Recent from talks
Contribute something
Nothing was collected or created yet.
Spanish flu
View on Wikipedia
| Spanish flu | |
|---|---|
 Soldiers sick with Spanish flu at a hospital ward at Camp Funston in Fort Riley, Kansas | |
| Disease | Influenza |
| Virus strain | Strains of A/H1N1 |
| Location | Worldwide |
| Index case | Haskell County, Kansas January 1918[a] |
| Date | February 1918 – April 1920[2] |
| Suspected cases‡ | 500 million (estimated)[3] |
Deaths | 25–50 million (generally accepted), other estimates range from 17 to 100 million[4][5][6] |
| ‡Suspected cases have not been confirmed by laboratory tests as being due to this strain, although some other strains may have been ruled out. | |
| Influenza (flu) |
|---|
 |
The 1918–1920 flu pandemic, also known as the Great Influenza epidemic or by the common misnomer Spanish flu, was an exceptionally deadly global influenza pandemic caused by the H1N1 subtype of the influenza A virus. The earliest documented case was March 1918 in Haskell County, Kansas, United States, with further cases recorded in France, Germany and the United Kingdom in April. Two years later, nearly a third of the global population, or an estimated 500 million people, had been infected. Estimates of deaths range from 17 million to 50 million,[7][8] and possibly as high as 100 million,[9] making it the deadliest pandemic in history.
The pandemic broke out near the end of World War I, when wartime censors in the belligerent countries suppressed bad news to maintain morale, but newspapers freely reported the outbreak in neutral Spain, creating a false impression of Spain as the epicenter and leading to the "Spanish flu" misnomer.[10] Limited historical epidemiological data make the pandemic's geographic origin indeterminate, with competing hypotheses on the initial spread.[3]
Most influenza outbreaks disproportionately kill the young and old, but this pandemic had unusually high mortality for young adults.[11] Scientists offer several explanations for the high mortality, including a six-year climate anomaly affecting migration of disease vectors with increased likelihood of spread through bodies of water.[12] However, the claim that young adults had a high mortality during the pandemic has been contested.[13] Malnourishment, overcrowded medical camps and hospitals, and poor hygiene, exacerbated by the war, promoted bacterial superinfection, killing most of the victims after a typically prolonged death bed.[14][15]
Etymologies
[edit]
This pandemic was known by many different names depending on place, time, and context. The etymology of alternative names historicises the scourge and its effects on people who would only learn years later that viruses caused influenza.[16] The lack of scientific answers led the Sierra Leone Weekly News (Freetown) to suggest a biblical framing in July 1918, using an interrogative from Exodus 16 in ancient Hebrew:[b] "One thing is for certain—the doctors are at present flabbergasted; and we suggest that rather than calling the disease influenza they should for the present until they have it in hand, say Man hu—'What is it?'"[18][19][20]
Descriptive names
[edit]Outbreaks of influenza-like illness were documented in 1916–17 at British military hospitals in Étaples, France,[21] and just across the English Channel at Aldershot, England. Clinical indications in common with the 1918 pandemic included rapid symptom progression to a "dusky" heliotrope face. This characteristic blue-violet cyanosis in expiring patients led to the name 'purple death'.[22][23][24]
The Aldershot physicians later wrote in The Lancet, "the influenza pneumococcal purulent bronchitis we and others described in 1916 and 1917 is fundamentally the same condition as the influenza of this present pandemic."[25] This "purulent bronchitis" is not yet linked to the same A/H1N1 virus,[26] but it may be a precursor.[25][27][28]
In 1918, 'epidemic influenza',[29] also known at the time as 'the grip' (French: la grippe, grasp),[30] appeared in Kansas, U.S., during late spring, and early reports from Spain began appearing on 21 May.[31][32] Reports from both places called it 'three-day fever'.[33][34][35]
Associative names
[edit]Many alternative names are exonyms in the practice of making new infectious diseases seem foreign.[36][37][38] This pattern was observed even before the 1889–1890 pandemic, also known as the 'Russian flu', when the Russians already called epidemic influenza the 'Chinese catarrh', the Germans called it the 'Russian pest', and the Italians called it the 'German disease'.[39][40] These epithets were re-used in the 1918 pandemic, along with new ones.[41]
'Spanish' influenza
[edit]
Outside Spain, the disease was soon misnamed 'Spanish influenza'.[42][43] In a 2 June 1918 The Times of London dispatch titled, "The Spanish Epidemic," a correspondent in Madrid reported over 100,000 victims of, "The unknown disease...clearly of a gripal character," without referring to "Spanish influenza" directly.[44] Three weeks later The Times reported that, "Everybody thinks of it as the 'Spanish' influenza to-day."[45] Three days after that an advertisement appeared in The Times for Formamint tablets to prevent "Spanish influenza".[46][47] When it reached Moscow, Pravda announced, "Ispánka (the Spanish lady) is in town," making 'the Spanish lady' another common name.[48]
The outbreak did not originate in Spain,[49] but reporting did, due to wartime censorship in belligerent nations. Spain was a neutral country unconcerned with appearances of combat readiness, and without a wartime propaganda machine to prop up morale,[50][51] so its newspapers freely reported epidemic effects, making Spain the apparent locus of the epidemic.[52] The censorship was so effective that Spain's health officials were unaware its neighboring countries were similarly affected.[53] In an October 1918 "Madrid Letter" to the Journal of the American Medical Association, a Spanish official protested, "we were surprised to learn that the disease was making ravages in other countries, and that people there were calling it the 'Spanish grip'. And wherefore Spanish? ...this epidemic was not born in Spain, and this should be recorded as a historic vindication."[54]

Other exonyms
[edit]French press initially used 'American flu', but adopted 'Spanish flu' in lieu of antagonizing an ally.[55] In the spring of 1918, British soldiers called it 'Flanders flu', while German soldiers used 'Flandern-Fieber' (Flemish fever), both after a battlefield in Belgium where many soldiers on both sides fell ill.[41][38][56][57] In Senegal it was named 'Brazilian flu', and in Brazil, 'German flu'.[58] In Spain it was also known as the 'French flu' (gripe francesa),[49][10] or the 'Naples Soldier' (Soldado de Nápoles), after a popular song from a zarzuela.[c][55] Spanish flu (gripe española) is now a common name in Spain,[60] but remains controversial there.[61][62]
Othering derived from geopolitical borders and social boundaries.[63][64] In Poland it was the 'Bolshevik disease',[58][65] while in Russia it was referred to it as the 'Kirghiz disease'.[57] Some Africans called it a 'white man's sickness', but in South Africa, white men also used the ethnophaulism 'kaffersiekte' (lit. 'negro disease').[41][66] Japan blamed sumo wrestlers for bringing the disease home from Taiwan, calling it 'sumo flu' (Sumo Kaze).[67][68]
World Health Organization 'best practices' first published in 2015 now aim to prevent social stigma by not associating culturally significant names with new diseases, listing "Spanish flu" under "examples to be avoided".[69][37][70] Many authors now eschew calling this the Spanish flu,[55] instead using variations of '1918–19/20 flu/influenza pandemic'.[71][72][73]
Local names
[edit]Some language endonyms did not name specific regions or groups of people. Examples specific to this pandemic include: Northern Ndebele: 'Malibuzwe' (let enquiries be made concerning it), Swahili: 'Ugonjo huo kichwa na kukohoa na kiuno' (the disease of head and coughing and spine),[74] Yao: 'chipindupindu' (disease from seeking to make a profit in wartime), Otjiherero: 'kaapitohanga' (disease which passes through like a bullet),[75] and Persian: nakhushi-yi bad (disease of the wind).[76][77]
Other names
[edit]This outbreak was also commonly known as the 'great influenza epidemic',[78][79] after the 'great war', a common name for World War I before World War II.[80] French military doctors originally called it 'disease 11' (maladie onze).[38] German doctors downplayed the severity by calling it 'pseudo influenza' (Greek: pseudo, false), while in Africa, doctors tried to get patients to take it more seriously by calling it 'influenza vera' (Latin: vera, true).[81]
A children's song from the 1889–90 flu pandemic[82] was shortened and adapted into a skipping-rope rhyme popular in 1918.[83][84] It is a metaphor for the transmissibility of 'Influenza', where that name was clipped to 'Enza':[85][86][87]
I had a little bird,
its name was Enza.
