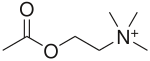
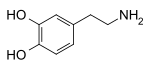
Recent from talks
Nothing was collected or created yet.
Psychoactive drug
View on Wikipedia

- Cocaine
- Crack cocaine
- Methylphenidate (Ritalin)
- Ephedrine
- MDMA (ecstasy)
- Peyote (mescaline)
- LSD blotter
- Psilocybin mushroom (Psilocybe cubensis)
- Diphenhydramine (Benadryl) (Unscheduled drug)
- Amanita muscaria mushroom (muscimol) (Unscheduled drug)
- Salvia divinorum (salvinorin A)
- Tylenol 3 (acetaminophen/codeine)
- Codeine with muscle relaxant
- Pipe tobacco (nicotine) (Unscheduled drug)
- Bupropion (Unscheduled drug)
- Cannabis (THC)
- Hashish (THC)


A psychoactive drug, psychopharmaceutical,[2] mind-altering drug, consciousness-altering drug, psychoactive substance,[3] or psychotropic substance[3] is a chemical substance that alters psychological functioning by modulating central nervous system (CNS) activity.[4][3] Psychoactive and psychotropic drugs both affect the brain, with psychotropics sometimes referring to psychiatric drugs or high-abuse substances, while “drug” can have negative connotations. Novel psychoactive substances are designer drugs made to mimic illegal ones and bypass laws.
Psychoactive drug use dates back to prehistory for medicinal and consciousness-altering purposes, with evidence of widespread cultural use. Many animals intentionally consume psychoactive substances, and some traditional legends suggest animals first introduced humans to their use. Psychoactive substances are used across cultures for purposes ranging from medicinal and therapeutic treatment of mental disorders and pain, to performance enhancement. Their effects are influenced by the drug itself, the environment, and individual factors. Psychoactive drugs are categorized by their pharmacological effects into types such as anxiolytics (reduce anxiety), empathogen–entactogens (enhance empathy), stimulants (increase CNS activity), depressants (decrease CNS activity), and hallucinogens (alter perception and emotions). Psychoactive drugs are administered through various routes—including oral ingestion, injection, rectal use, and inhalation—with the method and efficiency differing by drug.
Psychoactive drugs alter brain function by interacting with neurotransmitter systems—either enhancing or inhibiting activity—which can affect mood, perception, cognition, behavior, and potentially lead to dependence or long-term neural adaptations such as sensitization or tolerance. Addiction and dependence involve psychological and physical reliance on psychoactive substances, with treatments ranging from psychotherapy and medication to emerging psychedelic therapies; global prevalence is highest for alcohol, cannabis, and opioid use disorders.
The legality of psychoactive drugs has long been controversial, shaped by international treaties like the 1961 Single Convention on Narcotic Drugs and national laws such as the United States Controlled Substances Act. Distinctions are made between recreational and medical use. Enforcement varies across countries. While the 20th century saw global criminalization, recent shifts favor harm reduction and regulation over prohibition. Widely used psychoactive drugs include legal substances like caffeine, alcohol, and nicotine; prescribed medications such as SSRIs, opioids, and benzodiazepines; and illegal recreational drugs like cocaine, LSD, and MDMA.
History
[edit]Psychoactive drug use can be traced to prehistory. Archaeological evidence of the use of psychoactive substances, mostly plants, dates back at least 10,000 years; historical evidence indicates cultural use 5,000 years ago.[5] There is evidence of the chewing of coca leaves, for example, in Peruvian society 8,000 years ago.[6][7]
Psychoactive substances have been used medicinally and to alter consciousness. Consciousness altering may be a primary drive, akin to the need to satiate thirst, hunger, or sexual desire.[8] This may be manifest in the long history of drug use, and even in children's desire for spinning, swinging, or sliding, suggesting that the drive to alter one's state of mind is universal.[9]
In The Hasheesh Eater (1857), American author Fitz Hugh Ludlow was one of the first to describe in modern terms the desire to change one's consciousness through drug use:
[D]rugs are able to bring humans into the neighborhood of divine experience and can thus carry us up from our personal fate and the everyday circumstances of our life into a higher form of reality. It is, however, necessary to understand precisely what is meant by the use of drugs. We do not mean the purely physical craving ... That of which we speak is something much higher, namely the knowledge of the possibility of the soul to enter into a lighter being, and to catch a glimpse of deeper insights and more magnificent visions of the beauty, truth, and the divine than we are normally able to spy through the cracks in our prison cell. But there are not many drugs which have the power of stilling such craving. The entire catalog, at least to the extent that research has thus far written it, may include only opium, hashish, and in rarer cases alcohol, which has enlightening effects only upon very particular characters.[10]
During the 20th century, the majority of countries initially responded to the use of recreational drugs by prohibiting production, distribution, or use through criminalization.[citation needed] A notable example occurred with Prohibition in the United States, where early in the century alcohol was made illegal for 13 years. In recent decades, an emerging perspective among governments and law enforcement holds that illicit drug use cannot be stopped through prohibition.[citation needed] One organization holding that view, Law Enforcement Against Prohibition (LEAP), concluded that "[in] fighting a war on drugs the government has increased the problems of society and made them far worse. A system of regulation rather than prohibition is a less harmful, more ethical and a more effective public policy."[11][failed verification]
In some countries, there has been a move toward harm reduction, where the use of illicit drugs is neither condoned nor promoted, but services and support are provided to ensure users have adequate factual information readily available, and that the negative effects of their use be minimized. Such is the case with Portugal's drug policy of decriminalization, with a primary goal of reducing the adverse health effects of drug use.[12]
Terminology
[edit]Psychoactive and psychotropic are often used interchangeably in general and academic sources, to describe substances that act on the brain to alter cognition and perception; some sources make a distinction between the terms. One narrower definition of psychotropic refers to drugs used to treat mental disorders, such as anxiolytic sedatives, antidepressants, antimanic agents, and neuroleptics. Another usage of psychotropic refers to substances determined to pose "high abuse liability", including stimulants, hallucinogens, opioids, and sedatives/hypnotics including alcohol. In international drug control, psychotropic substances refers to the substances specified in the Convention on Psychotropic Substances, which does not include narcotics.[13]
The term "drug" has become a skunked term. "Drugs" can have a negative connotation, often associated with illegal substances like cocaine or heroin, despite the fact that the terms "drug" and "medicine" are sometimes used interchangeably.[14]
Novel psychoactive substances (NPS)[note 1], also known as "designer drugs" are a category of psychoactive drugs (substances) that are designed to mimic the effects of often illegal drugs, usually in efforts to circumvent existing drug laws.[15]
Types
[edit]Psychoactive drugs are divided according to their pharmacological effects. Common subtypes include:
- Anxiolytics are medicinally used to reduce the symptoms of anxiety, and sometimes insomnia.
- Example: benzodiazepines such as Xanax and Valium; barbiturates
- Empathogen–entactogens alter emotional state, often resulting in an increased sense of empathy, closeness, and emotional communication.
- Stimulants increase activity, or arousal, of the central nervous system. They can enhance alertness, attention, cognition, mood and physical performance. Some stimulants are used medicinally to treat individuals with ADHD and narcolepsy.
- Examples: amphetamines, caffeine, cocaine, nicotine
- Depressants reduce, or depress, activity and stimulation in the central nervous system. This category encompasses a spectrum of substances with sedative, soporific, and anesthetic properties, and include sedatives, hypnotics, and opioids.
- Examples: ethanol (alcohol), opioids such as morphine, fentanyl, and codeine, barbiturates, and benzodiazepines
- Hallucinogens, including psychedelics, dissociatives and deliriants, encompass substances that produce distinct alterations in perception, sensation of space and time, and emotional state.[16]
- Examples, psychedelics: Psilocybin, LSD, DMT (N,N-Dimethyltryptamine), mescaline, cannabis
- Examples, dissociatives: Dextromethorphan, Salvia divinorum
- Examples, deliriants: Datura, scopolamine
Uses
[edit]
The ways in which psychoactive substances are used vary widely between cultures. Some substances may have controlled or illegal uses, others may have shamanic purposes, and others are used medicinally. Examples would be social drinking, nootropic supplements, and sleep aids. Caffeine is the world's most widely consumed psychoactive substance, and is legal and unregulated in nearly all jurisdictions; in North America, 90% of adults consume caffeine daily.[17]
Mental disorders
[edit]
Psychiatric medications are psychoactive drugs prescribed for the management of mental and emotional disorders, or to aid in overcoming challenging behavior.[18] There are six major classes of psychiatric medications:
- Antidepressants treat disorders such as clinical depression, dysthymia, anxiety, eating disorders, and borderline personality disorder.[19]
- Stimulants, used to treat disorders such as attention deficit hyperactivity disorder and narcolepsy, and for weight reduction.
- Antipsychotics, used to treat psychotic symptoms, such as those associated with schizophrenia or severe mania, or as adjuncts to relieve clinical depression.
- Mood stabilizers, used to treat bipolar disorder and schizoaffective disorder.
- Anxiolytics, used to treat anxiety disorders.
- Depressants, used as hypnotics, sedatives, and anesthetics, depending upon dosage.
In addition, several psychoactive substances are currently employed to treat various addictions. These include acamprosate or naltrexone in the treatment of alcoholism, or methadone or buprenorphine maintenance therapy in the case of opioid addiction.[20]
Exposure to psychoactive drugs can cause changes to the brain that counteract or augment some of their effects; these changes may be beneficial or harmful. However, there is a significant amount of evidence that the relapse rate of mental disorders negatively corresponds with the length of properly followed treatment regimens (that is, relapse rate substantially declines over time), and to a much greater degree than placebo.[21]
Military
[edit]Drugs used by militaries
[edit]
Militaries worldwide have used or are using various psychoactive drugs to treat pain and to improve performance of soldiers by suppressing hunger, increasing the ability to sustain effort without food, increasing and lengthening wakefulness and concentration, suppressing fear, reducing empathy, and improving reflexes and memory-recall among other things.[22][23]
Both military and civilian American intelligence officials are known to have used psychoactive drugs while interrogating captives apprehended in its "war on terror". In July 2012 Jason Leopold and Jeffrey Kaye, psychologists and human rights workers, had a Freedom of Information Act request fulfilled that confirmed that the use of psychoactive drugs during interrogation was a long-standing practice.[24][25] Captives and former captives had been reporting medical staff collaborating with interrogators to drug captives with powerful psychoactive drugs prior to interrogation since the very first captives release.[26][27] In May 2003 recently released Pakistani captive Sha Mohammed Alikhel described the routine use of psychoactive drugs. He said that Jihan Wali, a captive kept in a nearby cell, was rendered catatonic through the use of these drugs.[citation needed]
Alcohol has a long association of military use, and has been called "liquid courage" for its role in preparing troops for battle, anaesthetize injured soldiers, and celebrate military victories. It has also served as a coping mechanism for combat stress reactions and a means of decompression from combat to everyday life. However, this reliance on alcohol can have negative consequences for physical and mental health.[28]
The first documented case of a soldier overdosing on methamphetamine during combat, was the Finnish corporal Aimo Koivunen, a soldier who fought in the Winter War and the Continuation War.[29][30]
Psychochemical warfare
[edit]Psychoactive drugs have been used in military applications as non-lethal weapons.
Pain management
[edit]Psychoactive drugs are often prescribed to manage pain. The subjective experience of pain is primarily regulated by endogenous opioid peptides. Thus, pain can often be managed using psychoactives that operate on this neurotransmitter system, also known as opioid receptor agonists. This class of drugs can be highly addictive, and includes opiate narcotics, like morphine and codeine.[31] NSAIDs, such as aspirin and ibuprofen, are also analgesics. These agents also reduce eicosanoid-mediated inflammation by inhibiting the enzyme cyclooxygenase.
Anesthesia
[edit]General anesthetics are a class of psychoactive drug used on people to block physical pain and other sensations. Most anesthetics induce unconsciousness, allowing the person to undergo medical procedures like surgery, without the feelings of physical pain or emotional trauma.[32] To induce unconsciousness, anesthetics affect the GABA and NMDA systems. For example, Propofol is a GABA agonist,[33] and ketamine is an NMDA receptor antagonist.[34]
Performance-enhancement
[edit]Performance-enhancing substances, also known as performance-enhancing drugs (PEDs),[35] are substances that are used to improve any form of activity performance in humans. A well-known example of cheating in sports involves doping in sport, where banned physical performance-enhancing drugs are used by athletes and bodybuilders. Athletic performance-enhancing substances are sometimes referred as ergogenic aids.[36][37] Cognitive performance-enhancing drugs, commonly called nootropics,[38] are sometimes used by students to improve academic performance. Performance-enhancing substances are also used by military personnel to enhance combat performance.[39]
Recreation
[edit]
Many psychoactive substances are used for their mood and perception altering effects, including those with accepted uses in medicine and psychiatry. Examples of psychoactive substances include caffeine, alcohol, cocaine, LSD, nicotine, cannabis, and dextromethorphan.[40] Classes of drugs frequently used recreationally include:
- Stimulants, which activate the central nervous system. These are used recreationally for their euphoric effects.
- Hallucinogens (psychedelics, dissociatives and deliriants), which induce perceptual and cognitive alterations.
- Hypnotics, which depress the central nervous system.
- Opioid analgesics, which also depress the central nervous system. These are used recreationally because of their euphoric effects.
- Inhalants, in the forms of gas aerosols, or solvents, which are inhaled as a vapor because of their stupefying effects. Many inhalants also fall into the above categories (such as nitrous oxide which is also an analgesic).
