Recent from talks
Contribute something
Nothing was collected or created yet.
Heavy metals
View on Wikipedia
| Part of a series on the |
| Periodic table |
|---|
 |
Heavy metals is a controversial and ambiguous term[2] for metallic elements with relatively high densities, atomic weights, or atomic numbers. The criteria used, and whether metalloids are included, vary depending on the author and context, and arguably, the term "heavy metal" should be avoided.[3][4] A heavy metal may be defined on the basis of density, atomic number, or chemical behaviour. More specific definitions have been published, none of which has been widely accepted. The definitions surveyed in this article encompass up to 96 of the 118 known chemical elements; only mercury, lead, and bismuth meet all of them. Despite this lack of agreement, the term (plural or singular) is widely used in science. A density of more than 5 g/cm3 is sometimes quoted as a commonly used criterion and is used in the body of this article.
The earliest known metals—common metals such as iron, copper, and tin, and precious metals such as silver, gold, and platinum—are heavy metals. From 1809 onward, light metals, such as magnesium, aluminium, and titanium, were discovered, as well as less well-known heavy metals, including gallium, thallium, and hafnium.
Some heavy metals are either essential nutrients (typically iron, cobalt, copper, and zinc), or relatively harmless (such as ruthenium, silver, and indium), but can be toxic in larger amounts or certain forms. Other heavy metals, such as arsenic, cadmium, mercury, and lead, are highly poisonous. Potential sources of heavy-metal poisoning include mining, tailings, smelting, industrial waste, agricultural runoff, occupational exposure, paints, and treated timber.
Physical and chemical characterisations of heavy metals need to be treated with caution, as the metals involved are not always consistently defined. Heavy metals, as well as being relatively dense, tend to be less reactive than lighter metals, and have far fewer soluble sulfides and hydroxides. While distinguishing a heavy metal such as tungsten from a lighter metal such as sodium is relatively easy, a few heavy metals, such as zinc, mercury, and lead, have some of the characteristics of lighter metals, and lighter metals, such as beryllium, scandium, and titanium, have some of the characteristics of heavier metals.
Heavy metals are relatively rare in the Earth's crust, but are present in many aspects of modern life. They are used in, for example, golf clubs, cars, antiseptics, self-cleaning ovens, plastics, solar panels, mobile phones, and particle accelerators.
Definitions
[edit]Controversial terminology
[edit]The International Union of Pure and Applied Chemistry (IUPAC), which standardizes nomenclature, says "the term 'heavy metals' is both meaningless and misleading".[2] The IUPAC report focuses on the legal and toxicological implications of describing "heavy metals" as toxins when no scientific evidence supports a connection. The density implied by the adjective "heavy" has almost no biological consequences, and pure metals are rarely the biologically active form.[5] This characterization has been echoed by numerous reviews.[6][7][8] The most widely used toxicology textbook, Casarett and Doull’s Toxicology[9] uses "toxic metal", not "heavy metal".[5] Nevertheless, there are scientific and science related articles which continue to use "heavy metal" as a term for toxic substances.[10][11] To be an acceptable term in scientific papers, a strict definition has been encouraged.[12]
Use outside toxicology
[edit]Even in applications other than toxicity, no widely agreed criterion-based definition of a heavy metal exists. Reviews have recommended that it not be used.[10][13] Different meanings may be attached to the term, depending on the context. For example, a heavy metal may be defined on the basis of density,[14] and the distinguishing criterion might be atomic number[15] or chemical behaviour.[16]
Density criteria range from above 3.5 g/cm3 to above 7 g/cm3.[17] Atomic weight definitions can range from greater than sodium (atomic weight 22.98);[17] greater than 40 (excluding s- and f-block metals, hence starting with scandium);[18] or more than 200, i.e. from mercury onwards.[19] Atomic numbers are sometimes capped at 92 (uranium).[20] Definitions based on atomic number have been criticised for including metals with low densities. For example, rubidium in group (column) 1 of the periodic table has an atomic number of 37, but a density of only 1.532 g/cm3, which is below the threshold figure used by other authors.[21] The same problem may occur with definitions which are based on atomic weight.[22]
| Heat map of heavy metals in the periodic table | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||
| 1 | H | He | ||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||
| 6 | Cs | Ba | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| 7 | Fr | Ra | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||||||||||
| La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | |||||||||||||||
| Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | |||||||||||||||
| ||||||||||||||||||||||||||||
| This table shows the number of heavy metal criteria met by each metal, out of the ten criteria listed in this section i.e. two based on density, three on atomic weight, two on atomic number, and three on chemical behaviour.[n 1] It illustrates the lack of agreement surrounding the concept, with the possible exception of mercury, lead, and bismuth. Six elements near the end of periods (rows) 4 to 7 sometimes considered metalloids are treated here as metals: they are germanium (Ge), arsenic (As), selenium (Se), antimony (Sb), tellurium (Te), and astatine (At).[31][n 2] Oganesson (Og) is treated as a nonmetal.
| ||||||||||||||||||||||||||||
The United States Pharmacopeia includes a test for heavy metals that involves precipitating metallic impurities as their coloured sulfides.[23] On the basis of this type of chemical test, the group would include the transition metals and post-transition metals.[16]
A different chemistry-based approach advocates replacing the term "heavy metal" with two groups of metals and a gray area. Class A metal ions prefer oxygen donors; class B ions prefer nitrogen or sulfur donors; and borderline or ambivalent ions show either class A or B characteristics, depending on the circumstances.[32] The distinction between the class A metals and the other two categories is sharp. The class A and class B terminology is analogous to the "hard acid" and "soft base" terminology sometimes used to refer to the behaviour of metal ions in inorganic systems.[33] The system groups the elements by where is the metal ion electronegativity and is its ionic radius. This index gauges the importance of covalent interactions vs ionic interactions for a given metal ion.[34] This scheme has been applied to analyze biologically active metals in sea water for example,[12] but it has not been widely adopted.[35]
Origins and use of the term
[edit]The heaviness of naturally occurring metals such as gold, copper, and iron may have been noticed in prehistory and, in light of their malleability, led to the first attempts to craft metal ornaments, tools, and weapons.[36]
In 1817, German chemist Leopold Gmelin divided the elements into nonmetals, light metals, and heavy metals.[37] Light metals had densities of 0.860–5.0 g/cm3; heavy metals 5.308–22.000.[38] The term heavy metal is sometimes used interchangeably with the term "heavy element". For example, in discussing the history of nuclear chemistry, Magee[39] noted that the actinides were once thought to represent a new heavy-element transition group, whereas Seaborg and co-workers "favoured ... a heavy metal rare-earth like series ...".
The counterparts to the heavy metals, the light metals, are defined by the Minerals, Metals and Materials Society as including "the traditional (aluminium, magnesium, beryllium, titanium, lithium, and other reactive metals) and emerging light metals (composites, laminates, etc.)"[40]
Biological role
[edit]| Element | Milligrams[41] | |
|---|---|---|
| Iron | 4000 | |
| Zinc | 2500 | |
| Lead[n 3] | 120 | |
| Copper | 70 | |
| Tin[n 4] | 30 | |
| Vanadium | 20 | |
| Cadmium | 20 | |
| Nickel[n 5] | 15 | |
| Selenium[n 6] | 14 | |
| Manganese | 12 | |
| Other[n 7] | 200 | |
| Total | 7000 | |
Trace amounts of some heavy metals, mostly in period 4, are required for certain biological processes. These are iron and copper (oxygen and electron transport); cobalt (complex syntheses and cell metabolism); vanadium and manganese (enzyme regulation or functioning); chromium (glucose utilisation); nickel (cell growth); arsenic (metabolic growth in some animals and possibly in humans) and selenium (antioxidant functioning and hormone production).[46] Periods 5 and 6 contain fewer essential heavy metals, consistent with the general pattern that heavier elements tend to be less abundant and that scarcer elements are less likely to be nutritionally essential.[47] In period 5, molybdenum is required for the catalysis of redox reactions; cadmium is used by some marine diatoms for the same purpose; and tin may be required for growth in a few species.[48] In period 6, tungsten is required by some archaea and bacteria for metabolic processes.[49] A deficiency of any of these period 4–6 essential heavy metals may increase susceptibility to heavy metal poisoning[50] (conversely, an excess may also have adverse biological effects).
An average 70 kg (150 lb) human body is about 0.01% heavy metals (~7 g (0.25 oz), equivalent to the weight of two dried peas, with iron at 4 g (0.14 oz), zinc at 2.5 g (0.088 oz), and lead at 0.12 g (0.0042 oz) comprising the three main constituents), 2% light metals (~1.4 kg (3.1 lb), the weight of a bottle of wine) and nearly 98% nonmetals (mostly water).[51][n 8]
A few non-essential heavy metals have been observed to have biological effects. Gallium, germanium (a metalloid), indium, and most lanthanides can stimulate metabolism, and titanium promotes growth in plants[52] (though it is not always considered a heavy metal).
Toxicity
[edit]Heavy metals are often assumed to be highly toxic or damaging to the environment[53] and while some are, certain others are toxic only when taken in excess or encountered in certain forms. Inhalation of certain metals, either as fine dust or most commonly as fumes, can also result in a condition called metal fume fever.
Environmental heavy metals
[edit]Chromium, arsenic, cadmium, mercury, and lead have the greatest potential to cause harm on account of their extensive use, the toxicity of some of their combined or elemental forms, and their widespread distribution in the environment.[54] Hexavalent chromium, for example, is highly toxic[citation needed] as are mercury vapour and many mercury compounds.[55] These five elements have a strong affinity for sulfur; in the human body they usually bind, via thiol groups (–SH), to enzymes responsible for controlling the speed of metabolic reactions. The resulting sulfur-metal bonds inhibit the proper functioning of the enzymes involved; human health deteriorates, sometimes fatally.[56] Chromium (in its hexavalent form) and arsenic are carcinogens; cadmium causes a degenerative bone disease; and mercury and lead damage the central nervous system.[citation needed]
-
Chromium crystals
and 1 cm3 cube -
Arsenic, sealed in a
container to stop tarnishing -
Cadmium bar
and 1 cm3 cube -
Lead is the most prevalent heavy metal contaminant.[57] Levels in the aquatic environments of industrialised societies have been estimated to be two to three times those of pre-industrial levels.[58] As a component of tetraethyl lead, (CH
3CH
2)
4Pb, it was used extensively in gasoline from the 1930s until the 1970s.[59] Although the use of leaded gasoline was largely phased out in North America by 1996, soils next to roads built before this time retain high lead concentrations.[60] Later research demonstrated a statistically significant correlation between the usage rate of leaded gasoline and violent crime in the United States; taking into account a 22-year time lag (for the average age of violent criminals), the violent crime curve virtually tracked the lead exposure curve.[61]
Other heavy metals noted for their potentially hazardous nature, usually as toxic environmental pollutants, include manganese (central nervous system damage);[62] cobalt and nickel (carcinogens);[63] copper (toxic for plants),[64][65] zinc,[66] selenium[67] and silver[68] (endocrine disruption, congenital disorders, or general toxic effects in fish, plants, birds, or other aquatic organisms); tin, as organotin (central nervous system damage);[69] antimony (a suspected carcinogen);[70] and thallium (central nervous system damage).[65][n 9]
Other heavy metals
[edit]A few other non-essential heavy metals have one or more toxic forms. Kidney failure and fatalities have been recorded arising from the ingestion of germanium dietary supplements (~15 to 300 g in total consumed over a period of two months to three years).[65] Exposure to osmium tetroxide (OsO4) may cause permanent eye damage and can lead to respiratory failure[73] and death.[74] Indium salts are toxic if more than few milligrams are ingested and will affect the kidneys, liver, and heart.[75] Cisplatin (PtCl2(NH3)2), an important drug used to kill cancer cells, is also a kidney and nerve poison.[65] Bismuth compounds can cause liver damage if taken in excess; insoluble uranium compounds, as well as the dangerous radiation they emit, can cause permanent kidney damage.[76]
Exposure sources
[edit]Heavy metals can degrade air, water, and soil quality, and subsequently cause health issues in plants, animals, and people, when they become concentrated as a result of industrial activities.[77][78] Common sources of heavy metals in this context include vehicle emissions;[79] motor oil;[80] fertilisers;[81] glassworking;[82] incinerators;[83] treated timber;[84] aging water supply infrastructure;[85] and microplastics floating in the world's oceans.[86] Recent examples of heavy metal contamination and health risks include the occurrence of Minamata disease, in Japan (1932–1968; lawsuits ongoing as of 2016);[87] the Bento Rodrigues dam disaster in Brazil,[88] high levels of lead in drinking water supplied to the residents of Flint, Michigan, in the north-east of the United States[89] and 2015 Hong Kong heavy metal in drinking water incidents.
Formation, abundance, occurrence, and extraction
[edit]| Heavy metals in the Earth's crust: | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| abundance and main occurrence or source[n 10] | |||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
| 1 | H | He | |||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||
| 6 | Cs | Ba | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | ||||
| 7 | |||||||||||||||||||
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | |||||||
| Th | U | ||||||||||||||||||
Most abundant (56,300 ppm by weight)
|
Rare (0.01–0.99 ppm)
| ||||||||||||||||||
Abundant (100–999 ppm)
|
Very rare (0.0001–0.0099 ppm)
| ||||||||||||||||||
Uncommon (1–99 ppm)
|
|||||||||||||||||||
| Heavy metals left of the dividing line occur (or are sourced) mainly as lithophiles; those to the right, as chalcophiles except gold (a siderophile) and tin (a lithophile). | |||||||||||||||||||
Heavy metals up to the vicinity of iron (in the periodic table) are largely made via stellar nucleosynthesis. In this process, lighter elements from hydrogen to silicon undergo successive fusion reactions inside stars, releasing light and heat and forming heavier elements with higher atomic numbers.[93]
Heavier heavy metals are not usually formed this way since fusion reactions involving such nuclei would consume rather than release energy.[94] Rather, they are largely synthesised (from elements with a lower atomic number) by neutron capture, with the two main modes of this repetitive capture being the s-process and the r-process. In the s-process ("s" stands for "slow"), singular captures are separated by years or decades, allowing the less stable nuclei to beta decay,[95] while in the r-process ("rapid"), captures happen faster than nuclei can decay. Therefore, the s-process takes a more or less clear path: for example, stable cadmium-110 nuclei are successively bombarded by free neutrons inside a star until they form cadmium-115 nuclei which are unstable and decay to form indium-115 (which is nearly stable, with a half-life 30,000 times the age of the universe). These nuclei capture neutrons and form indium-116, which is unstable, and decays to form tin-116, and so on.[93][96][n 11] In contrast, there is no such path in the r-process. The s-process stops at bismuth due to the short half-lives of the next two elements, polonium and astatine, which decay to bismuth or lead. The r-process is so fast it can skip this zone of instability and go on to create heavier elements such as thorium and uranium.[98]
Heavy metals condense in planets as a result of stellar evolution and destruction processes. Stars lose much of their mass when it is ejected late in their lifetimes, and sometimes thereafter as a result of a neutron star merger,[99][n 12] thereby increasing the abundance of elements heavier than helium in the interstellar medium. When gravitational attraction causes this matter to coalesce and collapse, new stars and planets are formed.[101]
The Earth's crust is made of approximately 5% of heavy metals by weight, with iron comprising 95% of this quantity. Light metals (~20%) and nonmetals (~75%) make up the other 95% of the crust.[90] Despite their overall scarcity, heavy metals can become concentrated in economically extractable quantities as a result of mountain building, erosion, or other geological processes.[102]
Heavy metals are found primarily as lithophiles (rock-loving) or chalcophiles (ore-loving). Lithophile heavy metals are mainly f-block elements and the more reactive of the d-block elements. They have a strong affinity for oxygen and mostly exist as relatively low density silicate minerals.[103] Chalcophile heavy metals are mainly the less reactive d-block elements, and period 4–6 p-block metals and metalloids. They are usually found in (insoluble) sulfide minerals. Being denser than the lithophiles, hence sinking lower into the crust at the time of its solidification, the chalcophiles tend to be less abundant than the lithophiles.[104]
In contrast, gold is a siderophile, or iron-loving element. It does not readily form compounds with either oxygen or sulfur.[105] At the time of the Earth's formation, and as the most noble (inert) of metals, gold sank into the core due to its tendency to form high-density metallic alloys. Consequently, it is a relatively rare metal.[106][failed verification] Some other (less) noble heavy metals—molybdenum, rhenium, the platinum group metals (ruthenium, rhodium, palladium, osmium, iridium, and platinum), germanium, and tin—can be counted as siderophiles but only in terms of their primary occurrence in the Earth (core, mantle and crust), rather the crust. These metals otherwise occur in the crust, in small quantities, chiefly as chalcophiles (less so in their native form).[107][n 13]
Concentrations of heavy metals below the crust are generally higher, with most being found in the largely iron-silicon-nickel core. Platinum, for example, comprises approximately 1 part per billion of the crust whereas its concentration in the core is thought to be nearly 6,000 times higher.[108][109] Recent speculation suggests that uranium (and thorium) in the core may generate a substantial amount of the heat that drives plate tectonics and (ultimately) sustains the Earth's magnetic field.[110][n 14]
Broadly speaking, and with some exceptions, lithophile heavy metals can be extracted from their ores by electrical or chemical treatments, while chalcophile heavy metals are obtained by roasting their sulphide ores to yield the corresponding oxides, and then heating these to obtain the raw metals.[112][n 15] Radium occurs in quantities too small to be economically mined and is instead obtained from spent nuclear fuels.[115] The chalcophile platinum group metals (PGM) mainly occur in small (mixed) quantities with other chalcophile ores. The ores involved need to be smelted, roasted, and then leached with sulfuric acid to produce a residue of PGM. This is chemically refined to obtain the individual metals in their pure forms.[116] Compared to other metals, PGM are expensive due to their scarcity[117] and high production costs.[118]
Gold, a siderophile, is most commonly recovered by dissolving the ores in which it is found in a cyanide solution.[119] The gold forms a dicyanoaurate(I), for example: 2 Au + H2O +½ O2 + 4 KCN → 2 K[Au(CN)2] + 2 KOH. Zinc is added to the mix and, being more reactive than gold, displaces the gold: 2 K[Au(CN)2] + Zn → K2[Zn(CN)4] + 2 Au. The gold precipitates out of solution as a sludge, and is filtered off and melted.[120]
Uses
[edit]Some common uses of heavy metals depend on the general characteristics of metals such as electrical conductivity and reflectivity or the general characteristics of heavy metals such as density, strength, and durability. Other uses depend on the characteristics of the specific element, such as their biological role as nutrients or poisons or some other specific atomic properties. Examples of such atomic properties include: partly filled d- or f- orbitals (in many of the transition, lanthanide, and actinide heavy metals) that enable the formation of coloured compounds;[121] the capacity of heavy metal ions (such as platinum,[122] cerium[123] or bismuth[124]) to exist in different oxidation states and are used in catalysts;[125] strong exchange interactions in 3d or 4f orbitals (in iron, cobalt, and nickel, or the lanthanide heavy metals) that give rise to magnetic effects;[126] and high atomic numbers and electron densities that underpin their nuclear science applications.[127] Typical uses of heavy metals can be broadly grouped into the following categories.[128]
Weight- or density-based
[edit]
Some uses of heavy metals, including in sport, mechanical engineering, military ordnance, and nuclear science, take advantage of their relatively high densities. In underwater diving, lead is used as a ballast;[130] in handicap horse racing each horse must carry a specified lead weight, based on factors including past performance, so as to equalize the chances of the various competitors.[131] In golf, tungsten, brass, or copper inserts in fairway clubs and irons lower the centre of gravity of the club making it easier to get the ball into the air;[132] and golf balls with tungsten cores are claimed to have better flight characteristics.[133] In fly fishing, sinking fly lines have a PVC coating embedded with tungsten powder, so that they sink at the required rate.[134] In track and field sport, steel balls used in the hammer throw and shot put events are filled with lead in order to attain the minimum weight required under international rules.[135] Tungsten was used in hammer throw balls at least up to 1980; the minimum size of the ball was increased in 1981 to eliminate the need for what was, at that time, an expensive metal (triple the cost of other hammers) not generally available in all countries.[136] Tungsten hammers were so dense that they penetrated too deeply into the turf.[137]
The higher the projectile density, the more effectively it can penetrate heavy armor plate ... Os, Ir, Pt, and Re ... are expensive ... U offers an appealing combination of high density, reasonable cost and high fracture toughness.