I opened the window,
and in-flu-enza.
History
[edit]Potential origins
[edit]Despite its name, historical and epidemiological data cannot identify the geographic origin of the Spanish flu.[3] However, several theories have been proposed.
United States
[edit]The first confirmed cases originated in the United States. Historian Alfred W. Crosby stated in 2003 that the flu originated in Kansas,[88] and author John M. Barry described a January 1918 outbreak in Haskell County, Kansas, as the origin in his 2004 article.[80]
A 2018 study of tissue slides and medical reports led by professor Michael Worobey found evidence against the disease originating from Kansas, as those cases were milder and had fewer deaths compared to the infections in New York City in the same period. The study did find evidence through phylogenetic analyses that the virus likely had a North American origin, though it was not conclusive. In addition, the haemagglutinin glycoproteins of the virus suggest that it originated long before 1918, and other studies suggest that the reassortment of the H1N1 virus likely occurred in or around 1915.[89]
Europe
[edit]
The major UK troop staging and hospital camp in Étaples in France has been theorized by virologist John Oxford as being at the center of the Spanish flu.[1] His study found that in late 1916 the Étaples camp was hit by a new disease with high mortality that caused symptoms similar to the flu.[90][1] According to Oxford, a similar outbreak occurred in March 1917 at army barracks in Aldershot,[91] and military pathologists later recognized these early outbreaks as the same disease as the Spanish flu.[92][1]
The overcrowded camp and hospital at Étaples was an ideal environment for the spread of a respiratory virus. The hospital treated thousands of victims of poison gas attacks, and other casualties of war. It also was home to a piggery, and poultry was regularly brought in to feed the camp. Oxford and his team postulated that a precursor virus, harbored in birds, mutated and then migrated to pigs kept near the front.[91][92] A report published in 2016 in the Journal of the Chinese Medical Association found evidence that the 1918 virus had been circulating in the European armies for months and possibly years before the 1918 pandemic.[93] Political scientist Andrew Price-Smith published data from the Austrian archives suggesting the influenza began in Austria in early 1917.[94]
A 2009 study in Influenza and Other Respiratory Viruses found that Spanish flu mortality simultaneously peaked within the two-month period of October and November 1918 in all fourteen European countries analyzed, which is inconsistent with the pattern that researchers would expect if the virus had originated somewhere in Europe and then spread outwards.[95]
China
[edit]In 1993, Claude Hannoun, the leading expert on the Spanish flu at the Pasteur Institute, asserted the precursor virus was likely to have come from China and then mutated in the United States near Boston and from there spread to Brest, France, Europe's battlefields, and the rest of the world, with Allied forces as the main disseminators.[96] Hannoun considered several alternative hypotheses of origin, such as Spain, Kansas, and Brest, as being possible, but not likely.[96]
In 2014, historian Mark Humphries of the Memorial University of Newfoundland argued that the mobilization of 96,000 Chinese laborers to work behind the British and French lines might have been the source of the pandemic. Humphries found archival evidence that a respiratory illness that struck northern China (where the laborers came from) in November 1917 was identified a year later by Chinese health officials as identical to the Spanish flu.[97][98] No tissue samples have survived for modern comparison.[99] Nevertheless, there were some reports of respiratory illness on the path the laborers took to get to Europe, which also passed through North America.[99]
China was one of the few regions of the world seemingly less affected by the Spanish flu pandemic, where several studies have documented a comparatively mild flu season in 1918.[100][101][102] (This is disputed due to lack of data during the Warlord Period.) This has led to speculation that the Spanish flu pandemic originated in China,[102][103] as the lower mortality rates may be explained by the Chinese population's previously acquired immunity to the flu virus.[104][102] In the Guangdong Province it was reported that early outbreaks of influenza in 1918 disproportionately impacted young men. The June outbreak infected children and adolescents between 11 and 20 years of age, while the October outbreak was most common in those aged 11 to 15.[102]
A report published in 2016 in the Journal of the Chinese Medical Association found no evidence that the 1918 virus was imported to Europe via Chinese and Southeast Asian soldiers and workers and instead found evidence of its circulation in Europe before the pandemic.[93] The 2016 study found that the low flu mortality rate (an estimated one in a thousand) recorded among the Chinese and Southeast Asian workers in Europe suggests that the Asian units were not different from other Allied military units in France at the end of 1918 and, thus not a likely source of a new lethal virus.[93] Further evidence against the disease being spread by Chinese workers was that workers entered Europe through other routes that did not result in a detectable spread, making them unlikely to have been the original hosts.[89]
Timeline
[edit]First wave of early 1918
[edit]

The pandemic is conventionally marked as having begun on 4 March 1918 with the recording of the case of Albert Gitchell, an army cook at Camp Funston in Kansas despite there having been cases before him.[106] The disease had already been observed 200 miles (320 km) away in Haskell County as early as January 1918, prompting local doctor Loring Miner to warn the editors of the U.S. Public Health Service's journal Public Health Reports.[80] Within days of the 4 March case at Camp Funston, 522 men at the camp had reported sick.[107] By 11 March 1918, the virus had reached Queens, New York.[108] Failure to take preventive measures in March/April was later criticized.[109]
As the U.S. had entered World War I, the disease quickly spread from Camp Funston, a major training ground for troops of the American Expeditionary Forces, to other U.S. Army camps and Europe, becoming an epidemic in the Midwest, East Coast, and French ports by April 1918, and reaching the Western Front by mid-April.[106] It then quickly spread to the rest of France, Great Britain, Italy, and Spain and in May reached Wrocław and Odessa.[106] After the signing of the Treaty of Brest-Litovsk (March 1918), Germany started releasing Russian prisoners of war, who brought the disease to their country.[110] It reached North Africa, India, and Japan in May, and soon after had likely gone around the world as there had been recorded cases in Southeast Asia in April.[111] In June an outbreak was reported in China.[112] After reaching Australia in July, the wave started to recede.[111]
The first wave lasted from the first quarter of 1918 and was relatively mild.[113] Mortality rates were not appreciably above normal; in the United States ~75,000 flu-related deaths were reported in the first six months of 1918, compared to ~63,000 deaths during the same time period in 1915.[3][114] In Madrid, Spain, fewer than 1,000 people died from influenza between May and June 1918.[115] There were no reported quarantines; the first wave caused a significant disruption in the military operations of World War I, with three-quarters of French troops, half the British forces, and over 900,000 German soldiers sick.[116]
Deadly second wave of late 1918
[edit]

The second wave began in the second half of August 1918, probably spreading to Boston, Massachusetts and Freetown, Sierra Leone, by ships from Brest, where it had likely arrived with American troops or French recruits for naval training.[116] From the Boston Navy Yard and Camp Devens, about 30 miles (48 km) west of Boston, other U.S. military sites were soon afflicted, as were troops being transported to Europe.[117] Helped by troop movements, it spread over the next two months to all of North America, and then to Central and South America, also reaching Brazil and the Caribbean on ships.[118] In July 1918, the Ottoman Empire saw its first cases, in soldiers.[119] From Freetown, the pandemic spread through West Africa along the coast, rivers, and railways, and from railheads to more remote communities, while South Africa received it in September on ships bringing back members of the South African Native Labour Corps from France.[118] From there it spread around southern Africa and beyond the Zambezi, reaching Ethiopia in November.[120] On 15 September, New York City saw its first fatality from influenza.[121] The Philadelphia Liberty Loans Parade, held in Philadelphia, Pennsylvania, on 28 September 1918 to promote government bonds for World War I, resulted in an outbreak causing 12,000 deaths.[122]
From Europe, the second wave swept through Russia in a southwest–northeast diagonal front, as well as being brought to Arkhangelsk by the North Russia intervention, and then spread throughout Asia following the Russian Civil War and the Trans-Siberian railway, reaching Iran (where it spread through Mashhad), and then India in September and China and Japan in October.[123] The celebrations of the Armistice of 11 November 1918 also caused outbreaks in Lima and Nairobi, but by December the wave was mostly over.[124]
The second wave of the 1918 pandemic was much more deadly than the first. The first wave had resembled typical flu epidemics; those most at risk were the sick and elderly, while younger, healthier people recovered easily. October 1918 was the month with the highest fatality rate of the whole pandemic.[125] In the United States, ~292,000 deaths were reported between September–December 1918, compared to ~26,000 during the same time period in 1915.[114] The Netherlands reported over 40,000 deaths from influenza and acute respiratory disease. Bombay reported ~15,000 deaths in a population of 1.1 million.[126] The 1918 flu pandemic in India was especially deadly, as Historian David Arnold estimates at least 12 million dead, about 5% of the population.[127]