In some modern and ancient cultures, drug usage is seen as a status symbol. Recreational drugs are seen as status symbols in settings such as at nightclubs and parties.[41] For example, in ancient Egypt, gods were commonly pictured holding hallucinogenic plants.[42]
Because there is controversy about regulation of recreational drugs, there is an ongoing debate about drug prohibition. Critics of prohibition believe that regulation of recreational drug use is a violation of personal autonomy and freedom.[43] In the United States, critics have noted that prohibition or regulation of recreational and spiritual drug use might be unconstitutional, and causing more harm than is prevented.[44]
Some people who take psychoactive drugs experience drug- or substance-induced psychosis. A 2019 systematic review and meta-analysis by Murrie et al. found that the pooled proportion of transition from substance-induced psychosis to schizophrenia was 25% (95% CI 18%–35%), compared with 36% (95% CI 30%–43%) for brief, atypical and not otherwise specified psychoses.[45] Type of substance was the primary predictor of transition from drug-induced psychosis to schizophrenia, with highest rates associated with cannabis (6 studies, 34%, CI 25%–46%), hallucinogens (3 studies, 26%, CI 14%–43%) and amphetamines (5 studies, 22%, CI 14%–34%). Lower rates were reported for opioid (12%), alcohol (10%) and sedative (9%) induced psychoses. Transition rates were slightly lower in older cohorts but were not affected by sex, country of the study, hospital or community location, urban or rural setting, diagnostic methods, or duration of follow-up.[45]
Ritual and spiritual
[edit]Offerings
[edit]Alcohol and tobacco (nicotine) have been and are used as offerings in various religions and spiritual practices.[citation needed] Coca leaves have been used as offerings in rituals.[46]
Alcohol
[edit]According to the Catholic Church, the sacramental wine used in the Eucharist must contain alcohol. Canon 924 of the present Code of Canon Law (1983) states:
§3 The wine must be natural, made from grapes of the vine, and not corrupt.[47]
Psychoactive use
[edit]Entheogen
[edit]
Certain psychoactives, particularly hallucinogens, have been used for religious purposes since prehistoric times. Native Americans have used peyote cacti containing mescaline for religious ceremonies for as long as 5700 years.[48] The muscimol-containing Amanita muscaria mushroom was used for ritual purposes throughout prehistoric Europe.[49]
The use of entheogens for religious purposes resurfaced in the West during the counterculture movements of the 1960s and 70s. Under the leadership of Timothy Leary, new spiritual and intention-based movements began to use LSD and other hallucinogens as tools to access deeper inner exploration. In the United States, the use of peyote for ritual purposes is protected only for members of the Native American Church, which is allowed to cultivate and distribute peyote. However, the genuine religious use of peyote, regardless of one's personal ancestry, is protected in Colorado, Arizona, New Mexico, Nevada, and Oregon.[50]
Psychedelic therapy
[edit]Psychedelic therapy (or psychedelic-assisted therapy) refers to the proposed use of psychedelic drugs, such as psilocybin, MDMA,[note 2] LSD, and ayahuasca, to treat mental disorders.[52][53] As of 2021, psychedelic drugs are controlled substances in most countries and psychedelic therapy is not legally available outside clinical trials, with some exceptions.[53][54]
Psychonautics
[edit]The aims and methods of psychonautics, when state-altering substances are involved, is commonly distinguished from recreational drug use by research sources.[55] Psychonautics as a means of exploration need not involve drugs, and may take place in a religious context with an established history. Cohen considers psychonautics closer in association to wisdom traditions and other transpersonal and integral movements.[56]
Self-medication
[edit]Self-medication, sometime called do-it-yourself (DIY) medicine, is a human behavior in which an individual uses a substance or any exogenous influence to self-administer treatment for physical or psychological conditions, for example headaches or fatigue.
The substances most widely used in self-medication are over-the-counter drugs and dietary supplements, which are used to treat common health issues at home. These do not require a doctor's prescription to obtain and, in some countries, are available in supermarkets and convenience stores.[57]
Sex
[edit]Sex and drugs date back to ancient humans and have been interlocked throughout human history. Both legal and illegal, the consumption of drugs and their effects on the human body encompasses all aspects of sex, including desire, performance, pleasure, conception, gestation, and disease.
There are many different types of drugs that are commonly associated with their effects on sex, including alcohol, cannabis, cocaine, MDMA, GHB, amphetamines, opioids, antidepressants, and many others.
Social movements
[edit]Cannabis
[edit]In the US, NORML (National Organization for the Reform of Marijuana Laws) has led since the 1970s a movement to legalize cannabis nationally.[58] The so-called "420 movement" is the global association of the number 420 with cannabis consumption: April 20th – fourth month, twentieth day – has become an international counterculture holiday based on the celebration and consumption of cannabis;[59][60][61] 4:20 pm on any day is a time to consume cannabis.[62][63]
Operation Overgrow
[edit]Operation Overgrow is the name, given by cannabis activists, of an "operation" to spread marijuana seeds wildly "so it grows like weed".[64] The thought behind the operation is to draw attention to the debate about legalization/decriminalization of marijuana.
Suicide
[edit]A drug overdose involves taking a dose of a drug that exceeds safe levels. In the UK (England and Wales) until 2013, a drug overdose was the most common suicide method in females.[65] In 2019 in males the percentage is 16%. Self-poisoning accounts for the highest number of non-fatal suicide attempts. In the United States about 60% of suicide attempts and 14% of suicide deaths involve drug overdoses.[66] The case fatality rate of suicide attempts involving overdose is about 2%.[66]
Most people are under the influence of sedative-hypnotic drugs (such as alcohol or benzodiazepines) when they die by suicide,[67] with alcoholism present in between 15% and 61% of cases.[68] Countries that have higher rates of alcohol use and a greater density of bars generally also have higher rates of suicide.[69] About 2.2–3.4% of those who have been treated for alcoholism at some point in their life die by suicide.[69] Alcoholics who attempt suicide are usually male, older, and have tried to take their own lives in the past.[68] In adolescents who misuse alcohol, neurological and psychological dysfunctions may contribute to the increased risk of suicide.[70]
Overdose attempts using painkillers are among the most common, due to their easy availability over-the-counter.[71]
Route of administration
[edit]Psychoactive drugs are administered via oral ingestion as a tablet, capsule, powder, liquid, and beverage; via injection by subcutaneous, intramuscular, and intravenous route; via rectum by suppository and enema; and via inhalation by smoking, vaporizing, and snorting. The efficiency of each method of administration varies from drug to drug.[72]
The psychiatric drugs fluoxetine, quetiapine, and lorazepam are ingested orally in tablet or capsule form. Alcohol and caffeine are ingested in beverage form; nicotine and cannabis are smoked or vaporized; peyote and psilocybin mushrooms are ingested in botanical form or dried; and crystalline drugs such as cocaine and methamphetamine are usually inhaled or snorted.
Determinants of effects
[edit]The theory of dosage, set, and setting is a useful model in dealing with the effects of psychoactive substances, especially in a controlled therapeutic setting as well as in recreational use. Dr. Timothy Leary, based on his own experiences and systematic observations on psychedelics, developed this theory along with his colleagues Ralph Metzner, and Richard Alpert (Ram Dass) in the 1960s.[73]
- Dosage
The first factor, dosage, has been a truism since ancient times, or at least since Paracelsus who said, "Dose makes the poison." Some compounds are beneficial or pleasurable when consumed in small amounts, but harmful, deadly, or evoke discomfort in higher doses.
- Set
The set is the internal attitudes and constitution of the person, including their expectations, wishes, fears, and sensitivity to the drug. This factor is especially important for the hallucinogens, which have the ability to make conscious experiences out of the unconscious. In traditional cultures, set is shaped primarily by the worldview, health and genetic characteristics that all the members of the culture share.
- Setting
The third aspect is setting, which pertains to the surroundings, the place, and the time in which the experiences transpire.
This theory clearly states that the effects are equally the result of chemical, pharmacological, psychological, and physical influences. The model that Timothy Leary proposed applied to the psychedelics, although it also applies to other psychoactives.[74]
Effects
[edit]
Psychoactive drugs operate by temporarily affecting a person's neurochemistry, which in turn causes changes in a person's mood, cognition, perception and behavior. There are many ways in which psychoactive drugs can affect the brain. Each drug has a specific action on one or more neurotransmitter or neuroreceptor in the brain.
Drugs that increase activity in particular neurotransmitter systems are called agonists. They act by increasing the synthesis of one or more neurotransmitters, by reducing its reuptake from the synapses, or by mimicking the action by binding directly to the postsynaptic receptor. Drugs that reduce neurotransmitter activity are called antagonists, and operate by interfering with synthesis or blocking postsynaptic receptors so that neurotransmitters cannot bind to them.[75]
Exposure to a psychoactive substance can cause changes in the structure and functioning of neurons, as the nervous system tries to re-establish the homeostasis disrupted by the presence of the drug (see also, neuroplasticity). Exposure to antagonists for a particular neurotransmitter can increase the number of receptors for that neurotransmitter or the receptors themselves may become more responsive to neurotransmitters; this is called sensitization. Conversely, overstimulation of receptors for a particular neurotransmitter may cause a decrease in both number and sensitivity of these receptors, a process called desensitization or tolerance. Sensitization and desensitization are more likely to occur with long-term exposure, although they may occur after only a single exposure. These processes are thought to play a role in drug dependence and addiction.[76] Physical dependence on antidepressants or anxiolytics may result in worse depression or anxiety, respectively, as withdrawal symptoms. Unfortunately, because clinical depression (also called major depressive disorder) is often referred to simply as depression, antidepressants are often requested by and prescribed for patients who are depressed, but not clinically depressed.
Affected neurotransmitter systems
[edit]The following is a brief table of notable drugs and their primary neurotransmitter, receptor or method of action. Many drugs act on more than one transmitter or receptor in the brain.[77]
Addiction and dependence
[edit]| Addiction and dependence glossary[86][87][88] | |
|---|---|
| |

Psychoactive drugs are often associated with addiction or drug dependence. Dependence can be divided into two types: psychological dependence, by which a user experiences negative psychological or emotional withdrawal symptoms (e.g., depression) and physical dependence, by which a user must use a drug to avoid physically uncomfortable or even medically harmful physical withdrawal symptoms.[90] Drugs that are both rewarding and reinforcing are addictive; these properties of a drug are mediated through activation of the mesolimbic dopamine pathway, particularly the nucleus accumbens. Not all addictive drugs are associated with physical dependence, e.g., amphetamine, and not all drugs that produce physical dependence are addictive drugs, e.g., oxymetazoline.
Globally, as of 2016, alcohol use disorders were the most prevalent of all substance use disorders (SUD) worldwide; cannabis dependence and opioid dependence were the next most prevalent SUDs.[91]
Many professionals, self-help groups, and businesses specialize in drug rehabilitation, with varying degrees of success, and many parents attempt to influence the actions and choices of their children regarding psychoactives.[92]
Common forms of rehabilitation include psychotherapy, support groups and pharmacotherapy, which uses psychoactive substances to reduce cravings and physiological withdrawal symptoms while a user is going through detox. Methadone, itself an opioid and a psychoactive substance, is a common treatment for heroin addiction, as is another opioid, buprenorphine. Recent research on addiction has shown some promise in using psychedelics such as ibogaine to treat and even cure drug addictions, although this has yet to become a widely accepted practice.[93][94]
Legality
[edit]
The legality of psychoactive drugs has been controversial through most of recent history; the Second Opium War and Prohibition are two historical examples of legal controversy surrounding psychoactive drugs. However, in recent years, the most influential document regarding the legality of psychoactive drugs is the Single Convention on Narcotic Drugs, an international treaty signed in 1961 as an Act of the United Nations. Signed by 73 nations including the United States, the USSR, Pakistan, India, and the United Kingdom, the Single Convention on Narcotic Drugs established Schedules for the legality of each drug and laid out an international agreement to fight addiction to recreational drugs by combatting the sale, trafficking, and use of scheduled drugs.[95] All countries that signed the treaty passed laws to implement these rules within their borders. However, some countries that signed the Single Convention on Narcotic Drugs, such as the Netherlands, are more lenient with their enforcement of these laws.[96]
In the United States, the Food and Drug Administration (FDA) has authority over all drugs, including psychoactive drugs. The FDA regulates which psychoactive drugs are over the counter and which are only available with a prescription.[97] However, certain psychoactive drugs, like alcohol, tobacco, and drugs listed in the Single Convention on Narcotic Drugs are subject to criminal laws. The Controlled Substances Act of 1970 regulates the recreational drugs outlined in the Single Convention on Narcotic Drugs.[98] Alcohol is regulated by state governments, but the federal National Minimum Drinking Age Act penalizes states for not following a national drinking age.[99] Tobacco is also regulated by all fifty state governments.[100] Most people accept such restrictions and prohibitions of certain drugs, especially the "hard" drugs, which are illegal in most countries.[101][102][103]
In the medical context, psychoactive drugs as a treatment for illness is widespread and generally accepted. Little controversy exists concerning over the counter psychoactive medications in antiemetics and antitussives. Psychoactive drugs are commonly prescribed to patients with psychiatric disorders. However, certain critics [who?] believe that certain prescription psychoactives, such as antidepressants and stimulants, are overprescribed and threaten patients' judgement and autonomy.[104][105]
Effect on animals
[edit]A number of animals consume different psychoactive plants, animals, berries and even fermented fruit, becoming intoxicated. An example of this is cats after consuming catnip. Traditional legends of sacred plants often contain references to animals that introduced humankind to their use.[106] Animals and psychoactive plants appear to have co-evolved, possibly explaining why these chemicals and their receptors exist within the nervous system.[107]
Widely used psychoactive drugs
[edit]This is a list of commonly used drugs that contain psychoactive ingredients. Please note that the following lists contains legal and illegal drugs (based on the country's laws).
Common legal drugs
[edit]The most widely consumed psychotropic drugs worldwide are:[108]
Common prescribed drugs
[edit]Common street drugs
[edit]See also
[edit]- Contact high
- Counterculture of the 1960s
- Demand reduction
- Designer drug
- Drug
- Drug addiction
- Drug checking
- Drug rehabilitation
- Hamilton's Pharmacopeia
- Hard and soft drugs
- Harm reduction
- Neuropsychopharmacology
- Psychopharmacology
- Poly drug use
- Project MKULTRA
- Psychedelic plants
- Psychoactive fish
- Recreational drug use
- Responsible drug use
- Self-medication
Notes
[edit]- ^ "New Psychoactive Substance", and "Novel Psychoactive Substance" (NPS) are often used interchangeably.
