[4][5] | ||
| −18.6·10−6 cm3/mol | |||
| 2.330 D[6] | |||
| Structure | |||
| C2v | |||
| Trigonal planar | |||
| Thermochemistry[8] | |||
Heat capacity (C)
|
35.387 J·mol−1·K−1 | ||
Std molar
entropy (S⦵298) |
218.760 J·mol−1·K−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−108.700 kJ·mol−1 | ||
Gibbs free energy (ΔfG⦵)
|
−102.667 kJ·mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
571 kJ·mol−1 | ||
| Pharmacology | |||
| QP53AX19 (WHO) | |||
| Hazards | |||
| GHS labelling: | |||
   [9] [9]
| |||
| Danger | |||
| H301+H311+H331, H314, H317, H335, H341, H350, H370[9] | |||
| P201, P280, P303+P361+P353, P304+P340+P310, P305+P351+P338, P308+P310[9] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 64 °C (147 °F; 337 K) | ||
| 430 °C (806 °F; 703 K) | |||
| Explosive limits | 7–73% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
100 mg/kg (oral, rat)[12] | ||
LC50 (median concentration)
|
333 ppm (mouse, 2 h) 815 ppm (rat, 30 min)[13] | ||
LCLo (lowest published)
|
333 ppm (cat, 2 h)[13] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 0.75 ppm ST 2 ppm (as formaldehyde and formalin)[10][11] | ||
REL (Recommended)
|
Ca TWA 0.016 ppm C 0.1 ppm [15-minute][10] | ||
IDLH (Immediate danger)
|
Ca [20 ppm][10] | ||
| Safety data sheet (SDS) | MSDS(Archived) | ||
| Related compounds | |||
Related aldehydes
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
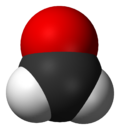
Formaldehyde (/fɔːrˈmældɪhaɪd/ ⓘ for-MAL-di-hide, US also /fər-/ ⓘ fər-) (systematic name methanal) is an organic compound with the chemical formula CH2O and structure H2C=O. The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as aqueous solutions (formalin), which consists mainly of the hydrate CH2(OH)2. It is the simplest of the aldehydes (R−CHO). As a precursor to many other materials and chemical compounds, in 2006 the global production of formaldehyde was estimated at 12 million tons per year.[14] It is mainly used in the production of industrial resins, e.g., for particle board and coatings.
Formaldehyde also occurs naturally. It is derived from the degradation of serine, dimethylglycine, and lipids. Demethylases act by converting N-methyl groups to formaldehyde.[15]
Formaldehyde is classified as a group 1 carcinogen[note 1][17] and can cause respiratory and skin irritation upon exposure.[16]
Forms
[edit]Formaldehyde is more complicated than many simple carbon compounds in that it adopts several diverse forms. These compounds can often be used interchangeably and can be interconverted.[citation needed]
- Molecular formaldehyde. A colorless gas with a characteristic pungent, irritating odor. It is stable at about 150 °C, but it polymerizes when condensed to a liquid.
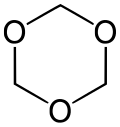
- 1,3,5-Trioxane, with the formula (CH2O)3. It is a white solid that dissolves without degradation in organic solvents. It is a trimer of molecular formaldehyde.
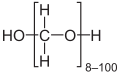
- Paraformaldehyde, with the formula HO(CH2O)nH. It is a white solid that is insoluble in most solvents.
- Methanediol, with the formula CH2(OH)2. This compound also exists in equilibrium with various oligomers (short polymers), depending on the concentration and temperature. A saturated water solution, of about 40% formaldehyde by volume or 37% by mass, is called "100% formalin".
A small amount of stabilizer, such as methanol, is usually added to suppress oxidation and polymerization. A typical commercial-grade formalin may contain 10–12% methanol in addition to various metallic impurities.
"Formaldehyde" was first used as a generic trademark in 1893 following a previous trade name, "formalin".[18]
- Main forms of formaldehyde
-
Monomeric formaldehyde (subject of this article)
-
Trioxane is a stable cyclic trimer of formaldehyde.
-
Paraformaldehyde is a common form of formaldehyde for industrial applications.
-
Methanediol, the predominant species in dilute aqueous solutions of formaldehyde
Structure and bonding
[edit]Molecular formaldehyde contains a central carbon atom with a double bond to the oxygen atom and a single bond to each hydrogen atom. This structure is summarised by the condensed formula H2C=O.[19] The molecule is planar, Y-shaped and its molecular symmetry belongs to the C2v point group.[20] The precise molecular geometry of gaseous formaldehyde has been determined by gas electron diffraction[19][21] and microwave spectroscopy.[22][23] The bond lengths are 1.21 Å for the carbon–oxygen bond[19][21][22][23][24] and around 1.11 Å for the carbon–hydrogen bond,[19][21][22][23] while the H–C–H bond angle is 117°,[22][23] close to the 120° angle found in an ideal trigonal planar molecule.[19] Some excited electronic states of formaldehyde are pyramidal rather than planar as in the ground state.[24]
Occurrence
[edit]Processes in the upper atmosphere contribute more than 80% of the total formaldehyde in the environment.[25] Formaldehyde is an intermediate in the oxidation (or combustion) of methane, as well as of other carbon compounds, e.g. in forest fires, automobile exhaust, and tobacco smoke. When produced in the atmosphere by the action of sunlight and oxygen on atmospheric methane and other hydrocarbons, it becomes part of smog. Formaldehyde has also been detected in outer space.
Formaldehyde and its adducts are ubiquitous in nature. Food may contain formaldehyde at levels 1–100 mg/kg.[26] Formaldehyde, formed in the metabolism of the amino acids serine and threonine, is found in the bloodstream of humans and other primates at concentrations of approximately 50 micromolar.[27] Experiments in which animals are exposed to an atmosphere containing isotopically labeled formaldehyde have demonstrated that even in deliberately exposed animals, the majority of formaldehyde-DNA adducts found in non-respiratory tissues are derived from endogenously produced formaldehyde.[28]
Formaldehyde does not accumulate in the environment, because it is broken down within a few hours by sunlight or by bacteria present in soil or water. Humans metabolize formaldehyde quickly, converting it to formic acid.[29][30] It nonetheless presents significant health concerns, as a contaminant.
Interstellar formaldehyde
[edit]Formaldehyde appears to be a useful probe in astrochemistry due to prominence of the 110←111 and 211←212 K-doublet transitions. It was the first polyatomic organic molecule detected in the interstellar medium.[31] Since its initial detection in 1969, it has been observed in many regions of the galaxy. Because of the widespread interest in interstellar formaldehyde, it has been extensively studied, yielding new extragalactic sources.[32] A proposed mechanism for the formation is the hydrogenation of CO ice:[33]
- H + CO → HCO
- HCO + H → CH2O
HCN, HNC, H2CO, and dust have also been observed inside the comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON).[34][35]
Synthesis and industrial production
[edit]Laboratory synthesis
[edit]Formaldehyde was discovered in 1859 by the Russian chemist Aleksandr Butlerov (1828–1886) when he attempted to synthesize methanediol ("methylene glycol") from iodomethane and silver oxalate.[36] In his paper, Butlerov referred to formaldehyde as "dioxymethylen" (methylene dioxide) because his empirical formula for it was incorrect, as atomic weights were not precisely determined until the Karlsruhe Congress.
The compound was identified as an aldehyde by August Wilhelm von Hofmann, who first announced its production by passing methanol vapor in air over hot platinum wire.[37][38] With modifications, Hofmann's method remains the basis of the present day industrial route.
Solution routes to formaldehyde also entail oxidation of methanol or iodomethane.[39]
Industry
[edit]Formaldehyde is produced industrially by the catalytic oxidation of methanol. The most common catalysts are silver metal (i.e. the FASIL process), iron(III) oxide,[40] iron molybdenum oxides (e.g. iron(III) molybdate) with a molybdenum-enriched surface,[41] or vanadium oxides. In the commonly used formox process, methanol and oxygen react at c. 250–400 °C in presence of iron oxide in combination with molybdenum and/or vanadium to produce formaldehyde according to the chemical equation:[42]
- 2 CH3OH + O2 → 2 CH2O + 2 H2O
The silver-based catalyst usually operates at a higher temperature, about 650 °C. Two chemical reactions on it simultaneously produce formaldehyde: that shown above and the dehydrogenation reaction:
- CH3OH → CH2O + H2
In principle, formaldehyde could be generated by oxidation of methane, but this route is not industrially viable because the methanol is more easily oxidized than methane.[42]
Biochemistry
[edit]Formaldehyde is produced via several enzyme-catalyzed routes.[43] Living beings, including humans, produce formaldehyde as part of their metabolism. Formaldehyde is key to several bodily functions (e.g. epigenetics[27]), but its amount must also be tightly controlled to avoid self-poisoning.[44]
- Serine hydroxymethyltransferase can decompose serine into formaldehyde and glycine, according to this reaction: HOCH2CH(NH2)CO2H → CH2O + H2C(NH2)CO2H.
- Methylotrophic microbes convert methanol into formaldehyde and energy via methanol dehydrogenase: CH3OH → CH2O + 2e− + 2H+
- Other routes to formaldehyde include oxidative demethylations, semicarbazide-sensitive amine oxidases, dimethylglycine dehydrogenases, lipid peroxidases, P450 oxidases, and N-methyl group demethylases.[43]
Formaldehyde is catabolized by alcohol dehydrogenase ADH5 and aldehyde dehydrogenase ALDH2.[45]
Organic chemistry
[edit]Formaldehyde is a building block in the synthesis of many other compounds of specialised and industrial significance. It exhibits most of the chemical properties of other aldehydes but is more reactive.[46]
Polymerization and hydration
[edit]Monomeric CH2O is a gas and is rarely encountered in the laboratory. Aqueous formaldehyde, unlike some other small aldehydes (which need specific conditions to oligomerize through aldol condensation) oligomerizes spontaneously at a common state. The trimer 1,3,5-trioxane, (CH2O)3, is a typical oligomer. Many cyclic oligomers of other sizes have been isolated. Similarly, formaldehyde hydrates to give the geminal diol methanediol, which condenses further to form hydroxy-terminated oligomers HO(CH2O)nH. The polymer is called paraformaldehyde. The higher concentration of formaldehyde—the more equilibrium shifts towards polymerization. Diluting with water or increasing the solution temperature, as well as adding alcohols (such as methanol or ethanol) lowers that tendency.
Gaseous formaldehyde polymerizes at active sites on vessel walls, but the mechanism of the reaction is unknown.[47] Small amounts of hydrogen chloride, boron trifluoride, or stannic chloride present in gaseous formaldehyde provide the catalytic effect and make the polymerization rapid.[48]
Cross-linking reactions
[edit]Formaldehyde forms cross-links by first combining with a protein to form methylol, which loses a water molecule to form a Schiff base.[49] The Schiff base can then react with DNA or protein to create a cross-linked product.[49] This reaction is the basis for the most common process of chemical fixation.
Oxidation and reduction
[edit]Formaldehyde is readily oxidized by atmospheric oxygen into formic acid. For this reason, commercial formaldehyde is typically contaminated with formic acid. Formaldehyde can be hydrogenated into methanol.
In the Cannizzaro reaction, formaldehyde and base react to produce formic acid and methanol, a disproportionation reaction.
Hydroxymethylation and chloromethylation
[edit]Formaldehyde reacts with many compounds, resulting in hydroxymethylation:
- X-H + CH2O → X-CH2OH (X = R2N, RC(O)NR', SH).