Third wave of 1919
[edit]
Pandemic activity persisted into 1919 in many places, possibly attributable to climate, specifically in the Northern Hemisphere, where it was winter and thus the usual time for influenza activity.[128][129] The pandemic nonetheless continued into 1919 largely independent of region and climate.[128]
Cases began to rise again in some parts of the U.S. as early as late November 1918,[130] with the Public Health Service issuing its first report of a "recrudescence of the disease" in "widely scattered localities" in early December.[131] This resurgent activity varied across the country, however, possibly on account of differing restrictions.[129] Michigan, for example, experienced a swift resurgence of influenza that reached its peak in December, possibly as a result of the lifting of the ban on public gatherings.[132] Pandemic interventions, such as bans on public gatherings and the closing of schools, were reimposed in many places in an attempt to suppress the spread.[131]
There was "a very sudden and very marked rise in general death rate" in most cities in January 1919; nearly all experienced "some degree of recrudescence" of the flu in January and February.[133]: 153–154 Significant outbreaks occurred in cities including Los Angeles,[134] New York City,[2] Memphis, Nashville, San Francisco,[135] and St. Louis.[136] By 21 February, with some local variation, influenza activity was reported to have been declining since mid-January in all parts of the country.[137] Following this "first great epidemic period" that had commenced in October 1918, deaths from pneumonia and influenza were "somewhat below average" in large U.S. cities between May 1919 and January 1920.[133]: 158 Nonetheless, nearly 160,000 deaths were attributed to these causes in the first six months of 1919.[138]
It was not until later in the winter and into the spring that a clearer resurgence appeared in Europe. A significant third wave had developed in England and Wales by mid-February, peaking in early March, though it did not fully subside until May.[139] France also experienced a significant wave that peaked in February, alongside the Netherlands. Norway, Finland, and Switzerland saw recrudescences of pandemic activity in March, and Sweden in April.[95]
Much of Spain was affected by "a substantial recrudescent wave" of influenza between January and April 1919.[140] Portugal experienced a resurgence in pandemic activity that lasted from March to September 1919, with the greatest impact being felt on the west coast and in the north of the country; all districts were affected between April and May specifically.[141]
Influenza entered Australia for the first time in January 1919 after a strict maritime quarantine had shielded the country through 1918.[142] It assumed epidemic proportions first in Melbourne, peaking in mid-February.[143] The flu soon appeared in neighboring New South Wales and South Australia.[142] New South Wales experienced its first wave of infection between mid-March and late May,[144] while a second, more severe wave occurred in Victoria between April and June.[143] Land quarantine measures hindered the spread of the disease. Queensland was not infected until late April; Western Australia avoided the disease until early June, and Tasmania remained free from it until mid-August.[142] Out of the six states, Victoria and New South Wales experienced generally more extensive epidemics. Each experienced another significant wave of illness over the winter. The second epidemic in New South Wales was more severe than the first,[144] while Victoria saw a third wave that was somewhat less extensive than its second, more akin to its first.[143]
The disease also reached other parts of the world for the first time in 1919, such as Madagascar, which saw its first cases in April; the outbreak had spread to practically all sections of the island by June.[145] In other parts, influenza recurred in the form of a true "third wave". Hong Kong experienced another outbreak in June,[146] as did South Africa during its fall and winter months in the Southern Hemisphere.[147][148][149] New Zealand experienced some cases in May.[150]
Parts of South America experienced a resurgence of pandemic activity throughout 1919. A third wave hit Brazil between January and June.[128] Between July 1919 and February 1920, Chile, which had been affected for the first time in October 1918, experienced a severe second wave, with mortality peaking in August 1919.[151] Montevideo similarly experienced a second outbreak between July and September.[152]
The third wave particularly affected Spain, Serbia, Mexico and Great Britain, resulting in hundreds of thousands of deaths.[153]
Fourth wave of 1920
[edit]
In the Northern Hemisphere, fears of a "recurrence" of the flu grew as fall approached. Experts cited past flu epidemics, such as that of 1889–1890, to predict that such a recurrence a year later was not unlikely,[154][155] though not all agreed.[156] In September 1919, U.S. Surgeon General Rupert Blue said a return of the flu later in the year would "probably, but by no means certainly," occur.[157] France had readied a public information campaign before the end of the summer,[158] and Britain began preparations in the autumn with the manufacture of vaccine.[159]
In Japan, the flu broke out again in December and spread rapidly throughout the country, a fact attributed at the time to cold weather.[160][161] Pandemic-related measures were renewed to check the outbreak, and health authorities recommended the use of masks.[161] The epidemic intensified in the latter part of December before swiftly peaking in January.[162]
Between October 1919 and 23 January 1920, 780,000 cases were reported across the country, with at least 20,000 deaths recorded by that date. This apparently reflected "a condition of severity three times greater than for the corresponding period of" 1918–1919.[162] Nonetheless, the disease was regarded as being milder than it had been the year before, albeit more infectious.[163] Despite its rapid peak at the beginning of the year, the outbreak persisted throughout the winter, before subsiding in the spring.[164]
In the United States, there were "almost continuously isolated or solitary cases" of flu throughout the spring and summer of 1919.[165] An increase in scattered cases became apparent as early as September,[166] but Chicago experienced one of the first major outbreaks of the flu beginning in the middle of January.[167] The Public Health Service announced it would take steps to "localize the epidemic",[168] but the disease was already causing a simultaneous outbreak in Kansas City and quickly spread outward from the center of the country.[165] A few days after its first announcement, PHS issued another assuring that the disease was under the control of state health authorities and that an outbreak of epidemic proportions was not expected.[169]
It became apparent within days of the start of Chicago's explosive growth in cases that the flu was spreading in the city at an even faster rate than in winter 1919, though fewer were dying.[170] Within a week, new cases in the city had surpassed its peak during the 1919 wave.[171] Around the same time, New York City began to see its own sudden increase in cases,[172] and other cities around the country were soon to follow.[173] Certain pandemic restrictions, such as the closing of schools and theaters and the staggering of business hours to avoid congestion, were reimposed in cities like Chicago,[174] Memphis,[175] and New York City.[176] As they had during the epidemic in fall 1918, schools in New York City remained open,[176] while those in Memphis were shuttered as part of restrictions on public gatherings.[175]
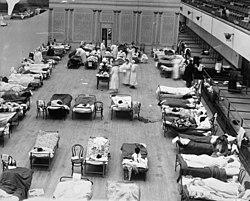
The fourth wave in the United States subsided as swiftly as it had appeared, reaching a peak in early February.[177] "An epidemic of considerable proportions marked the early months of 1920", the U.S. Mortality Statistics would later note; according to data at this time, the epidemic resulted in one third as many deaths as the 1918–1919 experience.[178] New York City alone reported 6,374 deaths between December 1919 and April 1920, almost twice the number of the first wave in spring 1918.[2] Detroit, Milwaukee, Kansas City, Minneapolis, and St. Louis were hit particularly hard, with death rates higher than all of 1918.[136] Hawaii experienced its peak of the pandemic in early 1920, recording 1,489 deaths from flu-related causes, compared with 615 in 1918 and 796 in 1919.[179]
Poland experienced a devastating outbreak during the winter months, with its capital Warsaw reaching a peak of 158 deaths in a single week, compared to the peak of 92 in December 1918; however, the 1920 epidemic passed in a matter of weeks, while the 1918–1919 wave had developed over the entire second half of 1918.[180] By contrast, the outbreak in western Europe was considered "benign", with the age distribution of deaths beginning to take on that of seasonal flu.[115] Spain, Denmark, Finland, Germany and Switzerland recorded a late peak between January–April 1920.[95]
Mexico experienced a fourth wave between February and March. In South America, Peru experienced "asynchronous recrudescent waves" throughout the year. A severe third wave hit Lima, the capital city, between January and March, resulting in an all-cause excess mortality rate approximately four times greater than that of the 1918–1919 wave. Ica similarly experienced another severe pandemic wave in 1920, between July and October.[181] A fourth wave also occurred in Brazil, in February.[128]
Korea and Taiwan experienced pronounced outbreaks in late 1919 and early 1920.[182][183]
Post-pandemic