- ^ MDMA and Ketamine are not a classical psychedelics but are sometimes discussed alongside classical psychedelics due to similarities in their psychoactive and potentially therapeutic effects.[51]
References
[edit]- ^ Nehlig A, Daval JL, Debry G (1992). "Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects". Brain Research. Brain Research Reviews. 17 (2): 139–170. doi:10.1016/0165-0173(92)90012-B. PMID 1356551. S2CID 14277779.
- ^ Raman R, Jarrett RT, Cull MJ, Gracey K, Shaffer AM, Epstein RA (2021-03-01). "Psychopharmaceutical Prescription Monitoring for Children in the Child Welfare System". Psychiatric Services. 72 (3): 295–301. doi:10.1176/appi.ps.202000077. ISSN 1075-2730.
- ^ a b c "psychoactive substance". www.cancer.gov. 2011-02-02. Retrieved 2025-05-24.
- ^ "CHAPTER 1 Alcohol and Other Drugs". The Public Health Bush Book: Facts & approaches to three key public health issues. ISBN 0-7245-3361-3. Archived from the original on 2015-03-28.
- ^ Merlin, M.D. (2003). "Archaeological Evidence for the Tradition of Psychoactive Plant Use in the Old World". Economic Botany. 57 (3): 295–323. doi:10.1663/0013-0001(2003)057[0295:AEFTTO]2.0.CO;2. S2CID 30297486.
- ^ Early Holocene coca chewing in northern Peru Volume: 84 Number: 326 Page: 939–953
- ^ "Coca leaves first chewed 8,000 years ago, says research". BBC News. December 2, 2010. Archived from the original on May 23, 2014.
- ^ Siegel, Ronald K (2005). Intoxication: The Universal Drive for Mind-Altering Substances. Park Street Press, Rochester, Vermont. ISBN 1-59477-069-7.
- ^ Weil A (2004). The Natural Mind: A Revolutionary Approach to the Drug Problem (Revised ed.). Houghton Mifflin. p. 15. ISBN 0-618-46513-8.
- ^ The Hashish Eater (1857) pg. 181
- ^ "LEAP's Mission Statement". Law Enforcement Against Prohibition. Archived from the original on 2008-09-13. Retrieved 2013-05-30.
- ^ "5 Years After: Portugal's Drug Decriminalization Policy Shows Positive Results". Scientific American. Archived from the original on 2013-08-15. Retrieved 2013-05-30.
- ^ "HRB National Drugs Library: Psychoactive drug or substance". Health Research Board. 2024. Retrieved Jul 30, 2024.
- ^ Zanders ED (2011). "Introduction to Drugs and Drug Targets". The Science and Business of Drug Discovery. pp. 11–27. doi:10.1007/978-1-4419-9902-3_2. ISBN 978-1-4419-9901-6. PMC 7120710.
- ^ "New psychoactive substances (NPS) | www.emcdda.europa.eu". www.emcdda.europa.eu. Retrieved 2024-06-09.
- ^ Bersani FS, Corazza O, Simonato P, Mylokosta A, Levari E, Lovaste R, Schifano F (2013). "Drops of madness? Recreational misuse of tropicamide collyrium; early warning alerts from Russia and Italy". General Hospital Psychiatry. 35 (5): 571–3. doi:10.1016/j.genhosppsych.2013.04.013. PMID 23706777.
- ^ Lovett R (24 September 2005). "Coffee: The demon drink?" (fee required). New Scientist (2518). Archived from the original on 24 October 2007. Retrieved 2007-11-19.
- ^ Matson JL, Neal D (2009). "Psychotropic medication use for challenging behaviors in persons with intellectual disabilities: An overview". Research in Developmental Disabilities. 30 (3): 572–86. doi:10.1016/j.ridd.2008.08.007. PMID 18845418.
- ^ Schatzberg AF (2000). "New indications for antidepressants". The Journal of Clinical Psychiatry. 61 (11): 9–17. PMID 10926050.
- ^ Swift RM (2016). "Pharmacotherapy of Substance Use, Craving, and Acute Abstinence Syndromes". In Sher KJ (ed.). The Oxford Handbook of Substance Use and Substance Use Disorders. Oxford University Press. pp. 601–603, 606. ISBN 978-0-19-938170-8. Archived from the original on 2018-05-09.
- ^ Hirschfeld RM (2001). "Clinical importance of long-term antidepressant treatment". The British Journal of Psychiatry. 179 (42): S4–8. doi:10.1192/bjp.179.42.s4. PMID 11532820.
- ^ Stoker L (14 April 2013). "Analysis Creating Supermen: battlefield performance enhancing drugs". Army Technology. Verdict Media Limited. Retrieved 22 June 2018.
- ^ Kamienski L (2016-04-08). "The Drugs That Built a Super Soldier". The Atlantic. Retrieved 22 June 2018.
- ^
Jason Leopold, Jeffrey Kaye (2011-07-11). "EXCLUSIVE: DoD Report Reveals Some Detainees Interrogated While Drugged, Others "Chemically Restrained"". Truthout. Archived from the original on 2020-03-28.
Truthout obtained a copy of the report - "Investigation of Allegations of the Use of Mind-Altering Drugs to Facilitate Interrogations of Detainees" - prepared by the DoD's deputy inspector general for intelligence in September 2009, under a Freedom of Information Act (FOIA) request we filed nearly two years ago.
- ^
Robert Beckhusen (2012-07-11). "U.S. Injected Gitmo Detainees With 'Mind Altering' Drugs". Wired magazine. Archived from the original on 2012-07-13. Retrieved 2012-07-14.
That's according to a recently declassified report (.pdf) from the Pentagon's inspector general, obtained by Truthout's Jeffrey Kaye and Jason Leopold after a Freedom of Information Act Request. In it, the inspector general concludes that 'certain detainees, diagnosed as having serious mental health conditions being treated with psychoactive medications on a continuing basis, were interrogated.' The report does not conclude, though, that anti-psychotic drugs were used specifically for interrogation purposes.
- ^
Haroon Rashid (2003-05-23). "Pakistani relives Guantanamo ordeal". BBC News. Archived from the original on 2012-10-31. Retrieved 2009-01-09.
Mr Shah alleged that the Americans had given him injections and tablets prior to interrogations. "They used to tell me I was mad," the 23-year-old told the BBC in his native village in Dir district near the Afghan border. I was given injections at least four or five times as well as different tablets. I don't know what they were meant for."
- ^
"People the law forgot". The Guardian. 2003-12-03. Archived from the original on 2013-08-27. Retrieved 2012-07-14.
The biggest damage is to my brain. My physical and mental state isn't right. I'm a changed person. I don't laugh or enjoy myself much.
- ^ Jones E, Fear NT (April 2011). "Alcohol use and misuse within the military: a review". International Review of Psychiatry. 23 (2): 166–172. doi:10.3109/09540261.2010.550868. PMID 21521086.
- ^ "Aimo Allan Koivunen". www.sotapolku.fi (in Finnish). 2016. Retrieved January 17, 2022.
- ^ Rantanen M (28 May 2002). "Finland: History: Amphetamine Overdose In Heat Of Combat". www.mapinc.org. Helsingin Sanomat. Retrieved January 17, 2022.
- ^ Quiding H, Lundqvist G, Boréus LO, Bondesson U, Ohrvik J (1993). "Analgesic effect and plasma concentrations of codeine and morphine after two dose levels of codeine following oral surgery". European Journal of Clinical Pharmacology. 44 (4): 319–23. doi:10.1007/BF00316466. PMID 8513842. S2CID 37268044.
- ^ Medline Plus. Anesthesia. Accessed on July 16, 2007.
- ^ Li X, Pearce RA (2000). "Effects of halothane on GABA(A) receptor kinetics: evidence for slowed agonist unbinding". The Journal of Neuroscience. 20 (3): 899–907. doi:10.1523/JNEUROSCI.20-03-00899.2000. PMC 6774186. PMID 10648694.
- ^ Harrison NL, Simmonds MA (1985). "Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex". British Journal of Pharmacology. 84 (2): 381–91. doi:10.1111/j.1476-5381.1985.tb12922.x. PMC 1987274. PMID 2858237.
- ^ "Effects of Performance-Enhancing Drugs | USADA". May 2019.
- ^ Pesta DH, Angadi SS, Burtscher M, Roberts CK (December 2013). "The effects of caffeine, nicotine, ethanol, and tetrahydrocannabinol on exercise performance". Nutrition & Metabolism. 10 (1) 71. doi:10.1186/1743-7075-10-71. PMC 3878772. PMID 24330705.
- ^ Liddle DG, Connor DJ (June 2013). "Nutritional supplements and ergogenic AIDS". Primary Care. 40 (2): 487–505. doi:10.1016/j.pop.2013.02.009. PMID 23668655.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training ...
Physiologic and performance effects [of amphetamines]
• Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
• Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
• Improved reaction time
• Increased muscle strength and delayed muscle fatigue
• Increased acceleration
• Increased alertness and attention to task - ^ Frati P, Kyriakou C, Del Rio A, Marinelli E, Vergallo GM, Zaami S, Busardò FP (January 2015). "Smart drugs and synthetic androgens for cognitive and physical enhancement: revolving doors of cosmetic neurology". Current Neuropharmacology. 13 (1): 5–11. doi:10.2174/1570159X13666141210221750. PMC 4462043. PMID 26074739.
Cognitive enhancement can be defined as the use of drugs and/or other means with the aim to improve the cognitive functions of healthy subjects in particular memory, attention, creativity and intelligence in the absence of any medical indication. ... The first aim of this paper was to review current trends in the misuse of smart drugs (also known as Nootropics) presently available on the market focusing in detail on methylphenidate, trying to evaluate the potential risk in healthy individuals, especially teenagers and young adults.
- ^ Better Fighting Through Chemistry? The Role of FDA Regulation in Crafting the Warrior of the Future (Report). 8 March 2004. Archived from the original on 3 February 2016.
- ^ Neuroscience of Psychoactive Substance Use and Dependence Archived 2006-10-03 at the Wayback Machine by the World Health Organization. Retrieved 5 July 2007.
- ^ Anderson TL (1998). "Drug identity change processes, race, and gender. III. Macrolevel opportunity concepts". Substance Use & Misuse. 33 (14): 2721–35. doi:10.3109/10826089809059347. PMID 9869440.
- ^ Bertol E, Fineschi V, Karch SB, Mari F, Riezzo I (2004). "Nymphaea cults in ancient Egypt and the New World: a lesson in empirical pharmacology". Journal of the Royal Society of Medicine. 97 (2): 84–5. doi:10.1177/014107680409700214. PMC 1079300. PMID 14749409.
- ^ Hayry M (2004). "Prescribing cannabis: freedom, autonomy, and values". Journal of Medical Ethics. 30 (4): 333–6. doi:10.1136/jme.2002.001347. PMC 1733898. PMID 15289511.
- ^ Barnett, Randy E. "The Presumption of Liberty and the Public Interest: Medical Marijuana and Fundamental Rights" Archived 2007-07-11 at the Wayback Machine. Retrieved 4 July 2007.
- ^ a b Murrie B, Lappin J, Large M, Sara G (16 October 2019). "Transition of Substance-Induced, Brief, and Atypical Psychoses to Schizophrenia: A Systematic Review and Meta-analysis". Schizophrenia Bulletin. 46 (3): 505–516. doi:10.1093/schbul/sbz102. PMC 7147575. PMID 31618428.
- ^ Quilter J (2022). The Ancient Central Andes (2nd ed.). New York, NY: Routledge World Archaeology. pp. 38–39, 279. ISBN 978-0-367-48151-3.
- ^ Code of Canon Law, 1983 Archived 19 June 2006 at the Wayback Machine
- ^ El-Seedi HR, De Smet PA, Beck O, Possnert G, Bruhn JG (2005). "Prehistoric peyote use: alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas". Journal of Ethnopharmacology. 101 (1–3): 238–42. doi:10.1016/j.jep.2005.04.022. PMID 15990261.
- ^ Vetulani J (2001). "Drug addiction. Part I. Psychoactive substances in the past and presence". Polish Journal of Pharmacology. 53 (3): 201–14. PMID 11785921.
- ^ Bullis RK (1990). "Swallowing the scroll: legal implications of the recent Supreme Court peyote cases". Journal of Psychoactive Drugs. 22 (3): 325–32. doi:10.1080/02791072.1990.10472556. PMID 2286866.
- ^ Nutt D (2019). "Psychedelic drugs-a new era in psychiatry?". Dialogues in Clinical Neuroscience. 21 (2): 139–147. doi:10.31887/DCNS.2019.21.2/dnutt. PMC 6787540. PMID 31636488.
- ^ Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, et al. (May 2020). "Psychedelics and Psychedelic-Assisted Psychotherapy". The American Journal of Psychiatry. 177 (5): 391–410. doi:10.1176/appi.ajp.2019.19010035. PMID 32098487. S2CID 211524704.
- ^ a b Marks M, Cohen IG (October 2021). "Psychedelic therapy: a roadmap for wider acceptance and utilization". Nature Medicine. 27 (10): 1669–1671. doi:10.1038/s41591-021-01530-3. PMID 34608331. S2CID 238355863.
- ^ Pilecki B, Luoma JB, Bathje GJ, Rhea J, Narloch VF (April 2021). "Ethical and legal issues in psychedelic harm reduction and integration therapy". Harm Reduction Journal. 18 (1) 40. doi:10.1186/s12954-021-00489-1. PMC 8028769. PMID 33827588.
- ^ Blom JD (2009). A Dictionary of Hallucinations. Springer. p. 434. ISBN 978-1-4419-1222-0. Retrieved 2010-03-05.