The resulting hydroxymethyl derivatives typically react further. Thus, amines give hexahydro-1,3,5-triazines:
- 3 RNH2 + 3 CH2O → (RNCH2)3 + 3 H2O
Similarly, when combined with hydrogen sulfide, it forms trithiane:[50]
- 3 CH2O + 3 H2S → (CH2S)3 + 3 H2O
In the presence of acids, it participates in electrophilic aromatic substitution reactions with aromatic compounds resulting in hydroxymethylated derivatives:
- ArH + CH2O → ArCH2OH
When conducted in the presence of hydrogen chloride, the product is the chloromethyl compound, as described in the Blanc chloromethylation. If the arene is electron-rich, as in phenols, elaborate condensations ensue. With 4-substituted phenols one obtains calixarenes.[51] Phenol results in polymers.
Other reactions
[edit]Many amino acids react with formaldehyde.[43] Cysteine converts to thioproline.
Uses
[edit]Industrial applications
[edit]Formaldehyde is a common precursor to more complex compounds and materials. In approximate order of decreasing consumption, products generated from formaldehyde include urea formaldehyde resin, melamine resin, phenol formaldehyde resin, polyoxymethylene plastics, 1,4-butanediol, and methylene diphenyl diisocyanate.[42] The textile industry uses formaldehyde-based resins as finishers to make fabrics crease-resistant.[52]

When condensed with phenol, urea, or melamine, formaldehyde produces, respectively, hard thermoset phenol formaldehyde resin, urea formaldehyde resin, and melamine resin. These polymers are permanent adhesives used in plywood and carpeting. They are also foamed to make insulation, or cast into moulded products. Production of formaldehyde resins accounts for more than half of formaldehyde consumption.
Formaldehyde is also a precursor to polyfunctional alcohols such as pentaerythritol, which is used to make paints and explosives. Other formaldehyde derivatives include methylene diphenyl diisocyanate, an important component in polyurethane paints and foams, and hexamine, which is used in phenol-formaldehyde resins as well as the explosive RDX.
Condensation with acetaldehyde affords pentaerythritol, a chemical necessary in synthesizing PETN, a high explosive:[53]

Niche uses
[edit]Disinfectant and biocide
[edit]An aqueous solution of formaldehyde can be useful as a disinfectant as it kills most bacteria and fungi (including their spores). It is used as an additive in vaccine manufacturing to inactivate toxins and pathogens.[54] Formaldehyde releasers are used as biocides in personal care products such as cosmetics. Although present at levels not normally considered harmful, they are known to cause allergic contact dermatitis in certain sensitised individuals.[55]
Aquarists use formaldehyde as a treatment for the parasites Ichthyophthirius multifiliis and Cryptocaryon irritans.[56] Formaldehyde is one of the main disinfectants recommended for destroying anthrax.[57]
Formaldehyde is also approved for use in the manufacture of animal feeds in the US. It is an antimicrobial agent used to maintain complete animal feeds or feed ingredients Salmonella negative for up to 21 days.[58]
Tissue fixative and embalming agent
[edit]
Formaldehyde preserves or fixes tissue or cells. The process involves cross-linking of primary amino groups. The European Union has banned the use of formaldehyde as a biocide (including embalming) under the Biocidal Products Directive (98/8/EC) due to its carcinogenic properties.[59][60] Countries with a strong tradition of embalming corpses, such as Ireland and other colder-weather countries, have raised concerns. Despite reports to the contrary,[61] no decision on the inclusion of formaldehyde on Annex I of the Biocidal Products Directive for product-type 22 (embalming and taxidermist fluids) had been made as of September 2009[update].[62]
Formaldehyde-based crosslinking is exploited in ChIP-on-chip or ChIP-sequencing genomics experiments, where DNA-binding proteins are cross-linked to their cognate binding sites on the chromosome and analyzed to determine what genes are regulated by the proteins. Formaldehyde is also used as a denaturing agent in RNA gel electrophoresis, preventing RNA from forming secondary structures. A solution of 4% formaldehyde fixes pathology tissue specimens at about one mm per hour at room temperature.
Drug testing
[edit]Formaldehyde and 18 M (concentrated) sulfuric acid makes Marquis reagent—which can identify alkaloids and other compounds.
Photography
[edit]In photography, formaldehyde is used in low concentrations for the process C-41 (color negative film) stabilizer in the final wash step,[63] as well as in the process E-6 pre-bleach step, to make it unnecessary in the final wash. Due to improvements in dye coupler chemistry, more modern (2006 or later) E-6 and C-41 films do not need formaldehyde, as their dyes are already stable.
Safety
[edit]In view of its widespread use, toxicity, and volatility, formaldehyde poses a significant danger to human health.[64][65] In 2011, the US National Toxicology Program described formaldehyde as "known to be a human carcinogen".[66][67][68]
Chronic inhalation
[edit]This section may require cleanup to meet Wikipedia's quality standards. The specific problem is: A little too scattered among different types of risks. Needs some reorganization. (November 2023) |
Concerns are associated with chronic (long-term) exposure by inhalation as may happen from thermal or chemical decomposition of formaldehyde-based resins and the production of formaldehyde resulting from the combustion of a variety of organic compounds (for example, exhaust gases). As formaldehyde resins are used in many construction materials,[69] it is one of the more common indoor air pollutants.[70][71] At concentrations above 0.1 ppm in air, formaldehyde can irritate the eyes and mucous membranes.[72] Formaldehyde inhaled at this concentration may cause headaches, a burning sensation in the throat, and difficulty breathing, and can trigger or aggravate asthma symptoms.[73][74]
The CDC considers formaldehyde as a systemic poison. Formaldehyde poisoning can cause permanent changes in the nervous system's functions.[75]
A 1988 Canadian study of houses with urea-formaldehyde foam insulation found that formaldehyde levels as low as 0.046 ppm were positively correlated with eye and nasal irritation.[76] A 2009 review of studies has shown a strong association between exposure to formaldehyde and the development of childhood asthma.[77]
A theory was proposed for the carcinogenesis of formaldehyde in 1978.[78] In 1987 the United States Environmental Protection Agency (EPA) classified it as a probable human carcinogen, and after more studies the WHO International Agency for Research on Cancer (IARC) in 1995 also classified it as a probable human carcinogen. Further information and evaluation of all known data led the IARC to reclassify formaldehyde as a known human carcinogen[79] associated with nasal sinus cancer and nasopharyngeal cancer.[80] Studies in 2009 and 2010 have also shown a positive correlation between exposure to formaldehyde and the development of leukemia, particularly myeloid leukemia.[81][82] Nasopharyngeal and sinonasal cancers are relatively rare, with a combined annual incidence in the United States of < 4,000 cases.[83][84] About 30,000 cases of myeloid leukemia occur in the United States each year.[85][86] Some evidence suggests that workplace exposure to formaldehyde contributes to sinonasal cancers.[87] Professionals exposed to formaldehyde in their occupation, such as funeral industry workers and embalmers, showed an increased risk of leukemia and brain cancer compared with the general population.[88] Other factors are important in determining individual risk for the development of leukemia or nasopharyngeal cancer.[87][89][90] In yeast, formaldehyde is found to perturb pathways for DNA repair and cell cycle.[91]
In the residential environment, formaldehyde exposure comes from a number of routes; formaldehyde can be emitted by treated wood products, such as plywood or particle board, but it is produced by paints, varnishes, floor finishes, and cigarette smoking as well.[92] In July 2016, the U.S. EPA released a prepublication version of its final rule on Formaldehyde Emission Standards for Composite Wood Products.[93] These new rules impact manufacturers, importers, distributors, and retailers of products containing composite wood, including fiberboard, particleboard, and various laminated products, who must comply with more stringent record-keeping and labeling requirements.[94]
| External videos | |
|---|---|
 | |
The U.S. EPA allows no more than 0.016 ppm formaldehyde in the air in new buildings constructed for that agency.[95][failed verification] A U.S. EPA study found a new home measured 0.076 ppm when brand new and 0.045 ppm after 30 days.[96] The Federal Emergency Management Agency (FEMA) has also announced limits on the formaldehyde levels in trailers purchased by that agency.[97] The EPA recommends the use of "exterior-grade" pressed-wood products with phenol instead of urea resin to limit formaldehyde exposure, since pressed-wood products containing formaldehyde resins are often a significant source of formaldehyde in homes.[80]
The eyes are most sensitive to formaldehyde exposure: The lowest level at which many people can begin to smell formaldehyde ranges between 0.05 and 1 ppm. The maximum concentration value at the workplace is 0.3 ppm.[98][need quotation to verify] In controlled chamber studies, individuals begin to sense eye irritation at about 0.5 ppm; 5 to 20 percent report eye irritation at 0.5 to 1 ppm; and greater certainty for sensory irritation occurred at 1 ppm and above. While some agencies have used a level as low as 0.1 ppm as a threshold for irritation, the expert panel found that a level of 0.3 ppm would protect against nearly all irritation. In fact, the expert panel found that a level of 1.0 ppm would avoid eye irritation—the most sensitive endpoint—in 75–95% of all people exposed.[99]

Formaldehyde levels in building environments are affected by a number of factors. These include the potency of formaldehyde-emitting products present, the ratio of the surface area of emitting materials to volume of space, environmental factors, product age, interactions with other materials, and ventilation conditions. Formaldehyde emits from a variety of construction materials, furnishings, and consumer products. The three products that emit the highest concentrations are medium density fiberboard, hardwood plywood, and particle board. Environmental factors such as temperature and relative humidity can elevate levels because formaldehyde has a high vapor pressure. Formaldehyde levels from building materials are the highest when a building first opens because materials would have less time to off-gas. Formaldehyde levels decrease over time as the sources suppress.