[edit]By mid-1920, the pandemic was largely considered to be "over" by the public as well as governments.[184] Though parts of Chile experienced a third, milder wave between November 1920 and March 1921,[151] the flu seemed to be mostly absent through the winter of 1920–1921.[133]: 167 In the United States, for example, deaths from pneumonia and influenza were "very much lower than for many years".[133]: 167
Seasonal influenza began to be reported again from many places in 1921.[133]: 168 Influenza continued to be felt in Chile, where a post-pandemic fourth wave affected 7 of its 24 provinces between June and December 1921.[151] The winter of 1921–1922 was the first major reappearance of seasonal influenza in the Northern Hemisphere, in many parts its most significant occurrence since the main pandemic in late 1918. Northwestern Europe was particularly affected. All-cause mortality in the Netherlands approximately doubled in January 1922 alone.[133]: 168 In Helsinki, a major epidemic (the fifth since 1918) prevailed between November and December 1921.[185] The flu was also widespread in the United States, its prevalence in California reportedly greater in early March 1922 than at any point since the pandemic ended in 1920.[133]: 172
In the years after 1920, the disease came to represent the "seasonal flu". The virus, H1N1, remained endemic, occasionally causing more severe or otherwise notable outbreaks.[186] The period since its initial appearance in 1918 has been termed a "pandemic era", in which all flu pandemics have been caused by its own descendants.[187] Following the first of these post-1918 pandemics, in 1957, the virus was totally displaced by the novel H2N2, the reassortant product of the human H1N1 and an avian influenza virus, which thereafter became the active influenza A virus in humans.[186]
In 1977, an influenza virus bearing a very close resemblance to the seasonal H1N1, which had not been seen since the 1950s, appeared in Russia and subsequently initiated a "technical" pandemic that principally affected those 26 and under.[188][189] While some natural explanations, such as the virus remaining in some frozen state for 20 years,[189] have been proposed to explain this unprecedented[190] phenomenon, the nature of influenza itself has been cited in favor of human involvement of some kind, such as an accidental leak from a lab where the old virus had been preserved for research purposes.[189] Following this miniature pandemic, the reemerged H1N1 became endemic again but did not displace the other active influenza A virus, H3N2 (which itself had displaced H2N2 through a pandemic in 1968).[188][186] For the first time, two influenza A viruses were observed in cocirculation.[104] This state has persisted even after 2009, when a novel H1N1 virus emerged, sparked a pandemic, and thereafter took the place of the seasonal H1N1 to circulate alongside H3N2.[104]
Epidemiology and pathology
[edit]Transmission and mutation
[edit]
The basic reproduction number of the virus was between 2 and 3.[191] The close quarters and massive troop movements of World War I hastened the pandemic, and probably both increased transmission and augmented mutation. The war may also have reduced people's resistance to the virus. Some speculate the soldiers' immune systems were weakened by malnourishment and the stresses of combat and chemical attacks, increasing their susceptibility.[192][193] Modern transportation systems and increased travel was a significant factor in the worldwide occurrence.[194] Another was lies and denial by governments, leaving the population ill-prepared to handle the outbreaks.[195]
The severity of the second wave has been attributed to the First World War.[196] In civilian life, natural selection favors a mild strain. Those who get very ill stay home, and those mildly ill continue with their lives, preferentially spreading the mild strain. In the trenches, natural selection was reversed. Soldiers with a mild strain stayed where they were, while the severely ill were sent on crowded trains to crowded field hospitals, spreading the deadlier virus. The second wave began, and the flu quickly spread around the world again. (During modern pandemics, health officials look for deadlier strains of a virus when it reaches places with social upheaval.[197]) The fact that most of those who recovered from first-wave infections had become immune showed that it must have been the same strain of flu. This was most dramatically illustrated in Copenhagen, which escaped with a combined mortality rate of 0.29% (0.02% in the first wave and 0.27% in the second wave) because of exposure to the less-lethal first wave.[198]
After the lethal second wave, new cases dropped abruptly. In Philadelphia, for example, 4,597 people died in the week ending 16 October, but by 11 November, influenza had almost disappeared from the city. One explanation for the rapid decline in lethality is that doctors became more effective in the prevention and treatment of pneumonia that developed after the victims had contracted the virus. However, John Barry stated in his 2004 book The Great Influenza: The Epic Story of the Deadliest Plague In History that researchers have found no evidence to support this position.[80] Another theory holds that the 1918 virus mutated extremely rapidly to a less lethal strain. Such evolution of influenza is a common occurrence: there is a tendency for pathogenic viruses to become less lethal with time, as the hosts of more dangerous strains tend to die out.[80] Fatal cases did continue into 1919, however. One notable example was that of ice hockey player Joe Hall, who died of the flu in April after an outbreak that resulted in the cancellation of the 1919 Stanley Cup Finals.[199]
Signs and symptoms
[edit]
The majority of the infected experienced only the typical flu symptoms of sore throat, headache, and fever, especially during the first wave.[200] However, during the second wave, the disease was much more serious, often complicated by bacterial pneumonia, which was often the cause of death.[200] This more serious type would cause heliotrope cyanosis to develop, whereby the skin would first develop two mahogany spots over the cheekbones which would then over a few hours color the entire face blue, followed by black coloration first in the extremities and then the limbs and the torso.[200] Death would follow within hours or days due to the lungs being filled with fluids.[200] Other signs and symptoms reported included spontaneous mouth and nosebleeds, miscarriages for pregnant women, a peculiar smell, teeth and hair falling out, delirium, dizziness, insomnia, loss of hearing or smell, and impaired vision.[200] One observer wrote, "One of the most striking of the complications was hemorrhage from mucous membranes, especially from the nose, stomach, and intestine. Bleeding from the ears and petechial hemorrhages in the skin also occurred".[201]
The majority of deaths were from bacterial pneumonia,[202][203][204] a common secondary infection associated with influenza. This pneumonia was itself caused by common upper respiratory-tract bacteria, which were able to get into the lungs via the damaged bronchial tubes.[205] The virus also killed people directly by causing massive hemorrhages and edema in the lungs.[204] Modern analysis has shown the virus to be particularly deadly because in animal trials it triggers an overreaction of the body's immune system (cytokine storm).[80] The strong immune reactions of young adults were postulated to have ravaged the body, whereas the weaker immune reactions of children and middle-aged adults resulted in fewer deaths.[206]
Misdiagnosis
[edit]Because the virus that caused the disease was too small to be seen under a microscope at the time, there were problems with correctly diagnosing it.[207] The bacterium Haemophilus influenzae was instead mistakenly thought to be the cause, as it was big enough to be seen and was present in many, though not all, patients.[207] For this reason, a vaccine that was used against that bacillus did not make infection rarer but did decrease the death rate.[208]
During the deadly second wave there were also fears that it was in fact plague, dengue fever, or cholera.[209] Another misdiagnosis was typhus, which was common in circumstances of social upheaval, and was therefore also affecting Russia in the aftermath of the October Revolution.[209] In Chile, the view of the country's elite was that the nation was in severe decline, and therefore doctors assumed that the disease was typhus caused by poor hygiene, causing a mismanaged response which did not ban mass gatherings.[209]
Role of climate conditions
[edit]
Studies have shown that the immune system of Spanish flu victims could have been weakened by unseasonably cold and wet weather for extended periods during the pandemic. This affected especially troops exposed to incessant rains and lower-than-average temperatures during World War I, and especially during the second wave of the pandemic. Climate data and mortality records analyzed at Harvard University and the Climate Change Institute at the University of Maine identified a severe climate anomaly that impacted Europe from 1914 to 1919, with several environmental indicators directly influencing the severity and spread of the pandemic.[12] Specifically, a significant increase in precipitation affected all of Europe during the second wave of the pandemic, from September to December 1918. Mortality figures follow closely the concurrent increase in precipitation and decrease in temperatures. Several explanations have been proposed for this, including that lower temperatures and increased precipitation provided ideal conditions for virus replication and transmission, while also negatively affecting peoples' immune systems, a factor proven to increase likelihood of infection by both viruses and pneumococcal co-morbid infections documented to have affected a large percentage of pandemic victims (one fifth of them, with a 36% mortality rate).[210][211][212][213][214] The climate anomaly likely influenced the migration of H1N1 avian vectors which contaminate bodies of water with their droppings, reaching 60% infection rates in autumn.[215][216][217] The climate anomaly has been associated with an anthropogenic increase in atmospheric dust, due to the incessant bombardment; increased nucleation due to dust particles (cloud condensation nuclei) contributed to increased precipitation.[218][219][220]