Structure–property relations
in nonferrous metals (2005, p. 16)
Heavy metals are used for ballast in boats,[138] aeroplanes,[139] and motor vehicles;[140] or in balance weights on wheels and crankshafts,[141] gyroscopes, and propellers,[142] and centrifugal clutches,[143] in situations requiring maximum weight in minimum space (for example in watch movements).[139]
In military ordnance, tungsten or uranium is used in armour plating[144] and armour piercing projectiles,[145] as well as in nuclear weapons to increase efficiency (by reflecting neutrons and momentarily delaying the expansion of reacting materials).[146] In the 1970s, tantalum was found to be more effective than copper in shaped charge and explosively formed anti-armour weapons on account of its higher density, allowing greater force concentration, and better deformability.[147] Less-toxic heavy metals, such as copper, tin, tungsten, and bismuth, and probably manganese (as well as boron, a metalloid), have replaced lead and antimony in the green bullets used by some armies and in some recreational shooting munitions.[148] Doubts have been raised about the safety (or green credentials) of tungsten.[149]
Biological and chemical
[edit]
The biocidal effects of some heavy metals have been known since antiquity.[151] Platinum, osmium, copper, ruthenium, and other heavy metals, including arsenic, are used in anti-cancer treatments, or have shown potential.[152] Antimony (anti-protozoal), bismuth (anti-ulcer), gold (anti-arthritic), and iron (anti-malarial) are also important in medicine.[153] Copper, zinc, silver, gold, or mercury are used in antiseptic formulations;[154] small amounts of some heavy metals are used to control algal growth in, for example, cooling towers.[155] Depending on their intended use as fertilisers or biocides, agrochemicals may contain heavy metals such as chromium, cobalt, nickel, copper, zinc, arsenic, cadmium, mercury, or lead.[156]
Selected heavy metals are used as catalysts in fuel processing (rhenium, for example), synthetic rubber and fibre production (bismuth), emission control devices (palladium and platinum), and in self-cleaning ovens (where cerium(IV) oxide in the walls of such ovens helps oxidise carbon-based cooking residues).[157] In soap chemistry, heavy metals form insoluble soaps that are used in lubricating greases, paint dryers, and fungicides (apart from lithium, the alkali metals and the ammonium ion form soluble soaps).[158]
Colouring and optics
[edit]
The colours of glass, ceramic glazes, paints, pigments, and plastics are commonly produced by the inclusion of heavy metals (or their compounds) such as chromium, manganese, cobalt, copper, zinc, zirconium, molybdenum, silver, tin, praseodymium, neodymium, erbium, tungsten, iridium, gold, lead, or uranium.[160] Tattoo inks may contain heavy metals, such as chromium, cobalt, nickel, and copper.[161] The high reflectivity of some heavy metals is important in the construction of mirrors, including precision astronomical instruments. Headlight reflectors rely on the excellent reflectivity of a thin film of rhodium.[162]
Electronics, magnets, and lighting
[edit]Heavy metals or their compounds can be found in electronic components, electrodes, and wiring and solar panels. Molybdenum powder is used in circuit board inks.[163] Home electrical systems, for the most part, are wired with copper wire for its good conducting properties.[164] Silver and gold are used in electrical and electronic devices, particularly in contact switches, as a result of their high electrical conductivity and capacity to resist or minimise the formation of impurities on their surfaces.[165] Heavy metals have been used in batteries for over 200 years, at least since Volta invented his copper and silver voltaic pile in 1800.[166]
Magnets are often made of heavy metals such as manganese, iron, cobalt, nickel, niobium, bismuth, praseodymium, neodymium, gadolinium, and dysprosium. Neodymium magnets are the strongest type of permanent magnet commercially available. They are key components of, for example, car door locks, starter motors, fuel pumps, and power windows.[167]
Heavy metals are used in lighting, lasers, and light-emitting diodes (LEDs). Fluorescent lighting relies on mercury vapour for its operation. Ruby lasers generate deep red beams by exciting chromium atoms in aluminum oxide; the lanthanides are also extensively employed in lasers. Copper, iridium, and platinum are used in organic LEDs.[168]
Nuclear
[edit]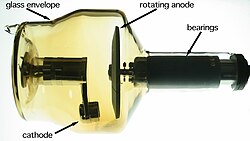
Because denser materials absorb more of certain types of radioactive emissions such as gamma rays than lighter ones, heavy metals are useful for radiation shielding and to focus radiation beams in linear accelerators and radiotherapy applications.
Niche uses of heavy metals with high atomic numbers occur in diagnostic imaging, electron microscopy, and nuclear science. In diagnostic imaging, heavy metals such as cobalt or tungsten make up the anode materials found in x-ray tubes.[172] In electron microscopy, heavy metals such as lead, gold, palladium, platinum, or uranium have been used in the past to make conductive coatings and to introduce electron density into biological specimens by staining, negative staining, or vacuum deposition.[173] In nuclear science, nuclei of heavy metals such as chromium, iron, or zinc are sometimes fired at other heavy metal targets to produce superheavy elements;[174] heavy metals are also employed as spallation targets for the production of neutrons[175] or isotopes of non-primordial elements such as astatine (using lead, bismuth, thorium, or uranium in the latter case).[176]
Notes
[edit]- ^ Criteria used were density:[17] (1) above 3.5 g/cm3; (2) above 7 g/cm3; atomic weight: (3) > 22.98;[17] (4) > 40 (excluding s- and f-block metals);[18] (5) > 200;[19] atomic number: (6) > 20; (7) 21–92;[20] chemical behaviour: (8) United States Pharmacopeia;[23][24][25] (9) Hawkes' periodic table-based definition (excluding the lanthanides and actinides);[16] and (10) Nieboer and Richardson's biochemical classifications.[26] Densities of the elements are mainly from Emsley.[27] Predicted densities have been used for At, Fr and Fm–Ts.[28] Indicative densities were derived for Fm, Md, No and Lr based on their atomic weights, estimated metallic radii,[29] and predicted close-packed crystalline structures.[30] Atomic weights are from Emsley,[27] inside back cover
- ^ Metalloids were, however, excluded from Hawkes' periodic table-based definition given he noted it was "not necessary to decide whether semimetals [i.e. metalloids] should be included as heavy metals."[16]
- ^ Lead, a cumulative poison, has a relatively high abundance due to its extensive historical use and human-caused discharge into the environment.[42]
- ^ Haynes shows an amount of < 17 mg for tin[43]
- ^ Iyengar records a figure of 5 mg for nickel;[44] Haynes shows an amount of 10 mg[43]
- ^ Selenium is a nonmetal.
- ^ Encompassing 45 heavy metals occurring in quantities of less than 10 mg each, including As (7 mg), Mo (5), Co (1.5), and Cr (1.4)[45]
- ^ Of the elements commonly recognised as metalloids, B and Si were counted as nonmetals; Ge, As, Sb, and Te as heavy metals.
- ^ Ni, Cu, Zn, Se, Ag and Sb appear in the United States Government's Toxic Pollutant List;[71] Mn, Co, and Sn are listed in the Australian Government's National Pollutant Inventory.[72]
- ^ Trace elements having an abundance much less than the one part per trillion of Ra and Pa (namely Tc, Pm, Po, At, Ac, Np, and Pu) are not shown. Abundances are from Lide[90] and Emsley;[91] occurrence types are from McQueen.[92]
- ^ In some cases, for example in the presence of high energy gamma rays or in a very high temperature hydrogen rich environment, the subject nuclei may experience neutron loss or proton gain resulting in the production of (comparatively rare) neutron deficient isotopes.[97]
- ^ The ejection of matter when two neutron stars collide is attributed to the interaction of their tidal forces, possible crustal disruption, and shock heating (which is what happens if you floor the accelerator in a car when the engine is cold).[100]
- ^ Iron, cobalt, nickel, germanium and tin are also siderophiles from a whole of Earth perspective.[92]
- ^ Heat escaping from the inner solid core is believed to generate motion in the outer core, which is made of liquid iron alloys. The motion of this liquid generates electrical currents which give rise to a magnetic field.[111]
- ^ Heavy metals that occur naturally in quantities too small to be economically mined (Tc, Pm, Po, At, Ac, Np and Pu) are instead produced by artificial transmutation.[113] The latter method is also used to produce heavy metals from americium onwards.[114]
- ^ Electrons impacting the tungsten anode generate X-rays;[170] rhenium gives tungsten better resistance to thermal shock;[171] molybdenum and graphite act as heat sinks. Molybdenum also has a density nearly half that of tungsten thereby reducing the weight of the anode.[169]
References
[edit]- ^ Emsley 2011, pp. 288, 374
- ^ a b Duffus 2002.
- ^ Pourret, Olivier; Bollinger, Jean-Claude; Hursthouse, Andrew (2021). "Heavy metal: a misused term?" (PDF). Acta Geochimica. 40 (3): 466–471. Bibcode:2021AcGch..40..466P. doi:10.1007/s11631-021-00468-0. S2CID 232342843.
- ^ Hübner, Astin & Herbert 2010
- ^ a b Duffus 2002, p. 795.
- ^ Ali & Khan 2018.
- ^ Nieboer & Richardson 1980.
- ^ Baldwin & Marshall 1999.
- ^ Goyer & Clarkson 1996, p. 839.
- ^ a b Pourret, Bollinger & Hursthouse 2021.
- ^ Hübner, Astin & Herbert 2010, p. 1513
- ^ a b Rainbow 1991, p. 416
- ^ Nieboer & Richardson 1980, p. 21
- ^ Morris 1992, p. 1001
- ^ Gorbachev, Zamyatnin & Lbov 1980, p. 5
- ^ a b c d Hawkes 1997
- ^ a b c d Duffus 2002, p. 798
- ^ a b Rand, Wells & McCarty 1995, p. 23
- ^ a b Baldwin & Marshall 1999, p. 267
- ^ a b Lyman 2003, p. 452
- ^ Duffus 2002, p. 797
- ^ Liens 2010, p. 1415
- ^ a b The United States Pharmacopeia 1985, p. 1189
- ^ Raghuram, Soma Raju & Sriramulu 2010, p. 15
- ^ Thorne & Roberts 1943, p. 534
- ^ Nieboer & Richardson 1980, p. 4
- ^ a b Emsley 2011
- ^ Hoffman, Lee & Pershina 2011, pp. 1691, 1723; Bonchev & Kamenska 1981, p. 1182
- ^ Silva 2010, pp. 1628, 1635, 1639, 1644
- ^ Fournier 1976, p. 243
- ^ Vernon 2013, p. 1703
- ^ Nieboer & Richardson 1980, p. 5
- ^ Nieboer & Richardson 1980, pp. 6–7
- ^ Nieboer & Richardson 1980, p. 9
- ^ Hübner, Astin & Herbert 2010, pp. 1511–1512
- ^ Raymond 1984, pp. 8–9
- ^ Habashi 2009, p. 31
- ^ Gmelin 1849, p. 2
- ^ Magee 1969, p. 14
- ^ The Minerals, Metals and Materials Society 2016
- ^ Emsley 2011, pp. 35, passim
- ^ Emsley 2011, pp. 280, 286; Baird & Cann 2012, pp. 549, 551
- ^ a b Haynes 2015, pp. 7–48
- ^ Iyengar 1998, p. 553
- ^ Emsley 2011, pp. 47, 331, 138, 133, passim
- ^ Emsley 2011, pp. 604, 31, 133, 358, 47, 475
- ^ Valkovic 1990, pp. 214, 218
- ^ Emsley 2011, pp. 331, 89, 552
- ^ Emsley 2011, p. 571
- ^ Venugopal & Luckey 1978, p. 307
- ^ Emsley 2011, pp. 24, passim
- ^ Emsley 2011, pp. 192, 197, 240, 120, 166, 188, 224, 269, 299, 423, 464, 549, 614, 559
- ^ Duffus 2002, pp. 794, 799
- ^ Baird & Cann 2012, p. 519
- ^ Kozin & Hansen 2013, p. 80
- ^ Baird & Cann 2012, pp. 519–520, 567; Rusyniak et al. 2010, p. 387
- ^ Di Maio 2001, p. 208
- ^ Perry & Vanderklein 1996, p. 208
- ^ Love 1998, p. 208
- ^ Hendrickson 2016, p. 42
- ^ Reyes 2007, pp. 1, 20, 35–36
- ^ Emsley 2011, p. 311
- ^ Wiberg 2001, pp. 1474, 1501
- ^ Colzi, I; Pignattelli, S; Giorni, E; Papini, A; Gonnelli, C (2015). "Linking root traits to copper exclusion mechanisms in Silene paradoxa L.(Caryophyllaceae)". Plant and Soil. 390 (1–2): 1–15. Bibcode:2015PlSoi.390....1C. doi:10.1007/s11104-014-2375-3.
- ^ a b c d Tokar et al. 2013
- ^ Eisler 1993, pp. 3, passim
- ^ Lemly 1997, p. 259; Ohlendorf 2003, p. 490
- ^ State Water Control Resources Board 1987, p. 63
- ^ Scott 1989, pp. 107–108
- ^ International Antimony Association 2016
- ^ United States Government 2014
- ^ Australian Government 2016
- ^ Cole & Stuart 2000, p. 315
- ^ Clegg 2014
- ^ Emsley 2011, p. 240
- ^ Emsley 2011, p. 595
- ^ Namla, Djadjiti; Mangse, George; Koleoso, Peter O.; Ogbaga, Chukwuma C.; Nwagbara, Onyinye F. (2022). "Assessment of Heavy Metal Concentrations of Municipal Open-Air Dumpsite: A Case Study of Gosa Dumpsite, Abuja". Innovations and Interdisciplinary Solutions for Underserved Areas. Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering. Vol. 449. pp. 165–174. doi:10.1007/978-3-031-23116-2_13. ISBN 978-3-031-23115-5.
- ^ Stankovic & Stankovic 2013, pp. 154–159
- ^ Ndiokwere, C.L. (January 1984). "A study of heavy metal pollution from motor vehicle emissions and its effect on roadside soil, vegetation and crops in Nigeria". Environmental Pollution Series B, Chemical and Physical. 7 (1): 35–42. doi:10.1016/0143-148X(84)90035-1.
- ^ https://blog.nationalgeographic.org/2015/08/03/heavy-metals-in-motor-oil-have-heavy-consequences/ Heavy Metals in Motor Oil Have Heavy Consequences
- ^ "Fear In The Fields -- How Hazardous Wastes Become Fertilizer -- Spreading Heavy Metals On Farmland Is Perfectly Legal, But Little Research Has Been Done To Find Out Whether It's Safe".
- ^ https://hazwastehelp.org/ArtHazards/glassworking.aspx Art Hazards
- ^ Wang, P.; Hu, Y.; Cheng, H. (2019). "Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China". Environmental Pollution. 252 (Pt A): 461–475. Bibcode:2019EPoll.252..461W. doi:10.1016/j.envpol.2019.04.082. PMID 31158674. S2CID 145832923.