- ^ UK Institute of Psychonautics and Somanautics page Archived 10 November 2010 at the Wayback Machine at his "Academy for Transpersonal Studies". Archived from the original on 23 September 2010. Retrieved 10 March 2010.
- ^ "What is self-Medication?". World Self-Medication Industry. Archived from the original on Jun 5, 2016. Retrieved 25 May 2016.
- ^ Joshua Clark Davis. (November 6, 2014). The Long Marijuana-Rights Movement. Archived September 11, 2016, at the Wayback Machine The Huffington Post. Retrieved August 3, 2016.
- ^ King M (April 24, 2007). "Thousands at UCSC burn one to mark cannabis holiday". Santa Cruz Sentinel. Archived from the original on April 26, 2007.
- ^ Halnon KB (April 11, 2005). "The power of 420". Archived from the original on May 13, 2013.
- ^ "420 event lists".
- ^ King M (April 24, 2007). "Thousands at UCSC burn one to mark cannabis holiday". Santa Cruz Sentinel. Archived from the original on April 26, 2007.
- ^ McCoy T (2014-04-18). "The strange story of how the pot holiday '4/20' got its name". The Washington Post. Retrieved 2020-04-18.
- ^ Karl Grauers (May 18, 2009). "Cannabis i Malmös blomlådor – igen" [Cannabis in Malmö flower boxes – again]. Metro. Stockholm, Sweden. Archived from the original on May 11, 2017.
- ^ "Suicides in England and Wales – Office for National Statistics". www.ons.gov.uk.
- ^ a b Conner A, Azrael D, Miller M (3 December 2019). "Suicide Case-Fatality Rates in the United States, 2007 to 2014" (PDF). Annals of Internal Medicine. 171 (12): 885–895. doi:10.7326/M19-1324. PMID 31791066. S2CID 208611916. Archived from the original (PDF) on 28 January 2021.
Table 1. Suicide Deaths, Nonfatal Suicide Attempts, and Total Suicidal Acts, Overall and by [...] Method [(Table contents' transcription:) Out of 3,657,886 recorded suicide attempts, 309,377 (8.46%) resulted in deaths; 2,171,482 of these suicidal acts are based on drug poisoning, 41,758 (1.92%) of which resulted in deaths.]
- ^ Youssef NA, Rich CL (2008). "Does acute treatment with sedatives/hypnotics for anxiety in depressed patients affect suicide risk? A literature review". Annals of Clinical Psychiatry. 20 (3): 157–169. doi:10.1080/10401230802177698. PMID 18633742.
- ^ a b Vijayakumar L, Kumar MS, Vijayakumar V (May 2011). "Substance use and suicide". Current Opinion in Psychiatry. 24 (3): 197–202. doi:10.1097/YCO.0b013e3283459242. PMID 21430536. S2CID 206143129.
- ^ a b Sher L (January 2006). "Alcohol consumption and suicide". QJM. 99 (1): 57–61. doi:10.1093/qjmed/hci146. PMID 16287907.
- ^ Sher L (2007). "Functional magnetic resonance imaging in studies of the neurobiology of suicidal behavior in adolescents with alcohol use disorders". International Journal of Adolescent Medicine and Health. 19 (1): 11–18. doi:10.1515/ijamh.2007.19.1.11. PMID 17458319. S2CID 42672912.
- ^ Brock A, Sini Dominy, Clare Griffiths (6 November 2003). "Trends in suicide by method in England and Wales, 1979 to 2001". Health Statistics Quarterly. 20: 7–18. ISSN 1465-1645. Retrieved 2007-06-25.
- ^ United States Food and Drug Administration. CDER Data Standards Manual Archived 2006-01-03 at the Wayback Machine. Retrieved on May 15, 2007.
- ^ Timothy Leary; Ralph Metzner; Richard Alpert. The Psychedelic Experience. New York: University Books. 1964
- ^ Ratsch, Christian (May 5, 2005). The Encyclopedia of Psychoactive Plants. Park Street Press. p. 944. ISBN 0-89281-978-2.
- ^ Seligma ME (1984). "4". Abnormal Psychology. W. W. Norton & Company. ISBN 0-393-94459-X.
- ^ "University of Texas, Addiction Science Research and Education Center". Archived from the original on August 14, 2011. Retrieved May 14, 2007.
- ^ Lüscher C, Ungless MA (2006). "The mechanistic classification of addictive drugs". PLOS Medicine. 3 (11): e437. doi:10.1371/journal.pmed.0030437. PMC 1635740. PMID 17105338.
- ^ Ford, Marsha. Clinical Toxicology. Philadelphia: Saunders, 2001. Chapter 36 – Caffeine and Related Nonprescription Sympathomimetics. ISBN 0-7216-5485-1[page needed]
- ^ "Supplements Accelerate Benzodiazepine Withdrawal: A Case Report and Biochemical Rationale". Archived from the original on 2016-08-16. Retrieved 2016-07-31.[full citation needed]
- ^ Di Carlo G, Borrelli F, Ernst E, Izzo AA (2001). "St John's wort: Prozac from the plant kingdom". Trends in Pharmacological Sciences. 22 (6): 292–7. doi:10.1016/S0165-6147(00)01716-8. PMID 11395157.
- ^ Glick SD, Maisonneuve IM (1998). "Mechanisms of Antiaddictive Actions of Ibogainea". Annals of the New York Academy of Sciences. 844 (1): 214–26. Bibcode:1998NYASA.844..214G. doi:10.1111/j.1749-6632.1998.tb08237.x. PMID 9668680. S2CID 11416176.
- ^ Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A (2003-03-20). "Modafinil increases histamine release in the anterior hypothalamus of rats". Neuroscience Letters. 339 (2): 143–146. doi:10.1016/s0304-3940(03)00006-5. ISSN 0304-3940. PMID 12614915. S2CID 42291148.
- ^ Herraiz T, Chaparro C (2006). "Human monoamine oxidase enzyme inhibition by coffee and β-carbolines norharman and harman isolated from coffee". Life Sciences. 78 (8): 795–802. doi:10.1016/j.lfs.2005.05.074. PMID 16139309.
- ^ Dhingra D, Kumar V (2008). "Evidences for the involvement of monoaminergic and GABAergic systems in antidepressant-like activity of garlic extract in mice". Indian Journal of Pharmacology. 40 (4): 175–9. doi:10.4103/0253-7613.43165. PMC 2792615. PMID 20040952.
- ^ Gerrard P, Malcolm R (June 2007). "Mechanisms of modafinil: A review of current research". Neuropsychiatric Disease and Treatment. 3 (3): 349–364. ISSN 1176-6328. PMC 2654794. PMID 19300566.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–375. ISBN 9780071481274.
- ^ Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues in Clinical Neuroscience. 15 (4): 431–443. PMC 3898681. PMID 24459410.
Despite the importance of numerous psychosocial factors, at its core, drug addiction involves a biological process: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type [nucleus accumbens] neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement ... Another ΔFosB target is cFos: as ΔFosB accumulates with repeated drug exposure it represses c-Fos and contributes to the molecular switch whereby ΔFosB is selectively induced in the chronic drug-treated state.41. ... Moreover, there is increasing evidence that, despite a range of genetic risks for addiction across the population, exposure to sufficiently high doses of a drug for long periods of time can transform someone who has relatively lower genetic loading into an addict.
- ^ Volkow ND, Koob GF, McLellan AT (January 2016). "Neurobiologic Advances from the Brain Disease Model of Addiction". New England Journal of Medicine. 374 (4): 363–371. doi:10.1056/NEJMra1511480. PMC 6135257. PMID 26816013.
Substance-use disorder: A diagnostic term in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) referring to recurrent use of alcohol or other drugs that causes clinically and functionally significant impairment, such as health problems, disability, and failure to meet major responsibilities at work, school, or home. Depending on the level of severity, this disorder is classified as mild, moderate, or severe.
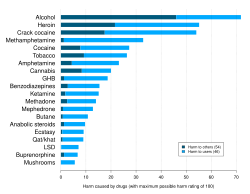
Addiction: A term used to indicate the most severe, chronic stage of substance-use disorder, in which there is a substantial loss of self-control, as indicated by compulsive drug taking despite the desire to stop taking the drug. In the DSM-5, the term addiction is synonymous with the classification of severe substance-use disorder. - ^ Nutt D, King LA, Saulsbury W, Blakemore C (2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831. S2CID 5903121.
- ^ Johnson B (2003). "Psychological Addiction, Physical Addiction, Addictive Character, and Addictive Personality Disorder: A Nosology of Addictive Disorders". Canadian Journal of Psychoanalysis. 11 (1): 135–60. OCLC 208052223.
- ^ Degenhardt L, et al. (GBD 2016 Alcohol and Drug Use Collaborators) (December 2018). "The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016". The Lancet. Psychiatry. 5 (12): 987–1012. doi:10.1016/S2215-0366(18)30337-7. PMC 6251968. PMID 30392731.
- ^ Hops H, Tildesley E, Lichtenstein E, Ary D, Sherman L (2009). "Parent-Adolescent Problem-Solving Interactions and Drug Use". The American Journal of Drug and Alcohol Abuse. 16 (3–4): 239–58. doi:10.3109/00952999009001586. PMID 2288323.
- ^ "Psychedelics Could Treat Addiction Says Vancouver Official". 2006-08-09. Archived from the original on December 2, 2006. Retrieved March 26, 2007.
- ^ "Ibogaine research to treat alcohol and drug addiction". Archived from the original on April 22, 2007. Retrieved March 26, 2007.
- ^ United Nations Single Convention on Narcotic Drugs. Archived 2008-05-10 at the Wayback Machine Retrieved on June 20, 2007.
- ^ MacCoun R, Reuter P (1997). "Interpreting Dutch Cannabis Policy: Reasoning by Analogy in the Legalization Debate". Science. 278 (5335): 47–52. doi:10.1126/science.278.5335.47. PMID 9311925.
- ^ History of the Food and Drug Administration. Retrieved at FDA's website Archived 2009-01-19 at the Wayback Machine on June 23, 2007.
- ^ United States Controlled Substances Act of 1970. Retrieved from the DEA's website Archived 2009-05-08 at the Wayback Machine on June 20, 2007.
- ^ Title 23 of the United States Code, Highways. Archived 2007-06-14 at the Wayback Machine Retrieved on June 20, 2007.
- ^ Taxadmin.org. State Excise Tax Rates on Cigarettes. Archived 2009-11-09 at the Wayback Machine Retrieved on June 20, 2007.
- ^ "What's your poison?". Caffeine. Archived from the original on July 26, 2009. Retrieved July 12, 2006.
- ^ Griffiths RR (1995). Psychopharmacology: The Fourth Generation of Progress (4th ed.). Lippincott Williams & Wilkins. p. 2002. ISBN 0-7817-0166-X.
- ^ Edwards G (2005). Matters of Substance: Drugs—and Why Everyone's a User. Thomas Dunne Books. p. 352. ISBN 0-312-33883-X.
- ^ Dworkin, Ronald. Artificial Happiness. New York: Carroll & Graf, 2006. pp.2–6. ISBN 0-7867-1933-8
- ^ Manninen BA (2006). "Medicating the mind: A Kantian analysis of overprescribing psychoactive drugs". Journal of Medical Ethics. 32 (2): 100–5. doi:10.1136/jme.2005.013540. PMC 2563334. PMID 16446415.
- ^ Samorini G (2002). Animals And Psychedelics: The Natural World & The Instinct To Alter Consciousness. Park Street Press. ISBN 0-89281-986-3.
- ^ Albert, David Bruce Jr. (1993). "Event Horizons of the Psyche". Archived from the original on September 27, 2006. Retrieved February 2, 2006.
- ^ Crocq MA (June 2003). "Alcohol, nicotine, caffeine, and mental disorders". Dialogues in Clinical Neuroscience. 5 (2): 175–185. doi:10.31887/DCNS.2003.5.2/macrocq. PMC 3181622. PMID 22033899.