Responses
[edit]Public health management
[edit]While systems for alerting public health authorities of infectious spread did exist in 1918, they did not generally include influenza, leading to a delayed response.[223] Nevertheless, actions were taken. Maritime quarantines were declared on islands such as Iceland, Australia, and American Samoa, saving many lives.[223] Social distancing measures were introduced, for example closing schools, theatres, and places of worship, limiting public transportation, and banning mass gatherings.[224] Wearing face masks became common in some places, such as Japan, though there were debates over their efficacy.[224][225] There was also some resistance to their use, as exemplified by the Anti-Mask League of San Francisco. Vaccines were developed, but as these were based on bacteria and not the actual virus, they could only help with secondary infections.[224] The enforcement of restrictions varied.[226] To a large extent, the New York City health commissioner ordered businesses to open and close on staggered shifts to avoid overcrowding on the subways.[227] A later study found that measures such as banning mass gatherings and requiring the wearing of face masks could cut the death rate up to 50 percent, but this was dependent on their being imposed early in the outbreak and not being lifted prematurely.[228]
Medical treatment
[edit]As there were no antiviral drugs to treat the virus, and no antibiotics to treat the secondary bacterial infections, doctors would rely on an assortment of medicines with varying degrees of effectiveness, such as aspirin, quinine, arsenics, digitalis, strychnine, epsom salts, castor oil, and iodine.[229] Traditional treatments, such as bloodletting, ayurveda, and kampo, were also applied.[230]
Information dissemination
[edit]Due to World War I, many countries engaged in wartime censorship, and suppressed reporting of the pandemic.[231] For example, the Italian newspaper Corriere della Sera was prohibited from reporting daily death tolls.[232] The newspapers of the time were also generally paternalistic and worried about mass panic.[232] Misinformation also spread along with the disease. In Ireland there was a belief that noxious gases were rising from the mass graves of Flanders Fields and being "blown all over the world by winds".[233] There were also rumors that the Germans were behind it, for example by poisoning the aspirin manufactured by Bayer, or by releasing poison gas from U-boats.[234]
Mortality
[edit]

The Spanish flu infected around 500 million people, about one-third of the world's population.[3] Estimates on deaths vary greatly, but it is considered to be one of the deadliest pandemics in history.[237][238] An early estimate from 1927 put global mortality at 21.6 million.[5] An estimate from 1991 states that the virus killed between 25 and 39 million people.[113] A 2005 estimate put the death toll at 50 million, and possibly as high as 100 million.[201][239] However, a 2018 reassessment in the American Journal of Epidemiology estimated the total to be about 17 million,[5] though this has been contested.[240] Estimates done in 2021 by John M. Barry have the total death toll alone at well above 100 million.[9] With a world population of 1.8 to 1.9 billion,[241] these estimates correspond to between 1 and 6 percent of the population.
A 2009 study in Influenza and Other Respiratory Viruses based on data from fourteen European countries estimated a total of 2.64 million excess deaths in Europe attributable to the Spanish flu during the 1918–1919 phase of the pandemic. This represents a mortality rate of about 1.1% of the European population (c. 250 million in 1918), considerably higher than the mortality rate in the U.S., which the authors hypothesize is likely due to the severe effects of the war in Europe.[95] The excess mortality rate in the U.K. has been estimated at 0.28%–0.4%, far below this European average.[5]
Some 12–17 million people died in India, about 5% of the population.[242] The death toll in India's British-ruled districts was 13.88 million.[243] Another estimate gives at least 12 million dead.[244] The decade between 1911 and 1921 was the only census period in which India's population fell, mostly due to devastation of the pandemic.[245][246] While India is generally described as the country most severely affected by the Spanish flu, at least one study argues that other factors may partially account for the very high excess mortality rates observed in 1918, citing unusually high 1917 mortality and wide regional variation (ranging from 0.47% to 6.66%).[5] A 2006 study in The Lancet also noted that Indian provinces had excess mortality rates ranging from 2.1% to 7.8%, stating: "Commentators at the time attributed this huge variation to differences in nutritional status and diurnal fluctuations in temperature."[247]
In Finland, 20,000 died out of 210,000 infected.[248] In Sweden, 34,000 died.[249]
In Japan, the flu killed nearly 500,000 people over two waves between 1918 and 1920, with nearly 300,000 excess deaths between October 1918 and May 1919 and 182,000 between December 1919 and May 1920.[164]
In the Dutch East Indies (now Indonesia), 1.5 million were assumed to have died among 30 million inhabitants.[250] In Tahiti, 13% of the population died in one month. Similarly, in Western Samoa 22% of the population of 38,000 died within two months.[251]
In Istanbul, capital of the Ottoman Empire, 6,403[252] to 10,000[119] died, giving the city a mortality rate of at least 0.56%.[252]
In New Zealand, the flu killed an estimated 6,400 Pākehā (or "New Zealanders primarily of European descent") and 2,500 Māori in six weeks, with Māori dying at eight times the rate of Pākehā.[253][254]
In Australia, the flu killed around 12,000[255] to 20,000 people.[256] The country's death rate, 2.7 per 1,000 people, was one of the lowest recorded; however, as much as 40 percent of the population were infected, and a mortality rate of 50 percent was recorded by some Aboriginal communities.[257][256] New South Wales and Victoria saw the greatest relative mortality, with 3.19 and 2.40 deaths per 1,000 people respectively, while Western Australia, Queensland, Southern Australia, and Tasmania experienced rates of 1.70, 1.14, 1.13, and 1.09 per 1,000 respectively. In Queensland, at least one-third of deaths recorded were in the Aboriginal population.[143]
In the U.S., about 20 million out of a population of 105 million became infected in the 1918–1919 season, and an estimated 500,000 to 850,000 died (0.5 to 0.8 percent of the U.S. population).[258][259][260] Native Americans tribes were particularly affected. In the Four Corners area, there were 3,293 registered deaths among Native Americans.[261] Entire Inuit and Alaskan Native village communities died in Alaska.[262] In Canada, 50,000 died.[263]
In Brazil, 300,000 died, including president Rodrigues Alves.[264]
In the UK, as many as 250,000 died; in France, more than 400,000.[265]
In Ghana, the influenza epidemic killed at least 100,000 people.[266] Tafari Makonnen (the future Emperor of Ethiopia) was one of the first Ethiopians who contracted influenza but survived.[267][268] Estimates for fatalities in the capital city, Addis Ababa, range from 5,000 to 10,000, or higher.[269]
The death toll in Russia has been estimated at 450,000, though the epidemiologists who suggested this number called it a "shot in the dark".[113] If it is correct, Russia lost roughly 0.4% of its population, meaning it suffered the lowest influenza-related mortality in Europe. Another study considers this number unlikely, given that the country was in the grip of a civil war, and the infrastructure of daily life had broken down; the study suggests that Russia's death toll was closer to 2%, or 2.7 million people.[270]
Devastated communities
[edit]
Even in areas where mortality was low, so many adults were incapacitated that daily life was hampered. Some communities closed all stores or required customers to leave orders outside. There were reports that healthcare workers could not tend the sick nor the gravediggers bury the dead because they too were ill. Mass graves were dug by steam shovel and bodies buried without coffins in many places.[271]
Bristol Bay, a region of Alaska populated by Indigenous people, suffered a death rate of 40 percent, with some villages entirely disappearing.[272] Nenana, Alaska, avoided the extent of the pandemic between 1918 and 1919, but the flu at last reached the town in spring 1920. Reports suggested that during the first two weeks of May, the majority of the town's population became infected; 10% of the population were estimated to have died, most of whom were Alaska Natives.[273]
Several Pacific island territories were hit particularly hard. The pandemic reached them from New Zealand, which was too slow to implement measures to prevent ships carrying the flu from leaving its ports. From New Zealand, the flu reached Tonga (killing 8% of the population), Nauru (16%), and Fiji (5%, 9,000 people).[274] Worst affected was Western Samoa, which had been occupied by New Zealand in 1914. 90% of the population was infected; 30% of adult men, 22% of adult women, and 10% of children died.[274] The disease spread fastest through the higher social classes among the Indigenous peoples, because of the custom of gathering oral tradition from chiefs on their deathbeds; many community elders were infected through this process.[275]
In Iran, the mortality was estimated at between 902,400 and 2,431,000, or 8% to 22% of the total population.[276] The country was going through the Persian famine of 1917–1919 concurrently.