- ^ Bradl 2005, pp. 15, 17–20
- ^ Harvey, Handley & Taylor 2015, p. 12276
- ^ Howell et al. 2012; Cole et al. 2011, pp. 2589–2590
- ^ Amasawa et al. 2016, pp. 95–101
- ^ Massarani 2015
- ^ Torrice 2016
- ^ a b Lide 2004, pp. 14–17
- ^ Emsley 2011, pp. 29, passim
- ^ a b McQueen 2009, p. 74
- ^ a b Cox 1997, pp. 73–89
- ^ Cox 1997, pp. 32, 63, 85
- ^ Podosek 2011, p. 482
- ^ Padmanabhan 2001, p. 234
- ^ Rehder 2010, pp. 32, 33
- ^ Hofmann 2002, pp. 23–24
- ^ Hadhazy 2016
- ^ Choptuik, Lehner & Pretorias 2015, p. 383
- ^ Cox 1997, pp. 83, 91, 102–103
- ^ Berry & Mason 1959, pp. 210–211; Rankin 2011, p. 69
- ^ Hartmann 2005, p. 197
- ^ Yousif 2007, pp. 11–12
- ^ Berry & Mason 1959, p. 214
- ^ Yousif 2007, p. 11
- ^ Wiberg 2001, p. 1511
- ^ Emsley 2011, p. 403
- ^ Litasov & Shatskiy 2016, p. 27
- ^ Sanders 2003; Preuss 2011
- ^ Natural Resources Canada 2015
- ^ MacKay, MacKay & Henderson 2002, pp. 203–204
- ^ Emsley 2011, pp. 525–528, 428–429, 414, 57–58, 22, 346–347, 408–409; Keller, Wolf & Shani 2012, p. 98
- ^ Emsley 2011, pp. 32 et seq.
- ^ Emsley 2011, p. 437
- ^ Chen & Huang 2006, p. 208; Crundwell et al. 2011, pp. 411–413; Renner et al. 2012, p. 332; Seymour & O'Farrelly 2012, pp. 10–12
- ^ Crundwell et al. 2011, p. 409
- ^ International Platinum Group Metals Association n.d., pp. 3–4
- ^ McLemore 2008, p. 44
- ^ Wiberg 2001, p. 1277
- ^ Jones 2001, p. 3
- ^ Berea, Rodriguez-lbelo & Navarro 2016, p. 203
- ^ Alves, Berutti & Sánchez 2012, p. 94
- ^ Yadav, Antony & Subba Reddy 2012, p. 231
- ^ Masters 1981, p. 5
- ^ Wulfsberg 1987, pp. 200–201
- ^ Bryson & Hammond 2005, p. 120 (high electron density); Frommer & Stabulas-Savage 2014, pp. 69–70 (high atomic number)
- ^ Landis, Sofield & Yu 2011, p. 269
- ^ Prieto 2011, p. 10; Pickering 1991, pp. 5–6, 17
- ^ Emsley 2011, p. 286
- ^ Berger & Bruning 1979, p. 173
- ^ Jackson & Summitt 2006, pp. 10, 13
- ^ Shedd 2002, p. 80.5; Kantra 2001, p. 10
- ^ Spolek 2007, p. 239
- ^ White 2010, p. 139
- ^ Dapena & Teves 1982, p. 78
- ^ Burkett 2010, p. 80
- ^ Moore & Ramamoorthy 1984, p. 102
- ^ a b National Materials Advisory Board 1973, p. 58
- ^ Livesey 2012, p. 57
- ^ VanGelder 2014, pp. 354, 801
- ^ National Materials Advisory Board 1971, pp. 35–37
- ^ Frick 2000, p. 342
- ^ Rockhoff 2012, p. 314
- ^ Russell & Lee 2005, pp. 16, 96
- ^ Morstein 2005, p. 129
- ^ Russell & Lee 2005, pp. 218–219
- ^ Lach et al. 2015; Di Maio 2016, p. 154
- ^ Preschel 2005; Guandalini et al. 2011, p. 488
- ^ Emsley 2011, p. 123
- ^ Weber & Rutula 2001, p. 415
- ^ Dunn 2009; Bonetti et al. 2009, pp. 1, 84, 201
- ^ Desoize 2004, p. 1529
- ^ Atlas 1986, p. 359; Lima et al. 2013, p. 1
- ^ Volesky 1990, p. 174
- ^ Nakbanpote, Meesungnoen & Prasad 2016, p. 180
- ^ Emsley 2011, pp. 447, 74, 384, 123
- ^ Elliot 1946, p. 11; Warth 1956, p. 571
- ^ McColm 1994, p. 215
- ^ Emsley 2011, pp. 135, 313, 141, 495, 626, 479, 630, 334, 495, 556, 424, 339, 169, 571, 252, 205, 286, 599
- ^ Everts 2016
- ^ Emsley 2011, p. 450
- ^ Emsley 2011, p. 334
- ^ Moselle 2004, pp. 409–410
- ^ Russell & Lee 2005, p. 323
- ^ Tretkoff 2006
- ^ Emsley 2011, pp. 73, 141, 141, 141, 355, 73, 424, 340, 189, 189
- ^ Baranoff 2015, p. 80; Wong et al. 2015, p. 6535
- ^ a b Ball, Moore & Turner 2008, p. 177
- ^ Ball, Moore & Turner 2008, pp. 248–249, 255
- ^ Russell & Lee 2005, p. 238
- ^ Tisza 2001, p. 73
- ^ Chandler & Roberson 2009, pp. 47, 367–369, 373; Ismail, Khulbe & Matsuura 2015, p. 302
- ^ Ebbing & Gammon 2017, p. 695
- ^ Pan & Dai 2015, p. 69
- ^ Brown 1987, p. 48
Sources
[edit]- Ahrland S., Liljenzin J. O. & Rydberg J. 1973, "Solution chemistry," in J. C. Bailar & A. F. Trotman-Dickenson (eds), Comprehensive Inorganic Chemistry, vol. 5, The Actinides, Pergamon Press, Oxford.
- Albutt M. & Dell R. 1963, The nitrites and sulphides of uranium, thorium and plutonium: A review of present knowledge, UK Atomic Energy Authority Research Group, Harwell, Berkshire.
- Ali H, Khan E (2018-01-02). "What are heavy metals? Long-standing controversy over the scientific use of the term 'heavy metals' – proposal of a comprehensive definition". Toxicological & Environmental Chemistry. 100 (1): 6–19. Bibcode:2018TxEC..100....6A. doi:10.1080/02772248.2017.1413652. ISSN 0277-2248.
- Alves A. K., Berutti, F. A. & Sánche, F. A. L. 2012, "Nanomaterials and catalysis", in C. P. Bergmann & M. J. de Andrade (ads), Nanonstructured Materials for Engineering Applications, Springer-Verlag, Berlin, ISBN 978-3-642-19130-5.
- Amasawa E., Yi Teah H., Yu Ting Khew, J., Ikeda I. & Onuki M. 2016, "Drawing Lessons from the Minamata Incident for the General Public: Exercise on Resilience, Minamata Unit AY2014", in M. Esteban, T. Akiyama, C. Chen, I. Ikea, T. Mino (eds), Sustainability Science: Field Methods and Exercises, Springer International, Switzerland, pp. 93–116, doi:10.1007/978-3-319-32930-7_5 ISBN 978-3-319-32929-1.
- Ariel E., Barta J. & Brandon D. 1973, "Preparation and properties of heavy metals", Powder Metallurgy International, vol. 5, no. 3, pp. 126–129.
- Atlas R. M. 1986, Basic and Practical Microbiology, Macmillan Publishing Company, New York, ISBN 978-0-02-304350-5.
- Australian Government 2016, National Pollutant Inventory, Department of the Environment and Energy, accessed 16 August 2016.
- Baird C. & Cann M. 2012, Environmental Chemistry, 5th ed., W. H. Freeman and Company, New York, ISBN 978-1-4292-7704-4.
- Baldwin D. R. & Marshall W. J. 1999, "Heavy metal poisoning and its laboratory investigation", Annals of Clinical Biochemistry, vol. 36, no. 3, pp. 267–300, doi:10.1177/000456329903600301.
- Ball J. L., Moore A. D. & Turner S. 2008, Ball and Moore's Essential Physics for Radiographers, 4th ed., Blackwell Publishing, Chichester, ISBN 978-1-4051-6101-5.
- Bánfalvi G. 2011, "Heavy metals, trace elements and their cellular effects", in G. Bánfalvi (ed.), Cellular Effects of Heavy Metals, Springer, Dordrecht, pp. 3–28, ISBN 978-94-007-0427-5.
- Baranoff E. 2015, "First-row transition metal complexes for the conversion of light into electricity and electricity into light", in W-Y Wong (ed.), Organometallics and Related Molecules for Energy Conversion, Springer, Heidelberg, pp. 61–90, ISBN 978-3-662-46053-5.
- Berea E., Rodriguez-lbelo M. & Navarro J. A. R. 2016, "Platinum Group Metal—Organic frameworks" in S. Kaskel (ed.), The Chemistry of Metal-Organic Frameworks: Synthesis, Characterisation, and Applications, vol. 2, Wiley-VCH Weinheim, pp. 203–230, ISBN 978-3-527-33874-0.
- Berger A. J. & Bruning N. 1979, Lady Luck's Companion: How to Play ... How to Enjoy ... How to Bet ... How to Win, Harper & Row, New York, ISBN 978-0-06-014696-2.
- Berry L. G. & Mason B. 1959, Mineralogy: Concepts, Descriptions, Determinations, W. H. Freeman and Company, San Francisco.
- Biddle H. C. & Bush G. L 1949, Chemistry Today, Rand McNally, Chicago.
- Bonchev D. & Kamenska V. 1981, "Predicting the properties of the 113–120 transactinide elements", The Journal of Physical Chemistry, vo. 85, no. 9, pp. 1177–1186, doi:10.1021/j150609a021.
- Bonetti A., Leone R., Muggia F. & Howell S. B. (eds) 2009, Platinum and Other Heavy Metal Compounds in Cancer Chemotherapy: Molecular Mechanisms and Clinical Applications, Humana Press, New York, ISBN 978-1-60327-458-6.
- Booth H. S. 1957, Inorganic Syntheses, vol. 5, McGraw-Hill, New York.
- Bradl H. E. 2005, "Sources and origins of heavy metals", in Bradl H. E. (ed.), Heavy Metals in the Environment: Origin, Interaction and Remediation, Elsevier, Amsterdam, ISBN 978-0-12-088381-3.
- Brady J. E. & Holum J. R. 1995, Chemistry: The Study of Matter and its Changes, 2nd ed., John Wiley & Sons, New York, ISBN 978-0-471-10042-3.
- Brephohl E. & McCreight T. (ed) 2001, The Theory and Practice of Goldsmithing, C. Lewton-Brain trans., Brynmorgen Press, Portland, Maine, ISBN 978-0-9615984-9-5.
- Brown I. 1987, "Astatine: Its organonuclear chemistry and biomedical applications," in H. J. Emeléus & A. G. Sharpe (eds), Advances in Inorganic Chemistry, vol. 31, Academic Press, Orlando, pp. 43–88, ISBN 978-0-12-023631-2.
- Bryson R. M. & Hammond C. 2005, "Generic methodologies for nanotechnology: Characterisation"', in R. Kelsall, I. W. Hamley & M. Geoghegan, Nanoscale Science and Technology, John Wiley & Sons, Chichester, pp. 56–129, ISBN 978-0-470-85086-2.
- Burkett B. 2010, Sport Mechanics for Coaches, 3rd ed., Human Kinetics, Champaign, Illinois, ISBN 978-0-7360-8359-1.
- Casey C. 1993, "Restructuring work: New work and new workers in post-industrial production", in R. P. Coulter & I. F. Goodson (eds), Rethinking Vocationalism: Whose Work/life is it?, Our Schools/Our Selves Education Foundation, Toronto, ISBN 978-0-921908-15-9.
- Chakhmouradian A.R., Smith M. P. & Kynicky J. 2015, "From "strategic" tungsten to "green" neodymium: A century of critical metals at a glance", Ore Geology Reviews, vol. 64, January, pp. 455–458, doi:10.1016/j.oregeorev.2014.06.008.
- Chambers E. 1743, "Metal", in Cyclopedia: Or an Universal Dictionary of Arts and Sciences (etc.), vol. 2, D. Midwinter, London.
- Chandler D. E. & Roberson R. W. 2009, Bioimaging: Current Concepts in Light & Electron Microscopy, Jones & Bartlett Publishers, Boston, ISBN 978-0-7637-3874-7.
- Chawla N. & Chawla K. K. 2013, Metal matrix composites, 2nd ed., Springer Science+Business Media, New York, ISBN 978-1-4614-9547-5.
- Chen J. & Huang K. 2006, "A new technique for extraction of platinum group metals by pressure cyanidation", Hydrometallurgy, vol. 82, nos. 3–4, pp. 164–171, doi:10.1016/j.hydromet.2006.03.041.
- Choptuik M. W., Lehner L. & Pretorias F. 2015, "Probing strong-field gravity through numerical simulation", in A. Ashtekar, B. K. Berger, J. Isenberg & M. MacCallum (eds), General Relativity and Gravitation: A Centennial Perspective, Cambridge University Press, Cambridge, ISBN 978-1-107-03731-1.
- Clegg B 2014, "Osmium tetroxide", Chemistry World, accessed 2 September 2016.
- Close F. 2015, Nuclear Physics: A Very Short Introduction, Oxford University Press, Oxford, ISBN 978-0-19-871863-5.
- Clugston M & Flemming R 2000, Advanced Chemistry, Oxford University, Oxford, ISBN 978-0-19-914633-8.
- Cole M., Lindeque P., Halsband C. & Galloway T. S. 2011, "Microplastics as contaminants in the marine environment: A review", Marine Pollution Bulletin, vol. 62, no. 12, pp. 2588–2597, doi:10.1016/j.marpolbul.2011.09.025.
- Cole S. E. & Stuart K. R. 2000, "Nuclear and cortical histology for brightfield microscopy", in D. J. Asai & J. D. Forney (eds), Methods in Cell Biology, vol. 62, Academic Press, San Diego, pp. 313–322, ISBN 978-0-12-544164-3.
- Cotton S. A. 1997, Chemistry of Precious Metals, Blackie Academic & Professional, London, ISBN 978-94-010-7154-3.
- Cotton S. 2006, Lanthanide and Actinide Chemistry, reprinted with corrections 2007, John Wiley & Sons, Chichester, ISBN 978-0-470-01005-1.
- Cox P. A. 1997, The elements: Their Origin, Abundance and Distribution, Oxford University Press, Oxford, ISBN 978-0-19-855298-7.
- Crundwell F. K., Moats M. S., Ramachandran V., Robinson T. G. & Davenport W. G. 2011, Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, Elsevier, Kidlington, Oxford, ISBN 978-0-08-096809-4.
- Cui X-Y., Li S-W., Zhang S-J., Fan Y-Y., Ma L. Q. 2015, "Toxic metals in children's toys and jewelry: Coupling bioaccessibility with risk assessment", Environmental Pollution, vol. 200, pp. 77–84, doi:10.1016/j.envpol.2015.01.035.
- Dapena J. & Teves M. A. 1982, "Influence of the diameter of the hammer head on the distance of a hammer throw", Research Quarterly for Exercise and Sport, vol. 53, no. 1, pp. 78–81, doi:10.1080/02701367.1982.10605229.
- De Zuane J. 1997, Handbook of Drinking Water Quality, 2nd ed., John Wiley & Sons, New York, ISBN 978-0-471-28789-6.
- Department of the Navy 2009, Gulf of Alaska Navy Training Activities: Draft Environmental Impact Statement/Overseas Environmental Impact Statement, U.S. Government, accessed 21 August 2016.
- Deschlag J. O. 2011, "Nuclear fission", in A. Vértes, S. Nagy, Z. Klencsár, R. G. Lovas, F. Rösch (eds), Handbook of Nuclear Chemistry, 2nd ed., Springer Science+Business Media, Dordrecht, pp. 223–280, ISBN 978-1-4419-0719-6.
- Desoize B. 2004, "Metals and metal compounds in cancer treatment", Anticancer Research, vol. 24, no. 3a, pp. 1529–1544, PMID 15274320.
- Dev N. 2008, 'Modelling Selenium Fate and Transport in Great Salt Lake Wetlands', PhD dissertation, University of Utah, ProQuest, Ann Arbor, Michigan, ISBN 978-0-549-86542-1.
- Di Maio V. J. M. 2001, Forensic Pathology, 2nd ed., CRC Press, Boca Raton, ISBN 0-8493-0072-X.
- Di Maio V. J. M. 2016, Gunshot Wounds: Practical Aspects of Firearms, Ballistics, and Forensic Techniques, 3rd ed., CRC Press, Boca Raton, Florida, ISBN 978-1-4987-2570-5.
- John H. Duffus 2002, 'Heavy metals'—A meaningless term?", Pure and Applied Chemistry, vol. 74, no. 5, pp. 793–807, doi:10.1351/pac200274050793.
- Dunn P. 2009, Unusual metals could forge new cancer drugs, University of Warwick, accessed 23 March 2016.
- Ebbing D. D. & Gammon S. D. 2017, General Chemistry, 11th ed., Cengage Learning, Boston, ISBN 978-1-305-58034-3.
- Edelstein N. M., Fuger J., Katz J. L. & Morss L. R. 2010, "Summary and comparison of properties of the actinde and transactinide elements," in L. R. Morss, N. M. Edelstein & J. Fuger (eds), The Chemistry of the Actinide and Transactinide Elements, 4th ed., vol. 1–6, Springer, Dordrecht, pp. 1753–1835, ISBN 978-94-007-0210-3.
- Eisler R. 1993, Zinc Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review, Biological Report 10, U. S. Department of the Interior, Laurel, Maryland, accessed 2 September 2016.
- Elliott S. B. 1946, The Alkaline-earth and Heavy-metal Soaps, Reinhold Publishing Corporation, New York.
- Emsley J. 2011, Nature's Building Blocks, new edition, Oxford University Press, Oxford, ISBN 978-0-19-960563-7.
- Everts S. 2016, "What chemicals are in your tattoo", Chemical & Engineering News, vol. 94, no. 33, pp. 24–26.