External links
[edit]Psychoactive drug
View on GrokipediaDefinition and Terminology
Core Concepts and Distinctions
A psychoactive drug is a chemical substance that alters brain function, resulting in temporary modifications to perception, mood, consciousness, cognition, or behavior, primarily through interactions with the central nervous system (CNS).[2][6] These effects arise from the drug's ability to cross the blood-brain barrier and modulate neuronal signaling, distinguishing psychoactive agents from non-psychoactive pharmaceuticals like antibiotics or peripheral analgesics, which exert minimal direct influence on mental processes.[7] At the neural level, core mechanisms involve interference with neurotransmitter systems—such as enhancing release, blocking reuptake, or agonizing/antagonizing receptors for dopamine, serotonin, GABA, or glutamate—leading to amplified or suppressed synaptic transmission.[8][9] This causal pathway underpins both therapeutic applications, like antidepressants modulating serotonin reuptake, and adverse outcomes, such as dependence from repeated dopamine surges induced by stimulants.[10] Effects are dose-dependent and context-sensitive, varying by individual neurochemistry, genetics, and environmental factors, with low doses often yielding subtle enhancements in alertness (e.g., caffeine via adenosine antagonism) and higher doses risking profound disruptions like hallucinations or sedation.[11][12] The terms "psychoactive" and "psychotropic" are frequently used interchangeably, though "psychotropic" more narrowly denotes substances prescribed for psychiatric conditions, such as antipsychotics or mood stabilizers that target specific psychopathologies rather than broadly recreational or cognitive alterations.[13][14] Unlike endogenous neuromodulators (e.g., endocannabinoids), psychoactive drugs are exogenous compounds, often synthetic or plant-derived, capable of inducing tolerance through adaptive receptor downregulation, which escalates required doses for equivalent effects over time.[15] This distinction highlights their potential for misuse, as repeated exposure can rewire reward circuits, fostering compulsive use independent of initial intent.[9] Psychoactive effects exclude mere physiological changes without CNS mediation, such as peripheral vasoconstriction from non-CNS-penetrating agents.[16]Etymology and Evolving Usage
The term "psychoactive" combines the prefix "psycho-," derived from the Greek psychē meaning "soul" or "mind," with "active," denoting substances that influence mental processes or states; it first appeared in English in 1959 in pharmacological contexts.[17] The word "drug" traces its origins to the Middle English drogge around the 14th century, referring to dried herbal substances used medicinally, with earlier roots possibly in Old French drogue or Middle Low German droppe implying barrel-stored commodities, though its foundational sense linked to desiccated plants for therapeutic application.[18] Together, "psychoactive drug" thus encapsulates agents—typically chemical compounds—that alter brain function, perception, mood, or cognition, distinguishing them from inert or purely physiological remedies. Prior to the mid-20th century, terminology for such substances emphasized observable effects rather than neural mechanisms, with terms like "narcotic" (from Greek narkōtikos, meaning "benumbing," coined in the 17th century for opium derivatives inducing stupor) or "stimulant" (emerging in the 18th century for coffee and tobacco's invigorating properties) dominating discourse.[18] Ancient and medieval references often employed broad descriptors such as the Greek pharmakon (ambiguously signifying remedy, poison, or spell-casting agent) or Latin venenum (poison with psychoactive implications), reflecting cultural views of mind-altering plants like mandrake or henbane as mystical or hazardous rather than categorically "psychoactive." These labels carried moral or empirical freight, with little standardization until pharmaceutical isolation of active principles, such as morphine from opium in 1804, shifted focus toward chemical specificity.[19] The modern usage of "psychoactive drug" crystallized in the 1950s amid psychopharmacology's rise, following discoveries like lysergic acid diethylamide (LSD) in 1943 and chlorpromazine's antipsychotic effects in 1952, enabling classification by neurotransmitter interactions rather than crude phenomenology.[20] This evolution paralleled regulatory frameworks, as seen in the World Health Organization's adoption of the term to denote substances impacting mental processes like perception or consciousness, encompassing both therapeutic agents (e.g., antidepressants) and recreational ones (e.g., cannabis).[8] By the 1960s, amid LSD's cultural diffusion, narrower terms like "psychedelic" (coined in 1956 from Greek psychē + dēloun, "mind-revealing") emerged for hallucinogens, while "psychotomimetic" briefly described psychosis-mimicking effects before fading due to pejorative connotations; "psychoactive" persisted as the umbrella term, later extending to "new psychoactive substances" in European Union policy from 2005 for synthetic analogs evading bans.[21][22] This progression reflects a causal shift from anecdotal effect-labeling to evidence-based neuroscientific categorization, though legacy terms like "narcotic" endure in legal contexts despite imprecise overlap with true psychoactivity.[23]Historical Context
Ancient and Traditional Applications
Archaeological evidence indicates that the consumption of psychoactive substances began in the Neolithic period, with fermented alcoholic beverages produced as early as 7000 BCE in sites such as Jiahu, China, where residue analysis of pottery revealed rice, honey, and fruit-based brews likely used for ritual and social purposes.[24] Opium poppy cultivation emerged around 3400 BCE in Mesopotamia, where Sumerians referred to it as the "hul gil" or "plant of joy" in clay tablets, employing its latex for pain relief and possibly in priestly rituals, as evidenced by residue in ceremonial vessels from 1600–1000 BCE.[25][26] In ancient China, cannabis seeds and fibers appear in archaeological contexts dating to approximately 8000 BCE in the Oki Islands near Japan, though systematic medicinal use is documented from 2737 BCE in texts attributed to Emperor Shen Nung, who prescribed it for ailments like rheumatism and absent-mindedness; by the second century CE, surgeon Hua T'o combined cannabis resin with wine as an anesthetic.[27] Alcoholic fermentation, including rice wine, paralleled these developments, with residues confirming production around 7000 BCE for communal and ceremonial intoxication.[28] Vedic India featured soma, a ritual elixir central to Rigveda hymns from circa 1500 BCE, prepared from a plant (identity debated but likely psychoactive, possibly involving ephedra or mushrooms) pressed into juice, mixed with milk or honey, and consumed by priests for visionary states and divine communion; cannabis (bhang) later integrated into Ayurvedic medicine for treating epilepsy and mental disorders.[29][30] In Mesoamerica, psilocybin mushrooms (teonanácatl, or "flesh of the gods") were used by Aztecs in religious festivals for divination and prophecy, with artifacts like mushroom stones from pre-Columbian sites suggesting ritual continuity; peyote cactus, containing mescaline, appears in shamanic practices from at least 3000 BCE, though evidence ties it more firmly to North American indigenous groups for healing and spiritual visions.[31][32] Andean civilizations, including the Inca, revered coca leaves (Erythroxylum coca) from around 3000 BCE, chewing them with lime for stamina against altitude sickness, hunger suppression, and ritual offerings to deities, as mummies and textiles from 1000 BCE sites contain coca residues; this practice blended medicinal (for gastrointestinal issues) and spiritual roles, viewing the plant as a divine gift.[33][34]Modern Pharmacological Advancements
![Zoloft_bottles.jpg][float-right]Modern psychopharmacology originated with the synthesis of chlorpromazine in 1950, first demonstrated to have antipsychotic properties in clinical trials in 1952, and approved by the U.S. Food and Drug Administration (FDA) in 1954 as the inaugural effective treatment for schizophrenia.[35][36] This phenothiazine derivative acted primarily as a dopamine D2 receptor antagonist, profoundly alleviating positive symptoms like hallucinations and delusions, which facilitated the deinstitutionalization of hundreds of thousands of patients from psychiatric hospitals in subsequent decades.[37] The 1950s and 1960s saw further foundational developments, including the introduction of imipramine in 1959 as the prototype tricyclic antidepressant (TCA), which inhibited reuptake of serotonin and norepinephrine to treat major depressive disorder.[38] Lithium carbonate, recognized for stabilizing mood in bipolar disorder around the same period, provided the first specific pharmacological option for manic episodes, though its narrow therapeutic index necessitated careful monitoring.[39] Benzodiazepines, such as chlordiazepoxide approved in 1960, advanced anxiolytic therapy by enhancing GABA_A receptor activity, offering safer alternatives to barbiturates for short-term anxiety and insomnia management.[39] The 1980s and 1990s brought selective serotonin reuptake inhibitors (SSRIs), exemplified by fluoxetine's FDA approval in December 1987, which selectively targeted serotonin transporters with fewer anticholinergic and cardiovascular side effects than TCAs, transforming depression treatment into a more accessible outpatient paradigm.[40] Atypical antipsychotics like clozapine, reintroduced in the U.S. in 1990 after earlier European use, improved outcomes for treatment-resistant schizophrenia by additionally blocking serotonin 5-HT2A receptors, thereby mitigating extrapyramidal symptoms while addressing negative symptoms more effectively than typical agents.[36] These second-generation drugs dominated the market due to lower tardive dyskinesia risk, though metabolic adverse effects emerged as a concern.[39] Into the 21st century, rapid-acting agents like esketamine, an NMDA receptor antagonist approved by the FDA in 2019 for treatment-resistant depression, demonstrated antidepressant effects within hours via glutamatergic modulation and synaptic plasticity enhancement, diverging from monoamine-based mechanisms.[41] Concurrently, pharmacogenomic insights, such as CYP2D6 polymorphisms influencing antipsychotic metabolism, enabled personalized dosing to minimize adverse reactions and optimize efficacy.[42] A renaissance in psychedelic research since the early 2000s has yielded promising data on serotonergic hallucinogens; for instance, psilocybin, studied in landmark trials from 2006 onward at institutions like Johns Hopkins, produced rapid, durable reductions in depression symptoms through 5-HT2A agonism and neuroplasticity promotion, earning FDA breakthrough therapy status in 2018.[43][44] MDMA similarly received breakthrough designation in 2017 for PTSD, with phase 3 trials indicating substantial symptom relief via enhanced emotional processing and oxytocin release.[45] In 2024, the FDA approved xanomeline-trospium (Cobenfy), the first antipsychotic in over 30 years to eschew primary dopamine antagonism in favor of muscarinic M1/M4 receptor agonism, offering efficacy against positive and negative symptoms with potentially fewer weight gain and sedation issues associated with prior classes.[46][36] These innovations underscore a shift toward mechanism-specific, multimodal targeting informed by neuroimaging and receptor polypharmacology, though challenges persist in translating preclinical promise to broad clinical utility amid regulatory and ethical hurdles.[47][48]
Regulatory and Cultural Shifts
In the early 20th century, regulatory frameworks emerged to curb unregulated access to psychoactive substances amid growing concerns over addiction and public health. The U.S. Pure Food and Drug Act of 1906 required labeling of active ingredients like opiates and cocaine in patent medicines, marking the first federal oversight of drug contents.[49] This was followed by the Harrison Narcotics Tax Act of 1914, which imposed taxes and registration on opium and coca products, effectively restricting medical and non-medical distribution while prioritizing enforcement against recreational use.[49] Internationally, the 1912 Hague Opium Convention laid groundwork for global controls, influencing subsequent treaties by committing signatories to limit narcotic exports and domestic trafficking.[50] Post-World War II, multilateral efforts intensified through United Nations conventions, standardizing prohibitions while allowing limited medical exceptions. The 1961 Single Convention on Narcotic Drugs consolidated prior agreements, classifying substances like opium, cannabis, and cocaine into schedules based on abuse potential and therapeutic value, ratified by over 180 countries and binding production quotas to estimated medical needs.[51] The 1971 Convention on Psychotropic Substances extended controls to synthetics such as LSD, amphetamines, and barbiturates, scheduling them similarly despite varying evidence of harm, with compliance enforced via international monitoring.[52] The 1988 Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances further criminalized money laundering and precursor chemicals, emphasizing extradition and asset seizure, though critics note these treaties prioritized supply suppression over demand reduction, correlating with persistent global production levels.[53][52] In the United States, the 1970 Controlled Substances Act categorized drugs into five schedules under federal law, prohibiting non-medical possession and distribution, with cannabis placed in Schedule I alongside heroin despite prior medical endorsements.[54] President Richard Nixon's 1971 declaration of a "War on Drugs" escalated enforcement, allocating billions to interdiction and treatment while tying federal aid to anti-drug policies, resulting in over 1.5 million annual arrests by the 2020s and disproportionate incarceration rates among minorities, though usage rates remained stable or rose.[55][56] Subsequent administrations amplified this via mandatory minimums and asset forfeiture, peaking U.S. prison populations at 2.3 million by 2008, yet empirical data indicate limited impact on prevalence, with overdose deaths climbing from 6,000 in 1980 to over 100,000 by 2022.[55][56] Cultural attitudes oscillated between acceptance and prohibition, reflecting evolving perceptions of risk and utility. Ancient societies integrated substances like opium and cannabis into rituals and medicine with minimal stigma, but 19th-century temperance movements vilified alcohol and narcotics as moral failings, influencing early bans.[57] The 1960s counterculture, fueled by figures like Timothy Leary advocating LSD for consciousness expansion, challenged taboos and spurred recreational experimentation, yet provoked backlash framing psychedelics as societal threats.[58] Recent decades have witnessed liberalization, driven by evidence of therapeutic potential and critiques of prohibition's costs. California's 1996 Proposition 215 pioneered state-level medical cannabis legalization, defying federal law and expanding to 38 states by 2023; recreational markets followed in Colorado and Washington in 2012, reaching 24 states plus D.C. by 2025, generating $30 billion in annual sales while reducing arrests by 90% in some jurisdictions.[59] Psychedelics have seen decriminalization in Denver (2019), Oregon (2020 via Measure 109 for psilocybin therapy), and cities like Oakland and Seattle, with clinical trials showing efficacy for depression and PTSD, though long-term societal impacts remain understudied amid rising non-medical use.[60] These shifts parallel a cultural pivot toward harm reduction and medicalization, with public support for cannabis reform surpassing 70% in U.S. polls by 2023, yet raising concerns over youth access and dependency normalization.[61][62]Classification and Pharmacology
Major Chemical and Functional Classes