In Ireland, during the worst 12 months, the Spanish flu accounted for one-third of all deaths.[277][278]
In South Africa it is estimated that about 300,000 people amounting to 6% of the population died within six weeks. Government actions in the early stages of the virus' arrival in the country in September 1918 are believed to have unintentionally accelerated its spread.[279] Almost a quarter of the working population of Kimberley, consisting of workers in the diamond mines, died.[280] In British Somaliland, one official estimated that 7% of the native population died.[281] This huge death toll resulted from an extremely high infection rate of up to 50% and the extreme severity of the symptoms.[113]
Other areas
[edit]In the Pacific, American Samoa[282] and the French colony of New Caledonia[283] succeeded in preventing even a single death from influenza through effective quarantines. However, the outbreak was delayed into 1926 for American Samoa and 1921 for New Caledonia as the quarantine period ended.[284] On American Samoa, at least 25% of the island residents were clinically attacked and 0.1% died, and on New Caledonia, there was widespread illness and 0.1% population died.[284] Australia also managed to avoid the first two waves with a quarantine.[223] Iceland protected a third of its population from exposure by blocking the main road of the island.[223] By the end of the pandemic, the isolated island of Marajó, in Brazil's Amazon River Delta had not reported an outbreak.[285] Saint Helena also reported no deaths.[286]

Estimates for the death toll in China have varied widely,[287][113] reflecting the lack of centralized collection of health data at the time due to the Warlord period. China may have experienced a relatively mild flu season in 1918 compared to other areas of the world.[102][104][288] However, some reports from its interior suggest that mortality rates from influenza were perhaps higher in at least a few locations in 1918.[270] At the very least, there is little evidence that China as a whole was seriously affected by the flu compared to other countries.[289]
The first estimate of the Chinese death toll was made in 1991 by Patterson and Pyle, which estimated a toll of between 5 and 9 million. However, this study was criticized by later studies due to flawed methodology, and newer studies have published estimates of a far lower mortality rate in China.[100][290] For instance, Iijima in 1998 estimates the death toll in China to be between 1 and 1.28 million based on data available from Chinese port cities.[291] The lower estimates of the Chinese death toll are based on the low mortality rates that were found in Chinese port cities and on the assumption that poor communications prevented the flu from penetrating the interior of China.[287] However, some contemporary newspaper and post office reports, as well as reports from missionary doctors, suggest that the flu did penetrate the Chinese interior and that influenza was severe in at least some locations in the countryside of China.[270]
Although medical records from China's interior are lacking, extensive medical data were recorded in Chinese port cities, such as then British-controlled Hong Kong, Canton, Peking, Harbin and Shanghai. These data were collected by the Chinese Maritime Customs Service, which was largely staffed by non-Chinese foreigners.[292] As a whole, data from China's port cities show low mortality rates compared to other cities in Asia.[292] For example, the British authorities at Hong Kong and Canton reported a mortality rate from influenza at a rate of 0.25% and 0.32%, much lower than the reported mortality rate of other cities in Asia, such as Calcutta or Bombay, where influenza was much more devastating.[292] Similarly, in the city of Shanghai – which had a population of over 2 million – there were only 266 recorded deaths from influenza among the Chinese population in 1918.[292] If extrapolated from the extensive data recorded from Chinese cities, the suggested mortality rate from influenza in China as a whole in 1918 was likely lower than 1% – much lower than the world average (which was around 3–5%).[292] In contrast, Japan and Taiwan had reported a mortality rate from influenza around 0.45% and 0.69% respectively, higher than the mortality rate collected from data in Chinese port cities, such as Hong Kong (0.25%), Canton (0.32%), and Shanghai.[292]
However, the influenza mortality rate in Hong Kong and Canton are under-recorded, because only the deaths that occurred in colony hospitals were counted.[292] Similarly, in Shanghai, these statistics are limited to that area of the city under the control of the health section of the Shanghai International Settlement; the actual death toll in Shanghai was much higher.[292] The medical records from China's interior indicate that, compared to cities, rural communities have substantially higher mortality rate.[293] A published influenza survey in Houlu County, Hebei Province, found that the case fatality rate was 9.77% and 0.79% of county population died from influenza in October and November 1918.[294]
Patterns of fatality
[edit]
The pandemic mostly killed young adults. In 1918–1919, 99% of pandemic influenza deaths in the U.S. occurred in people under 65, and nearly half of deaths were in young adults 20 to 40 years old. In 1920, the mortality rate among people under 65 had decreased sixfold to half the mortality rate of people over 65, but 92% of deaths still occurred in people under 65.[295] This is unusual since influenza is typically most deadly to weak individuals, such as infants under age two, adults over age 70, and the immunocompromised. In 1918, older adults may have had partial protection caused by exposure to the 1889–1890 flu pandemic.[296] According to historian John M. Barry, the most vulnerable of all were pregnant women. He reported that in thirteen studies of hospitalized women in the pandemic, the death rate ranged from 23% to 71%.[297] Of the pregnant women who survived childbirth, over one-quarter (26%) lost the child.[298] Another oddity was that the outbreak was widespread in the summer and autumn (in the Northern Hemisphere); influenza is usually worse in winter.[299]
There were also geographic patterns to the disease's fatality. Some parts of Asia had 30 times higher death rates than some parts of Europe, and generally, Africa and Asia had higher rates, while Europe and North America had lower ones.[300] There was also great variation within continents, with three times higher mortality in Hungary and Spain compared to Denmark, two to three times higher chance of death in Sub-Saharan Africa compared to North Africa, and possibly up to ten times higher rates between the extremes of Asia.[300] Cities were affected worse than rural areas.[300] There were also differences between cities, which might have reflected exposure to the milder first wave giving immunity, as well as the introduction of social distancing measures.[301]
Another major pattern was the differences between social classes. In Oslo, death rates were inversely correlated with apartment size, as the poorer people living in smaller apartments died at a higher rate.[302] Social status was also reflected in the higher mortality among immigrant communities, with Italian Americans, a recently arrived group at the time, nearly twice as likely to die compared to the average Americans.[300] These disparities reflected worse diets, crowded living conditions, and problems accessing healthcare.[300] Paradoxically, however, African Americans were relatively spared.[300]
More men than women were killed by the flu, as they were more likely to go out and be exposed, while women tended to stay at home.[301] For the same reason men also were more likely to have pre-existing tuberculosis, which severely worsened the chances of recovery.[301] However, in India the opposite was true, potentially because Indian women were neglected with poorer nutrition, and were expected to care for the sick.[301]
A study conducted by He et al. (2011) examined the factors that underlie variability in temporal patterns and their correlation to patterns of mortality and morbidity. Their analysis suggests that temporal variations in transmission rate provide the best explanation, and the variation in transmission required to generate these three waves is within biologically plausible values.[303] Another study by He et al. (2013) used a simple epidemic model incorporating three factors to infer the cause of the three waves of the 1918 influenza pandemic. These factors were school opening and closing, temperature changes throughout the outbreak, and human behavioral changes in response to the outbreak. Their modeling results showed that all three factors are important, but human behavioral responses showed the most significant effects.[304]
Effects
[edit]World War I
[edit]Academic Andrew Price-Smith has argued that the virus helped tip the balance of power in the latter days of the war towards the Allied cause. He provides data that the viral waves hit the Central Powers before the Allied powers and that morbidity and mortality in Germany and Austria were considerably higher than in Britain and France.[94] A 2006 Lancet study corroborates higher excess mortality rates in Germany (0.76%) and Austria (1.61%) compared to Britain (0.34%) and France (0.75%).[247]
Kenneth Kahn at Oxford University Computing Services writes that "Many researchers have suggested that the conditions of the war significantly aided the spread of the disease. And others have argued that the course of the war (and subsequent peace treaty) was influenced by the pandemic." Kahn has developed a model that can be used to test these theories.[305]