- Fournier J. 1976, "Bonding and the electronic structure of the actinide metals," Journal of Physics and Chemistry of Solids, vol 37, no. 2, pp. 235–244, doi:10.1016/0022-3697(76)90167-0.
- Frick J. P. (ed.) 2000, Woldman's Engineering Alloys, 9th ed., ASM International, Materials Park, Ohio, ISBN 978-0-87170-691-1.
- Frommer H. H. & Stabulas-Savage J. J. 2014, Radiology for the Dental Professional, 9th ed., Mosby Inc., St. Louis, Missouri, ISBN 978-0-323-06401-9.
- Gidding J. C. 1973, Chemistry, Man, and Environmental Change: An Integrated Approach, Canfield Press, New York, ISBN 978-0-06-382790-5.
- Gmelin L. 1849, Hand-book of chemistry, vol. III, Metals, translated from the German by H. Watts, Cavendish Society, London.
- Goldsmith R. H. 1982, "Metalloids", Journal of Chemical Education, vol. 59, no. 6, pp. 526–527, doi:10.1021/ed059p526.
- Gorbachev V. M., Zamyatnin Y. S. & Lbov A. A. 1980, Nuclear Reactions in Heavy Elements: A Data Handbook, Pergamon Press, Oxford, ISBN 978-0-08-023595-0.
- Gordh G. & Headrick D. 2003, A Dictionary of Entomology, CABI Publishing, Wallingford, ISBN 978-0-85199-655-4.
- * Goyer RA, Clarkson TW (1996). "Toxic effects of metals". Casarett and Doull's toxicology: the basic science of poisons 5. McGraw-Hill.
- Greenberg B. R. & Patterson D. 2008, Art in Chemistry; Chemistry in Art, 2nd ed., Teachers Ideas Press, Westport, Connecticut, ISBN 978-1-59158-309-7.
- Gribbon J. 2016, 13.8: The Quest to Find the True Age of the Universe and the Theory of Everything, Yale University Press, New Haven, ISBN 978-0-300-21827-5.
- Gschneidner Jr., K. A. 1975, Inorganic compounds, in C. T. Horowitz (ed.), Scandium: Its Occurrence, Chemistry, Physics, Metallurgy, Biology and Technology, Academic Press, London, pp. 152–251, ISBN 978-0-12-355850-3.
- Guandalini G. S., Zhang L., Fornero E., Centeno J. A., Mokashi V. P., Ortiz P. A., Stockelman M. D., Osterburg A. R. & Chapman G. G. 2011, "Tissue distribution of tungsten in mice following oral exposure to sodium tungstate," Chemical Research in Toxicology, vol. 24, no. 4, pp 488–493, doi:10.1021/tx200011k.
- Guney M. & Zagury G. J. 2012, "Heavy metals in toys and low-cost jewelry: Critical review of U.S. and Canadian legislations and recommendations for testing", Environmental Science & Technology, vol. 48, pp. 1238–1246, doi:10.1021/es4036122.
- Habashi F. 2009, "Gmelin and his Handbuch" Archived 2016-04-15 at the Wayback Machine, Bulletin for the History of Chemistry, vol. 34, no. 1, pp. 30–1.
- Hadhazy A. 2016, "Galactic 'gold mine' explains the origin of nature's heaviest elements Archived 2016-05-24 at the Wayback Machine", Science Spotlights, 10 May 2016, accessed 11 July 2016.
- Hartmann W. K. 2005, Moons & Planets, 5th ed., Thomson Brooks/Cole, Belmont, California, ISBN 978-0-534-49393-6.
- Harvey P. J., Handley H. K. & Taylor M. P. 2015, "Identification of the sources of metal (lead) contamination in drinking waters in north-eastern Tasmania using lead isotopic compositions," Environmental Science and Pollution Research, vol. 22, no. 16, pp. 12276–12288, doi:10.1007/s11356-015-4349-2 PMID 25895456.
- Hasan S. E. 1996, Geology and Hazardous Waste Management, Prentice Hall, Upper Saddle River, New Jersey, ISBN 978-0-02-351682-5.
- Hawkes S. J. 1997, "What is a "heavy metal"?", Journal of Chemical Education, vol. 74, no. 11, p. 1374, doi:10.1021/ed074p1374.
- Haynes W. M. 2015, CRC Handbook of Chemistry and Physics, 96th ed., CRC Press, Boca Raton, Florida, ISBN 978-1-4822-6097-7.
- Hendrickson D. J. 2916, "Effects of early experience on brain and body", in D. Alicata, N. N. Jacobs, A. Guerrero and M. Piasecki (eds), Problem-based Behavioural Science and Psychiatry 2nd ed., Springer, Cham, pp. 33–54, ISBN 978-3-319-23669-8.
- Hermann A., Hoffmann R. & Ashcroft N. W. 2013, "Condensed astatine: Monatomic and metallic Archived 2016-03-16 at the Wayback Machine", Physical Review Letters, vol. 111, pp. 11604–1−11604-5, doi:10.1103/PhysRevLett.111.116404.
- Herron N. 2000, "Cadmium compounds," in Kirk-Othmer Encyclopedia of Chemical Technology, vol. 4, John Wiley & Sons, New York, pp. 507–523, ISBN 978-0-471-23896-6.
- Hoffman D. C., Lee D. M. & Pershina V. 2011, "Transactinide elements and future elements," in L. R. Morss, N. Edelstein, J. Fuger & J. J. Katz (eds), The Chemistry of the Actinide and Transactinide Elements, 4th ed., vol. 3, Springer, Dordrecht, pp. 1652–1752, ISBN 978-94-007-0210-3.
- Hofmann S. 2002, On Beyond Uranium: Journey to the End of the Periodic Table, Taylor & Francis, London, ISBN 978-0-415-28495-0.
- Housecroft J. E. 2008, Inorganic Chemistry, Elsevier, Burlington, Massachusetts, ISBN 978-0-12-356786-4.
- Howell N., Lavers J., Paterson D., Garrett R. & Banati R. 2012, Trace metal distribution in feathers from migratory, pelagic birds, Australian Nuclear Science and Technology Organisation, accessed 3 May 2014.
- Hübner R., Astin K. B. & Herbert R. J. H. 2010, "'Heavy metal'—time to move on from semantics to pragmatics?", Journal of Environmental Monitoring, vol. 12, pp. 1511–1514, doi:10.1039/C0EM00056F.
- Ikehata K., Jin Y., Maleky N. & Lin A. 2015, "Heavy metal pollution in water resources in China—Occurrence and public health implications", in S. K. Sharma (ed.), Heavy Metals in Water: Presence, Removal and Safety, Royal Society of Chemistry, Cambridge, pp. 141–167, ISBN 978-1-84973-885-9.
- International Antimony Association 2016, Antimony compounds, accessed 2 September 2016.
- International Platinum Group Metals Association n.d., The Primary Production of Platinum Group Metals (PGMs) Archived 2016-08-04 at the Wayback Machine, accessed 4 September 2016.
- Ismail A. F., Khulbe K. & Matsuura T. 2015, Gas Separation Membranes: Polymeric and Inorganic, Springer, Cham, Switzerland, ISBN 978-3-319-01095-3.
- IUPAC 2016, "IUPAC is naming the four new elements nihonium, moscovium, tennessine, and oganesson" accessed 27 August 2016.
- Iyengar G. V. 1998, "Reevaluation of the trace element content in Reference Man", Radiation Physics and Chemistry, vol. 51, nos 4–6, pp. 545–560, doi:10.1016/S0969-806X(97)00202-8
- Jackson J. & Summitt J. 2006, The Modern Guide to Golf Clubmaking: The Principles and Techniques of Component Golf Club Assembly and Alteration, 5th ed., Hireko Trading Company, City of Industry, California, ISBN 978-0-9619413-0-7.
- Järup L 2003, "Hazards of heavy metal contamination", British Medical Bulletin, vol. 68, no. 1, pp. 167–182, doi:10.1093/bmb/ldg032.
- Jones C. J. 2001, d- and f-Block Chemistry, Royal Society of Chemistry, Cambridge, ISBN 978-0-85404-637-9.
- Kantra S. 2001, "What's new", Popular Science, vol. 254, no. 4, April, p. 10.
- Keller C., Wolf W. & Shani J. 2012, "Radionuclides, 2. Radioactive elements and artificial radionuclides", in F. Ullmann (ed.), Ullmann's Encyclopedia of Industrial Chemistry, vol. 31, Wiley-VCH, Weinheim, pp. 89–117, doi:10.1002/14356007.o22_o15.
- King R. B. 1995, Inorganic Chemistry of Main Group Elements, Wiley-VCH, New York, ISBN 978-1-56081-679-9.
- Kolthoff I. M. & Elving P. J. FR 1964, Treatise on Analytical Chemistry, part II, vol. 6, Interscience Encyclopedia, New York, ISBN 978-0-07-038685-3.
- Korenman I. M. 1959, "Regularities in properties of thallium", Journal of General Chemistry of the USSR, English translation, Consultants Bureau, New York, vol. 29, no. 2, pp. 1366–90, ISSN 0022-1279.
- Kozin L. F. & Hansen S. C. 2013, Mercury Handbook: Chemistry, Applications and Environmental Impact, RSC Publishing, Cambridge, ISBN 978-1-84973-409-7.
- Kumar R., Srivastava P. K., Srivastava S. P. 1994, "Leaching of heavy metals (Cr, Fe, and Ni) from stainless steel utensils in food simulates and food materials", Bulletin of Environmental Contamination and Toxicology, vol. 53, no. 2, doi:10.1007/BF00192942, pp. 259–266.
- Lach K., Steer B., Gorbunov B., Mička V. & Muir R. B. 2015, "Evaluation of exposure to airborne heavy metals at gun shooting ranges", The Annals of Occupational Hygiene, vol. 59, no. 3, pp. 307–323, doi:10.1093/annhyg/meu097.
- Landis W., Sofield R. & Yu M-H. 2010, Introduction to Environmental Toxicology: Molecular Substructures to Ecological Landscapes, 4th ed., CRC Press, Boca Raton, Florida, ISBN 978-1-4398-0411-7.
- Lane T. W., Saito M. A., George G. N., Pickering, I. J., Prince R. C. & Morel F. M. M. 2005, "Biochemistry: A cadmium enzyme from a marine diatom", Nature, vol. 435, no. 7038, p. 42, doi:10.1038/435042a.
- Lee J. D. 1996, Concise Inorganic Chemistry, 5th ed., Blackwell Science, Oxford, ISBN 978-0-632-05293-6.
- Leeper G. W. 1978, Managing the Heavy Metals on the Land Marcel Dekker, New York, ISBN 0-8247-6661-X.
- Lemly A. D. 1997, "A teratogenic deformity index for evaluating impacts of selenium on fish populations", Ecotoxicology and Environmental Safety, vol. 37, no. 3, pp. 259–266, doi:10.1006/eesa.1997.1554.
- Lide D. R. (ed.) 2004, CRC Handbook of Chemistry and Physics, 85th ed., CRC Press, Boca Raton, Florida, ISBN 978-0-8493-0485-9.
- Liens J. 2010, "Heavy metals as pollutants", in B. Warf (ed.), Encyclopaedia of Geography, Sage Publications, Thousand Oaks, California, pp. 1415–1418, ISBN 978-1-4129-5697-0.
- Lima E., Guerra R., Lara V. & Guzmán A. 2013, "Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi". Chemistry Central, vol. 7:11, doi:10.1186/1752-153X-7-11 PMID 23331621 PMC 3556127.
- Litasov K. D. & Shatskiy A. F. 2016, "Composition of the Earth's core: A review", Russian Geology and Geophysics, vol. 57, no. 1, pp. 22–46, doi:10.1016/j.rgg.2016.01.003.
- Livesey A. 2012, Advanced Motorsport Engineering, Routledge, London, ISBN 978-0-7506-8908-3.
- Livingston R. A. 1991, "Influence of the Environment on the Patina of the Statue of Liberty", Environmental Science & Technology, vol. 25, no. 8, pp. 1400–1408, doi:10.1021/es00020a006.
- Longo F. R. 1974, General Chemistry: Interaction of Matter, Energy, and Man, McGraw-Hill, New York, ISBN 978-0-07-038685-3.
- Love M. 1998, Phasing Out Lead from Gasoline: Worldwide Experience and Policy Implications, World Bank Technical Paper volume 397, The World Bank, Washington DC, ISBN 0-8213-4157-X.
- Lyman W. J. 1995, "Transport and transformation processes", in Fundamentals of Aquatic Toxicology, G. M. Rand (ed.), Taylor & Francis, London, pp. 449–492, ISBN 978-1-56032-090-6.
- Macintyre J. E. 1994, Dictionary of inorganic compounds, supplement 2, Dictionary of Inorganic Compounds, vol. 7, Chapman & Hall, London, ISBN 978-0-412-49100-9.
- MacKay K. M., MacKay R. A. & Henderson W. 2002, Introduction to Modern Inorganic Chemistry, 6th ed., Nelson Thornes, Cheltenham, ISBN 978-0-7487-6420-4.
- Magee R. J. 1969, Steps to Atomic Power, Cheshire for La Trobe University, Melbourne.
- Magill F. N. I (ed.) 1992, Magill's Survey of Science, Physical Science series, vol. 3, Salem Press, Pasadena, ISBN 978-0-89356-621-0.
- Martin M. H. & Coughtrey P. J. 1982, Biological Monitoring of Heavy Metal Pollution, Applied Science Publishers, London, ISBN 978-0-85334-136-9.
- Massarani M. 2015, "Brazilian mine disaster releases dangerous metals," Chemistry World, November 2015, accessed 16 April 2016.
- Masters C. 1981, Homogenous Transition-metal Catalysis: A Gentle Art, Chapman and Hall, London, ISBN 978-0-412-22110-1.
- Matyi R. J. & Baboian R. 1986, "An X-ray Diffraction Analysis of the Patina of the Statue of Liberty", Powder Diffraction, vol. 1, no. 4, pp. 299–304, doi:10.1017/S0885715600011970.
- McColm I. J. 1994, Dictionary of Ceramic Science and Engineering, 2nd ed., Springer Science+Business Media, New York, ISBN 978-1-4419-3235-8.
- McCurdy, Richard M. (1975). Qualities and quantities: preparation for college chemistry. New York: Harcourt Brace Jovanovich. ISBN 978-0-15-574100-3.
- McLemore V. T. (ed.) 2008, Basics of Metal Mining Influenced Water, vol. 1, Society for Mining, Metallurgy, and Exploration, Littleton, Colorado, ISBN 978-0-87335-259-8.
- McQueen K. G. 2009, Regolith geochemistry, in K. M. Scott & C. F. Pain (eds), Regolith Science, CSIRO Publishing, Collingwood, Victoria, ISBN 978-0-643-09396-6.
- Mellor J. W. 1924, A comprehensive Treatise on Inorganic and Theoretical Chemistry, vol. 5, Longmans, Green and Company, London.
- Moore J. W. & Ramamoorthy S. 1984, Heavy Metals in Natural Waters: Applied Monitoring and Impact Assessment, Springer Verlag, New York, ISBN 978-1-4612-9739-0.
- Morris C. G. 1992, Academic Press Dictionary of Science and Technology, Harcourt Brace Jovanovich, San Diego, ISBN 978-0-12-200400-1.
- Morstein J. H. 2005, "Fat Man", in E. A. Croddy & Y. Y. Wirtz (eds), Weapons of Mass Destruction: An Encyclopedia of Worldwide Policy, Technology, and History, ABC-CLIO, Santa Barbara, California, ISBN 978-1-85109-495-0.
- Moselle B. (ed.) 2005, 2004 National Home Improvement Estimator, Craftsman Book Company, Carlsbad, California, ISBN 978-1-57218-150-2.
- Naja G. M. & Volesky B. 2009, "Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides", in L. K. Wang, J. P. Chen, Y. Hung & N. K. Shammas, Heavy Metals in the Environment, CRC Press, Boca Raton, Florida, ISBN 978-1-4200-7316-4.
- Nakbanpote W., Meesungneon O. & Prasad M. N. V. 2016, "Potential of ornamental plants for phytoremediation of heavy metals and income generation", in M. N. V. Prasad (ed.), Bioremediation and Bioeconomy, Elsevier, Amsterdam, pp. 179–218, ISBN 978-0-12-802830-8.
- Nathans M. W. 1963, Elementary Chemistry, Prentice Hall, Englewood Cliffs, New Jersey.
- National Materials Advisory Board 1971, Trends in the Use of Depleted Uranium, National Academy of Sciences – National Academy of Engineering, Washington DC.
- National Materials Advisory Board 1973, Trends in Usage of Tungsten, National Academy of Sciences – National Academy of Engineering, Washington DC.
- National Organization for Rare Disorders 2015, Heavy metal poisoning, accessed 3 March 2016.
- Natural Resources Canada 2015, "Generation of the Earth's magnetic field", accessed 30 August 2016.
- Nieboer E. & Richardson D. 1978, "Lichens and 'heavy metals' ", International Lichenology Newsletter, vol. 11, no. 1, pp. 1–3.
- Nieboer E. & Richardson D. H. S. 1980, "The replacement of the nondescript term 'heavy metals' by a biologically and chemically significant classification of metal ions", Environmental Pollution Series B, Chemical and Physical, vol. 1, no. 1, pp. 3–26, doi:10.1016/0143-148X(80)90017-8.
- Nzierżanowski K. & Gawroński S. W. 2012, "Heavy metal concentration in plants growing on the vicinity of railroad tracks: a pilot study", Challenges of Modern Technology, vol. 3, no. 1, pp. 42–45, ISSN 2353-4419, accessed 21 August 2016.
- Ohlendorf H. M. 2003, "Ecotoxicology of selenium", in D. J. Hoffman, B. A. Rattner, G. A. Burton & J. Cairns, Handbook of Ecotoxicology, 2nd ed., Lewis Publishers, Boca Raton, pp. 466–491, ISBN 978-1-56670-546-2.