Psychoactive drugs are categorized into major functional classes based on their predominant effects on the central nervous system (CNS), such as sedation, stimulation, analgesia, or perceptual alteration, often tied to specific neurotransmitter modulation.[63] These functional groupings reflect empirical observations of behavioral and physiological outcomes, with mechanisms verified through pharmacological studies.[18] Chemical classifications complement this by grouping compounds sharing structural similarities, which predict shared pharmacodynamics, as seen in peer-reviewed analyses of substance classes.[64] Depressants, also termed sedatives or anxiolytics, primarily enhance inhibitory neurotransmission, particularly via gamma-aminobutyric acid (GABA) receptor agonism, leading to reduced neuronal excitability, slowed cognition, and motor impairment.[63] Common examples include ethanol, which potentiates GABA-A receptor activity while inhibiting glutamate; barbiturates like phenobarbital, which prolong GABA channel opening; and benzodiazepines such as diazepam, which bind allosteric sites on GABA-A receptors to increase chloride influx.[65] Chemically, many fall into barbiturate or 1,4-benzodiazepine structures, though alcohol represents a distinct organic solvent class.[64] Stimulants elevate CNS arousal by promoting catecholamine release or reuptake inhibition, primarily dopamine and norepinephrine, resulting in heightened alertness, euphoria, and cardiovascular activation.[3] Amphetamines, such as methamphetamine, are phenethylamine derivatives that reverse monoamine transporters to flood synapses with neurotransmitters.[66] Cocaine, a tropane alkaloid from coca leaves, blocks dopamine reuptake via transporter inhibition, with effects peaking within minutes of administration.[63] Caffeine, a xanthine alkaloid, antagonizes adenosine receptors to indirectly boost arousal, distinct from sympathomimetics but sharing stimulant outcomes./06:_The_Effects_of_Psychoactive_Drugs/6.01:_Psychopharmacology_and_Psychoactive_Drug_Classification) Opioids mimic endogenous endorphins by agonizing mu, delta, or kappa receptors, suppressing pain signals and inducing respiratory depression alongside euphoria./11:_Nervous_System/11.8:_Psychoactive_Drugs) Natural alkaloids like morphine and codeine derive from opium poppy (Papaver somniferum), while semi-synthetics such as heroin (diacetylmorphine) and synthetics like fentanyl feature benzylisoquinoline or piperidine cores.[64] These bind G-protein-coupled receptors to inhibit adenylyl cyclase and hyperpolarize neurons, with fentanyl's potency—up to 100 times that of morphine—stemming from its high lipophilicity and receptor affinity.[63] Hallucinogens disrupt sensory processing, often through serotonin 5-HT2A receptor agonism, producing perceptual distortions and altered thought patterns without primary sedation or stimulation.[67] Classic psychedelics like lysergic acid diethylamide (LSD), a semi-synthetic ergoline from ergot alkaloids, and psilocybin, an indole alkaloid from mushrooms, activate cortical 5-HT2A sites to enhance glutamatergic signaling.[64] Dissociative hallucinogens, such as ketamine (an arylcyclohexylamine), antagonize NMDA glutamate receptors, inducing detachment and analgesia via non-competitive channel blockade.[66]| Functional Class | Primary Mechanism | Key Chemical Subgroups | Examples |
|---|---|---|---|
| Depressants | GABA enhancement, glutamate inhibition | Barbiturates, benzodiazepines, alcohols | Ethanol, diazepam, phenobarbital[65] |
| Stimulants | Catecholamine release/reuptake block | Phenethylamines, tropanes, xanthines | Amphetamine, cocaine, caffeine[66] |
| Opioids | Mu-opioid receptor agonism | Opium alkaloids, piperidines | Morphine, fentanyl, heroin[64] |
| Hallucinogens | 5-HT2A agonism or NMDA antagonism | Ergolines, indoles, arylcyclohexylamines | LSD, psilocybin, ketamine[67] |
Mechanisms of Action on the Brain
Psychoactive drugs exert their effects primarily by modulating synaptic transmission between neurons in the brain, interfering with the release, reuptake, or receptor binding of neurotransmitters.[9] These substances can mimic neurotransmitters, block their transporters, activate or inhibit receptors, or alter ion channel function, thereby disrupting normal neural signaling pathways essential for perception, mood, cognition, and behavior.[69] Such interactions often target key brain regions like the mesolimbic dopamine pathway, prefrontal cortex, and limbic system, leading to amplified or suppressed activity in reward, inhibitory, or excitatory circuits.[70] Drugs acting on monoaminergic systems, including dopamine, serotonin, and norepinephrine, commonly enhance extracellular neurotransmitter levels by inhibiting reuptake transporters or promoting release. For example, cocaine binds to the dopamine transporter (DAT), preventing dopamine reuptake and causing accumulation in the synaptic cleft, which heightens dopaminergic signaling in the nucleus accumbens and contributes to euphoria and reinforcement.[71] Amphetamines similarly elevate dopamine by reversing DAT function and blocking vesicular monoamine transporter 2 (VMAT2), facilitating cytoplasmic dopamine release.[72] Serotonergic drugs like selective serotonin reuptake inhibitors (SSRIs) block the serotonin transporter (SERT), prolonging serotonin availability at synapses to modulate mood regulation, though chronic use induces adaptive receptor downregulation.[73] Inhibitory neurotransmitter systems, particularly GABAergic pathways, are targeted by anxiolytics and sedatives that potentiate GABA_A receptor function, increasing chloride influx and hyperpolarizing neurons to suppress excitability. Benzodiazepines bind at the GABA_A receptor's benzodiazepine site, enhancing GABA affinity without directly activating the receptor, resulting in reduced neural firing across widespread circuits.[9] Opioids, such as morphine, activate mu-opioid receptors to inhibit presynaptic neurotransmitter release via G-protein-coupled mechanisms, diminishing pain signaling and inducing analgesia alongside respiratory depression.[69] Excitatory glutamatergic transmission is modulated by dissociative anesthetics like ketamine, which non-competitively antagonize NMDA receptors, blocking glutamate-induced calcium influx and disrupting cortical integration, potentially underlying hallucinatory effects and rapid antidepressant actions through enhanced AMPA signaling.[74] Psychedelics, including LSD and psilocybin, primarily agonize serotonin 5-HT_{2A} receptors in the cortex, altering sensory processing and promoting neural plasticity via downstream beta-arrestin and mTOR pathways.[74] Cannabinoids like THC bind CB1 receptors to inhibit adenylate cyclase and voltage-gated calcium channels, reducing neurotransmitter release in a retrograde manner and affecting memory and appetite circuits.[75] Cholinergic systems are influenced by nicotine, which activates nicotinic acetylcholine receptors, depolarizing postsynaptic neurons and enhancing dopamine release in reward areas, fostering dependence through repeated stimulation.[73] Across these systems, drugs often converge on dopamine modulation for reinforcing effects, but specificity arises from receptor subtype selectivity and regional distribution, explaining diverse pharmacological profiles from stimulation to sedation.[70] Long-term exposure triggers neuroadaptations, such as receptor desensitization or altered gene expression, which underpin tolerance and withdrawal.[76]Pharmacokinetics and Routes of Administration
Psychoactive drugs undergo absorption, distribution, metabolism, and excretion (ADME), processes that govern their concentration in the brain and thus the onset, intensity, and duration of psychoactive effects.[77] Absorption efficiency depends on the route of administration and drug formulation, with bioavailability ranging from near 100% for intravenous routes to lower values for oral due to first-pass hepatic metabolism.[78] Distribution to the central nervous system requires crossing the blood-brain barrier, a selective endothelial layer permeable primarily to lipophilic substances, enabling rapid equilibration for most psychoactive agents via passive diffusion.[77] Metabolism, chiefly in the liver via cytochrome P450 enzymes, biotransforms these drugs into polar metabolites for excretion, though individual variability from genetic polymorphisms can prolong effects in slow metabolizers.[77] Excretion occurs predominantly through renal filtration, with half-lives varying widely; for instance, many stimulants exhibit short durations under 1 hour, while some antidepressants persist for days.[77] Routes of administration profoundly influence pharmacokinetics by altering absorption speed and bypassing barriers like first-pass metabolism, thereby affecting abuse liability through rapid brain delivery.[78] Intravenous injection provides immediate bioavailability and onset within seconds, ideal for potent effects but carrying risks of infection and overdose.[78] Inhalation, common for volatiles like nicotine or cannabinoids, leverages the lung's vast surface for rapid absorption (10-30 seconds to brain), achieving high bioavailability (up to 50-90% for some gases) while avoiding gastrointestinal degradation.[77][78] Oral ingestion, used for many pharmaceuticals like SSRIs or benzodiazepines, offers convenience but delayed onset (30 minutes to hours) and reduced bioavailability (20-70%) from acidic stomach breakdown and hepatic first-pass.[78] Intranasal insufflation, prevalent for stimulants like cocaine, enables quick mucosal absorption (5-15 minutes onset) and partial first-pass avoidance, though nasal irritation limits dosing.[78] Sublingual or buccal routes, as with some opioids, provide faster onset than oral by direct vascular uptake, bypassing first-pass for higher efficiency.[78] Rectal administration, occasionally for sedatives, yields variable but often rapid absorption with partial hepatic bypass.[78] Transdermal patches, applied to opioids like fentanyl, sustain slow release over days, minimizing peaks but requiring skin permeability.[78]| Route | Typical Onset to Brain | Bioavailability | Key Considerations for Psychoactives |
|---|---|---|---|
| Intravenous | <10 seconds | 100% | Highest abuse potential due to immediacy; used in medical settings for anesthetics.[78][77] |
| Inhalation | 10-30 seconds | 10-90% (route-dependent) | Rapid for smoked or vaporized substances; pulmonary deposition variability.[78] |
| Intranasal | 5-15 minutes | High (bypasses first-pass) | Common for powders; mucosal damage risk.[78] |
| Oral | 30 minutes-2 hours | 20-70% | Slowest; food and pH affect absorption.[78] |
| Sublingual/Buccal | 5-15 minutes | High | Avoids GI; taste issues.[78] |
Physiological and Psychological Effects
Acute and Short-Term Impacts
Psychoactive drugs induce acute effects by altering neurotransmitter activity in the brain, leading to immediate changes in psychological states such as mood elevation or sedation, and physiological responses including variations in heart rate and respiration.[11] These impacts typically onset within minutes to hours depending on the route of administration and peak shortly thereafter, with duration varying by substance half-life.[72] Stimulants, such as amphetamines and cocaine, acutely enhance dopamine transmission, producing psychological effects like euphoria, heightened alertness, excitement, and reduced fatigue.[72] Physiologically, they increase heart rate, blood pressure, body temperature, and pupil dilation, while suppressing appetite.[72] Short-term consequences following the peak include a "crash" phase characterized by fatigue, depression, and irritability due to depleted neurotransmitter stores.[72] Central nervous system depressants, including alcohol and benzodiazepines, acutely inhibit neural activity via enhancement of GABA signaling, resulting in psychological relaxation, reduced anxiety, and impaired cognition and judgment.[79] Physiological effects encompass slowed reaction times, slurred speech, ataxia, and at higher doses, respiratory depression and unconsciousness.[79] Short-term rebound may involve anxiety, insomnia, or seizures upon cessation after brief exposure.[80] Opioids, like morphine and fentanyl, bind to mu-opioid receptors to acutely induce psychological euphoria, drowsiness, and analgesia, alongside physiological slowed breathing, constipation, nausea, and pinpoint pupils.[81] Overdose risks manifest rapidly as severe respiratory depression potentially leading to hypoxia and death.[81] Short-term effects post-administration include lingering sedation and nausea persisting for hours.[81] Hallucinogens, such as LSD and psilocybin, acutely disrupt serotonin signaling to cause psychological perceptual distortions, hallucinations, altered sense of time, and intensified emotions.[82] Physiological responses feature elevated blood pressure, heart rate, dilated pupils, and potential nausea or tremors.[82] Short-term aftereffects can include psychological distress or "bad trips" with anxiety and paranoia lasting hours.[82]Chronic and Long-Term Consequences
Chronic exposure to psychoactive drugs induces neuroadaptations in brain reward circuits, particularly involving dopamine systems, leading to tolerance, dependence, and heightened relapse risk, as evidenced by reduced D2 dopamine receptor availability in imaging studies of addicted individuals.[69] These changes manifest as compulsive drug-seeking behavior despite adverse outcomes, characterizing addiction as a relapsing brain disorder with potential for partial recovery but persistent vulnerability.[83] Physical harms vary by substance class; alcohol consumption exceeding 30 grams daily over years correlates with cirrhosis in 10-20% of cases and increased hepatocellular carcinoma risk, while opioids like heroin cause chronic respiratory suppression and infectious complications from injection, contributing to 75,000 annual U.S. overdose deaths as of 2023.[84] Stimulants such as methamphetamine induce cardiovascular toxicity, including cardiomyopathy and stroke, with chronic users showing 2-4 times higher myocardial infarction rates.[85] Neurological consequences include structural brain alterations; long-term cocaine use reduces prefrontal cortex gray matter volume by up to 10%, impairing executive function and impulse control, per MRI studies.[86] Cannabis heavy use in adolescents links to persistent IQ declines of 6-8 points and hippocampal volume reductions, though causality remains debated due to confounding factors like polysubstance use.[87] Psychedelics exhibit lower chronic harm profiles; systematic reviews of psilocybin and LSD report rare persistent perceptual disorders (HPPD) in under 5% of users, with some evidence of enduring reductions in depression and substance misuse following therapeutic doses.[88] Multi-criteria assessments rank heroin and alcohol highest for overall harm, including dependence (scores 3.0/3.0) and physical damage (2.8/3.0 for heroin), while ecstasy and psychedelics score lowest.[89][90]Factors Influencing Variability
The variability in individual responses to psychoactive drugs arises from interactions among genetic, physiological, pharmacokinetic, and environmental factors, leading to differences in efficacy, side effects, and risk of adverse outcomes. Genetic polymorphisms in drug-metabolizing enzymes such as cytochrome P450 isoforms (e.g., CYP2D6) can significantly alter metabolism rates, resulting in rapid or poor metabolizer phenotypes that affect plasma concentrations and therapeutic windows for drugs like antidepressants and opioids.[91] Heritability estimates for substance use disorder vulnerability range from 40-60%, with specific variants in dopamine-related genes (e.g., DRD2) influencing reward sensitivity and reinforcing effects of stimulants.[92] Sex differences further contribute, as evidenced by studies showing females exhibit greater variability in morphine analgesia due to hormonal influences on mu-opioid receptor expression and pharmacokinetics.[93] Pharmacokinetic parameters, including absorption, distribution, metabolism, and excretion, modulate drug onset, duration, and intensity; for instance, hepatic first-pass metabolism reduces bioavailability of orally administered drugs like codeine, while intravenous routes bypass this for rapid central nervous system effects.[94] Body composition, age, and organ function impact distribution volumes—elderly individuals often experience prolonged effects from benzodiazepines due to reduced hepatic clearance and increased adipose tissue accumulation.[95] Concurrent medications or substances can induce enzyme inhibition or competition, as seen with grapefruit juice elevating serum levels of certain antipsychotics via CYP3A4 inhibition, heightening toxicity risks.[96] Psychological and contextual elements, particularly "set" (mindset, expectations) and "setting" (physical and social environment), exert pronounced influence on subjective effects, especially for psychedelics like psilocybin, where positive expectations correlate with therapeutic outcomes in controlled trials, while adverse settings amplify anxiety or hallucinations.[97] Prior exposure induces tolerance through receptor downregulation, diminishing responses over time; for example, chronic cannabis use alters endocannabinoid signaling, reducing acute euphoric effects in tolerant users.[98] These factors interact causally: genetic predispositions may amplify environmental triggers, underscoring the need for personalized dosing to mitigate inter-individual discrepancies.[99]Therapeutic Applications