Economic
[edit]
Many businesses in the entertainment and service industries suffered losses in revenue, while the healthcare industry reported profit gains.[306] Historian Nancy Bristow has argued that the pandemic, when combined with the increasing number of women attending college, contributed to the success of women in nursing. This was due in part to the failure of medical doctors, who were predominantly men, to contain the illness. Nursing staff, who were mainly women, celebrated the success of their patient care and did not associate the spread of the disease with their work.[307]
A 2020 study found that U.S. cities that implemented early and extensive non-medical measures (quarantine, etc.) suffered no additional adverse economic effects due to implementing those measures.[308][309] However, the validity of this study has been questioned because of the coincidence of WWI and other problems with data reliability.[310]
Long-term effects
[edit]A 2006 study in the Journal of Political Economy found that "cohorts in utero during the pandemic displayed reduced educational attainment, increased rates of physical disability, lower income, lower socioeconomic status, and higher transfer payments received compared with other birth cohorts."[311] A 2018 study found that the pandemic reduced educational attainment in populations.[312] The flu has also been linked to the outbreak of encephalitis lethargica in the 1920s.[313]
Survivors faced an elevated mortality risk. Some survivors did not fully recover from physiological conditions resulting from infection.[314]
Legacy
[edit]
The Spanish flu began to fade from public awareness over the decades until the bird flu and other pandemics in the 1990s and 2000s.[315][316] This has led some historians to label the Spanish flu a "forgotten pandemic".[88] However, this label has been challenged by the historian Guy Beiner, who demonstrated how the pandemic was overshadowed by the commemoration of the First World War and mostly neglected in mainstream historiography, yet was remembered in private and local traditions across the globe.[316]
There are various theories of why the Spanish flu was "forgotten". The rapid pace of the pandemic, which killed most of its victims in the United States within less than nine months, resulted in limited media coverage. The general population was familiar with patterns of pandemic disease in the late 19th and early 20th centuries: typhoid, yellow fever, diphtheria, and cholera all occurred near the same time. These outbreaks probably lessened the significance of the influenza pandemic for the public.[317] In some areas, the flu was not reported on, the only mention being that of advertisements for medicines claiming to cure it.[318]
Additionally, the outbreak coincided with the deaths and media focus on the First World War.[319] The majority of fatalities, from both the war and the epidemic, were among young adults. The high number of war-related deaths of young adults may have overshadowed the deaths caused by flu.[295] Particularly in Europe, where the war's toll was high, the flu may not have had a tremendous psychological impact or may have seemed an extension of the war's tragedies.[295]
In literature and other media
[edit]
Despite the toll of the pandemic, it was never a large theme in American literature.[320] Alfred Crosby suspects it was overshadowed by World War I.[321] Katherine Anne Porter's 1939 novella Pale Horse, Pale Rider is one of the most well-known fictional accounts of the pandemic.[320] The 2006 novel The Last Town on Earth focuses on a town which attempts to limit the spread of the flu by preventing people from entering or leaving.[322] The Pull of the Stars is a 2020 novel by Emma Donoghue set in Dublin during the Spanish flu. Publishers fast-tracked publication because of the then ongoing COVID-19 pandemic.[323]
Comparison with other pandemics
[edit]
| Name | Date | World pop. | Subtype | Reproduction number[326] | Infected (est.) | Deaths worldwide | Case fatality rate | Pandemic severity |
|---|---|---|---|---|---|---|---|---|
| Spanish flu[327] | 1918–20 | 1.80 billion | H1N1 | 1.80 (IQR, 1.47–2.27) | 33% (500 million)[328] or >56% (>1 billion)[329] | 17[330]–100[331][332] million | 2–3%,[329] or ~4%, or ~10%[333] | 5 |
| Asian flu | 1957–58 | 2.90 billion | H2N2 | 1.65 (IQR, 1.53–1.70) | >17% (>500 million)[329] | 1–4 million[329] | <0.2%[329] | 2 |
| Hong Kong flu | 1968–69 | 3.53 billion | H3N2 | 1.80 (IQR, 1.56–1.85) | >14% (>500 million)[329] | 1–4 million[329] | <0.2%[329][334] | 2 |
| 1977 Russian flu | 1977–79 | 4.21 billion | H1N1 | ? | ? | 0.7 million[335] | ? | ? |
| 2009 swine flu pandemic[336][337] | 2009–10 | 6.85 billion | H1N1/09 | 1.46 (IQR, 1.30–1.70) | 11–21% (0.7–1.4 billion)[338] | 151,700–575,400[339] | 0.01%[340][341] | 1 |
| Typical seasonal flu[t 1] | Every year | 7.75 billion | A/H3N2, A/H1N1, B, ... | 1.28 (IQR, 1.19–1.37) | 5–15% (340 million – 1 billion)[342] 3–11% or 5–20%[343][344] (240 million – 1.6 billion) |
290,000–650,000/year[345] | <0.1%[346] | 1 |
Notes
| ||||||||
Research
[edit]
Similarities between a reconstruction of the virus and avian viruses, combined with the human pandemic preceding the first reports of influenza in swine, led researchers to conclude the influenza virus jumped directly from birds to humans, and swine caught the disease from humans.[347][348] More recent research has suggested the strain may have originated in a nonhuman, mammalian species,[349] in 1882–1913.[350] This ancestor virus diverged about 1913–1915 into the classical swine and human H1N1 influenza lineages. The last common ancestor of human strains dates between February 1917 and April 1918. Because pigs are more readily infected with avian influenza viruses than are humans, they were suggested as the original recipients of the virus, passing the virus to humans sometime between 1913 and 1918.[350]

An effort to recreate the Spanish flu strain (a strain of influenza A subtype H1N1) was a collaboration among the Armed Forces Institute of Pathology, the USDA ARS Southeast Poultry Research Laboratory, and Mount Sinai School of Medicine in New York City. The effort resulted in the announcement in 2005 that the group had successfully determined the virus' genetic sequence, using historic tissue samples recovered by pathologist Johan Hultin from an Inuit female flu victim buried in the Alaskan permafrost and samples preserved from American soldiers.[351][352][353][348] This enabled researchers at the Centers for Disease Control and Prevention (CDC) and the Mount Sinai School of Medicine, led by Dr Terrence Tumpey, to synthesize RNA segments from the H1N1 virus and reconstruct infective virus particles.[354] These were subsequently used to experimentally infect mice, ferrets, and macaques, providing information about how to prevent and control future pandemics.[355]
In 2007, Kobasa et al. reported that monkeys (Macaca fascicularis) infected with the recreated flu strain exhibited classic symptoms of the 1918 pandemic, and died from an overreaction of the immune system.[356] This may explain why the Spanish flu had its surprising effect on younger, healthier people, as a person with a stronger immune system would potentially have a stronger overreaction.[357]
In December 2008, research by Yoshihiro Kawaoka of the University of Wisconsin linked three specific genes (termed PA, PB1, and PB2) and a nucleoprotein derived from Spanish flu samples to the ability of the 1918 flu virus to invade the lungs and cause pneumonia. These genes were inserted into a modern H1N1 strain and triggered similar symptoms in animal testing.[358]
In 2008 an investigation used the virus sequence to obtain the Hemagglutinin (HA) antigen and observe the adaptive immunity in 32 survivors of the 1918 pandemic; all of them presented seroreactivity and 7 of 8 further tested presented memory B cells able to produce antibodies that bound to the HA antigen, highlighting the ability of immunological memory.[359][348]
In June 2010, a team at the Mount Sinai School of Medicine reported the 2009 flu pandemic vaccine provided some cross-protection against the Spanish flu pandemic strain.[360]
In 2013, the AIR Worldwide Research and Modeling Group "estimated the effects of a similar pandemic occurring today using the AIR Pandemic Flu Model". In the model, "a modern-day 'Spanish flu' event would result in additional life insurance losses of between US$15.3–27.8 billion in the United States alone", with 188,000–337,000 deaths in the United States.[361]
In 2018, Michael Worobey, a professor at the University of Arizona who is examining the history of the 1918 pandemic, revealed that he obtained tissue slides created by William Rolland, a physician who reported on a respiratory illness likely to be the virus while a pathologist in the British military during World War One.[362][363][364] Worobey extracted tissue from the slides to potentially reveal more about the origin of the pathogen.[26]
See also
[edit]- 1918 flu pandemic in India – Known in India as "Bombay Fever"
- 2009 swine flu pandemic – 2009–2010 pandemic of swine influenza caused by H1N1 influenza virus
- Anti-Mask League of San Francisco – 1919 San Francisco organization
- COVID-19 pandemic – Pandemic caused by SARS-CoV-2 (2019–2023)
- Pandemic – Widespread, often global, epidemic of severe infectious disease
- List of epidemics and pandemics
- List of Spanish flu cases
Footnotes
[edit]References
[edit]Citations
[edit]- ^ a b c d Valentine V (20 February 2006). "Origins of the 1918 Pandemic: The Case for France". National Public Radio. Archived from the original on 30 April 2009. Retrieved 13 April 2020.