- Ondreička R., Kortus J. & Ginter E. 1971, "Aluminium, its absorption, distribution, and effects on phosphorus metabolism", in S. C. Skoryna & D. Waldron-Edward (eds), Intestinal Absorption of Metal Ions, Trace Elements and Radionuclides, Pergamon press, Oxford.
- Ong K. L., Tan T. H. & Cheung W. L. 1997, "Potassium permanganate poisoning—a rare cause of fatal poisoning", Journal of Accident & Emergency Medicine, vol. 14, no. 1, pp. 43–45, PMC 1342846.
- Oxford English Dictionary 1989, 2nd ed., Oxford University Press, Oxford, ISBN 978-0-19-861213-1.
- Pacheco-Torgal F., Jalali S. & Fucic A. (eds) 2012, Toxicity of building materials, Woodhead Publishing, Oxford, ISBN 978-0-85709-122-2.
- Padmanabhan T. 2001, Theoretical Astrophysics, vol. 2, Stars and Stellar Systems, Cambridge University Press, Cambridge, ISBN 978-0-521-56241-6.
- Pan W. & Dai J. 2015, "ADS based on linear accelerators", in W. Chao & W. Chou (eds), Reviews of accelerator science and technology, vol. 8, Accelerator Applications in Energy and Security, World Scientific, Singapore, pp. 55–76, ISBN 981-3108-89-4.
- Parish R. V. 1977, The Metallic Elements, Longman, New York, ISBN 978-0-582-44278-8.
- Perry J. & Vanderklein E. L. Water Quality: Management of a Natural Resource, Blackwell Science, Cambridge, Massachusetts ISBN 0-86542-469-1.
- Pickering N. C. 1991, The Bowed String: Observations on the Design, Manufacture, Testing and Performance of Strings for Violins, Violas and Cellos, Amereon, Mattituck, New York.
- Podosek F. A. 2011, "Noble gases", in H. D. Holland & K. K. Turekian (eds), Isotope Geochemistry: From the Treatise on Geochemistry, Elsevier, Amsterdam, pp. 467–492, ISBN 978-0-08-096710-3.
- Podsiki C. 2008, "Heavy metals, their salts, and other compounds", AIC News, November, special insert, pp. 1–4.
- Preschel J. July 29, 2005, "Green bullets not so eco-friendly", CBS News, accessed 18 March 2016.
- Preuss P. 17 July 2011, "What keeps the Earth cooking?," Berkeley Lab, accessed 17 July 2016.
- Prieto C. 2011, The Adventures of a Cello: Revised Edition, with a New Epilogue, University of Texas Press, Austin, ISBN 978-0-292-72393-1
- Raghuram P., Soma Raju I. V. & Sriramulu J. 2010, "Heavy metals testing in active pharmaceutical ingredients: an alternate approach", Pharmazie, vol. 65, no. 1, pp. 15–18, doi:10.1691/ph.2010.9222.
- Rainbow P. S. 1991, "The biology of heavy metals in the sea", in J. Rose (ed.), Water and the Environment, Gordon and Breach Science Publishers, Philadelphia, pp. 415–432, ISBN 978-2-88124-747-7.
- Rand G. M., Wells P. G. & McCarty L. S. 1995, "Introduction to aquatic toxicology", in G. M. Rand (ed.), Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment, 2nd ed., Taylor & Francis, London, pp. 3–70, ISBN 978-1-56032-090-6.
- Rankin W. J. 2011, Minerals, Metals and Sustainability: Meeting Future Material Needs, CSIRO Publishing, Collingwood, Victoria, ISBN 978-0-643-09726-1.
- Rasic-Milutinovic Z. & Jovanovic D. 2013, "Toxic metals", in M. Ferrante, G. Oliveri Conti, Z. Rasic-Milutinovic & D. Jovanovic (eds), Health Effects of Metals and Related Substances in Drinking Water, IWA Publishing, London, ISBN 978-1-68015-557-0.
- Raymond R. 1984, Out of the Fiery Furnace: The Impact of Metals on the History of Mankind, Macmillan, South Melbourne, ISBN 978-0-333-38024-6.
- Rebhandl W., Milassin A., Brunner L., Steffan I., Benkö T., Hörmann M., Burschen J. 2007, "In vitro study of ingested coins: Leave them or retrieve them?", Journal of Paediatric Surgery, vol. 42, no. 10, pp. 1729–1734, doi:10.1016/j.jpedsurg.2007.05.031.
- Rehder D. 2010, Chemistry in Space: From Interstellar Matter to the Origin of Life, Wiley-VCH, Weinheim, ISBN 978-3-527-32689-1.
- Renner H., Schlamp G., Kleinwächter I., Drost E., Lüchow H. M., Tews P., Panster P., Diehl M., Lang J., Kreuzer T., Knödler A., Starz K. A., Dermann K., Rothaut J., Drieselmann R., Peter C. & Schiele R. 2012, "Platinum Group Metals and compounds", in F. Ullmann (ed.), Ullmann's Encyclopedia of Industrial Chemistry, vol. 28, Wiley-VCH, Weinheim, pp. 317–388, doi:10.1002/14356007.a21_075.
- Reyes J. W. 2007, Environmental Policy as Social Policy? The Impact of Childhood Lead Exposure on Crime, National Bureau of Economic Research Working Paper 13097, accessed 16 October 2016.
- Ridpath I. (ed.) 2012, Oxford Dictionary of Astronomy, 2nd ed. rev., Oxford University Press, New York, ISBN 978-0-19-960905-5.
- Rockhoff H. 2012, America's Economic Way of War: War and the US Economy from the Spanish–American War to the Persian Gulf War, Cambridge University Press, Cambridge, ISBN 978-0-521-85940-0.
- Roe J. & Roe M. 1992, "World's coinage uses 24 chemical elements", World Coinage News, vol. 19, no. 4, pp. 24–25; no. 5, pp. 18–19.
- Russell A. M. & Lee K. L. 2005, Structure–Property Relations in Nonferrous Metals, John Wiley & Sons, Hoboken, New Jersey, ISBN 978-0-471-64952-6.
- Rusyniak D. E., Arroyo A., Acciani J., Froberg B., Kao L. & Furbee B. 2010, "Heavy metal poisoning: Management of intoxication and antidotes", in A. Luch (ed.), Molecular, Clinical and Environmental Toxicology, vol. 2, Birkhäuser Verlag, Basel, pp. 365–396, ISBN 978-3-7643-8337-4.
- Ryan J. 2012, Personal Financial Literacy, 2nd ed., South-Western, Mason, Ohio, ISBN 978-0-8400-5829-4.
- Samsonov G. V. (ed.) 1968, Handbook of the Physicochemical Properties of the Elements, IFI-Plenum, New York, ISBN 978-1-4684-6066-7.
- Sanders R. 2003, "Radioactive potassium may be major heat source in Earth's core," UCBerkelyNews, 10 December, accessed 17 July 20016.
- Schweitzer P. A. 2003, Metallic materials: Physical, Mechanical, and Corrosion properties, Marcel Dekker, New York, ISBN 978-0-8247-0878-8.
- Schweitzer G. K. & Pesterfield L. L. 2010, The Aqueous Chemistry of the Elements, Oxford University Press, Oxford, ISBN 978-0-19-539335-4.
- Scott R. M. 1989, Chemical Hazards in the Workplace, CRC Press, Boca Raton, Orlando, ISBN 978-0-87371-134-0.
- Scoullos M. (ed.), Vonkeman G. H., Thornton I. & Makuch Z. 2001, Mercury — Cadmium — Lead Handbook for Sustainable Heavy Metals Policy and Regulation, Kluwer Academic Publishers, Dordrecht, ISBN 978-1-4020-0224-3.
- Selinger B. 1978, Chemistry in the Market Place, 2nd ed., Australian National University Press, Canberra, ISBN 978-0-7081-0728-7.
- Seymour R. J. & O'Farrelly J. 2012, "Platinum Group Metals", Kirk-Other Encyclopaedia of Chemical Technology, John Wiley & Sons, New York, doi:10.1002/0471238961.1612012019052513.a01.pub3.
- Shaw B. P., Sahu S. K. & Mishra R. K. 1999, "Heavy metal induced oxidative damage in terrestrial plants", in M. N. V. Prased (ed.), Heavy Metal Stress in Plants: From Biomolecules to Ecosystems Springer-Verlag, Berlin, ISBN 978-3-540-40131-5.
- Shedd K. B. 2002, "Tungsten" Archived 2019-01-11 at the Wayback Machine, Minerals Yearbook, United States Geological Survey.
- Sidgwick N. V. 1950, The Chemical Elements and their Compounds, vol. 1, Oxford University Press, London.
- Silva R. J. 2010, "Fermium, mendelevium, nobelium, and lawrencium", in L. R. Morss, N. Edelstein & J. Fuger (eds), The Chemistry of the Actinide and Transactinide Elements, vol. 3, 4th ed., Springer, Dordrecht, pp. 1621–1651, ISBN 978-94-007-0210-3.
- Spolek G. 2007, "Design and materials in fly fishing", in A. Subic (ed.), Materials in Sports Equipment, Volume 2, Woodhead Publishing, Abington, Cambridge, pp. 225–247, ISBN 978-1-84569-131-8.
- Stankovic S. & Stankocic A. R. 2013, "Bioindicators of toxic metals", in E. Lichtfouse, J. Schwarzbauer, D. Robert 2013, Green materials for energy, products and depollution, Springer, Dordrecht, ISBN 978-94-007-6835-2, pp. 151–228.
- State Water Control Resources Board 1987, Toxic substances monitoring program, issue 79, part 20 of the Water Quality Monitoring Report, Sacramento, California.
- Technical Publications 1953, Fire Engineering, vol. 111, p. 235, ISSN 0015-2587.
- The Minerals, Metals and Materials Society, Light Metals Division 2016, accessed 22 June 2016.
- The United States Pharmacopeia 1985, 21st revision, The United States Pharmacopeial Convention, Rockville, Maryland, ISBN 978-0-913595-04-6.
- Thorne P. C. L. & Roberts E. R. 1943, Fritz Ephraim Inorganic Chemistry, 4th ed., Gurney and Jackson, London.
- Tisza M. 2001, Physical Metallurgy for Engineers, ASM International, Materials Park, Ohio, ISBN 978-0-87170-725-3.
- Tokar E. J., Boyd W. A., Freedman J. H. & Wales M. P. 2013, "Toxic effects of metals", in C. D. Klaassen (ed.), Casarett and Doull's Toxicology: the Basic Science of Poisons, 8th ed., McGraw-Hill Medical, New York, ISBN 978-0-07-176923-5, accessed 9 September 2016 (subscription required).
- Tomasik P. & Ratajewicz Z. 1985, Pyridine metal complexes, vol. 14, no. 6A, The Chemistry of Heterocyclic Compounds, John Wiley & Sons, New York, ISBN 978-0-471-05073-5.
- Topp N. E. 1965, The Chemistry of the Rare-earth Elements, Elsevier Publishing Company, Amsterdam.
- Torrice M. 2016, "How lead ended up in Flint's tap water," Chemical & Engineering News, vol. 94, no. 7, pp. 26–27.
- Tretkoff E. 2006, "March 20, 1800: Volta describes the Electric Battery", APS News, This Month in Physics History, American Physical Society, accessed 26 August 2016.
- Uden P. C. 2005, 'Speciation of Selenium,' in R. Cornelis, J. Caruso, H. Crews & K. Heumann (eds), Handbook of Elemental Speciation II: Species in the Environment, Food, Medicine and Occupational Health, John Wiley & Sons, Chichester, pp. 346–65, ISBN 978-0-470-85598-0.
- United States Environmental Protection Agency 1988, Ambient Aquatic Life Water Quality Criteria for Antimony (III), draft, Office of Research and Development, Environmental Research Laboratories, Washington.
- United States Environmental Protection Agency 2014, Technical Fact Sheet–Tungsten, accessed 27 March 2016.
- United States Government 2014, Toxic Pollutant List, Code of Federal Regulations, 40 CFR 401.15., accessed 27 March 2016.
- Valkovic V. 1990, "Origin of trace element requirements by living matter", in B. Gruber & J. H. Yopp (eds), Symmetries in Science IV: Biological and biophysical systems, Plenum Press, New York, pp. 213–242, ISBN 978-1-4612-7884-9.
- VanGelder K. T. 2014, Fundamentals of Automotive Technology: Principles and Practice, Jones & Bartlett Learning, Burlington MA, ISBN 978-1-4496-7108-2.
- Venner M., Lessening M., Pankani D. & Strecker E. 2004, Identification of Research Needs Related to Highway Runoff Management, Transportation Research Board, Washington DC, ISBN 978-0-309-08815-2, accessed 21 August 2016.
- Venugopal B. & Luckey T. D. 1978, Metal Toxicity in Mammals, vol. 2, Plenum Press, New York, ISBN 978-0-306-37177-6.
- Vernon R. E. 2013, "Which elements are metalloids", Journal of Chemical Education, vol. 90, no. 12, pp. 1703–1707, doi:10.1021/ed3008457.
- Volesky B. 1990, Biosorption of Heavy Metals, CRC Press, Boca Raton, ISBN 978-0-8493-4917-1.
- von Gleich A. 2013, "Outlines of a sustainable metals industry", in A. von Gleich, R. U. Ayres & S. Gößling-Reisemann (eds), Sustainable Metals Management, Springer, Dordrecht, pp. 3–40, ISBN 978-1-4020-4007-8.
- von Zeerleder A. 1949, Technology of Light Metals, Elsevier Publishing Company, New York.
- Warth A. H. 1956, The Chemistry and Technology of Waxes, Reinhold Publishing Corporation, New York.
- Weart S. R. 1983, "The discovery of nuclear fission and a nuclear physics paradigm", in W. Shea (ed.), Otto Hahn and the Rise of Nuclear Physics, D. Reidel Publishing Company, Dordrecht, pp. 91–133, ISBN 978-90-277-1584-5.
- Weber D. J. & Rutula W. A. 2001, "Use of metals as microbicides in preventing infections in healthcare", in Disinfection, Sterilization, and Preservation, 5th ed., S. S. Block (ed.), Lippincott, Williams & Wilkins, Philadelphia, ISBN 978-0-683-30740-5.
- Welter G. 1976, Cleaning and Preservation of Coins and Medals, S. J. Durst, New York, ISBN 978-0-915262-03-8.
- White C. 2010, Projectile Dynamics in Sport: Principles and Applications, Routledge, London, ISBN 978-0-415-47331-6.
- Wiberg N. 2001, Inorganic Chemistry, Academic Press, San Diego, ISBN 978-0-12-352651-9.
- Wijayawardena M. A. A., Megharaj M. & Naidu R. 2016, "Exposure, toxicity, health impacts and bioavailability of heavy metal mixtures", in D. L. Sparks, Advances in Agronomy, vol. 138, pp. 175–234, Academic Press, London, ISBN 978-0-12-804774-3.
- Wingerson L. 1986, "America cleans up Liberty[permanent dead link]", New Scientist, 25 December/1 January 1987, pp. 31–35, accessed 1 October 2016.
- Wong M. Y., Hedley G. J., Xie G., Kölln L. S, Samuel I. D. W., Pertegaś A., Bolink H. J., Mosman-Colman, E., "Light-emitting electrochemical cells and solution-processed organic light-emitting diodes using small molecule organic thermally activated delayed fluorescence emitters", Chemistry of Materials, vol. 27, no. 19, pp. 6535–6542, doi:10.1021/acs.chemmater.5b03245.
- Wulfsberg G. 1987, Principles of Descriptive Inorganic Chemistry, Brooks/Cole Publishing Company, Monterey, California, ISBN 978-0-534-07494-4.
- Wulfsberg G. 2000, Inorganic Chemistry, University Science Books, Sausalito, California, ISBN 978-1-891389-01-6.
- Yadav J. S., Antony A., Subba Reddy, B. V. 2012, "Bismuth(III) salts as synthetic tools in organic transformations", in T. Ollevier (ed.), Bismuth-mediated Organic Reactions, Topics in Current Chemistry 311, Springer, Heidelberg, ISBN 978-3-642-27238-7.
- Yang D. J., Jolly W. L. & O'Keefe A. 1977, "Conversion of hydrous germanium(II) oxide to germynyl sesquioxide, (HGe)2O3", 'Inorganic Chemistry, vol. 16, no. 11, pp. 2980–2982, doi:10.1021/ic50177a070.
- Yousif N. 2007, Geochemistry of stream sediment from the state of Colorado using NURE data, ETD Collection for the University of Texas, El Paso, paper AAI3273991.
Further reading
[edit]Definition and usage
- Ali H. & Khan E. 2017, "What are heavy metals? Long-standing controversy over the scientific use of the term 'heavy metals'—proposal of a comprehensive definition", Toxicological & Environmental Chemistry, pp. 1–25, doi:10.1080/02772248.2017.1413652. Suggests defining heavy metals as "naturally occurring metals having atomic number (Z) greater than 20 and an elemental density greater than 5 g cm−3".
- Duffus J. H. 2002, "'Heavy metals'—A meaningless term?", Pure and Applied Chemistry, vol. 74, no. 5, pp. 793–807, doi:10.1351/pac200274050793. Includes a survey of the term's various meanings.
- Hawkes S. J. 1997, "What is a 'heavy metal'?", Journal of Chemical Education, vol. 74, no. 11, p. 1374, doi:10.1021/ed074p1374. A chemist's perspective.
- Hübner R., Astin K. B. & Herbert R. J. H. 2010, "'Heavy metal'—time to move on from semantics to pragmatics?", Journal of Environmental Monitoring, vol. 12, pp. 1511–1514, doi:10.1039/C0EM00056F. Finds that, despite its lack of specificity, the term appears to have become part of the language of science.