In operating rooms, formaldehyde is produced as a byproduct of electrosurgery and is present in surgical smoke, exposing surgeons and healthcare workers to potentially unsafe concentrations.[100]
Formaldehyde levels in air can be sampled and tested in several ways, including impinger, treated sorbent, and passive monitors.[101] The National Institute for Occupational Safety and Health (NIOSH) has measurement methods numbered 2016, 2541, 3500, and 3800.[102]
In June 2011, the twelfth edition of the National Toxicology Program (NTP) Report on Carcinogens (RoC) changed the listing status of formaldehyde from "reasonably anticipated to be a human carcinogen" to "known to be a human carcinogen."[66][67][68] Concurrently, a National Academy of Sciences (NAS) committee was convened and issued an independent review of the draft U.S. EPA IRIS assessment of formaldehyde, providing a comprehensive health effects assessment and quantitative estimates of human risks of adverse effects.[103]
Acute irritation and allergic reaction
[edit]
For most people, irritation from formaldehyde is temporary and reversible, although formaldehyde can cause allergies and is part of the standard patch test series. In 2005–06, it was the seventh-most-prevalent allergen in patch tests (9.0%).[104] People with formaldehyde allergy are advised to avoid formaldehyde releasers as well (e.g., Quaternium-15, imidazolidinyl urea, and diazolidinyl urea).[105] People who suffer allergic reactions to formaldehyde tend to display lesions on the skin in the areas that have had direct contact with the substance, such as the neck or thighs (often due to formaldehyde released from permanent press finished clothing) or dermatitis on the face (typically from cosmetics).[55] Formaldehyde has been banned in cosmetics in both Sweden[106] and Japan.[107]
Other routes
[edit]In humans, ingestion of as little as 30 millilitres (1.0 US fl oz) of 37% formaldehyde solution can cause death. Other symptoms associated with ingesting such a solution include gastrointestinal damage (vomiting, abdominal pain), and systematic damage (dizziness).[75] Testing for formaldehyde is by blood and/or urine by gas chromatography–mass spectrometry. Other methods to detect formaldehyde include infrared detection, gas detector tubes, gas detectors using electrochemical sensors, and high-performance liquid chromatography (HPLC). HPLC is the most sensitive.[108]
The fifteenth edition (2021) of the U.S. National Toxicology Program Report on Carcinogens notes that currently in the U.S. "The general population can be exposed to formaldehyde primarily from breathing indoor or outdoor air, from tobacco smoke, from use of cosmetic products containing formaldehyde, and, to a more limited extent, from ingestion of food and water." Affected water includes groundwater, surface water, and bottled water. It also notes that occupational exposure can be significant.[109]
Contaminant in food
[edit]Formaldehyde in food can be present naturally, added as an inadvertent contaminant, or intentionally added as a preservative, disinfectant, or bacteriostatic agent. Cooking and smoking food can also result in formaldehyde being produced in food. Foods that the U.S. National Toxicology Program has reported to have higher levels compared to other foods are fish, seafood, and smoked ham. It also notes formaldehyde in food generally occurs in a bound form and that formaldehyde is unstable in an aqueous solution. [109]
Scandals have broken in both the 2005 Indonesia food scare and 2007 Vietnam food scare regarding the addition of formaldehyde to foods to extend shelf life. In 2011, after a four-year absence, Indonesian authorities found foods with formaldehyde being sold in markets in a number of regions across the country.[110] In August 2011, at least at two Carrefour supermarkets, the Central Jakarta Livestock and Fishery Sub-Department found cendol containing 10 parts per million of formaldehyde.[111] In 2014, the owner of two noodle factories in Bogor, Indonesia, was arrested for using formaldehyde in noodles.[112] Foods known to be contaminated included noodles, salted fish, and tofu. Chicken and beer were also rumored to be contaminated. In some places, such as China, manufacturers still use formaldehyde illegally as a preservative in foods, which exposes people to formaldehyde ingestion.[113]
In 2011 in Nakhon Ratchasima, Thailand, truckloads of rotten chicken were treated with formaldehyde for sale in which "a large network", including 11 slaughterhouses run by a criminal gang, were implicated.[114] In 2012, 1 billion rupiah (almost US$100,000) of fish imported from Pakistan to Batam, Indonesia, were found laced with formaldehyde.[115]
Formalin contamination of foods has been reported in Bangladesh, with stores and supermarkets selling fruits, fishes, and vegetables that have been treated with formalin to keep them fresh.[116] However, in 2015, a Formalin Control Bill was passed in the Parliament of Bangladesh with a provision of life-term imprisonment as the maximum punishment as well as a maximum fine of 2,000,000 BDT but not less than 500,000 BDT for importing, producing, or hoarding formalin without a license.[117]
In the early 1900s, formaldehyde was frequently added by US milk plants to milk bottles as a method of pasteurization due to the lack of knowledge and concern[118] regarding formaldehyde's toxicity.[119][120]
Formaldehyde was one of the chemicals used in 19th century industrialised food production that was investigated by Dr. Harvey W. Wiley with his famous 'Poison Squad' as part of the US Department of Agriculture. This led to the 1906 Pure Food and Drug Act, a landmark event in the early history of food regulation in the United States.[121]
Regulation
[edit]Formaldehyde is banned from use in certain applications (preservatives for liquid-cooling and processing systems, slimicides, metalworking-fluid preservatives, and antifouling products) under the Biocidal Products Directive.[122][123] In the EU, the maximum allowed concentration of formaldehyde in finished products is 0.2%, and any product that exceeds 0.05% has to include a warning that the product contains formaldehyde.[55]
In the United States, Congress passed a bill July 7, 2010, regarding the use of formaldehyde in hardwood plywood, particle board, and medium density fiberboard. The bill limited the allowable amount of formaldehyde emissions from these wood products to 0.09 ppm, and required companies to meet this standard by January 2013.[124] The final U.S. EPA rule specified maximum emissions of "0.05 ppm formaldehyde for hardwood plywood, 0.09 ppm formaldehyde for particleboard, 0.11 ppm formaldehyde for medium-density fiberboard, and 0.13 ppm formaldehyde for thin medium-density fiberboard."[125]
Formaldehyde was declared a toxic substance by the 1999 Canadian Environmental Protection Act.[126]
The FDA is proposing a ban on hair relaxers with formaldehyde due to cancer concerns.[127]
See also
[edit]References
[edit]- ^ a b "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: Royal Society of Chemistry. 2014. p. 908. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b "SIDS Initial Assessment Report" (PDF). International Programme on Chemical Safety. Archived from the original (PDF) on 2019-03-28. Retrieved 2019-04-21.
- ^ Spence, Robert; Wild, William (1935). "114. The vapour-pressure curve of formaldehyde, and some related data". Journal of the Chemical Society (Resumed): 506–509. doi:10.1039/jr9350000506.
- ^ "PubChem Compound Database; CID=712". National Center for Biotechnology Information. Archived from the original on 2019-04-12. Retrieved 2017-07-08.
- ^ "Acidity of aldehydes". Chemistry Stack Exchange. Archived from the original on 2018-09-01. Retrieved 2019-04-21.
- ^ Nelson, R. D. Jr.; Lide, D. R.; Maryott, A. A. (1967). "Selected Values of electric dipole moments for molecules in the gas phase (NSRDS-NBS10)" (PDF). Archived (PDF) from the original on 2018-06-08. Retrieved 2019-04-21.
- ^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, Florida: CRC Press. pp. C–301, E–61. ISBN 0-8493-0462-8.
- ^ CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ a b c Record of Formaldehyde in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 13 March 2020.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0293". National Institute for Occupational Safety and Health (NIOSH).
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0294". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Substance Name: Formaldehyde [USP]". ChemlDplus. US National Library of Medicine. Archived from the original on 2017-09-18.
- ^ a b "Formaldehyde". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Humans, IARC Working Group on the Evaluation of Carcinogenic Risks to (2006). Summary of Data Reported and Evaluation. International Agency for Research on Cancer. Archived from the original on 2024-02-02. Retrieved 2023-03-06.
- ^ Kamps, Jos J. A. G.; Hopkinson, Richard J.; Schofield, Christopher J.; Claridge, Timothy D. W. (2019). "How formaldehyde reacts with amino acids". Communications Chemistry. 2 (1): 126. Bibcode:2019CmChe...2..126K. doi:10.1038/s42004-019-0224-2.
- ^ a b "Formaldehyde and Cancer Risk". 10 June 2011. Archived from the original on 2023-09-20. Retrieved 2023-09-21.
- ^ Zhang, Luoping (2018). "CH 5. Formaldehyde Carcinogenesis". Formaldehyde: Exposure, Toxicity and Health Effects (1st ed.). Cambridge: Royal Society of Chemistry, The. ISBN 978-1-78262-973-3.
- ^ Formalin, Merriam-Webster, Inc., 15 January 2020, archived from the original on 18 April 2020, retrieved 18 February 2020
- ^ a b c d e Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. pp. 915–917, 926. ISBN 978-0-19-965763-6.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1291. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ a b c Chuichi, Kato; Shigehiro, Konaka; Takao, Iijima; Masao, Kimura (1969). "Electron Diffraction Studies of Formaldehyde, Acetaldehyde and Acetone". Bull. Chem. Soc. Jpn. 42 (8): 2148–2158. doi:10.1246/bcsj.42.2148.
- ^ a b c d William M. Haynes, ed. (2012). CRC Handbook of Chemistry and Physics (93rd ed.). CRC Press. pp. 9–39. ISBN 978-1-4398-8050-0.
- ^ a b c d Duncan, J. L. (1974). "The ground-state average and equilibrium structures of formaldehyde and ethylene". Mol. Phys. 28 (5): 1177–1191. Bibcode:1974MolPh..28.1177D. doi:10.1080/00268977400102501.
- ^ a b Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). John Wiley & Sons. pp. 24–25, 335. ISBN 978-0-471-72091-1.
- ^ Luecken, D. J.; Hutzell, W. T.; Strum, M. L.; Pouliot, G. A. (2012-02-01). "Regional sources of atmospheric formaldehyde and acetaldehyde, and implications for atmospheric modeling". Atmospheric Environment. 47: 477–490. Bibcode:2012AtmEn..47..477L. doi:10.1016/j.atmosenv.2011.10.005. ISSN 1352-2310.
- ^ "Chapter 5.8 Formaldehyde" (PDF). Air Quality Guidelines (2nd ed.). Copenhagen, Denmark: WHO Regional Office for Europe. 2001. Archived (PDF) from the original on 2023-02-18. Retrieved 2023-02-18.
- ^ a b Pham, Vanha N.; Bruemmer, Kevin J.; Toh, Joel D. W.; Ge, Eva J.; Tenney, Logan; Ward, Carl C.; Dingler, Felix A.; Millington, Christopher L.; Garcia-Prieto, Carlos A.; Pulos-Holmes, Mia C.; Ingolia, Nicholas T.; Pontel, Lucas B.; Esteller, Manel; Patel, Ketan J.; Nomura, Daniel K.; Chang, Christopher J. (2023). "Formaldehyde regulates S -adenosylmethionine biosynthesis and one-carbon metabolism". Science. 382 (6670) eabp9201. Bibcode:2023Sci...382P9201P. doi:10.1126/science.abp9201. PMC 11500418. PMID 37917677. S2CID 264935787.
- ^ Swenberg, J. A.; Lu, K.; Moeller, B. C.; Gao, L.; Upton, P. B.; Nakamura, J.; Starr, T. B. (2011). "Endogenous versus Exogenous DNA Adducts: Their Role in Carcinogenesis, Epidemiology, and Risk Assessment". Toxicological Sciences. 120 (Suppl 1): S130 – S145. doi:10.1093/toxsci/kfq371. PMC 3043087. PMID 21163908.
- ^ "Formaldehyde Is Biodegradable, Quickly Broken Down in the Air By Sunlight or By Bacteria in Soil or Water" (Press release). Formaldehyde Panel of the American Chemistry Council. 2014-01-29. Archived from the original on 2019-03-28. Retrieved 2017-04-22.
- ^ "Toxicological Profile for Formaldehyde" (PDF). 2019-03-28. Archived from the original (PDF) on 2019-03-28. Retrieved 2023-02-18.
- ^ Zuckerman, B.; Buhl, D.; Palmer, P.; Snyder, L. E. (1970). "Observation of interstellar formaldehyde". Astrophys. J. 160: 485–506. Bibcode:1970ApJ...160..485Z. doi:10.1086/150449.
- ^ Mangum, Jeffrey G.; Darling, Jeremy; Menten, Karl M.; Henkel, Christian (2008). "Formaldehyde Densitometry of Starburst Galaxies". Astrophys. J. 673 (2): 832–46. arXiv:0710.2115. Bibcode:2008ApJ...673..832M. doi:10.1086/524354. S2CID 14035123.
- ^ Woon, David E. (2002). "Modeling Gas-Grain Chemistry with Quantum Chemical Cluster Calculations. I. Heterogeneous Hydrogenation of CO and H2CO on Icy Grain Mantles". Astrophys. J. 569 (1): 541–48. Bibcode:2002ApJ...569..541W. doi:10.1086/339279.
- ^ Zubritsky, Elizabeth; Neal-Jones, Nancy (11 August 2014). "RELEASE 14-038 - NASA's 3-D Study of Comets Reveals Chemical Factory at Work". NASA. Archived from the original on 12 August 2014. Retrieved 12 August 2014.