Management of Psychiatric Conditions
Psychoactive drugs constitute a primary pharmacological approach for managing psychiatric conditions, targeting neurotransmitter imbalances to alleviate symptoms in disorders such as major depressive disorder, schizophrenia, bipolar disorder, anxiety disorders, and attention-deficit/hyperactivity disorder (ADHD).[100] These agents modulate brain chemistry, with evidence from randomized controlled trials demonstrating symptom reduction, though effect sizes vary and full remission remains elusive for many patients.32802-7/fulltext) Antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs) like fluoxetine and sertraline, are first-line treatments for major depressive disorder. A 2018 meta-analysis of 522 trials involving 116,477 participants found all 21 evaluated antidepressants more efficacious than placebo for response rates, with odds ratios ranging from 1.37 (reboxetine) to 2.13 (amitriptyline).32802-7/fulltext) However, the overall effect size is small, with a Hedges' g of 0.32 for SSRIs and SNRIs versus placebo in adults.[101] Efficacy in adolescents is more limited, with only fluoxetine showing superiority over placebo in some analyses.[102] Antipsychotics are essential for schizophrenia, reducing acute positive symptoms like hallucinations and delusions. A review of meta-analyses confirms their efficacy in short-term treatment, with clozapine demonstrating particular benefit in treatment-resistant cases, though long-term adherence is challenged by side effects such as weight gain and extrapyramidal symptoms.[100] In maintenance therapy, antipsychotics like olanzapine and risperidone lower relapse rates compared to placebo, but real-world effectiveness studies show high discontinuation rates, around 50-70% within one year.[103] For bipolar disorder, lithium serves as a mood stabilizer with robust evidence for preventing manic episodes. Long-term trials indicate lithium reduces relapse risk by approximately 40%, from 24% to 14% in manic phases, and exhibits anti-suicidal effects, lowering mortality in treated cohorts.[104] Comparable efficacy to anticonvulsants like valproate has been observed in acute mania, though lithium's neuroprotective properties may confer additional benefits over alternatives like quetiapine.[105] Benzodiazepines provide rapid anxiolytic effects for generalized anxiety and panic disorders, outperforming antidepressants in acute somatic symptom relief.[106] Short-term use yields moderate effect sizes (Cohen's d ≈ 0.62), but guidelines caution against long-term administration due to tolerance, dependence, and withdrawal risks, with evidence supporting intermittent dosing for sustained management in select cases.[107]00934-X/fulltext) Stimulant medications, including methylphenidate and amphetamines, effectively diminish ADHD core symptoms of inattention and hyperactivity. Meta-analyses confirm stimulants yield larger improvements than non-stimulants, with standardized mean differences indicating clinically meaningful reductions in symptom severity across age groups.[108] Both stimulants and non-stimulants like atomoxetine also enhance quality of life metrics, though benefits plateau with chronic use and require monitoring for cardiovascular risks.[109] Overall, while psychoactive drugs offer symptomatic relief supported by empirical trials, their modest average effects underscore the need for individualized treatment and adjunctive therapies like psychotherapy.32802-7/fulltext)Analgesia and Anesthesia
Psychoactive drugs play a central role in analgesia and anesthesia by altering central nervous system activity to suppress pain signals and induce states of reduced awareness or dissociation. Opioids, a primary class, achieve analgesia through binding to mu-opioid receptors in the brain and spinal cord, which inhibits nociceptive transmission and modulates emotional responses to pain, often accompanied by euphoria and sedation.[81] [110] Morphine, derived from opium, has been used since the 19th century for severe acute pain, such as postoperative recovery, with intravenous doses of 2-10 mg providing effective relief for 3-4 hours.[110] Fentanyl, a synthetic opioid 50-100 times more potent than morphine, is administered via transdermal patches or injections for chronic cancer pain management, demonstrating superior efficacy in randomized trials for breakthrough pain control.[110] Dissociative anesthetics like ketamine offer analgesia and anesthesia through non-competitive antagonism of NMDA receptors, blocking glutamate-mediated excitatory neurotransmission and producing a trance-like state with preserved respiratory drive, distinguishing it from traditional agents.[111] Approved for clinical use since 1970, ketamine is employed for short procedures in emergency settings or pediatrics at doses of 1-2 mg/kg intravenously, providing rapid onset analgesia and amnesia while inducing dissociative hallucinations that resolve post-administration.[112] [111] Its psychoactive properties, including perceptual distortions, stem from these same mechanisms but enable utility in resource-limited environments where full general anesthesia risks are high.[112] Nitrous oxide, an inhalational agent, contributes to balanced anesthesia as an adjunct at 50-70% concentrations, exerting mild analgesic effects via opioid receptor agonism and NMDA inhibition, alongside euphoric and mildly psychedelic sensations such as perceptual alterations.[113] [114] In dental and minor surgical contexts, it reduces the minimum alveolar concentration of other anesthetics by 20-30%, enhancing overall efficacy while minimizing cardiovascular depression.[114] These applications underscore the therapeutic leverage of psychoactive modulation, though empirical data from controlled studies emphasize dose-dependent separation of beneficial sensory blockade from adverse psychoactive experiences.[113]Cognitive and Performance Enhancement
Psychoactive stimulants such as methylphenidate and amphetamines have been investigated for their potential to enhance cognitive functions like attention and working memory in healthy adults without attention-deficit/hyperactivity disorder (ADHD).[115] A meta-analysis of randomized controlled trials found that methylphenidate improved performance in novel and attention-based tasks, with reduced planning latency in complex activities, though effects were modest and domain-specific.[115] Similarly, prescription stimulants demonstrated small positive impacts on inhibitory control and memory consolidation in non-clinical populations, but results varied by dosage and individual baseline performance.[116] Modafinil, a wakefulness-promoting agent, shows evidence of enhancing executive functions including planning and decision-making in healthy, non-sleep-deprived individuals.[117] Systematic reviews indicate modafinil's benefits are more pronounced in sleep-deprived states, with limited generalizability to rested adults, where improvements in attention occur but working memory and cognitive flexibility remain unaffected.[118] In contrast, amphetamines like dextroamphetamine yield inconsistent enhancements across cognitive domains, with some studies reporting gains in episodic memory but potential impairments in divergent thinking.[119] Caffeine, a widely consumed psychoactive xanthine, reliably boosts alertness and reaction times at doses of 100-200 mg, equivalent to 1-2 cups of coffee, through adenosine receptor antagonism.[120] However, its effects on higher-order cognition like sustained attention wane with habitual use due to tolerance, limiting long-term enhancement potential.[120] Nicotine, acting via nicotinic acetylcholine receptors, acutely improves fine motor skills and attentional orienting in non-smokers, but chronic exposure in healthy users risks dependence without proportional cognitive gains.[121] Overall efficacy remains tempered by factors such as genetic variability in drug metabolism and baseline cognitive ability, where low performers may benefit more than high performers.[122] While these substances are increasingly used off-label by students and professionals— with surveys reporting up to 20% prevalence among university populations—longitudinal data underscore risks including cardiovascular strain, anxiety, and diminished returns over time.[123] Peer-reviewed evidence cautions against broad endorsement for enhancement, emphasizing that benefits seldom exceed placebo in ecologically valid settings and may exacerbate inequalities in access and safety.[124]Non-Medical Uses
Recreational Consumption Patterns
Recreational use of psychoactive drugs primarily involves seeking euphoria, social enhancement, or sensory alteration, with patterns dominated by cannabis and alcohol globally. Cannabis is the most prevalent illicit substance for recreational purposes, with an estimated 244 million past-year users worldwide in 2023, outpacing other categories like opioids (approximately 60 million users) and amphetamines (around 30 million).[125][126] This equates to cannabis comprising over 75% of total past-year illicit drug use among the 316 million global users recorded in 2023.[127] Alcohol, a legal psychoactive drug, exhibits the highest recreational consumption volume, particularly in high-income countries where per capita intake averages 5-10 liters of pure alcohol annually, often in social settings like taverns or parties.[8] Regional variations highlight cultural and availability factors: in the Americas and Europe, cannabis and cocaine dominate recreational scenes, with cocaine use surging in Europe due to increased purity and supply, affecting nightlife polydrug patterns.[128] In Asia and the Middle East, opioids and cannabis prevail recreationally, while Africa sees high cannabis prevalence alongside emerging methamphetamine use.[129] In the United States, the 2024 National Survey on Drug Use and Health reported 16.7% of individuals aged 12 and older engaging in past-month illicit drug use, predominantly marijuana, alongside 46.6% past-month alcohol consumption, underscoring alcohol's role in routine recreational contexts.[130] Demographic trends show males comprising 75% of users for cannabis, cocaine, and opioids, with peak recreational initiation among young adults aged 18-25.[125] Common administration routes include smoking or vaping for cannabis, oral ingestion for alcohol and MDMA, and intranasal for cocaine, often combined in polydrug regimens at festivals or clubs to extend effects or mitigate comedowns.[131] Recent shifts include rising synthetic cannabinoid and high-potency cannabis experimentation, driven by online markets, alongside microdosing psychedelics for subtle recreational enhancement among professionals.[132]| Drug Category | Estimated Past-Year Users (millions, 2023) | Primary Recreational Regions |
|---|---|---|
| Cannabis | 244 | Global, esp. Americas, Europe |
| Opioids | 60 | Asia, Middle East |
| Amphetamines | 30 | East/Southeast Asia, Africa |
| Cocaine | 22 | Europe, Americas |
Ritualistic and Spiritual Contexts
Psychoactive substances, often termed entheogens when used in spiritual contexts, have been employed in rituals to facilitate altered states of consciousness believed to enable communion with the divine, ancestral spirits, or heightened insight. Archaeological and ethnobotanical evidence indicates such practices date back millennia, with chemical residues of psychotropics like harmine and dimethyltryptamine found in a 1000-year-old shamanic bundle from Bolivia, suggesting ritual ingestion for visionary experiences. These uses contrast with recreational patterns by emphasizing structured ceremonial frameworks, where set, setting, and cultural narratives shape outcomes, as observed in indigenous shamanism.[133][134] In ancient Vedic rituals of India, around 1500 BCE, soma—a prepared plant extract praised in the Rigveda for inducing ecstasy and immortality—was central to sacrificial ceremonies honoring gods like Indra. Descriptions in the texts portray soma as a psychoactive elixir granting visionary states and rejuvenation, though its botanical identity remains debated among scholars, with candidates including ephedra or fly agaric mushrooms; not all experts concur on its hallucinogenic potency, attributing effects partly to ritual fervor. Similarly, the Eleusinian Mysteries in ancient Greece, from circa 1500 BCE to 392 CE, involved kykeon, a barley-based beverage possibly laced with ergot alkaloids akin to LSD precursors, which initiates like Plato credited with profound spiritual revelations of life's mysteries. Evidence for psychedelics here is circumstantial, drawn from historical accounts and mycological analysis, but contested by some as overstated folklore rather than empirical pharmacology.[135][136][137] Indigenous Amazonian practices feature ayahuasca, a decoction of Banisteriopsis caapi vine and Psychotria viridis leaves containing DMT and MAO inhibitors, ingested in shamanic ceremonies for healing, prophecy, and spirit communication; ethnobotanical studies document its role in maintaining cultural equilibria of spiritual and physical health, with ceremonial use linked to reduced substance dependence in participants. In Mesoamerica, psilocybin mushrooms (teonanácatl) and peyote cacti have been ritual staples since pre-Columbian times, evidenced by codices and archaeological finds, aiding divination and communal rites. The Native American Church, formalized in the late 19th century in Oklahoma from earlier Plains traditions, incorporates peyote—containing mescaline—as a sacrament for moral guidance and Christ-like visions, securing U.S. legal exemption in 1994 after peyote's introduction from Mexico around the 1870s. These traditions persist amid modernization, with ayahuasca ceremonies in groups like Santo Daime showing sustained spiritual applications, though Western adaptations risk diluting indigenous protocols.[138][139][31][140][141]Self-Medication and Coping Mechanisms
Individuals with untreated or inadequately managed psychiatric conditions frequently resort to psychoactive substances as a means of alleviating distressing symptoms, a behavior encapsulated by the self-medication hypothesis. This hypothesis posits that the selection of specific drugs corresponds to the psychopharmacologic properties that temporarily mitigate particular affective states, such as anxiety or dysphoria, rather than random choice.[142] Originating from clinical observations, it suggests that substance use serves as an attempt at self-regulation when professional interventions are absent or insufficient.[143] Prevalence data indicate substantial overlap between mental health disorders and self-medication practices. For instance, among adults with mood disorders, approximately 24.1% report using alcohol or other drugs to cope with their symptoms, with similar patterns observed in anxiety disorders.[144] Cannabis self-medication for mood or anxiety issues is reported by a notable subset, with one survey finding 27% of lifetime cannabis users citing medical purposes, including symptom management, though rates are higher among those with preexisting conditions.[145] Alcohol remains a primary agent for self-medicating low mood, often escalating in frequency among those avoiding formal care.[146] This coping mechanism extends to broader stressors, including trauma and chronic stress, where substances like opioids or stimulants provide short-term relief from hyperarousal or emotional numbing.[147] However, empirical evidence underscores causal risks: self-medication correlates with heightened dependence liability, as initial symptom relief reinforces use patterns, potentially exacerbating underlying psychopathology through tolerance and withdrawal effects that mimic or intensify original distress.[148] Longitudinal studies reveal that such practices increase the odds of comorbid substance use disorders, with self-medicators showing elevated rates of paranoia, depression persistence, and impaired treatment adherence compared to non-self-medicating individuals.[149][150] Causal realism highlights that while psychoactive agents may interrupt acute discomfort via neurotransmitter modulation—e.g., alcohol enhancing GABAergic inhibition to dampen anxiety—the absence of targeted dosing and monitoring leads to dysregulation, where adaptive coping devolves into maladaptive cycles.[143] Peer-reviewed analyses consistently find no sustained therapeutic benefit without clinical oversight, with self-medication often delaying diagnosis and intervention for root causes like untreated depression or PTSD.[151] In high-stress populations, such as military personnel, substance misuse as a coping strategy temporarily numbs psychological pain but elevates long-term vulnerability to addiction and relapse.[152] Thus, while intuitively appealing, this approach empirically undermines resilience by prioritizing pharmacological palliation over evidence-based psychological or pharmacological strategies.Risks and Dependencies