Toxicity and biological role
- Baird C. & Cann M. 2012, Environmental Chemistry, 5th ed., chapter 12, "Toxic heavy metals", W. H. Freeman and Company, New York, ISBN 1-4292-7704-1. Discusses the use, toxicity, and distribution of Hg, Pb, Cd, As, and Cr.
- Nieboer E. & Richardson D. H. S. 1980, "The replacement of the nondescript term 'heavy metals' by a biologically and chemically significant classification of metal ions", Environmental Pollution Series B, Chemical and Physical, vol. 1, no. 1, pp. 3–26, doi:10.1016/0143-148X(80)90017-8. A widely cited paper, focusing on the biological role of heavy metals.
- Pyatha, S., Kim, H., Lee, D., & Kim, K. (2022). "Association Between Heavy Metal Exposure and Parkinson's Disease: A Review of the Mechanisms Related to Oxidative Stress". Antioxidants 11(12): 2467.
Formation
- Hadhazy A. 2016, "Galactic 'gold mine' explains the origin of nature's heaviest elements Archived 2016-05-24 at the Wayback Machine", Science Spotlights, 10 May, accessed 11 July 2016
Uses
- Koehler C. S. W. 2001, "Heavy metal medicine", Chemistry Chronicles, American Chemical Society, accessed 11 July 2016
- Morowitz N. 2006, "The heavy metals", Modern Marvels, season 12, episode 14, HistoryChannel.com
- Öhrström L. 2014, "Tantalum oxide", Chemistry World, 24 September, accessed 4 October 2016. The author explains how tantalum(V) oxide banished brick-sized mobile phones. Also available as a podcast.
External links
[edit] Media related to Heavy metals at Wikimedia Commons
Media related to Heavy metals at Wikimedia Commons
Heavy metals
View on GrokipediaHeavy metals denote a heterogeneous class of metallic elements and metalloids distinguished by their elevated densities—typically at least five times that of water—and high atomic masses, though the designation remains imprecise and context-dependent in scientific usage, often prioritizing toxicity over strict physicochemical criteria.[1][2] Common exemplars include mercury, lead, cadmium, arsenic, and chromium, which persist in the environment due to low biodegradability and accumulate via industrial emissions, mining, and agricultural applications.[3] While certain heavy metals like iron and zinc fulfill essential physiological roles in trace quantities, non-essential variants exert toxicity through mechanisms such as oxidative stress, enzyme inhibition, and genotoxicity, manifesting in neurological deficits, renal damage, and carcinogenesis upon chronic exposure.[4][5] Defining characteristics encompass lustrous appearances, malleability, ductility, and conductivity, alongside applications in electronics, alloys, pigments, and batteries, which have amplified anthropogenic dispersal and prompted regulatory scrutiny over bioaccumulation in ecosystems and human tissues.[6][7] Empirical assessments underscore speciation's role in hazard profiles—e.g., inorganic versus organic mercury—challenging blanket characterizations of "heaviness" as causal for detriment, with causal chains linking exposure dosage, duration, and bioavailability to outcomes rather than inherent elemental mass alone.[8][9]
Definitions and Classification
Physical and Chemical Criteria
Heavy metals are classified based on physical properties such as high relative density and atomic mass, with density commonly exceeding 5 g/cm³ (five times that of water) serving as a primary empirical threshold to distinguish them from lighter elements.[1] [10] This criterion emphasizes measurable mass per unit volume under standard conditions, excluding low-density metals like aluminum at 2.70 g/cm³.[11] Exemplary densities include lead at 11.34 g/cm³ and mercury at 13.57 g/cm³, reflecting the compact atomic packing typical of these elements.[12] [13] Atomic mass provides a complementary chemical criterion, often ranging from approximately 63.5 u (copper) to 200.6 u (mercury), aligning with elements in the d-block (transition metals) and heavier p-block post-transition metals.[14] These positions in the periodic table correlate with increased electron shells and nuclear charge, contributing to elevated atomic weights and densities without invoking biological effects.[15] Empirical measurements, such as those from standard reference data, confirm this range for metallic elements exhibiting these traits, excluding non-metals or gases regardless of mass.[16] Borderline cases, such as iron (density 7.87 g/cm³, atomic mass 55.85 u), copper (8.96 g/cm³, 63.55 u), and zinc (7.14 g/cm³, 65.38 u), are included in some classifications due to densities surpassing the 5 g/cm³ threshold, though stricter cutoffs near 7 g/cm³ may exclude lighter transition metals.[17] [18] This empirical approach prioritizes verifiable physical data over arbitrary lists, ensuring classification rests on atomic structure and measurable properties like lattice stability in solid phases.[19]| Element | Atomic Mass (u) | Density (g/cm³) |
|---|---|---|
| Iron | 55.85 | 7.87 |
| Copper | 63.55 | 8.96 |
| Zinc | 65.38 | 7.14 |
| Lead | 207.2 | 11.34 |
| Mercury | 200.59 | 13.57 |
Toxicological and Regulatory Perspectives
Regulatory bodies and toxicological frameworks classify heavy metals primarily through lists of environmental pollutants of concern, prioritizing attributes like persistence, bioaccumulation, and dose-dependent toxicity over density or atomic weight. The U.S. Environmental Protection Agency (EPA) designates arsenic, cadmium, chromium, lead, and mercury as key priority pollutants under the Clean Water Act, selected for their potential to bioaccumulate in aquatic ecosystems and cause chronic health effects at elevated exposures, irrespective of physical properties.[20][1] Copper, nickel, and zinc are also included in expanded EPA monitoring due to observed ecosystem risks, though their essentiality complicates uniform treatment.[21] International standards exhibit variations in thresholds, reflecting differences in risk assessment methodologies and data interpretations. The World Health Organization (WHO) establishes guideline values for drinking water based on toxicological endpoints, such as 10 μg/L for arsenic (provisional, accounting for analytical limits and carcinogenicity), 3 μg/L for cadmium (to prevent renal damage), 50 μg/L for total chromium, 10 μg/L for lead (neurodevelopmental risks), and 6 μg/L for inorganic mercury (methylmercury neurotoxicity). In contrast, the European Union (EU) enforces stricter soil limits via Directive 86/278/EEC for sewage sludge application, capping cadmium at 1–3 mg/kg dry matter depending on soil pH and crop type to avert long-term accumulation, while water standards align closely with WHO but incorporate additional bioavailability factors.[22] These discrepancies arise from region-specific exposure models and policy priorities, such as the EU's emphasis on agricultural soil protection.[23] Such regulatory categorizations often impose broad-brush approaches that conflate non-essential toxins with essential elements, potentially fostering overregulation without nuanced consideration of exposure thresholds. For example, zinc—critical for immune function and DNA synthesis—is regulated alongside mercury despite its lower inherent toxicity at nutritional levels, as evidenced by WHO's 3 mg/L provisional guideline driven by taste rather than acute risk.[24] Chromium further illustrates valence-specific discrepancies: trivalent Cr(III) supports glucose metabolism as a trace nutrient, whereas hexavalent Cr(VI) induces carcinogenicity via reactive oxygen species and DNA adducts, yet many standards regulate total chromium without differentiating oxidation states, overlooking natural detoxification pathways that convert Cr(VI) to less harmful Cr(III).[25][26] This imprecision stems from policy needs for simplified enforcement, but it deviates from toxicological principles like Paracelsus' dose-response axiom, where toxicity hinges on concentration rather than elemental identity alone.[27] Critics argue the "heavy metals" label itself lacks scientific rigor, arbitrarily grouping disparate elements and metalloids, which undermines precise risk communication and may inflate perceived hazards for benign exposures.[28]Debates on Terminology
The term "heavy metals" originated in 19th-century metallurgical contexts to describe metals with high densities, such as those used in ores separable by gravity methods, but lacked a precise chemical definition from the outset.[29] By the mid-20th century, its usage expanded into analytical chemistry and toxicology, influenced by environmental concerns emerging in the 1960s and 1970s, where it increasingly connoted toxicity rather than purely physical properties, leading to conflation of density with biological harm.[27] This shift, accelerated by regulatory and media emphasis on pollutants like lead and mercury following events such as the 1970s Minamata Bay disaster, embedded the term in policy and public discourse, often without rigorous boundaries.[30] Critics, including the International Union of Pure and Applied Chemistry (IUPAC), have deemed "heavy metals" meaningless due to its inconsistent application across scientific fields; definitions vary from density thresholds (e.g., >5 g/cm³) to atomic number (>20 or >63), yet encompass essential non-toxic elements like iron (density 7.87 g/cm³, vital for hemoglobin) while excluding lighter toxicants such as beryllium (density 1.85 g/cm³, a known carcinogen) or arsenic (a metalloid often grouped in despite not being a true metal).[27] The term's ambiguity fosters misleading generalizations, as toxicity arises from bioavailability, dose, and chemical form rather than density alone—e.g., copper is essential at trace levels but toxic in excess, defying a unified "heavy" category.[31] Nieboer and Richardson argued in 1980 that it obscures chemically significant distinctions, proposing classification by metal ion affinity (class A oxygen-seekers like calcium vs. class B sulfur-seekers like mercury) over vague gravimetric criteria. Proposals for alternatives emphasize precision to disentangle physical traits from toxicological implications; IUPAC recommends avoiding the term entirely in favor of specific element lists or context-defined groups like "transition metals" for d-block elements with variable oxidation states.[27] Recent suggestions include "potentially toxic elements" (PTEs) to highlight risk without implying uniform density or metalloid inclusion, as advanced by Pourret and Hursthouse in 2019, arguing it better aligns with empirical ecotoxicology where selenium (density 4.79 g/cm³) poses threats akin to cadmium despite borderline "heaviness."[28] Ali and Khan's 2018 attempt to redefine it as metals with atomic number >20 and density >5 g/cm³ retains utility for broad geochemical surveys but fails to resolve core inconsistencies, per subsequent critiques.[30] These debates underscore a preference for first-principles categorization—e.g., by periodic group or speciation—over legacy terms that prioritize convenience amid environmental narratives.[32]Physical and Chemical Properties
Density and Atomic Characteristics
Heavy metals are distinguished by atomic numbers typically greater than 20, corresponding to high atomic masses that underpin their dense structures.[2] These elements, primarily from the d- and f-blocks, feature electron configurations with partially filled orbitals, such as the d^6 configuration in iron enabling paramagnetism from unpaired electrons, or the relativistic effects in gold's 6s^1 5d^10 setup contributing to its malleability and ductility. Densities of heavy metals generally exceed 5 g/cm³, markedly higher than water's 1 g/cm³, with extremes like osmium at 22.59 g/cm³ and tungsten at 19.25 g/cm³ facilitating applications demanding substantial mass per volume, including radiation shielding.[33][34] Lanthanides exemplify variations, classified as heavy due to atomic masses from 140 to 173 u despite densities of 6.1–9.8 g/cm³, lower than peak transition metals but elevated relative to lighter elements.[35] [36][33][34]Reactivity, Bonding, and Stability
Heavy metals, particularly those in the d-block, display variable oxidation states arising from the comparable energies of (n-1)d and ns orbitals, enabling the loss of electrons from both valence shells. Manganese exemplifies this, exhibiting states from +2 to +7, with the +2 state being the most thermodynamically stable due to the half-filled d^5 configuration that confers electronic stability.[37][37] Higher states like +7 in permanganate (MnO_4^-) are stabilized by strong π-bonding with oxygen ligands, reflecting the influence of ligand field effects on orbital energies.[37] In coordination chemistry, these metals form complexes with high coordination numbers—often 6 or higher—facilitated by their larger atomic radii and diffuse d-orbitals, which accommodate multiple ligands via σ- and π-bonding. Platinum(II), a d^8 ion, preferentially adopts square planar geometry due to crystal field stabilization energy (CFSE) that favors this arrangement over tetrahedral, with splitting parameters (Δ) exceeding 20,000 cm^{-1} for strong-field ligands like CN^-.[38][38] This geometry promotes selective reactivity, such as trans influence in substitution reactions, where axial ligands are labilized by strong trans donors like phosphines. Relativistic quantum effects become pronounced in heavier atoms, causing s-orbital contraction and d-orbital expansion; for gold, the 6s orbital contracts by approximately 16-20%, elevating its ionization energy to 890 kJ/mol and rendering it chemically inert relative to silver or copper analogs, as the stabilized s electrons resist oxidation.[39][40] This contrasts with lighter 3d metals, where such effects are negligible, leading to greater reactivity. The thermodynamic stability of metal-ligand bonds in chelates is governed by formation constants (β_n), where log β values for heavy metal-EDTA complexes often exceed 15-20, driven by ΔG = -RT ln β and enhanced by the chelate effect's entropic contribution (ΔS > 0 from monodentate ligand displacement).[41][41] For multidentate ligands, stability increases with metal ion charge and decreases with size in softer acids per HSAB theory, though kinetic inertness in octahedral d^3 or d^6 low-spin complexes arises from high activation barriers for ligand dissociation.[42]Geological Occurrence and Extraction
Natural Abundance and Formation
Heavy metals form primarily through processes of stellar nucleosynthesis, with elements lighter than and including iron produced via nuclear fusion in the cores of massive stars during their main-sequence and advanced evolutionary phases.[43] Heavier elements, such as lead and those beyond, arise predominantly from the rapid neutron-capture process (r-process), which occurs in extreme astrophysical events like core-collapse supernovae and neutron star mergers, where neutron fluxes enable the synthesis of neutron-rich isotopes that decay into stable heavy nuclei.[44] These cosmic processes dispersed heavy metals into the interstellar medium, from which they were accreted into the protoplanetary disk and incorporated into Earth's formation approximately 4.54 billion years ago, establishing their primordial distribution throughout the planet's mantle, core, and crust.[45] In the Earth's crust, heavy metals exhibit varying degrees of abundance, reflecting both primordial endowments and subsequent geochemical differentiation, with iron being highly ubiquitous while others like copper occur at trace levels. Iron comprises about 5.6% by weight of the continental crust, ranking it among the most abundant elements after oxygen, silicon, and aluminum. Copper, zinc, and lead are present at much lower concentrations, typically 50–60 ppm, 70–80 ppm, and 10–15 ppm, respectively, underscoring their dispersion rather than rarity in geological contexts. These baseline crustal levels, derived from petrogenic analyses, highlight the natural prevalence of heavy metals independent of anthropogenic concentration. Geological processes concentrate these dispersed elements into economically viable ore deposits through magmatic, hydrothermal, and sedimentary mechanisms. Hydrothermal deposits, such as porphyry copper systems, form via hot, metal-laden fluids emanating from cooling magmatic intrusions, precipitating sulfides like chalcopyrite in stockwork veins within altered host rocks.[46] In contrast, sedimentary deposits include marine manganese nodules, polymetallic concretions on ocean floors enriched in manganese, nickel, and copper through slow precipitation from seawater under low-oxygen conditions over millions of years. Volcanic activity further contributes to natural enrichment, with degassing and eruptions releasing mercury at rates of approximately 37–57 tons per year globally, establishing pre-industrial atmospheric and soil baselines that persist in non-anthropogenically influenced environments.[47]| Element | Crustal Abundance (wt%) | Primary Geological Concentration Process |
|---|---|---|
| Iron (Fe) | ~5.6% | Magmatic segregation and hydrothermal |
| Copper (Cu) | ~0.005% (50 ppm) | Hydrothermal (porphyry) |
| Zinc (Zn) | ~0.007% (70 ppm) | Sedimentary and hydrothermal |
| Lead (Pb) | ~0.001% (10 ppm) | Hydrothermal veins |
| Manganese (Mn) | ~0.095% (950 ppm) | Sedimentary nodules |
Mining Techniques and Production
Heavy metals are primarily extracted from sulfide ores through a combination of mining and beneficiation processes tailored to ore type and depth. Underground mining predominates for lead and zinc ores, such as galena (PbS) and sphalerite (ZnS), due to their occurrence in deep vein deposits, involving drilling, blasting, and haulage to surface mills.[48] Open-pit methods apply to shallower deposits, as seen in some copper sulfide operations, enabling large-scale excavation with lower labor intensity but higher material movement volumes.[49] Ore concentration typically begins with froth flotation for sulfide-bearing heavy metals, where crushed ore is mixed with water and reagents to selectively attach hydrophobic collectors like xanthates to mineral surfaces, forming a froth rich in target metals that is skimmed off. This method achieves high selectivity for chalcopyrite (CuFeS₂), the chief copper sulfide ore, separating it from gangue and pyrite through pH control and depressants, with recovery rates often exceeding 85% in optimized circuits.[50][51] Similar flotation sequences recover lead and zinc sulfides, followed by differential cleaning to produce separate concentrates.[52] Metal production from concentrates employs pyrometallurgical smelting for many sulfides, roasting ores to convert sulfides to oxides before reduction with carbon in furnaces, yielding matte intermediates refined electrolytically. Hydrometallurgical routes, involving acid leaching and solvent extraction, suit nickel laterite ores (oxidized deposits) via high-pressure acid leaching (HPAL) at 250°C, dissolving nickel into solution for precipitation as hydroxide, offering scalability for low-grade feeds where pyrometallurgy is energy-intensive.[53][54] Byproduct recovery enhances efficiency, as silver is co-extracted from lead-zinc flotation tailings through selective leaching or smelting, minimizing waste and utilizing trace concentrations (often 50-200 g/t) that would otherwise be uneconomic.[55][56] Recent advances include bioleaching, leveraging acidophilic bacteria like Acidithiobacillus ferrooxidans to oxidize sulfides in low-grade ores (<1% metal), enabling extraction from refractory or dilute deposits via heap or tank processes with lower energy demands than traditional roasting. Commercial applications for copper and zinc bioleaching expanded in the 2010s, recovering up to 80% of metals from tailings through microbial generation of ferric iron and sulfuric acid as lixiviants.[57][58] These techniques underscore engineering progress in processing diminishing high-grade reserves, prioritizing yield per unit ore over conventional high-temperature methods.[59]Global Reserves and Supply Dynamics