- ^ Cordiner, M.A.; et al. (11 August 2014). "Mapping the Release of Volatiles in the Inner Comae of Comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) Using the Atacama Large Millimeter/Submillimeter Array". The Astrophysical Journal. 792 (1): L2. arXiv:1408.2458. Bibcode:2014ApJ...792L...2C. doi:10.1088/2041-8205/792/1/L2. S2CID 26277035.
- ^ A. Butlerow (1859). "Ueber einige Derivate des Jodmethylens". Annalen der Chemie und Pharmacie. 111 (2): 242–252. doi:10.1002/JLAC.18591110219. ISSN 0075-4617. Wikidata Q55881565.
- ^ See: A. W. Hofmann (14 October 1867) "Zur Kenntnis des Methylaldehyds" ([Contributions] to our knowledge of methylaldehyde), Monatsbericht der Königlich Preussischen Akademie der Wissenschaften zu Berlin (Monthly Report of the Royal Prussian Academy of Sciences in Berlin), vol. 8, pages 665–669. Reprinted in:
- A.W. Hofmann, (1868) "Zur Kenntnis des Methylaldehyds", Annalen der Chemie und Pharmacie (Annals of Chemistry and Pharmacy), vol. 145, no. 3, pages 357–361.
- A.W. Hofmann (1868) "Zur Kenntnis des Methylaldehyds", Journal für praktische Chemie (Journal for Practical Chemistry), vol. 103, no. 1, pages 246–250.
- Hofmann, A.W. (1869). "Beiträge zur Kenntnis des Methylaldehyds". Journal für Praktische Chemie. 107 (1): 414–424. doi:10.1002/prac.18691070161.
- A.W. Hofmann (1869) "Beiträge zur Kenntnis des Methylaldehyds," Berichte der Deutschen Chemischen Gesellschaft (Reports of the German Chemical Society), vol. 2, pages 152–159.
- ^ Read, J. (1935). Text-Book of Organic Chemistry. London: G Bell & Sons.
- ^ Hooker, Jacob M.; Schönberger, Matthias; Schieferstein, Hanno; Fowler, Joanna S. (2008). "A Simple, Rapid Method for the Preparation of [11C]Formaldehyde". Angewandte Chemie International Edition. 47 (32): 5989–5992. doi:10.1002/anie.200800991. PMC 2522306. PMID 18604787.
- ^ Wang, Chien-Tsung; Ro, Shih-Hung (2005-05-10). "Nanocluster iron oxide-silica aerogel catalysts for methanol partial oxidation". Applied Catalysis A: General. 285 (1): 196–204. Bibcode:2005AppCA.285..196W. doi:10.1016/j.apcata.2005.02.029. ISSN 0926-860X.
- ^ Dias, Ana Paula Soares; Montemor, Fátima; Portela, Manuel Farinha; Kiennemann, Alain (2015-02-01). "The role of the suprastoichiometric molybdenum during methanol to formaldehyde oxidation over Mo–Fe mixed oxides". Journal of Molecular Catalysis A: Chemical. 397: 93–98. doi:10.1016/j.molcata.2014.10.022. ISSN 1381-1169.
- ^ a b c Reuss, Günther; Disteldorf, Walter; Gamer, Armin Otto; Hilt, Albrecht (2000). "Formaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_619. ISBN 3527306730.
- ^ a b c Kamps, Jos J. A. G.; Hopkinson, Richard J.; Schofield, Christopher J.; Claridge, Timothy D. W. (2019). "How formaldehyde reacts with amino acids". Communications Chemistry. 2 (1): 126. Bibcode:2019CmChe...2..126K. doi:10.1038/s42004-019-0224-2. S2CID 207913561.
- ^ Chen, J; Chen, W; Zhang, J; Zhao, H; Cui, J; Wu, J; Shi, A (27 September 2023). "Dual effects of endogenous formaldehyde on the organism and drugs for its removal". Journal of Applied Toxicology. 44 (6): 798–817. doi:10.1002/jat.4546. PMID 37766419. S2CID 263125399.
- ^ Nakamura, Jun; Holley, Darcy W.; Kawamoto, Toshihiro; Bultman, Scott J. (December 2020). "The failure of two major formaldehyde catabolism enzymes (ADH5 and ALDH2) leads to partial synthetic lethality in C57BL/6 mice". Genes and Environment. 42 (1): 21. Bibcode:2020GeneE..42...21N. doi:10.1186/s41021-020-00160-4. PMC 7268536. PMID 32514323.
- ^ Cheng, Chan; Lu, Feng (2012). Formaldehyde: Chemistry, Applications and Role in Polymerization (Pollution Science, Technology and Abatement).
- ^ Boyles, James G.; Toby, Sidney (June 1966). "The mechanism of the polymerization of gaseous formaldehyde". Journal of Polymer Science Part B: Polymer Letters. 4 (6): 411–415. Bibcode:1966JPoSL...4..411B. doi:10.1002/pol.1966.110040608. Archived from the original on 2023-04-20. Retrieved 2023-04-20.
- ^ Bevington, J. C.; Norrish, R. G. W. (1951-03-07). "The catalyzed polymerization of gaseous formaldehyde". Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. 205 (1083): 516–529. Bibcode:1951RSPSA.205..516B. doi:10.1098/rspa.1951.0046. ISSN 0080-4630. S2CID 95395629. Archived from the original on 2019-10-25. Retrieved 2023-04-20.
- ^ a b Hoffman EA, Frey BL, Smith LM, Auble DT (2015). "Formaldehyde crosslinking: a tool for the study of chromatin complexes". Journal of Biological Chemistry. 290 (44): 26404–26411. doi:10.1074/jbc.R115.651679. PMC 4646298. PMID 26354429.
- ^ Bost, R. W.; Constable, E. W. (1936). "sym-Trithiane". Organic Syntheses. 16: 81; Collected Volumes, vol. 2, p. 610.
- ^ Gutsche, C. D.; Iqbal, M. (1993). "p-tert-Butylcalix[4]arene". Organic Syntheses; Collected Volumes, vol. 8, p. 75.
- ^ "Formaldehyde in Clothing and Textiles FactSheet". NICNAS. Australian National Industrial Chemicals Notification and Assessment Scheme. 2013-05-01. Archived from the original on 2019-03-19. Retrieved 2014-11-12.
- ^ Schurink, H. B. J. (1925). "Pentaerythritol". Organic Syntheses. 4: 53; Collected Volumes, vol. 1, p. 425.
- ^ "Ingredients of Vaccines - Fact Sheet". Center for Disease Control. Archived from the original on 2019-04-21. Retrieved 2018-08-04.
Formaldehyde is used to inactivate bacterial products for toxoid vaccines, (these are vaccines that use an inactive bacterial toxin to produce immunity.) It is also used to kill unwanted viruses and bacteria that might contaminate the vaccine during production. Most formaldehyde is removed from the vaccine before it is packaged.
- ^ a b c De Groot, Anton C; Flyvholm, Mari-Ann; Lensen, Gerda; Menné, Torkil; Coenraads, Pieter-Jan (2009). "Formaldehyde-releasers: relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde-releasers". Contact Dermatitis. 61 (2): 63–85. doi:10.1111/j.1600-0536.2009.01582.x. hdl:11370/c3ff7adf-9f21-4564-96e0-0b9c5d025b30. PMID 19706047. S2CID 23404196. Archived from the original on 2020-03-13. Retrieved 2019-07-08.
- ^ Francis-Floyd, Ruth (April 1996). "Use of Formalin to Control Fish Parasites". Institute of Food and Agricultural Sciences, University of Florida. Archived from the original on May 27, 2012.
- ^ "Disinfection, decontamination, fumigation, incineration", Anthrax in Humans and Animals. 4th edition, World Health Organization, 2008, archived from the original on 2022-07-06, retrieved 2023-11-20
- ^ "§573.460 Formaldehyde". U.S. Government Publishing Office. 2019-04-19. Archived from the original on 2017-05-05. Retrieved 2016-07-09.
- ^ Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market Archived 19 February 2008 at the Wayback Machine. OJEU L123, 24.04.1998, pp. 1–63. (consolidated version to 2008-09-26 (PDF) Archived 2010-01-27 at the Wayback Machine)
- ^ Commission Regulation (EC) No 2032/2003 of 4 November 2003 on the second phase of the 10-year work programme referred to in Article 16(2) of Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market, and amending Regulation (EC) No 1896/2000 Archived 12 June 2011 at the Wayback Machine. OJEU L307, 24.11.2003, p. 1–96. (consolidated version to 2007-01-04 (PDF) Archived 2011-06-14 at the Wayback Machine)
- ^ Patel, Alkesh (2007-07-04). "Formaldehyde Ban set for 22 September 2007". WebWire. Archived from the original on 2018-12-12. Retrieved 2012-05-19.
- ^ "European chemical Substances Information System (ESIS) entry for formaldehyde". Archived from the original on 2014-01-01. Retrieved 2009-09-01.
- ^ "Process C-41 Using Kodak Flexicolor Chemicals - Publication Z-131". Kodak. Archived from the original on 2016-06-15. Retrieved 2009-09-01.
- ^ "Formaldehyde", Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol (PDF), IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 88, Lyon, France: International Agency for Research on Cancer, 2006, pp. 39–325, ISBN 978-92-832-1288-1, archived (PDF) from the original on 2012-03-04, retrieved 2009-09-01
- ^ "Formaldehyde (gas)", Report on Carcinogens, Eleventh Edition (PDF), U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, 2005
- ^ a b Harris, Gardiner (2011-06-10). "Government Says 2 Common Materials Pose Risk of Cancer". The New York Times. Archived from the original on 2019-03-28. Retrieved 2011-06-11.
- ^ a b National Toxicology Program (2011-06-10). "12th Report on Carcinogens". National Toxicology Program. Archived from the original on 2011-06-08. Retrieved 2011-06-11.
- ^ a b National Toxicology Program (2011-06-10). "Report On Carcinogens - Twelfth Edition - 2011" (PDF). National Toxicology Program. Archived from the original on 2011-06-12. Retrieved 2011-06-11.
- ^ Kristak, Lubos; Antov, Petar; Bekhta, Pavlo; Lubis, Muhammad Adly Rahandi; Iswanto, Apri Heri; Reh, Roman; Sedliacik, Jan; Savov, Viktor; Taghiyari, Hamid R.; Papadopoulos, Antonios N.; Pizzi, Antonio; Hejna, Aleksander (2023-03-04). "Recent progress in ultra-low formaldehyde emitting adhesive systems and formaldehyde scavengers in wood-based panels: a review". Wood Material Science & Engineering. 18 (2): 763–782. doi:10.1080/17480272.2022.2056080. ISSN 1748-0272. Retrieved 2025-06-11.
- ^ "Formaldehyde in indoor air of new apartments in Greece" (PDF). Retrieved 2024-12-24. by George Mantanis, Eleni Vouli, Chariclea Gonitsioti and Georgios Ntalos; Presentation at the COST Action E49 Conference "Measurement and Control of VOC Emissions from Wood-Based Panels", 28-30 Nov. 2007, WKI, Braunschweig, Germany
- ^ "Indoor Air Pollution in California" (PDF). Air Resources Board, California Environmental Protection Agency. July 2005. pp. 65–70. Archived from the original (PDF) on 2019-03-01. Retrieved 2012-05-19.
- ^ "Formaldehyde". Occupational Safety and Health Administration. August 2008. Archived from the original on 2019-04-11. Retrieved 2009-09-01.
- ^ "Formaldehyde Reference Exposure Levels". California Office Of Health Hazard Assessment. December 2008. Archived from the original (PDF) on 2019-03-23. Retrieved 2012-05-19.