Addiction Mechanisms and Prevalence
Psychoactive drugs exert their addictive potential primarily through hijacking the brain's mesolimbic reward pathway, where they trigger supraphysiological releases of dopamine in the nucleus accumbens, far exceeding natural rewards like food or sex and thereby reinforcing drug-seeking behaviors via positive reinforcement.[153] This acute dopaminergic surge, observed across classes such as opioids, stimulants, and depressants, activates downstream signaling that promotes synaptic plasticity changes favoring habitual use.[154] Chronic administration leads to tolerance, characterized by diminished drug effects requiring escalating doses, due to adaptations like dopamine receptor downregulation (e.g., D2 autoreceptor desensitization) and homeostatic adjustments in the ventral tegmental area.[155] Physical dependence emerges as the brain compensates for persistent drug presence, resulting in withdrawal syndromes upon abstinence—manifesting as dysphoria, autonomic hyperactivity, or seizures depending on the substance—which function as negative reinforcement to perpetuate use and avert discomfort.[156] Long-term neuroadaptations underpin compulsive relapse vulnerability, including accumulation of transcription factor ΔFosB in striatal neurons, which epigenetically alters gene expression to heighten incentive salience for drug cues while blunting response to non-drug rewards.[155] Glutamatergic projections from prefrontal cortex and amygdala strengthen conditioned responses, shifting control from voluntary goal-directed actions to inflexible habits via dorsolateral striatum recruitment.[154] These mechanisms interact with genetic predispositions (e.g., variants in dopamine-related genes like DRD2) and environmental factors, but causal evidence from neuroimaging consistently implicates reward circuit dysregulation as central, rather than mere psychological weakness.[157] Not all psychoactive drugs equally induce addiction; those with rapid onset and high potency, like cocaine or heroin, show steeper trajectories due to pharmacokinetic profiles amplifying peak dopamine levels.[158] Globally, drug use disorders—affecting psychoactive substances excluding alcohol—affected an estimated 39.5 million people in 2021, or roughly 0.5% of the population aged 15-64, with opioids accounting for the majority of treatment-seeking cases at 60 million past-year users.[159] Including alcohol use disorders, the World Health Organization reports over 400 million people worldwide experience harmful patterns of psychoactive substance use, contributing to 5.1% of the global disease burden as measured by disability-adjusted life years in 2019 data updated through 2023.[160] Prevalence rates vary regionally, with higher burdens in the Americas (e.g., 2.4% SUD prevalence among adults) compared to Africa (1.3%), driven by availability and socioeconomic factors rather than inherent cultural resistance.[161] Only about 20% of those with disorders receive treatment, limited by access barriers and underreporting, though U.S. data from 2023 indicate 48.5 million individuals aged 12+ met criteria for SUD, underscoring underestimation in self-report surveys.[159][162] Cannabis dominates non-medical use disorders numerically (219 million users in 2021), but severe dependence is rarer than for stimulants or opioids, where lifetime addiction rates can exceed 20% among users.[159]Acute Dangers and Overdose
Acute overdose from psychoactive drugs primarily manifests through disruption of vital physiological functions, with lethality varying markedly by substance class and individual factors such as tolerance, polydrug use, and route of administration. Central nervous system (CNS) depressants, including opioids, benzodiazepines, and alcohol, pose the highest risk of fatal respiratory suppression, leading to hypoxia, coma, and cardiopulmonary arrest. In the United States, drug overdose deaths reached 105,007 in 2023, with opioids implicated in approximately 75% of cases, largely driven by synthetic fentanyl analogs that exhibit high potency and narrow therapeutic margins.[163][162] Stimulant psychoactive drugs, such as cocaine, amphetamines, and synthetic cathinones, induce acute toxicity via excessive catecholamine release, resulting in sympathomimetic effects like severe hypertension, tachycardia, arrhythmias, hyperthermia, seizures, and stroke. These cardiovascular and neurological complications contribute to sudden death, particularly in users with preexisting heart conditions or during binge consumption. Mechanisms involve blockade of monoamine reuptake transporters, amplifying dopamine and norepinephrine activity to toxic levels.[75][164] Hallucinogens and psychedelics, including LSD, psilocybin, and mescaline, generally exhibit low acute lethal potential due to high LD50 values relative to typical doses, with primary dangers stemming from behavioral disinhibition, panic-induced accidents, or serotonin syndrome when combined with monoamine oxidase inhibitors. Dissociative anesthetics like ketamine can cause respiratory depression and dissociative states leading to trauma at high doses. In contrast, certain novel psychoactive substances mimicking these classes may carry underestimated risks due to variable purity and unpredictable pharmacokinetics.[165][64] Polydrug interactions exacerbate acute dangers, as stimulants may mask depressant effects initially, delaying recognition of overdose until irreversible damage occurs; for instance, opioid-stimulant combinations have risen in prevalence and contribute to polysubstance fatalities. Supportive interventions, such as naloxone for opioid reversal, highlight the reversibility of some acute events if administered promptly, though outcomes depend on rapid medical access. Empirical assessments of drug harm, incorporating acute physical toxicity scores, rank heroin, methadone, and cocaine among the most dangerous for overdose potential.[5][166]Broader Health and Cognitive Harms
Chronic exposure to psychoactive drugs induces persistent alterations in brain structure and function, contributing to cognitive deficits such as impaired memory, executive dysfunction, and reduced processing speed, alongside elevated risks for neurodegenerative conditions and psychiatric comorbidities. These effects arise from mechanisms including neurotoxicity, disrupted synaptic plasticity, and inflammation, varying by substance class but consistently linked to dosage, duration, and individual vulnerability factors like genetics and co-occurring disorders. Empirical studies, often from longitudinal cohorts, demonstrate dose-dependent declines that may persist post-abstinence, challenging assumptions of full reversibility in mainstream narratives that downplay long-term sequelae.[167] For alcohol, prolonged heavy consumption causes cortical atrophy and white matter lesions, correlating with deficits in decision-making and impulse control; a 2025 analysis of over 1,700 participants found heavy drinkers exhibited a 133% increased prevalence of brain lesions predictive of cognitive impairment. Neuronal shrinkage and thiamine deficiency exacerbate these changes, leading to syndromes like Wernicke-Korsakoff with irreversible amnesia in severe cases.[168][169][170] Cannabis users engaging in persistent weekly consumption from adolescence show neuropsychological decline across learning, memory, and verbal fluency domains, with a mean IQ reduction of 5.5 points from childhood to midlife in a 45-year New Zealand cohort study of 1,037 individuals, independent of socioeconomic confounds. Heavy lifetime use further diminishes brain activation during cognitive tasks, as evidenced by fMRI data linking THC exposure to hypofrontality and attenuated performance.[171][172] Stimulants like methamphetamine induce moderate-to-severe cognitive impairments in attention, executive functions, and verbal fluency, per a meta-analysis of 49 studies involving over 1,400 users, with deficits persisting up to a year post-abstinence and correlating with gray matter volume loss in prefrontal regions. Chronic amphetamine-type stimulant use similarly disrupts interference control and decision-making, heightening vulnerability to relapse through eroded self-regulation.[173][174] Opioids exert neuroexcitatory effects via long-term dysregulation of mu-receptors, manifesting as myoclonus, hyperalgesia, and endocrine disruptions including hypogonadism and osteoporosis; a 2022 review highlights systemic impacts on immunity and respiration, with brain injury patterns like leukoencephalopathy observed in overdose survivors. These changes compound cognitive fog and motivational deficits, often overlooked in prescribing guidelines favoring short-term analgesia.[175][176] Hallucinogens and novel psychoactive substances carry risks of enduring derealization, depersonalization, and psychosis; case series document prolonged mania or existential distress in 10-20% of users post-single exposure, with polysubstance contexts amplifying vulnerability despite therapeutic optimism in biased academic trials.[177][178]Societal and Policy Dimensions
Legal Frameworks and Enforcement
The primary international legal framework governing psychoactive drugs stems from three United Nations treaties: the 1961 Single Convention on Narcotic Drugs, which establishes controls over narcotic substances like opium and cannabis through four schedules differentiated by medical utility and abuse risk, limiting production, trade, and use to medical and scientific purposes;[179] the 1971 Convention on Psychotropic Substances, which extends scheduling (I-IV) to synthetic psychoactive compounds such as amphetamines and benzodiazepines, emphasizing prevention of abuse while permitting regulated medical access;[180] and the 1988 United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, which mandates criminalization of production, possession, and trafficking while requiring signatories to cooperate on precursor chemical controls and extradition. These conventions, ratified by over 180 countries, classify substances based on empirical assessments of harm potential and therapeutic value, though implementation varies due to national sovereignty. In the United States, the Controlled Substances Act of 1970 implements these treaties domestically by categorizing psychoactive drugs into five schedules: Schedule I includes substances like heroin, LSD, and marijuana (at the federal level) with high abuse potential and no accepted medical use; Schedules II-V encompass drugs like cocaine, amphetamines, and certain antidepressants with graduated risks and medical applications, subjecting them to quotas, prescriptions, and penalties scaling with severity.[181] The Drug Enforcement Administration (DEA) administers scheduling, rescheduling (e.g., ongoing debates over cannabis reclassification from Schedule I), and enforcement, seizing over 379 million fentanyl-laced pills and 50 million pounds of methamphetamine in fiscal year 2023 amid a shift toward synthetic psychoactives.[182] Penalties under the Act include up to life imprisonment for trafficking Schedule I/II substances causing death, reflecting causal links between unregulated distribution and overdose mortality exceeding 100,000 annually.[183] Legal approaches diverge globally, with some nations adopting decriminalization models; Portugal's 2001 policy shifted personal possession of all psychoactive drugs from criminal to administrative offenses, emphasizing treatment over incarceration, resulting in a 80% drop in drug-induced deaths per capita from 2001 to 2019 and reduced HIV transmission among injectors from 1,400 cases in 2003 to 106 by 2012, though trafficking remains prosecutable and seizures of heroin/cocaine declined substantially post-reform.[184] Conversely, strict prohibition persists in countries like Singapore, where possession of small amounts of cannabis or MDMA incurs mandatory caning or death penalties for trafficking, correlating with low prevalence rates but raising enforcement costs and human rights critiques unsupported by comparable harm reductions elsewhere. Enforcement faces persistent challenges from novel psychoactive substances (NPS), which evade scheduling through chemical analogs—over 1,200 identified globally by 2023, complicating analog acts like the U.S. Federal Analogue Act of 1986—and online/dark web markets enabling precursor shipments. International bodies like Interpol and the DEA collaborate on operations yielding billions in asset forfeitures, yet cartels adapt via synthetic production (e.g., fentanyl from Chinese precursors routed through Mexico), sustaining supply despite interdictions; empirical data indicate enforcement reduces street prices but fails to curb overall consumption or innovation in clandestine labs.[182] Systemic biases in reporting, such as underemphasis on enforcement efficacy in academic sources favoring harm reduction, underscore the need for causal analysis prioritizing overdose and trafficking data over ideological narratives.[185]Economic and Public Health Costs
The use of psychoactive drugs generates extensive economic costs, including healthcare expenditures, reduced workforce productivity, premature deaths, and criminal justice involvement, alongside public health burdens measured in mortality, morbidity, and disability-adjusted life years (DALYs). In the United States, these costs for alcohol misuse alone reached $249 billion in 2010, with binge drinking accounting for approximately 75% of the total due to associated medical treatment, lost work, and criminal justice expenses. Cigarette smoking, a primary form of nicotine use, imposed over $600 billion in annual costs in 2018, comprising more than $240 billion in direct healthcare spending and nearly $151 billion in productivity losses from illness and early mortality. Illicit drug use contributed an additional $193 billion yearly, while prescription opioid abuse added $78.5 billion, encompassing treatment, emergency care, and societal impacts. The illicit opioid crisis, dominated by fentanyl since the mid-2010s, escalated to an estimated $2.7 trillion in 2023—equivalent to 9.7% of U.S. GDP—through overdose-related fatalities, healthcare demands, and broader economic disruptions. Public health impacts amplify these economic tolls via acute risks like overdoses and chronic conditions such as organ damage and dependence. Overdose deaths in the U.S. surpassed 106,000 annually by the early 2020s, with opioids involved in the majority, marking a sixfold rise since 2000 and straining emergency services and long-term care systems. Globally, psychoactive drug use (excluding alcohol and tobacco) causes around 600,000 deaths per year, disproportionately affecting males, while opioid disorders drive the highest DALY burden among drug types due to their potency and addiction potential. In 2016, alcohol use accounted for 99.2 million DALYs worldwide (4.2% of total disease burden), and other drug use for 31.8 million, reflecting impairments from dependence, infectious diseases transmitted via injection, and neuropsychiatric harms. Tobacco contributes over 480,000 U.S. deaths annually from cancers, cardiovascular disease, and respiratory failure, underscoring how legal psychoactive substances often exceed illicit ones in aggregate health tolls despite differing enforcement contexts.| Substance Category | Estimated Annual U.S. Economic Cost | Key Components | Year |
|---|---|---|---|
| Alcohol Misuse | $249 billion | Healthcare ($28B), lost productivity ($179B), crime ($27B) | 2010 |
| Tobacco (Cigarettes) | >$600 billion | Healthcare (>$240B), productivity losses (~$151B) | 2018 |
| Illicit Drugs | $193 billion | Healthcare, productivity, justice system | Recent |
| Prescription Opioids | $78.5 billion | Misuse treatment, overdoses | Recent |