World reserves of heavy metals, as estimated by geological surveys, indicate substantial proven quantities sufficient to meet current demand for decades to centuries when accounting for recycling and undiscovered resources, challenging claims of acute global shortages. For lead, proven reserves stand at approximately 85 million metric tons, with annual mine production around 4.5 million metric tons, yielding a static reserve-to-production ratio exceeding 18 years; however, extensive resources and secondary recovery extend viability far longer.[60] Similarly, copper reserves total about 890 million metric tons against production of roughly 22 million metric tons annually, supporting a ratio over 40 years, bolstered by recycling rates often surpassing 50% in developed economies.[61] Cobalt reserves are estimated at 8.3 million metric tons, with production at 170,000 metric tons per year, but resources exceed 25 million metric tons, mitigating short-term depletion risks despite concentrated supply sources.[62] Economic extraction hinges on ore concentrations far exceeding crustal abundances, typically by factors of 100 to 1,000 or more, enabling viability through geological enrichment processes. Crustal abundance for copper averages 50 parts per million, yet economically mineable deposits require grades of 0.5% to 1% (5,000–10,000 ppm), reflecting natural hydrothermal or sedimentary concentration.[63] For lead, crustal levels are about 10 ppm, but viable ores in Mississippi Valley-type deposits grade 1–5% or higher, often as galena (PbS). These thresholds determine feasibility, with declining grades increasing energy costs but not precluding long-term supply given technological advances in processing lower-grade ores. Supply dynamics are shaped by geopolitical concentrations, amplifying risks from political instability or export controls rather than absolute scarcity. The Democratic Republic of Congo accounts for over 70% of global cobalt production, tied to sediment-hosted copper-cobalt deposits, exposing supplies to regional conflict and governance issues.[62] Copper production is more diversified, led by Chile (28%) and Peru (10%), though processing dominance by China influences prices.[61] Recycling plays a critical role in resilience, with secondary lead comprising up to 50% of supply in the U.S. and copper scrap recycling averting the need for equivalent primary mining, reducing net import reliance.[64]| Metal | Proven Reserves (million metric tons) | Annual Production (million metric tons) | Reserve/Production Ratio (years) | Key Recycling Insight |
|---|---|---|---|---|
| Lead | 85 | 4.5 | ~19 | Secondary production ~50% of U.S. supply[60] |
| Copper | 890 | 22 | ~40 | Global recycling >50% in end-use sectors[65] |
| Cobalt | 8.3 | 0.17 | ~49 | Limited recycling (~10%), resources 25 Mt total[62] |
Biological Roles and Essentiality
Functions of Essential Heavy Metals
Essential heavy metals, including iron (Fe), zinc (Zn), copper (Cu), and manganese (Mn), serve as indispensable cofactors in enzymes and proteins central to fundamental life processes such as respiration, catalysis, and redox reactions. These elements, required in trace amounts due to their integration into ancient metabolic pathways, enable efficient electron transfer and substrate activation that non-metallic alternatives cannot replicate, underscoring their evolutionary conservation across organisms.[66][67] Iron functions primarily in oxygen transport via hemoglobin, where Fe²⁺ in the heme prosthetic group reversibly binds O₂, facilitating its delivery to tissues; this role extends to myoglobin and non-heme iron proteins like aconitase in the citric acid cycle.[68] Copper contributes to aerobic respiration as a component of cytochrome c oxidase (COX), the terminal complex in the mitochondrial electron transport chain, where its CuA and CuB centers mediate four-electron reduction of O₂ to water, preventing toxic superoxide formation.[69] Zinc acts as a structural and catalytic ion in over 300 enzymes, stabilizing active sites and polarizing substrates; for instance, in carbonic anhydrase, Zn²⁺ coordinates water to generate a nucleophilic hydroxide for CO₂ hydration to bicarbonate, essential for pH regulation and CO₂ transport.[70] Manganese supports enzymatic functions in metabolism and antioxidant defense, notably as Mn²⁺ or Mn³⁺ in arginase for urea cycle nitrogen disposal and in mitochondrial superoxide dismutase for dismutation of O₂⁻ to H₂O₂. These metals' synergies and competitions, such as Fe-Zn rivalry at intestinal transporters like DMT1, reflect coordinated homeostasis to maintain optimal cofactor loading without excess.[71] Daily trace requirements are minimal yet precise: RDAs for Fe are 8 mg for adult men and 18 mg for premenopausal women, while AIs for Mn are 2.3 mg for men and 1.8 mg for women, calibrated to support these biochemical imperatives across populations.[68][72] Genetic disruptions affirm indispensability; for example, ATP7A mutations in Menkes disease impair Cu efflux from enterocytes and delivery to COX, halting mitochondrial respiration and revealing Cu's non-redundant role in energy production. Such defects demonstrate how precise metal trafficking, evolved for cofactor assembly, underpins organismal viability.[73]Deficiency Effects and Requirements
Iron deficiency, the most prevalent micronutrient deficiency worldwide, manifests primarily as iron-deficiency anemia, characterized by reduced hemoglobin production leading to fatigue, weakness, and impaired cognitive and physical development. Globally, anemia affected approximately 1.92 billion people in 2021, representing 24.3% of the population, with dietary iron deficiency as the leading cause contributing to years lived with disability. In children under five and pregnant women, prevalence remains high, exacerbating risks of stunted growth and maternal mortality. The recommended dietary allowance (RDA) for iron is 8 mg/day for adult men and postmenopausal women, increasing to 18 mg/day for premenopausal women due to menstrual losses, and 27 mg/day during pregnancy, as established by the Institute of Medicine.[74] Zinc deficiency impairs immune function, DNA synthesis, and growth, particularly in children, where it contributes to stunting—a condition affecting height-for-age in over 155 million children under five globally. An estimated 17% of the world's population is at risk of inadequate zinc intake, with higher burdens in low-income regions reliant on plant-based diets low in bioavailable zinc. In the soil-plant-human chain, alkaline soils (pH >7) reduce zinc solubility and plant uptake, leading to lower concentrations in crops like cereals and subsequent dietary shortfalls in human populations dependent on those staples. The RDA for zinc is 11 mg/day for adult men and 8 mg/day for women, with higher needs (12 mg/day) during lactation to support enzymatic roles in metabolism.[75][76][77] Copper deficiency, though less common due to wider dietary distribution, causes hematological issues such as anemia and neutropenia, alongside neurological symptoms like myelopathy and ataxia from impaired myelin formation. It arises from prolonged inadequate intake or malabsorption, affecting red blood cell production and connective tissue integrity. The RDA for copper is 900 micrograms per day for adults, sufficient to prevent deficiency in most populations consuming varied diets including nuts, shellfish, and organ meats. Empirical trials demonstrate supplementation efficacy; for instance, iron fortification programs have reduced anemia prevalence by 20-50% in targeted groups, with meta-analyses confirming improved hemoglobin levels and lower deficiency rates in fortified versus unfortified communities. Similarly, zinc supplementation in deficient children has mitigated stunting risks by enhancing linear growth in controlled studies.[78][79][80]Interactions with Organisms
Heavy metals interact with organisms through incorporation into metalloproteins that perform critical enzymatic functions, such as superoxide dismutases (SODs) containing manganese (Mn-SOD), copper-zinc (Cu/Zn-SOD), or iron, which catalyze the dismutation of superoxide radicals (O₂⁻) into hydrogen peroxide (H₂O₂) and oxygen (O₂), thereby quenching reactive oxygen species (ROS) and mitigating oxidative stress.[81][82] These enzymes are ubiquitous across prokaryotes and eukaryotes, enabling cellular protection against endogenous ROS generated during metabolism.[83] Organisms maintain metal homeostasis via specialized transporters, exemplified by the ZIP (Zrt-/Irt-like protein) family, which facilitates influx of zinc (Zn²⁺) and other divalent cations across plasma and intracellular membranes to regulate cytosolic concentrations and prevent toxicity or deficiency.[84][85] ZIP transporters exhibit evolutionary conservation from bacteria and fungi to plants and animals, reflecting ancient adaptations for metal acquisition in nutrient-limited environments.[86][87] In microbial communities, heavy metals support adaptive mechanisms like dissimilatory reduction of hexavalent chromium (Cr(VI)) to trivalent chromium (Cr(III)) by bacteria such as Pseudomonas and Bacillus species, employing chromate reductases that utilize electron donors like NADH, thereby detoxifying the metalloid while potentially gaining energy or resistance advantages.[88][89] Facultative anaerobes enhance Cr(VI) reduction under low-oxygen conditions, linking metal transformation to microbial respiration strategies.[90] Symbiotic interactions extend to gut microbiota, where iron (Fe) modulates community dynamics by favoring proliferation of pathogenic bacteria like Salmonella and Escherichia coli, which upregulate siderophore production and virulence factors in response to host-derived Fe availability, altering competition with commensals.[91][92] Elevated luminal Fe from dietary sources disrupts microbiota balance, promoting pathobiont overgrowth via enhanced metabolic fitness.[93] Low-dose exposure to heavy metals can elicit hormesis, a biphasic response where subtoxic concentrations induce adaptive stress pathways, such as upregulated antioxidant enzyme expression or metabolic shifts, yielding benefits like enhanced growth or resilience in plants and microbes exposed to trace cadmium or arsenic.[94][95] This phenomenon arises from overcompensation to mild perturbations, conserved across taxa as a survival mechanism against fluctuating environmental metals.[96]Human Health Effects
Essentiality, Deficiency, and Optimal Intake
Certain heavy metals, including iron, zinc, copper, and manganese, are essential micronutrients required in trace amounts for human enzymatic functions, oxygen transport, immune response, and antioxidant defense.[68][97][78][98] Deficiency arises from inadequate dietary intake, malabsorption, or increased losses, leading to impaired health outcomes, while optimal physiological levels confer net benefits by supporting vital processes without toxicity risks at those doses.[99] The dose-response relationship follows a J- or U-shaped curve for mortality, where both sub-physiological deficiency and supra-physiological excess elevate risks, embodying Paracelsus' principle that "the dose makes the poison."[100][101] Iron is essential for hemoglobin synthesis and myoglobin function; the recommended dietary allowance (RDA) is 8 mg/day for adult men and postmenopausal women, rising to 18 mg/day for premenopausal women due to menstrual losses.[68] Deficiency, prevalent in populations with low heme iron intake or chronic blood loss, manifests as anemia, fatigue, cognitive impairment, and weakened immunity, with serum ferritin below 30 mcg/L indicating depleted stores and values under 10 mcg/L signaling severe depletion.[68] Optimal serum ferritin ranges from 30-300 mcg/L in adults to balance deficiency prevention against overload risks, as evidenced by population data showing higher morbidity from anemia in deficient groups compared to controlled supplementation benefits.[68][102] Zinc supports DNA synthesis, protein metabolism, and wound healing via over 300 enzymes; the RDA is 11 mg/day for men and 8 mg/day for women.[97] National Health and Nutrition Examination Survey (NHANES) data from 2005-2016 indicate that approximately 15% of U.S. adults aged 19 and older have zinc intakes below the estimated average requirement (EAR), correlating with elevated infection susceptibility, growth stunting in children, and dermatitis.[97] Plasma zinc below 70 mcg/dL serves as a biomarker for inadequacy, though inflammation-adjusted measures are preferred; optimal intake maintains serum levels of 80-120 mcg/dL to minimize deficiency without excess-induced copper antagonism.[97][103] Copper functions in cytochrome c oxidase for energy production and superoxide dismutase for antioxidation; the RDA is 900 mcg/day for adults.[78] Deficiency, though uncommon, presents with neutropenia, anemia unresponsive to iron, ataxia, and hypopigmentation, often linked to excessive zinc supplementation or malabsorption disorders like Menkes disease.[78][104] Serum copper of 70-140 mcg/dL and ceruloplasmin above 20 mg/dL indicate adequacy, with intakes calibrated to avoid the J-shaped risk where deficiency impairs collagen cross-linking and excess promotes oxidative damage.[78][105] Manganese aids in glycosylation and bone formation through enzymes like arginase; no RDA exists, but the adequate intake (AI) is 2.3 mg/day for men and 1.8 mg/day for women.[98] Human deficiency is rare due to ubiquitous dietary sources, but experimental cases show dermatological issues, hypocholesterolemia, and impaired glucose tolerance, with plasma levels below 4 mcg/L suggesting potential inadequacy in high-risk groups like total parenteral nutrition patients.[98][106] Optimal whole-blood manganese of 4-15 mcg/L supports metabolic roles without neurotoxicity thresholds exceeded at nutritional doses.[98]| Metal | RDA/AI (Adults) | Key Deficiency Biomarker | Optimal Range Example |
|---|---|---|---|
| Iron | 8-18 mg/day | Serum ferritin <30 mcg/L | Ferritin 30-300 mcg/L[68] |
| Zinc | 8-11 mg/day | Plasma zinc <70 mcg/dL | Serum 80-120 mcg/dL[97] |
| Copper | 900 mcg/day | Serum copper <70 mcg/dL | Serum 70-140 mcg/dL[78] |
| Manganese | 1.8-2.3 mg/day (AI) | Plasma manganese <4 mcg/L | Whole blood 4-15 mcg/L[98] |
Toxicity Mechanisms and Dose Dependency
Heavy metals primarily exert toxicity by binding to sulfhydryl (-SH) groups in proteins, displacing essential ions from metalloproteins, and catalyzing the formation of reactive oxygen species (ROS) through Fenton-like reactions, which overwhelm cellular antioxidant defenses and lead to lipid peroxidation, protein oxidation, and DNA damage.[9] [107] For non-essential metals like mercury (Hg), this involves high-affinity binding to thiols in enzymes and glutathione (GSH), disrupting antioxidant systems such as glutathione peroxidase and thioredoxin reductase, thereby amplifying oxidative stress and impairing mitochondrial function.[108] [109] Lead (Pb) similarly inhibits key enzymes, including δ-aminolevulinic acid dehydratase (ALAD) by displacing zinc at its active site, while mimicking calcium to interfere with neurotransmitter release and vascular signaling, concurrently generating ROS via auto-oxidation of accumulated intermediates.[110] [111] Cadmium (Cd), acting as a Zn/Ca analog, binds to metallothioneins and inhibits DNA repair proteins like O6-methylguanine-DNA methyltransferase, promoting genomic instability without direct DNA reactivity.[112] [113] Overloads of essential heavy metals, such as copper (Cu) or iron (Fe), exacerbate ROS via enhanced Fenton chemistry, where free Cu²⁺ or Fe²⁺ reduces H₂O₂ to hydroxyl radicals (•OH), damaging membranes and accelerating apoptosis.[107] These pathways demonstrate dose dependency, with toxicity thresholds varying by metal and endpoint; for Pb, the lowest observed adverse effect level (LOAEL) in humans is approximately 0.0012 mg/kg body weight/day, below which biochemical disruptions like ALAD inhibition may not manifest systemically, though subtle effects on heme synthesis occur at lower chronic intakes.[114] Genotoxic effects from Cd, such as unrepaired strand breaks, intensify above 1-10 µM cellular concentrations, reflecting saturation of repair inhibition.[115] Chelation therapy enhances excretion in overload states; dimercaptosuccinic acid (DMSA) mobilizes Pb and Hg from soft tissues with efficacy up to 50-70% burden reduction in acute poisoning, though redistribution risks necessitate monitoring.[116] At low doses, adaptive responses like metallothionein induction may confer protection, preventing ROS escalation.[117] Emerging 2024-2025 research links heavy metal-induced oxidative stress to accelerated cellular senescence, where persistent ROS activates p53-mediated pathways mimicking aging hallmarks like telomere attrition and epigenetic drift, yet low-exposure models show reversibility through antioxidant upregulation or chelation, averting irreversible mitochondrial dysfunction.[118] [117] This underscores causal thresholds where molecular harm transitions from reparable disequilibrium to cumulative pathology, informed by biochemical kinetics rather than linear no-threshold assumptions.[119]Epidemiological Data and Risk Assessment
Cohort studies and meta-analyses have identified associations between low-level lead exposure in children and reduced IQ scores, with blood lead concentrations below 5 μg/dL linked to measurable declines. A 2022 systematic review protocol examined this relationship, focusing on updated data from prospective cohorts, estimating potential IQ losses of 1-3 points per μg/dL increment in early childhood.[120] Earlier meta-analyses, such as one pooling 12 studies using multiple regression, reported a 2.6-point IQ drop for blood lead increases from 10 to 20 μg/dL, though these findings persist amid debates over residual confounding from socioeconomic status and parental cognition, which may inflate attributable fractions beyond causal contributions from lead alone.[121] A 2003 New England Journal of Medicine analysis of 172 children found inverse associations at levels under 10 μg/dL, but subsequent critiques highlight that adjusted models explain only a fraction of IQ variance, with environmental lead accounting for less than 5% in some recalibrations.[122] For cadmium, epidemiological evidence from population-based cohorts ties urinary or blood levels to chronic kidney disease (CKD) progression, particularly among smokers where tobacco serves as a primary exposure vector. A 2024 meta-analysis of environmental cadmium exposure reported increased CKD risk via reduced estimated glomerular filtration rate (eGFR), with odds ratios elevated by 1.2-1.5 in high-exposure quartiles.[123] The National Health and Nutrition Examination Survey (NHANES) data from over 13,000 adults showed current smokers with higher cadmium burdens exhibited 20-30% greater CKD prevalence compared to non-smokers, attributing roughly 10-15% of cases to cadmium after adjusting for age and hypertension, though dietary and occupational confounders limit precise fractions.[124] Community studies near smelters, controlling for comorbidities, confirmed associations with stage 3+ CKD at urinary cadmium exceeding 2 μg/g creatinine.[125] Risk assessments for heavy metals employ benchmark dose (BMD) modeling to derive regulatory limits, quantifying the dose associated with a 10% response rate (BMD10) over no-observed-adverse-effect levels, as per EPA guidelines updated in 2000 and refined through 2020s applications. For lead, BMD analyses of neurodevelopmental endpoints support action levels around 3.5-5 μg/dL blood lead, informing CDC reference values.[126] Cadmium BMDs for renal effects, drawn from Swedish cohorts, yield urinary thresholds of 0.5-1 μg/g creatinine, underpinning EPA's drinking water maximum contaminant level of 5 μg/L and reference doses of 0.0005 mg/kg/day.[127] These models emphasize population-level risks while critiquing over-reliance on correlation; for instance, global burden estimates attribute 1 million deaths annually to lead but note that natural soil baselines of 10-30 ppm—far exceeding urban "hazard" thresholds in unimpacted areas—suggest exaggerated attribution in low-contamination settings.[128][129] Therapeutic contexts illustrate managed risks outweighing toxicity in oncology, where platinum compounds like cisplatin yield high efficacy despite dose-dependent nephro- and neurotoxicity. In testicular cancer regimens, cisplatin achieves 90-95% cure rates for advanced stages at cumulative doses up to 400 mg/m², with CKD risks mitigated by hydration protocols reducing incidence to under 20%.[130] Meta-analyses confirm superior survival versus alternatives, though ototoxicity affects 40-60% of pediatric recipients, underscoring that attributable health burdens are context-specific and often lower than untreated malignancy progression.[131][132]Environmental Presence and Dynamics