- ^ "Formaldehyde and Indoor Air". Health Canada. 2012-03-29. Archived from the original on 2019-04-23. Retrieved 2019-04-23.
- ^ a b "Formaldehyde | Medical Management Guidelines | Toxic Substance Portal | ATSDR". Centres for Disease Control and Prevention. Archived from the original on 2021-08-25. Retrieved 2021-08-25.
- ^ Broder, I; Corey, P; Brasher, P; Lipa, M; Cole, P (1991). "Formaldehyde exposure and health status in households". Environmental Health Perspectives. 95: 101–4. Bibcode:1991EnvHP..95..101B. doi:10.1289/ehp.9195101. PMC 1568408. PMID 1821362.
- ^ McGwin, G; Lienert, J; Kennedy, JI (November 2009). "Formaldehyde Exposure and Asthma in Children: A Systematic Review". Environmental Health Perspectives. 118 (3): 313–7. doi:10.1289/ehp.0901143. PMC 2854756. PMID 20064771.
- ^ Lobachev, AN (1978). "РОЛЬ МИТОХОНДРИАЛЬНЫХ ПРОЦЕССОВ В РАЗВИТИИ И СТАРЕНИИ ОРГАНИЗМА. СТАРЕНИЕ И РАК" [Role of mitochondrial processes in the development and aging of organism. Aging and cancer] (PDF) (in Russian). VINITI. Archived from the original (PDF) on 2013-06-06. Retrieved 2012-08-01.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2006). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans - VOLUME 88 - Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol (PDF). WHO Press. ISBN 92-832-1288-6. Archived from the original (PDF) on 2018-07-12. Retrieved 2019-04-23.
- ^ a b "Formaldehyde and Cancer Risk". National Cancer Institute. 2011-06-10. Archived from the original on 2019-01-23.
- ^ Zhang, Luoping; Steinmaus, Craig; Eastmond, Eastmond; Xin, Xin; Smith, Smith (March–June 2009). "Formaldehyde exposure and leukemia: A new meta-analysis and potential mechanisms" (PDF). Mutation Research. 681 (2–3): 150–168. Bibcode:2009MRRMR.681..150Z. doi:10.1016/j.mrrev.2008.07.002. PMID 18674636. Archived from the original (PDF) on 27 March 2014.
- ^ Zhang, Luoping; Freeman, Laura E. Beane; Nakamura, Jun; Hecht, Stephen S.; Vandenberg, John J.; Smith, Martyn T.; Sonawane, Babasaheb R. (2010). "Formaldehyde and Leukemia: Epidemiology, Potential Mechanisms, and Implications for Risk Assessment". Environmental and Molecular Mutagenesis. 51 (3): 181–191. Bibcode:2010EnvMM..51..181Z. doi:10.1002/em.20534. PMC 2839060. PMID 19790261.
- ^ "Key Statistics for Nasopharyngeal Cancer". American Cancer Society. Archived from the original on 2019-01-11. Retrieved 2019-04-22.
- ^ Turner JH, Reh DD (June 2012). "Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data". Head Neck. 34 (6): 877–85. doi:10.1002/hed.21830. PMID 22127982. S2CID 205857872.
- ^ "Key Statistics for Chronic Myeloid Leukemia". American Cancer Society. Archived from the original on 2019-04-23. Retrieved 2019-04-22.
- ^ "What are the key statistics about acute myeloid leukemia?Key Statistics for Acute Myeloid Leukemia (AML)". American Cancer Society. Archived from the original on 2019-04-23. Retrieved 2019-04-22.
- ^ a b "Risk Factors for Nasopharyngeal Cancer". American Cancer Society. 24 September 2018. Archived from the original on 10 December 2016. Retrieved 17 September 2019.
- ^ Butticè, Claudio (2015). "Solvents". In Colditz, Graham A. (ed.). The SAGE Encyclopedia of Cancer and Society (Second ed.). Thousand Oaks: SAGE Publications. pp. 1089–1091. doi:10.4135/9781483345758.n530. ISBN 978-1-4833-4573-4. Archived from the original on 2021-10-14. Retrieved 2015-10-27.
- ^ "Risk Factors for Acute Myeloid Leukemia (AML)". American Cancer Society. 2018-08-21. Archived from the original on 2019-04-23.
- ^ "Risk Factors for Chronic Myeloid Leukemia". American Cancer Society. 2018-06-19. Archived from the original on 2018-12-12.
- ^ Ogbede, J. U., Giaever, G., & Nislow, C. (2021). A genome-wide portrait of pervasive drug contaminants. Scientific reports, 11(1), 12487. https://doi.org/10.1038/s41598-021-91792-1 Archived 2021-12-04 at the Wayback Machine
- ^ Dales, R; Liu, L; Wheeler, AJ; Gilbert, NL (July 2008). "Quality of indoor residential air and health". Canadian Medical Association Journal. 179 (2): 147–52. doi:10.1503/cmaj.070359. PMC 2443227. PMID 18625986.
- ^ "Formaldehyde Emission Standards for Composite Wood Products". EPA. 8 July 2016. Archived from the original on 2018-12-24. Retrieved 2019-04-24.
- ^ Passmore, Whitney; Sullivan, Michael J. (2016-08-04). "EPA Issues Final Rule on Formaldehyde Emission Standards for Composite Wood Products". The National Law Review. Womble Carlyle Sandridge & Rice, PLLC. Archived from the original on 2018-06-19. Retrieved 2016-08-24 – via Google News.
- ^ "Testing for Indoor Air Quality, Baseline IAQ, and Materials". Environmental Protection Agency. Archived from the original on October 15, 2006.
- ^ M. Koontz, H. Rector, D. Cade, C. Wilkes, and L. Niang. 1996. Residential Indoor Air Formaldehyde Testing Program: Pilot Study. Report No. IE-2814, prepared by GEOMET Technologies, Inc. for the USEPA Office of Pollution Prevention and Toxics under EPA Contract No. 68-D3-0013, Washington, DC
- ^ Evans, Ben (2008-04-11). "FEMA limits formaldehyde in trailers". The Boston Globe. Archived from the original on June 15, 2010. Retrieved 2008-09-04.
- ^ "Formaldehyde CAS 50-00-0" (PDF). United Nations Environment Programme. Archived from the original (PDF) on 2019-03-28. Retrieved 2019-04-25.
- ^ Formaldehyde Epidemiology, Toxicology and Environmental Group, Inc (August 2002). "Formaldehyde and Facts About Health Effects" (PDF). Archived from the original (PDF) on 2011-05-11.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Carroll, Gregory T.; Kirschman, David L. (2023). "Catalytic Surgical Smoke Filtration Unit Reduces Formaldehyde Levels in a Simulated Operating Room Environment". ACS Chemical Health & Safety. 30 (1): 21–28. doi:10.1021/acs.chas.2c00071. ISSN 1871-5532. S2CID 255047115. Archived from the original on 2023-05-14. Retrieved 2023-05-17.
- ^ "When Sampling Formaldehyde, The Medium Matters". Galson Labs. Archived from the original on 2011-03-23.
- ^ "NIOSH Pocket Gide to Chemical Hazards: Formaldehyde". National Institute for Occupational Safety and Health, CDC. 2018-11-29. Archived from the original on 2019-03-28.
- ^ Addendum to the 12th Report on Carcinogens (PDF) Archived 2011-06-08 at the Wayback Machine National Toxicology Program, U.S. Department of Health and Human Services. Retrieved 06-13-2011
- ^ Zug KA, Warshaw EM, Fowler JF, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J (2009). "Patch-test results of the North American Contact Dermatitis Group 2005-2006". Dermatitis. 20 (3): 149–60. doi:10.2310/6620.2009.08097. PMID 19470301. S2CID 24088485.
- ^ "Formaldehyde allergy". DermNet NZ. New Zealand Dermatological Society. 2002. Archived from the original on 2018-09-23. Retrieved 2019-04-25.
- ^ "Formaldehyde And Formaldehyde-Releasing Preservatives". Safe Cosmetics. Retrieved 2024-06-03.
- ^ Hayashida, Mike. "The Regulation of Cosmetics in Japan" (PDF). Archived from the original (PDF) on 2019-04-14. Retrieved 2019-04-25.
- ^ Ngwa, Moise (2010-10-25). "formaldehyde testing" (PDF). Cedar Rapids Gazette. Archived from the original (PDF) on 2018-10-25. Retrieved 2012-05-19.
- ^ a b RoC Profile: Formaldehyde (PDF) (Report). National Toxicology Program, Department of Health and Human Services. 2021.
- ^ "Formaldehyde-laced foods reemerge in Indonesian markets". antaranews.com. 2011-08-10. Archived from the original on 2018-10-25.
- ^ "Formaldehyde-Tainted Rice Drinks Found at Carrefour Markets". Jakarta Globe. 2011-08-22. Archived from the original on 2012-09-28.
- ^ "BPOM Uncovers Two Formaldehyde-Tainted Noodle Factories in Bogor". Jakarta Globe. 2014-10-12. Archived from the original on 2015-08-01.
- ^
- Tang, Xiaojiang; Bai, Yang; Duong, Anh; Smith, Martyn T.; Li, Laiyu; Zhang, Luoping (2009). "Formaldehyde in China: Production, consumption, exposure levels, and health effects". Environment International. 35 (8): 1210–1224. Bibcode:2009EnInt..35.1210T. doi:10.1016/j.envint.2009.06.002. ISSN 0160-4120. PMID 19589601.
- see references cited on p. 1216 above
- "Municipality sees red over bad blood processing". China Daily. 2011-03-18. Archived from the original on 2018-10-24.
- ^ "Illegal business 'being run by a gang'". The Nation. 2011-06-16. Archived from the original on 2011-06-16.
- ^ "Import of formaldehyde fish from Pakistan foiled in Batam". The Jakarta Post. 2012-02-23. Archived from the original on 2018-12-16.
- ^ "Trader Fined for Selling Fish Treated with Formalin". The Daily Star. 2009-08-31. Archived from the original on 2019-04-29.
- ^ "Formalin Control Bill 2015 passed". ntv online. 2015-02-16. Archived from the original on 2018-03-23. Retrieved 2015-03-04.
- ^ Blum, Deborah (2018-09-25). The poison squad: one chemist's single-minded crusade for food safety at the turn of the twentieth century. New York, New York: Penguin Press. ISBN 978-1-59420-514-9. OCLC 1024107182.
- ^ "Was Death in the Milk?". The Indianapolis News. 1900-07-31. p. 5. Archived from the original on 2018-11-17. Retrieved 2014-08-20 – via Newspapers.com.

- ^ "Wants New Law Enacted. Food Inspector Farnsworth Would Have Use of Formaldehyde in Milk Stopped". The Topeka Daily Capital. 1903-08-30. p. 8. Archived from the original on 2018-11-17. Retrieved 2014-08-20 – via Newspapers.com.

- ^ Meadows, Michelle (January 2006). "A Century of Ensuring Safe Foods and Cosmetics". FDA Consumer. 40 (1): 6–13. PMID 16528821. Archived from the original (PDF) on March 6, 2021.
- ^ "European Union Bans formaldehyde/formalin within Europe" (PDF). European Commission's Environment Directorate-General. 22 June 2007. pp. 1–3. Archived from the original (PDF) on 27 April 2013.
- ^ "ESIS (European Chemical Substances Information System)". European Commission Joint Research Centre Institute for Health and Consumer Protection. February 2009. Archived from the original on 1 January 2014. Retrieved 19 May 2012.
- ^ "S.1660 - Formaldehyde Standards for Composite Wood Products Act". GovTrack. 25 August 2010. Archived from the original on 29 April 2019.
- ^ "Formaldehyde Emission Standards for Composite Wood Products". Regulations.gov. United States Federal Register. 12 December 2016. Archived from the original on 10 August 2019. Retrieved 21 December 2019.
The emission standards will be 0.05 ppm formaldehyde for hardwood plywood, 0.09 ppm formaldehyde for particleboard, 0.11 ppm formaldehyde for medium-density fiberboard, and 0.13 ppm formaldehyde for thin medium-density fiberboard.
- ^ "Health Canada - Proposed residential indoor air quality guidelines for formaldehyde". Health Canada. April 2007. Archived from the original on 2013-05-30.
- ^ "View Rule". www.reginfo.gov. Archived from the original on 2023-10-20. Retrieved 2023-10-21.
Notes
[edit]- ^ Formaldehyde is classified as a carcinogen, according to the Environmental Protection Agency, International Agency for Research on Cancer (IARC), and U.S. National Toxicology Program.[16]
External links
[edit]- International Chemical Safety Card 0275 (gas)
- International Chemical Safety Card 0695 (solution)
- NIOSH Pocket Guide to Chemical Hazards. "#0293". National Institute for Occupational Safety and Health (NIOSH).
- Entry for "Formaldehyde" on the Australian National Pollutant Inventory
- Formaldehyde from ChemSub Online
- Prevention guide—Formaldehyde in the Workplace (PDF) from the IRSST
- Formaldehyde from the National Institute for Occupational Safety and Health
- IPCS Health and Safety Guide 57: Formaldehyde
- IPCS Environmental Health Criteria 89: Formaldehyde
- SIDS Initial Assessment Report for Formaldehyde from the Organisation for Economic Co-operation and Development (OECD)
- "Formaldehyde Added to 'Known Carcinogens' List Despite Lobbying by Chemical Industry"—video report by Democracy Now!
- Do you own a post-Katrina FEMA trailer? Check your VIN#
- So you're living in one of FEMA's Katrina trailers... What can you do?
- Formaldehyde in the Pesticide Properties DataBase (PPDB)
Formaldehyde
View on GrokipediaHistory
Discovery and Early Characterization
Formaldehyde was first reported in 1859 by Russian chemist Aleksandr Mikhailovich Butlerov during experiments aimed at synthesizing methylene glycol, where he observed a pungent, colorless gas as a byproduct.[11][12] Butlerov generated the compound through the hydrolysis of methylene acetate or related reactions, noting its irritating odor and reactivity, though he did not fully isolate or characterize it at the time.[13] This initial observation marked the recognition of formaldehyde as a distinct chemical entity, distinct from previously known aldehydes.[14] The compound's identity was conclusively established in 1868 by German chemist August Wilhelm von Hofmann, who synthesized it via the partial oxidation of methanol vapors mixed with air passed over a heated platinum spiral.[14][15] Hofmann identified formaldehyde as methylaldehyde (CH₂O), the simplest aldehyde, through detailed analysis of its chemical properties, including its solubility in water to form a solution that polymerized upon evaporation and its reactions forming characteristic derivatives like formaldehydemethylene acetal.[11] This work provided the first rigorous structural confirmation, distinguishing it from acetaldehyde and other homologs via empirical formula determination and comparative reactivity tests.[14] Early characterizations highlighted formaldehyde's volatility, with a boiling point around -19°C, high reactivity toward nucleophiles, and tendency to trimerize into trioxane or form paraformaldehyde polymers under certain conditions.[16] These properties were verified through distillation and combustion analyses, confirming its empirical formula and positioning it as a foundational carbonyl compound in organic chemistry.[14] Subsequent studies in the late 19th century built on Hofmann's methods, emphasizing its role in oxidation pathways from alcohols.[17]Industrial Development and Key Milestones
Commercial production of formaldehyde began in Germany in the 1880s through the partial oxidation of methanol, enabling the transition from laboratory-scale synthesis to industrial volumes.[11] [12] This development followed the identification of viable catalytic methods, with industrial feasibility achieved by 1882 via copper-based catalysis. A continuous commercial process was refined in Germany around 1889, improving efficiency and yield from methanol vapor oxidation over silver catalysts.[14] Production subsequently expanded internationally, reaching Belgium and France shortly thereafter, and the United States by the early 1900s.[11] In the U.S., Heyden Chemical Works established the first successful commercial facility in Garfield, New Jersey, in 1904, followed by an additional plant later that year.[14] The 1920s marked a pivotal advancement with the commercialization of high-pressure methanol synthesis, providing a low-cost feedstock that catalyzed widespread scaling of formaldehyde production via catalytic oxidation.[18] This period aligned with rising demand from resin applications, such as phenol-formaldehyde polymers introduced commercially around 1910, further driving process optimizations including the adoption of iron-molybdate catalysts in later decades for higher selectivity.[19]Physical and Chemical Properties
Molecular Structure and Bonding
Formaldehyde, with the molecular formula CH₂O, features a central carbon atom bonded to two hydrogen atoms via single bonds and to one oxygen atom via a double bond, resulting in a linear arrangement of the C=O unit flanked by the C-H bonds.[20] The double bond between carbon and oxygen comprises one sigma bond formed by end-to-end overlap of atomic orbitals and one pi bond from sideways overlap of p orbitals.[20] The carbon atom in formaldehyde adopts sp² hybridization, utilizing three sp² hybrid orbitals to form sigma bonds with the two hydrogens and the oxygen, while its unhybridized p orbital participates in the pi bond with oxygen's p orbital.[21] This hybridization configuration dictates the trigonal planar electron and molecular geometry, with the oxygen atom also sp² hybridized, its lone pairs occupying the remaining sp² orbitals and p orbital.[22] Experimental bond lengths measure approximately 1.11 Å for each C-H bond and 1.21 Å for the C=O bond, reflecting the partial double-bond character and electron density distribution.[20] Bond angles deviate slightly from the ideal 120° due to the influence of the double bond and lone pairs on oxygen: the H-C-H angle is about 118°, and each H-C-O angle is roughly 121°.[23] These structural parameters contribute to formaldehyde's polarity, evidenced by a dipole moment of 2.33 debye, arising from the electronegativity difference between carbon and oxygen that polarizes the C=O bond.[24]Physical Properties and Forms
Formaldehyde exists primarily as a colorless, flammable gas at standard temperature and pressure, characterized by a pungent, irritating odor detectable at concentrations as low as 1 ppm.[1][25] Its molecular weight is 30.03 g/mol, with a vapor density of 1.04 relative to air and a vapor pressure exceeding 1 atm, reaching approximately 3890 mmHg at 25°C, indicating high volatility.[26][6] The melting point is -92°C, and the boiling point is -19°C, allowing it to liquefy under moderate cooling or compression.[1][27] In the liquid state at -20°C, its density is 0.815 g/cm³.[28] Formaldehyde exhibits high solubility in water, with up to 400 g/L dissolving at 25°C, though it readily hydrates to form methanediol (CH2(OH)2), the predominant species in dilute aqueous solutions rather than the free aldehyde.[29][30] Commercially, it is often handled as an aqueous solution known as formalin, typically 37% by weight formaldehyde stabilized with 10-15% methanol to prevent polymerization, appearing as a clear liquid with a boiling point around 96°C and density near 1.08 g/mL at 25°C.[31][32] In solid forms, formaldehyde polymerizes to yield stable derivatives such as paraformaldehyde, a white, crystalline linear polyacetal (n=8-100) used in industrial applications for controlled release of the monomer upon heating or depolymerization.[33] Another form is trioxane, a cyclic trimer (C3H6O3) that forms as colorless crystals stable under anhydrous conditions, with a melting point of 61-62°C, serving as a latent source of formaldehyde in organic synthesis.[33] These polymeric forms arise from reversible condensation reactions, contrasting the monomeric gas's instability in concentrated solutions without stabilizers.[34]Natural Occurrence
Biochemical Production in Organisms
Formaldehyde is produced endogenously in bacteria, plants, animals, and humans as an intermediate in various metabolic processes, including one-carbon metabolism and demethylation reactions.[16][35] In microorganisms such as methylotrophs and methanotrophs, formaldehyde arises primarily from the oxidation of short-chain hydrocarbons like methane or methanol, serving as a key intermediate before assimilation or dissimilation.[36] These pathways enable bacteria to utilize C1 compounds as carbon and energy sources, with formaldehyde channeled into routes like the ribulose monophosphate or serine pathways for assimilation.[37] In plants, formaldehyde production occurs at low levels during processes such as the breakdown of certain volatile organic compounds or as a byproduct of photosynthetic metabolism, though plants more prominently absorb and metabolize exogenous formaldehyde via enzymatic pathways.[38] Historical studies suggested formaldehyde formation in green plants via photochemical reactions, but contemporary evidence emphasizes its transient role rather than significant net production.[39] Mammalian cells generate formaldehyde through demethylation of DNA, histones, and other substrates by enzymes like TET proteins; oxidative degradation of folates; serine catabolism; and methanol metabolism.[40] These reactions link to methionine-homocysteine cycles and one-carbon metabolism, where formaldehyde regulates S-adenosylmethionine biosynthesis.[41][42] Endogenous production rates in humans are estimated at 0.61–0.91 mg/kg body weight per minute, reflecting rapid turnover due to its reactivity, with daily totals around 878–1310 mg/kg body weight assuming a half-life of 1–1.5 minutes.[43] Despite its genotoxic potential, cells divert it into protective metabolic sinks like tetrahydrofolate-dependent pathways.[44]Environmental and Cosmic Presence
Formaldehyde is present in the Earth's atmosphere from natural sources including biomass combustion during forest fires, volcanic emissions, and photochemical oxidation of naturally emitted hydrocarbons such as methane and isoprene from vegetation.[45][46] In rural and suburban outdoor air, concentrations typically range from 0.0002 to 0.006 parts per million (ppm), while urban levels are higher at 0.001 to 0.02 ppm, reflecting contributions from both natural and anthropogenic processes, though natural background levels persist even in remote areas.[4] Atmospheric formaldehyde photodegrades rapidly, with a half-life of hours, primarily into formic acid and carbon monoxide via reaction with hydroxyl radicals.[47] In marine environments, formaldehyde arises from oceanic photochemical processes and biological activity, yielding average concentrations of 2.4 ± 0.9 parts per billion by volume (ppbv) in coastal atmospheres, with peaks up to 6.8 ppbv linked to air masses over water bodies.[48] It dissolves readily in water but degrades quickly through biological and chemical processes, resulting in low persistence in surface waters and oceans.[4] In soils, formaldehyde is rarely detected due to its rapid microbial degradation and volatilization upon release, maintaining negligible steady-state levels despite occasional natural inputs.[4] In cosmic environments, formaldehyde is ubiquitous in dense interstellar molecular clouds, where it serves as a tracer of nonequilibrium chemistry driven by cosmic rays, detectable via its ground-state rotational transition at 4830 MHz.[49][50] It has been observed in cometary comae and nuclei, including emissions from Comet C/2002 T7 (LINEAR) at radio wavelengths, and may form through cosmic ray irradiation of interstellar ices or gas-phase reactions involving precursors like methanol.[49] Formaldehyde's presence in such settings underscores its role as a simple organic building block in prebiotic interstellar chemistry, with detections extending to diffuse clouds and star-forming regions.[50]Synthesis and Production
Laboratory Synthesis