Natural Versus Anthropogenic Sources
Heavy metals are released into the environment through both natural geological processes and human activities, with natural sources establishing long-term baseline fluxes via mechanisms such as rock weathering, volcanic emissions, and erosion. These processes mobilize metals like mercury (Hg), lead (Pb), and zinc (Zn) at rates determined by Earth's crustal composition and tectonic activity, often depositing them into soils, sediments, and water bodies over millennia. Volcanic eruptions and degassing contribute significantly, with global volcanic Hg emissions estimated at approximately 700 metric tons per year, accounting for 20-40% of total primary natural Hg releases.[133] Weathering of metal-rich rocks and soils adds further inputs, with natural global Hg fluxes from such sources ranging from 100-300 metric tons annually, though these figures exclude secondary re-emission from legacy deposits.[134] Anthropogenic sources, including ore extraction, metal smelting, coal combustion, and industrial effluents, have intensified fluxes since the mid-19th century, often surpassing primary natural emissions on a global scale for bioaccumulative metals. For Hg, anthropogenic atmospheric emissions total about 2,220 metric tons per year, exceeding primary natural inputs but interacting with natural cycling processes like soil and ocean evasion, which amplify total atmospheric burdens to 6,500-8,200 metric tons annually.[135] [136] Similar patterns hold for Pb and cadmium (Cd), where pre-industrial fluxes were dominated by natural erosion and volcanism, but industrial activities elevated atmospheric deposition by factors of 10-100 in affected regions. Geochemical baselines from unpolluted sites underscore that natural fluxes can rival or exceed anthropogenic ones locally, particularly in geologically active or metal-enriched terrains, emphasizing the importance of site-specific assessments over generalized pollution narratives.[137] Proxy records from ice cores and marine sediments quantify pre-industrial levels, revealing naturally low atmospheric concentrations punctuated by volcanic spikes, with anthropogenic signals emerging post-1850. Antarctic ice cores indicate that volcanism supplied 83-99% of pre-anthropogenic deposition for Pb, thallium (Tl), bismuth (Bi), and Cd, with background fluxes orders of magnitude below modern industrialized rates.[137] Lake and ocean sediments act as primary sinks, sequestering metals through adsorption onto particles and burial, thereby modulating long-term environmental inventories; for instance, global sedimentary accumulation rates for Zn and other metals reflect steady natural inputs from riverine transport and aeolian dust, independent of recent human perturbations. Geological legacies in soils, such as Zn enrichment in glacial tills derived from sulfide-rich bedrock, demonstrate natural pedogenic concentrations that can reach levels comparable to moderate anthropogenic contamination, as observed in northeastern North American escarpment soils.[138] These baselines highlight that while anthropogenic mobilization perturbs cycles, natural geochemical dynamics provide substantial ongoing fluxes, varying by metal and locale.Bioaccumulation and Ecosystem Impacts
Bioaccumulation refers to the net accumulation of heavy metals in organisms over time through direct uptake from the environment or diet, while biomagnification describes the increasing concentration of these metals at higher trophic levels in food webs. In aquatic ecosystems, methylmercury (MeHg), a highly bioavailable form of mercury, exemplifies biomagnification, with concentrations amplifying by factors of 2.1 to 4.3 from predatory invertebrates to fish in freshwater systems. This process occurs primarily because MeHg binds strongly to sulfhydryl groups in proteins, resisting excretion and transferring efficiently across trophic boundaries, as observed in marine food chains where top predators like large fish exhibit tissue levels orders of magnitude higher than primary producers.[139][140][141] Organisms exhibit tolerance mechanisms that mitigate accumulation and ecosystem-wide impacts, such as ligand binding and sequestration. Phytoplankton, for instance, tolerate elevated copper (Cu) through extracellular ligands and intracellular binding via copper-containing particles or metallothioneins, reducing bioavailability to higher trophic levels. These adaptations, including enhanced efflux pumps and compartmentalization in vacuoles, enable resilience in metal-exposed microbial communities, preventing wholesale collapses predicted by some equilibrium-based models. Empirical food web studies reveal that such tolerances dampen trophic transfer rates compared to modeled scenarios assuming linear uptake, highlighting causal feedbacks like microbial demethylation of MeHg that limit propagation.[142][143][144] Case studies underscore real ecological risks tempered by recovery potential. In Minamata Bay, Japan, decades of industrial MeHg discharges led to severe trophic contamination, but post-1970 regulations and 1974-1990 dredging reduced sediment mercury below Japanese standards by 2014, allowing faunal recolonization and demonstrating ecosystem rebound absent in static risk models. Terrestrial synergies between soil salinity and heavy metals exacerbate crop uptake, as seen in 2024 assessments where saline conditions increased cadmium and lead mobility, synergistically stressing root systems and reducing biomass by up to 30% in staples like wheat, though adaptive microbial consortia partially offset bioavailability.[145][146][147] Biodiversity responses include hyperaccumulation in select flora, enhancing ecosystem resilience via phytoremediation potential. Thlaspi caerulescens, a zinc and cadmium hyperaccumulator, sequesters up to 30,000 mg/kg dry weight of these metals in shoots without toxicity, facilitating soil decontamination through harvestable biomass and altering local food web dynamics by immobilizing contaminants. Such species-specific traits, evolved under metalliferous pressures, contrast with generalized model predictions of uniform biodiversity loss, as field trials show co-occurring non-accumulators benefiting from reduced metal cycling.[148][149][150]Remediation Methods and Efficacy
Physical methods for heavy metal remediation include permeable reactive barriers (PRBs), which involve installing subsurface walls filled with reactive materials such as zero-valent iron (ZVI) to intercept and treat contaminated groundwater plumes.[151] These barriers promote sorption, precipitation, and reduction reactions, achieving removal efficiencies of 85-92% for metals like copper in controlled studies, though field performance varies due to clogging and porosity heterogeneity over time. [151] Case studies demonstrate sustained efficacy for multi-metal contamination when combined with pumice for enhanced permeability, but long-term monitoring reveals declines in reactivity after 5-10 years without maintenance.[152] Chemical stabilization techniques, such as phosphate amendments for lead (Pb), convert bioavailable forms into insoluble pyromorphite minerals, reducing leachability by up to 90% in treated soils.[153] Efficacy depends on soil pH, phosphate dosage, and co-contaminants; for instance, soluble phosphates like Na3PO4 immobilize Pb effectively in lab settings but show variable field results due to competition from arsenic or iron oxides.[154] [155] In situ applications at legacy sites have stabilized Pb without excavation, though widespread adoption remains limited by inconsistent long-term stability under fluctuating environmental conditions.[156] Biological remediation leverages microorganisms for heavy metal reduction and immobilization, with advances in 2024 highlighting bacterial consortia that enzymatically reduce Cr(VI) to less toxic Cr(III) via dissimilatory pathways.[157] Fungi and algae enhance biosorption, achieving 70-95% removal in batch tests, but field-scale efficacy is constrained by microbial survival in toxic environments and slower kinetics compared to chemical methods.[158] [159] Influential factors include soil organic matter and pH, which can boost performance by 20-50% through biofilm formation, yet bioremediation often requires augmentation with nutrients, extending timelines to months or years.[158] At major sites like U.S. Superfund locations with heavy metal legacies, integrated remediation has achieved 80-95% contaminant reduction in targeted zones, but total costs frequently exceed $100 million per site due to excavation, treatment, and monitoring.[160] [161] Technique selection balances removal rates against site-specific variables; for example, PRBs offer passive, lower operational costs post-installation (around $50-200 per cubic meter), while biological approaches reduce energy inputs by 50-70% relative to physical extraction but demand verification of endpoint stability.[162] Emerging nanomaterials, such as iron oxide nanoparticles, enable selective adsorption of metals like Cd and Pb with capacities exceeding 200 mg/g due to high surface area and tunable functionalization.[163] Lab demonstrations show 90-99% removal efficiencies, yet scalability challenges include aggregation in soils, potential secondary toxicity from nanoparticle release, and high synthesis costs limiting field trials to small pilots.[164] Regeneration via magnetic separation improves reusability, but real-world deployment requires addressing dispersion uniformity and long-term fate to avoid unintended ecosystem transfer.[165] Overall, hybrid approaches combining physical barriers with biological enhancements show promise for cost-effective, sustained efficacy, though empirical validation across diverse geologies remains essential.[162]Industrial and Technological Applications
Structural and Density-Based Uses
Heavy metals find structural applications where their elevated densities—often exceeding 10 g/cm³—confer advantages in shielding, ballast, and kinetic energy management, complemented by intrinsic mechanical properties like tensile strength and hardness. These uses prioritize engineering performance in environments demanding weight concentration or impact resistance, such as aerospace components and protective barriers.[6] Lead, density 11.34 g/cm³, serves extensively as radiation shielding material in medical imaging suites, nuclear reactors, and industrial X-ray facilities, where its high atomic number (82) and density enable effective attenuation of gamma rays and X-rays through photoelectric absorption and Compton scattering. Lead sheets, bricks, and aprons fabricated from it line walls, enclosures, and personnel protective gear, reducing exposure doses by factors of 10 or more depending on thickness; for instance, 1 mm of lead halves the intensity of 100 keV X-rays.[166][167][168] Tungsten-based alloys, with densities of 17-18.5 g/cm³, are integral to aerospace structures for counterweights in aircraft noses, rotor blades in helicopters, and vibration-damping components like bucking bars, exploiting their combined high specific gravity and yield strengths up to 1000 MPa to minimize volume while maintaining balance and durability under cyclic loads. In tooling, tungsten carbide composites provide cutting edges for drills and milling tools, enduring temperatures above 1000°C and wear rates orders of magnitude lower than steel equivalents due to hardness exceeding 1500 HV.[169][170][171] Depleted uranium (DU), density approximately 19.1 g/cm³, enhances military vehicle armor on tanks like the M1 Abrams, where layered DU plates absorb and disrupt incoming projectiles via adiabatic shear-induced fracturing, outperforming equivalent steel in multi-hit survivability tests. For penetrators, DU cores in kinetic energy rounds, such as 120 mm tank ammunition, achieve velocities over 1600 m/s and penetrate rolled homogeneous armor exceeding 800 mm thickness, aided by DU's pyrophoric ignition upon impact that erodes target interiors.[172][173][174] Tantalum alloys, often blended into nickel-based superalloys, bolster high-temperature structural elements in jet turbine blades and rocket nozzles, where additions of 3-12 wt% tantalum elevate creep rupture strength by 20-50% at 1000°C through solid-solution hardening and precipitate formation, enabling sustained operation under thermal gradients up to 1500°C. Ta-W variants further resist oxidation in aerospace thermal barriers, maintaining integrity in oxidizing atmospheres where pure tantalum would degrade rapidly.[175][176][177]Catalytic and Chemical Roles
Heavy metals leverage their variable oxidation states and d-orbital-mediated adsorption to catalyze key industrial reactions, often achieving turnover numbers orders of magnitude higher than uncatalyzed processes due to lowered activation energies. Palladium, for instance, excels in hydrogenation, dissociating H2 on its surface to enable selective reduction of alkenes and alkynes; in solvent-free squalene hydrogenation, Pd nanoparticles supported on carbon have demonstrated turnover numbers exceeding 300,000 while maintaining stability over extended operation.[178] Raney nickel, prepared by leaching aluminum from Ni-Al alloys to create high-surface-area nickel, facilitates efficient hydrogenation of aromatic compounds like benzene to cyclohexane, a precursor for nylon production, with industrial applications emphasizing its robustness under heterogeneous conditions.[179] In ammonia synthesis via the Haber-Bosch process, iron catalysts—typically magnetite (Fe3O4) promoted with alumina and potassium oxide—operate at 400–500°C and 150–300 atm to convert N2 and H2 into NH3, yielding conversion efficiencies of 10–20% per pass but enabling global production of approximately 180 million metric tons annually through recycling.[180] These promoters enhance iron's activity by stabilizing active sites and suppressing sintering, underscoring the causal role of heavy metal synergies in scaling endothermic equilibria.[181] Heavy metals also fulfill non-catalytic chemical roles in pigments, exploiting their electronic transitions for color stability. Cadmium sulfide (CdS), a semiconductor with a bandgap of 2.4 eV, produces an intense lemon-yellow pigment commercially available since the 1840s, valued for its opacity, lightfastness, and resistance to fading compared to organic alternatives; its adoption followed cadmium's isolation in 1817, enabling vibrant hues in paints and ceramics despite toxicity concerns.[182] Similarly, lead chromate (PbCrO4) forms chrome yellow, leveraging chromate's tetrahedral coordination for bright pigmentation in industrial coatings since the early 19th century.[183]Electronics, Energy, and Advanced Tech
Copper and silver serve critical roles in electronic interconnects and wiring due to their exceptional electrical conductivity, enabling efficient current flow in devices from circuit boards to power transmission lines. Silver possesses the highest conductivity of any metal at approximately 63 × 10⁶ S/m, surpassing copper's 59 × 10⁶ S/m, though copper dominates applications for its balance of performance, abundance, and lower cost.[184][185] In semiconductors, gallium arsenide (GaAs) is employed in high-frequency transistors, solar cells, and light-emitting diodes for its direct bandgap and superior electron mobility compared to silicon, facilitating faster signal processing and optoelectronic functions. Indium gallium arsenide (InGaAs), an alloy of GaAs and indium arsenide, extends capabilities into shortwave infrared detection for imaging sensors and telecommunications, with enhanced sensitivity in the 0.9–1.7 μm range. Indium also enables transparent conductive films like indium tin oxide in flat-panel displays and touchscreens.[186][187] Nickel and cobalt are integral to lithium-ion battery cathodes in electric vehicles, particularly in nickel-manganese-cobalt (NMC) formulations where elevated nickel content—up to ultra-high levels—boosts energy density and range. In 2024, innovations such as nickel-rich NMC cathodes and the 4680 cylindrical cell format increased capacity by up to fivefold while reducing cobalt reliance, addressing supply constraints amid rising EV demand. Neodymium-iron-boron (NdFeB) permanent magnets, incorporating rare earth elements like neodymium and praseodymium (with heavy rare earths such as dysprosium for thermal stability), power efficient synchronous motors in EVs and direct-drive generators in wind turbines, enhancing torque and reducing gearbox needs.[188][189] Lead halide perovskites drive next-generation solar cells, achieving certified tandem efficiencies of 34.6% in 2024 through integration with silicon, outpacing traditional silicon limits but hindered by degradation from moisture and ion migration. Recent 2024–2025 advancements, including protective coatings that triple operational stability under accelerated aging, mitigate these tradeoffs, though long-term durability remains below commercial silicon benchmarks.[190][191] Uranium-235 and plutonium-239 fuel nuclear fission reactors, where neutron-induced splitting of their nuclei releases sustained energy via chain reactions controlled by moderators and absorbers, powering over 400 global reactors as of 2025.[192]Economic and Geopolitical Significance
Production, Trade, and Market Values
Global production of major heavy metals, particularly those with significant industrial demand like copper and lead, reached substantial volumes in 2024. Copper mine output totaled 23 million metric tons, led by Chile and Peru as top producers, reflecting steady growth amid expanding applications in wiring and renewables.[193] Lead mine production approximated 4.5 million metric tons, often as a byproduct of zinc and silver mining, with China and Australia as primary contributors.[194] Zinc and nickel followed at 13 million and 3.3 million metric tons, respectively, underscoring the sector's reliance on polymetallic deposits.[194] Trade flows are heavily concentrated, with China exerting dominance in refining stages due to its extensive smelting capacity and state-supported infrastructure. For copper, China produced over 12 million tons of refined metal in recent years—more than half of global output—while relying on imports for the majority of concentrates, creating bottlenecks vulnerable to geopolitical tensions.[195] [196] Lead refining follows a similar pattern, with China processing a large share of global supply, amplifying its influence over export markets to Europe and North America.[197] Market values fluctuate with supply dynamics and demand signals. Copper prices averaged $4.15 per pound in 2024, up 8% from the prior year, driven by record highs above $5 per pound in May amid mine disruptions and energy transition needs, though later moderated by economic slowdowns.[198] [199] Recycling bolsters supply economics, contributing about 30% of global copper through secondary production of roughly 4.55 million tons annually, which is more energy-efficient and reduces reliance on primary mining.[200] [201]| Metal | Global Mine Production (2024, million metric tons) | Key Producers |
|---|---|---|
| Copper | 23 | Chile, Peru, China |
| Lead | 4.5 | China, Australia, United States |
| Zinc | 13 | China, Peru, Australia |
| Nickel | 3.3 | Indonesia, Philippines, Russia |