In laboratories, formaldehyde is commonly synthesized via the dehydrogenation of methanol, where methanol vapor is passed over a heated copper catalyst, typically maintained at 250–350 °C, producing formaldehyde gas and hydrogen according to the reaction CH₃OH → H₂C=O + H₂.[18][51] This endothermic process requires external heating to sustain the reaction, and the gaseous formaldehyde is often collected by absorption in cold water to form an aqueous solution known as formalin.[52] Yields in this method can exceed 80% under optimized conditions, though side reactions such as further oxidation to formic acid or carbon oxides may occur if temperatures fluctuate or catalyst purity is low.[53] An alternative laboratory approach employs partial oxidation of methanol by mixing its vapors with air (approximately 40–50% methanol in air by volume) and passing the mixture over a silver catalyst gauze at higher temperatures of 500–700 °C, facilitating both dehydrogenation and oxygen-mediated oxidation pathways to yield formaldehyde, water, and carbon dioxide as byproducts.[52][54] This exothermic variant allows for better heat management in small-scale setups and achieves selectivities up to 90%, but requires precise control of oxygen levels to minimize complete combustion to CO₂.[55] Catalysts like platinized asbestos have been used historically for similar oxidative preparations at around 300 °C, though copper and silver remain preferred for their efficiency and availability.[51] Less common methods include chemical oxidation of methanol using strong oxidants such as potassium dichromate in acidic media, which generates formaldehyde alongside reduced chromium species and requires distillation for isolation, but this is inefficient for pure product due to over-oxidation and waste generation.[56] Specialized syntheses, such as those for isotopically labeled formaldehyde (e.g., [¹¹C]formaldehyde from [¹¹C]methanol via oxidation), employ tailored catalysts or reagents like trimethylamine-N-oxide but are confined to radiochemistry applications rather than routine laboratory use.[57] All methods necessitate ventilation and safety precautions given formaldehyde's toxicity and flammability.[16]Industrial Production Methods
The predominant industrial production of formaldehyde occurs through the catalytic vapor-phase oxidation and dehydrogenation of methanol using air as the oxidant. This process accounts for over 99% of global formaldehyde output, with methanol serving as the primary feedstock due to its availability and cost-effectiveness.[52][58] Two principal catalytic methods are employed: the silver catalyst process and the metal oxide catalyst process. In the silver catalyst process, vaporized methanol (typically 30-40% concentration in air) is passed over polycrystalline silver catalysts at temperatures of 500-700°C and pressures near atmospheric. The reaction proceeds via partial oxidation (CH₃OH + ½O₂ → CH₂O + H₂O) and dehydrogenation (CH₃OH → CH₂O + H₂), with the latter being endothermic and supported by exothermic oxidation heat; excess methanol (up to 20-25% unconverted) is recycled after distillation. This method yields formaldehyde concentrations up to 40-50% in the reactor effluent, suitable for producing high-purity gas or concentrated solutions, but it consumes more methanol per ton of product (approximately 1.15-1.25 tons methanol per ton formaldehyde) compared to alternatives.[59][52][60] The metal oxide catalyst process, often utilizing iron-molybdate (Fe₂(MoO₄)₃-MoO₃) or similar formulations like the Formox system with vanadium and molybdenum promoters, operates at lower temperatures of 250-400°C to enhance selectivity and minimize over-oxidation to CO₂. Methanol vapor (15-20% in air) contacts the catalyst bed, favoring the oxidation pathway with near-complete oxygen utilization and methanol conversions exceeding 95%, yielding 88-92% formaldehyde based on methanol input—requiring about 15% less methanol than the silver process (roughly 1.0-1.1 tons per ton formaldehyde). The effluent is absorbed in water to form 37-50% aqueous solutions (formalin), followed by distillation to remove water, dimethyl ether, and trioxane byproducts. This process dominates modern large-scale production due to higher energy efficiency in feedstock use, though it may incur higher utility costs for compression and cooling in some configurations.[61][52][62] Both processes incorporate safety measures for handling flammable mixtures, maintaining methanol-air ratios below the explosion limit (typically 6-15% methanol by volume), and employ multitubular reactors with steam-cooled walls to manage exothermic heat. Global capacity exceeds 50 million tons annually, with metal oxide processes comprising the majority share owing to superior selectivity and scalability for integrated resin plants. Alternative routes, such as partial oxidation of hydrocarbons (e.g., methane or naphtha), were used historically but have been largely phased out due to lower yields and higher costs.[63][52][58]Chemical Reactivity
Polymerization and Hydration
In aqueous solutions, formaldehyde undergoes rapid hydration to form methanediol (CH₂(OH)₂), the gem-diol tautomer, via nucleophilic addition of water to the carbonyl group. This equilibrium strongly favors the hydrated form, with the ratio of methanediol to free formaldehyde concentrations reaching approximately 2,200 at 298 K in dilute solutions.[64] The hydration reaction is reversible, and the position of equilibrium shifts toward the diol at lower temperatures and higher water activity, reflecting the solvent's role in stabilizing the hydrate through hydrogen bonding.[65] Nuclear magnetic resonance studies confirm that in low-concentration aqueous formaldehyde, over 99% exists as methanediol, with free aldehyde detectable only via spectroscopic methods.[66] Polymerization of formaldehyde occurs under conditions of low water content, such as in concentrated solutions or anhydrous media, yielding polyoxymethylene structures through stepwise condensation or chain-growth mechanisms. Paraformaldehyde, a common linear oligomer (CH₂O)ₙ with n typically 8–100, forms by concentrating aqueous formaldehyde solutions and removing water, often accelerated by mild acidification or heating, resulting in a white, solid precipitate used as a convenient formaldehyde source.[67] This depolymerizes back to monomer upon heating or in basic/acidic aqueous media, enabling controlled release.[34] Cyclic polymerization produces 1,3,5-trioxane, a stable trimer, via acid-catalyzed trimerization of formaldehyde in concentrated aqueous solutions, typically employing mineral acids like sulfuric acid at elevated temperatures.[68] The reaction proceeds through protonation of the carbonyl, facilitating electrophilic attack and cyclization, with yields optimized by high formaldehyde concentrations (above 50 wt%) to suppress linear polymer formation.[69] High-molecular-weight polyoxymethylene (POM), a thermoplastic with degree of polymerization exceeding 1,000, requires anhydrous formaldehyde monomer and anionic initiators, such as alkali metal alkoxides or organometallic compounds, to propagate living polymerization chains with minimal termination.[70] This process, developed industrially in the mid-20th century, yields crystalline polymers stabilized against depolymerization by end-capping agents like acetate groups.[71] Unlike paraformaldehyde's thermal instability, stabilized POM resists reversion to monomer below its melting point of approximately 175°C.[72]Cross-Linking and Condensation Reactions
Formaldehyde cross-links proteins by reacting with nucleophilic side chains, particularly the ε-amino groups of lysine residues and the guanidino groups of arginine, forming methylene bridges (–CH₂–) that covalently link proximal residues within 2 Å due to the reagent's small size.[73] The initial step involves nucleophilic attack by an amine on the carbonyl carbon of formaldehyde, yielding a hemiaminal (methylol) intermediate, which dehydrates to an iminium ion (Schiff base) that serves as an electrophile for a second nucleophilic attack by another residue's amine, amide, or guanidyl group, with optimal efficiency at 1–2% formaldehyde concentration, room temperature, and 10–15 minute incubation.[74] Cross-links between amino and primary amide or guanidyl groups predominate, as evidenced by early studies on model peptides.[75] Mass spectrometry analyses have refined this model, revealing that apparent cross-links often manifest as +24 Da mass shifts between peptides, corresponding to the dimerization of two formaldehyde-modified amino acids (each gaining +12 Da via hydroxymethylation or formylation) rather than a simple direct methylene bridge (+14 Da); this occurs because the reactive iminium intermediates disproportionate or cyclize before bridging distant sites.[76] Such modifications vary by amino acid reactivity—lysine and cysteine form stable adducts fastest, while arginine and histidine yield slower, reversible ones—and are reversible under heat or mild base, enabling applications in chromatin immunoprecipitation where 1% formaldehyde fixes protein-DNA interactions in vivo.[77] In nucleic acids, formaldehyde cross-links exocyclic amines of bases (e.g., adenine N6, cytosine N4) to protein lysines, with reversal rates depending on the specific bridge (e.g., ~0.001–0.01 min⁻¹ at 65°C).[78][79] In condensation reactions, formaldehyde undergoes electrophilic aromatic substitution or nucleophilic addition-elimination with compounds bearing active methylene or amino groups, eliminating water to form methylene-linked polymers, as in the production of urea-formaldehyde (UF) and phenol-formaldehyde (PF) resins, which account for over 50% of global formaldehyde consumption.[80] UF resin synthesis begins with urea and formaldehyde (molar ratio ~1:2) in aqueous alkaline medium at 70–90°C to form mono- and dimethylolureas via addition, followed by acid-catalyzed (pH 4–5) polycondensation at 90–100°C, yielding branched networks of –NH–CH₂–NH– and –N(CH₂)–N– linkages; curing involves further condensation and crystallization, with free formaldehyde content controlled below 0.1% to minimize emissions.[81][82] PF resins form similarly: phenol reacts with excess formaldehyde under base catalysis to generate ortho- and para-hydroxymethylphenols (resoles), which condense under acid or heat to methylene (–CH₂–) and dibenzyl ether bridges, with resorcinol variants accelerating via quinone methide intermediates in Michael additions.[83] These reactions are stepwise, with self-condensation of methylolphenols dominating in formaldehyde-free stages, influenced by substituent electron density.[84] The Mannich reaction exemplifies a related condensation-cross-linking pathway, where formaldehyde, a primary or secondary amine, and an enolizable carbonyl (e.g., ketone) or activated arene (e.g., phenol) react to form β-amino methylene derivatives, enabling network formation in adhesives or ligands; for instance, hydroquinone with formaldehyde and ammonia yields aminomethylated products under acid catalysis.[85] In Betti variants, phenols and amines cross-link via methylene bridges, as seen in recent syntheses adding sulfur or nitrogen heteroatoms.[86] These processes underpin durable materials like particleboard binders but require precise stoichiometry to avoid brittleness from over-cross-linking.[87]Oxidation, Reduction, and Other Transformations
Formaldehyde is oxidized to formic acid (HCOOH) and further to carbon dioxide (CO₂) through multiple pathways, depending on conditions and oxidants. In the gas phase, autoxidation with molecular oxygen occurs via a radical chain mechanism initiated by triplet oxygen, involving H-atom abstraction or addition reactions and propagating radicals such as O, H, OH, and HO₂, with peroxides like H₂O₂ forming as intermediates.[88] In aqueous solution, the hydroxyl radical (OH) oxidizes formaldehyde at 293 K, following detailed kinetics where the rate-determining step involves HCHO• radical formation and subsequent reactions yielding hydrated formic acid.[89] A metal-mediated example is the copper(II)-catalyzed oxidation in water:with the reaction rate influenced by pH and copper concentration.[90] Reduction of formaldehyde primarily produces methanol (CH₃OH), achievable via hydrogenation with catalysts such as supported metals. In anaerobic microbial processes, formaldehyde serves as both oxidant and reductant in disproportionation, yielding formate and methane or methanol, as observed in experiments where HCHO provided all carbon and reducing equivalents under N₂, confirming balanced oxidation-reduction stoichiometry.[91] Other transformations include the Cannizzaro disproportionation, prominent for formaldehyde due to its lack of α-hydrogens; in concentrated alkali (e.g., NaOH), two molecules react without external oxidant or reductant:
a self-redox process where one equivalent is oxidized to formate and the other reduced to methanol, often quantitative under anhydrous conditions.[91] Metal-promoted variants, such as rhodium-catalyzed hydroformylation, convert formaldehyde to glycolaldehyde (HOCH₂CHO), involving CO insertion and hydrogenation steps.[92] In electrocatalytic contexts, formaldehyde undergoes multi-electron oxidation to formate or CO₂ at low potentials on Cu-based electrodes, bypassing oxygen evolution.[93] These reactions underscore formaldehyde's role as a versatile C1 synthon, though practical applications prioritize stability over transformation due to its reactivity.[94]
Uses and Applications
Primary Industrial Uses
Formaldehyde is predominantly utilized in the production of thermosetting resins, which represent the primary industrial application and account for the majority of global consumption. Resins comprised approximately 63% of worldwide formaldehyde use in 2009, with the construction industry alone consuming 60 to 70% of total production for engineered wood products.[95][96] These resins enable efficient bonding of wood fibers, particles, and veneers, facilitating the manufacture of durable, cost-effective materials essential for building and furniture sectors.[97] Urea-formaldehyde (UF) resins, the most common type, serve as adhesives in particleboard, medium-density fiberboard (MDF), and high-density fiberboard (HDF), binding wood particles under heat and pressure to form panels used in cabinetry, flooring, and shelving.[98][3] Phenol-formaldehyde (PF) resins are employed in plywood and oriented strand board (OSB), offering superior moisture resistance suitable for structural applications like exterior sheathing and subflooring.[97] Melamine-formaldehyde (MF) resins provide hard, scratch-resistant surfaces for laminates in countertops and decorative panels.[98] Beyond wood composites, formaldehyde-derived resins support automotive components, such as interior trim and under-hood parts via PF and polyoxymethylene (POM) plastics, and foundry applications through hexamine in epoxy and rubber formulations.[98] It also functions as an intermediate for pentaerythritol synthesis, used in alkyd resins for paints and varnishes, though these uses constitute smaller shares relative to wood product resins.[3] Paraformaldehyde, a polymeric form, is often preferred in industrial processes for controlled release in adhesive formulations.[98]
Specialized and Emerging Applications
In medical applications, formaldehyde functions as a disinfectant and sterilizing agent for equipment and surfaces in hospitals and laboratories, leveraging its ability to denature proteins and nucleic acids in microorganisms.[99] It is also employed to inactivate viruses and bacteria in vaccine production, such as in formulations for polio, diphtheria, and hepatitis A, where concentrations around 0.02-0.1% ensure pathogen neutralization while preserving antigenic properties.[100][101] In tissue fixation for pathology and embalming, aqueous solutions (typically 4-10% formalin) cross-link proteins to stabilize cellular structures, enabling long-term preservation of specimens for microscopic analysis or autopsy studies.[102][103] Biotechnological uses include formaldehyde's role in cross-linking DNA-protein complexes for chromatin immunoprecipitation (ChIP) assays, which map protein binding sites on genomes to elucidate gene regulation mechanisms, with typical exposure times of 10-30 minutes at 1% concentration.[104] It serves as a component in some anti-infective pharmaceuticals and enhances drug absorption in gelatin capsules by modifying polymer matrices.[102] Specialized industrial niches encompass leather tanning, where formaldehyde-based agents stabilize collagen fibers against degradation, improving durability in processes handling up to 10-20 kg per ton of hide.[105] In oil and gas extraction, it acts as a corrosion inhibitor and biocide in drilling fluids and well treatments, mitigating microbial-induced souring at dosages of 50-200 ppm.[106][105] Mining operations utilize it for dust suppression and reagent stabilization in flotation processes.[106] Emerging research explores formaldehyde's integration into advanced materials, such as epoxy resins for wind turbine components in renewable energy production, where it contributes to high-strength composites enduring mechanical stresses over 20-year lifespans.[98] In biotechnology, investigations into controlled-release systems for formaldehyde-derived cross-linkers aim to refine protein stabilization in enzyme immobilization for industrial biocatalysis, though scalability remains limited as of 2023.[104] These developments prioritize low-emission formulations to address environmental constraints while expanding utility in sustainable technologies.[98]Exposure and Health Effects
Routes of Exposure
The primary route of human exposure to formaldehyde is inhalation of its gas or vapor, which is readily absorbed through the respiratory tract due to its high water solubility and reactivity.[6][107] This pathway predominates in both occupational settings, such as manufacturing of resins or textiles, and environmental contexts like indoor air from off-gassing building materials or combustion sources including tobacco smoke.[3][45] Inhaled formaldehyde is primarily deposited in the upper airways, where concentrations above 0.1 ppm can cause sensory irritation.[6] Dermal exposure occurs through direct contact with formaldehyde-containing liquids or solutions, leading to absorption across intact skin, though systemic effects are limited by rapid local metabolism to formic acid.[6][108] Skin absorption is more significant in occupational scenarios involving handling of formalin (aqueous formaldehyde solutions) for embalming or sterilization, often resulting in localized irritation or allergic contact dermatitis rather than widespread distribution.[45] Eye contact, frequently concurrent with dermal exposure, causes immediate lacrimation and conjunctival irritation at concentrations as low as 0.5 ppm.[6] Ingestion represents a minor route of exposure, typically accidental or suicidal, though low natural levels occur in many foods such as fruits, vegetables, and meats, where they are rapidly metabolized without harm.[16] High exposure from illegal addition to food can cause acute effects including irritation and nausea, with potential long-term risks.[6] Formaldehyde is absorbed efficiently from the gastrointestinal tract after dilution in aqueous forms like formalin, and bound formaldehyde in cookware handles (e.g., phenolic resins) does not significantly leach into food during normal use. Oral uptake leads to rapid corrosive damage to mucosal surfaces in high doses, but chronic low-level dietary ingestion poses no widespread concerns in regulated markets.[109] Overall, while all routes contribute to total body burden, inhalation accounts for the majority of population-level exposures, with dermal and oral pathways more relevant in specific high-risk activities.[10][110]Acute and Irritant Effects
Formaldehyde gas causes acute irritation to the eyes, nose, and throat at airborne concentrations as low as 0.3 parts per million (ppm), manifesting as burning sensations, tearing, and nasal discharge.[6][2] At levels above 0.1 ppm, respiratory tract irritation occurs, with symptom severity increasing with higher concentrations, including cough, wheezing, and shortness of breath.[108] Eye irritation thresholds range from 0.3 to 0.9 ppm in occupational settings, while severe ocular effects develop between 4 and 20 ppm.[111] Higher acute exposures, exceeding 5 ppm, can lead to intense mucous membrane inflammation, pulmonary edema, and potentially fatal respiratory distress due to direct corrosive action on lung tissues.[6][112] Sensory irritation thresholds in controlled human studies indicate eye effects as the most sensitive, with general sensory irritation evident around 1 ppm.[113] Nasal irritation is reported at 0.5 ppm with peaks to 1.0 ppm or lower levels (0.3–0.5 ppm) in combination with other irritants.[114] Skin contact with liquid formaldehyde solutions, such as formalin, produces immediate irritant dermatitis characterized by erythema, edema, and vesiculation, particularly in sensitized individuals.[115] Acute ingestion results in corrosive gastrointestinal damage, vascular collapse, and systemic toxicity, often fatal without prompt intervention.[111] These effects stem from formaldehyde's reactivity as an electrophile, forming adducts with proteins and nucleic acids in biological tissues, triggering inflammatory cascades.[10] Symptoms typically resolve upon cessation of exposure, though severe cases may require medical management including bronchodilators and supportive care.[6]
Chronic Effects and Carcinogenicity Data
Chronic inhalation exposure to formaldehyde at low to moderate levels has been associated with persistent respiratory tract irritation, including symptoms such as coughing, wheezing, and decreased pulmonary function in occupational cohorts.[116] Studies of workers in industries like particleboard manufacturing and embalmers report chronic effects including nasal dryness, epithelial damage, and olfactory impairment, with some evidence of adaptation over time reducing symptom intensity after initial exposure periods of 4-6 weeks.[111] Sensitization leading to allergic responses, such as occupational asthma, occurs in a subset of exposed individuals, characterized by chest tightness, shortness of breath, and reversible airway obstruction upon re-exposure. These effects are primarily localized to the upper respiratory mucosa due to formaldehyde's high reactivity, with dose-dependent risks observed below 1 ppm in long-term studies.[2] Formaldehyde is classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC) in Group 1, based primarily on sufficient evidence from inhalation exposures linking high occupational levels to nasopharyngeal cancer and sinonasal cancer, with carcinogenicity mainly associated with inhalation rather than typical dietary intake.[117] Cohort studies of formaldehyde-exposed workers, such as those in chemical manufacturing and woodworking, show relative risks for nasopharyngeal cancer elevated by 1.3- to 3-fold at cumulative exposures exceeding 10 ppm-years, with risks concentrated in the highest exposure quartiles and supported by consistent findings across multiple international datasets.[9] The National Toxicology Program (NTP) lists formaldehyde as a known human carcinogen, citing mechanistic evidence of DNA-protein crosslinks and cytotoxicity in nasal tissues as precursors to tumorigenesis in rodent models mirroring human site-specific effects.[118] The U.S. Environmental Protection Agency's Integrated Risk Information System (IRIS) assessment affirms inhalation-related carcinogenicity for nasopharyngeal and sinonasal sites, deriving unit risk estimates from pooled occupational data indicating no safe threshold for these portal-of-entry cancers.[119] Associations with myeloid leukemia and other lymphohematopoietic cancers have been reported in some cohort analyses, particularly with peak or cumulative exposure metrics, but causal evidence remains limited and contested.[9] IARC and NTP include leukemia in their classifications based on positive findings in select studies, such as elevated standardized mortality ratios in embalmers and industrial workers; however, systematic reviews highlight inconsistencies, including lack of exposure-response trends, absence of bone marrow genotoxicity in humans, and formaldehyde's rapid local metabolism preventing systemic circulation to hematopoietic tissues.[120][121] Recent meta-analyses and re-evaluations, integrating negative findings from large updated cohorts, conclude no convincing causal link, attributing apparent associations to confounding factors like concurrent exposures or diagnostic biases rather than direct leukemogenesis.[122][123] Overall, while nasopharyngeal risks are empirically robust from high-exposure data, leukemia claims rely on weaker, non-localized evidence, underscoring the need for site-specific causality in assessments.[124]Safety, Regulation, and Controversies
Occupational and Consumer Safety Measures
Occupational safety measures for formaldehyde emphasize exposure monitoring, engineering controls, and personal protective equipment (PPE) to mitigate risks from inhalation, skin contact, and eye exposure. The Occupational Safety and Health Administration (OSHA) mandates a permissible exposure limit (PEL) of 0.75 parts per million (ppm) as an 8-hour time-weighted average (TWA), with a short-term exposure limit (STEL) of 2 ppm over 15 minutes and an action level of 0.5 ppm triggering monitoring and medical surveillance.[125] [108] The National Institute for Occupational Safety and Health (NIOSH) recommends a more stringent REL of 0.016 ppm TWA or 0.1 ppm 15-minute ceiling, reflecting concerns over carcinogenicity even at low levels.[126] Employers must implement feasible engineering controls such as local exhaust ventilation and enclosed systems before relying on PPE, alongside initial and periodic air monitoring to assess exposures.[125]| Agency | Exposure Limit | Description | Source |
|---|---|---|---|
| OSHA PEL | 0.75 ppm | 8-hour TWA | [125] |
| OSHA STEL | 2 ppm | 15-minute maximum | [125] |
| NIOSH REL | 0.016 ppm | TWA (carcinogen policy) | [126] |
| NIOSH Ceiling | 0.1 ppm | 15-minute limit | [126] |