- ^ a b c Yang W, Petkova E, Shaman J (March 2014). "The 1918 influenza pandemic in New York City: age-specific timing, mortality, and transmission dynamics". Influenza and Other Respiratory Viruses. 8 (2). National Institutes of Health: 177–188. doi:10.1111/irv.12217. PMC 4082668. PMID 24299150.
- ^ a b c d e Taubenberger & Morens 2006.
- ^ "Pandemic Influenza Risk Management WHO Interim Guidance" (PDF). World Health Organization. 2013. p. 25. Archived (PDF) from the original on 21 January 2021. Retrieved 21 August 2021.
- ^ a b c d e Spreeuwenberg P, Kroneman M, Paget J (December 2018). "Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic". American Journal of Epidemiology. 187 (12). Oxford University Press: 2561–2567. doi:10.1093/aje/kwy191. PMC 7314216. PMID 30202996.
- ^ Rosenwald MS (7 April 2020). "History's deadliest pandemics, from ancient Rome to modern America". The Washington Post. Archived from the original on 7 April 2020. Retrieved 11 April 2020.
- ^ CDC (17 December 2019). "The Discovery and Reconstruction of the 1918 Pandemic Virus". Centers for Disease Control and Prevention. Archived from the original on 25 March 2020. Retrieved 19 September 2022.
- ^ Spreeuwenberg (7 September 2018). "Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic". Our World in Data. 187 (12): 2561–2567. doi:10.1093/aje/kwy191. PMC 7314216. PMID 30202996. Archived from the original on 7 September 2021. Retrieved 17 September 2024.
- ^ a b Barry JM (2021). The Great Influenza: The Story of the Deadliest Pandemic in History. Penguin Books. pp. 397–398. ISBN 978-0-14-303649-4.
- ^ a b Mayer J (29 January 2019). "The Origin Of The Name 'Spanish Flu'". Science Friday. Archived from the original on 29 January 2019. Retrieved 30 July 2021.
Etymology: In ancient times, before epidemiology science, people believed the stars and "heavenly bodies" flowed into us and dictated our lives and health—influenza means 'to influence' in Italian, and the word stems from the Latin for 'flow in.' Sickness, like other unexplainable events, was attributed to the influence of the stars... But the name for the infamous 1918 outbreak, the Spanish flu, is actually a misnomer.
- ^ Gagnon, Miller & et al 2013, p. e69586.
- ^ a b More AF, Loveluck CP, Clifford H, Handley MJ, Korotkikh EV, Kurbatov AV, et al. (September 2020). "The Impact of a Six-Year Climate Anomaly on the "Spanish Flu" Pandemic and WWI". GeoHealth. 4 (9) e2020GH000277. Bibcode:2020GHeal...4..277M. doi:10.1029/2020GH000277. ISSN 2471-1403. PMC 7513628. PMID 33005839.
- ^ Marshall L (9 October 2023). "1918 flu pandemic myth debunked by skeletal remains". CU Boulder Today. Archived from the original on 26 March 2024. Retrieved 26 March 2024.
- ^ Brundage JF, Shanks GD (December 2007). "What really happened during the 1918 influenza pandemic? The importance of bacterial secondary infections". The Journal of Infectious Diseases. 196 (11): 1717–18, author reply 1718–19. doi:10.1086/522355. PMID 18008258.
- ^ Morens DM, Fauci AS (April 2007). "The 1918 influenza pandemic: insights for the 21st century". The Journal of Infectious Diseases. 195 (7): 1018–1028. doi:10.1086/511989. PMID 17330793.
- ^ Davis 2013, p. 7: "In short, although the Spanish flu 'had nothing "Spanish" about it,' from a strictly epidemiological perspective, its discursive link to the Iberian nation is beyond dispute. The importance of narrative for a historically informed appreciation of the epidemic stems from the importance of this discursive link and is tied directly to the scientific uncertainty about the etiological agent of the epidemic;"
- ^ "Biblical Hebrew Words and Meaning". hebrewversity. 14 March 2018. Retrieved 11 August 2021.
in the original Hebrew the people of Israel asked: "Ma'n Hu?" {?מן הוא} – English for 'what is it?' and that is the origin of the name 'manna'
- ^ Spinney 2018, p. 65: "In Freetown, a newspaper suggested that the disease be called manhu until more was known about it. Manhu, a Hebrew word meaning 'what is it?'
- ^ Cole F (1994), Sierra Leone and World War 1, University of London, School of Oriental and African Studies, p. 213, 10731720, archived from the original on 11 August 2021, retrieved 11 August 2021,
local interpretations of the crisis had become deeply reminiscent of the increasing disobedience of the Israelites in the wilderness of Sin. It was therefore urged that the epidemic be called 'Man hu,' (an obvious corruption of "manna") meaning 'what is it?'
- ^ Sierra Leone Weekly News, vol. XXXV, 9 July 1918, p. 6 quoted in Mueller JW (1998), p. 8.
- ^ Hammond JA, Rolland W, Shore TH (14 July 1917). "Purulent Bronchitis". The Lancet. 190 (4898): 41–46. doi:10.1016/s0140-6736(01)56229-7. ISSN 0140-6736. Archived from the original on 22 March 2023. Retrieved 30 July 2021. Alt URL Archived 26 August 2019 at the Wayback Machine
- ^ Radusin M (September 2012). "The Spanish flu – Part I: the first wave". Vojnosanitetski Pregled. 69 (9): 812–817. PMID 23050410.
the Purple Death ... name resulted from the specific skin colour in the most severe cases of the diseased, who by the rule also succumbed to the disease, and is also at the same time the only one which does not link this disease with the Iberian peninsula. This name is actually the most correct.
- ^ McCord CP (November 1966). "The Purple Death. Some things remembered about the influenza epidemic of 1918 at one army camp". Journal of Occupational Medicine. 8 (11). Industrial Medical Association: 593–598. PMID 5334191.
- ^ Getz D (2017). Purple death : the mysterious Spanish flu of 1918. Square Fish. ISBN 978-1-250-13909-2. OCLC 1006711971.
- ^ a b Oxford JS, Gill D (2 September 2019). "A possible European origin of the Spanish influenza and the first attempts to reduce mortality to combat superinfecting bacteria: an opinion from a virologist and a military historian". Human Vaccines & Immunotherapeutics. 15 (9): 2009–2012. doi:10.1080/21645515.2019.1607711. PMC 6773402. PMID 31121112.
Two papers were published in The Lancet in 1917 describing an outbreak of disease constituting 'almost a small epidemic'. The first paper was written by physicians at a hospital center in northern France,3 and the second by a team at an army hospital in Aldershot, in southern England. In both earlier instances and in the 1918 pandemic the disease was characterized by a 'dusky' cyanosis, a rapid progression from quite minor symptoms to death
- ^ a b Cox J, Gill D, Cox F, Worobey M (April 2019). "Purulent bronchitis in 1917 and pandemic influenza in 1918". The Lancet. Infectious Diseases. 19 (4): 360–361. doi:10.1016/s1473-3099(19)30114-8. PMID 30938298. S2CID 91189842.
- ^ Honigsbaum M (June 2018). "Spanish influenza redux: revisiting the mother of all pandemics". Lancet. 391 (10139): 2492–2495. doi:10.1016/S0140-6736(18)31360-6. PMID 29976462. S2CID 49709093.
Labelled 'purulent bronchitis' for want of a better term, the disease proved fatal in half the cases and many soldiers also developed cyanosis. 2 years later, British respiratory experts, also writing in The Lancet, but this time in the wake of the pandemic, would decide the disease had been 'fundamentally the same condition' as 'Spanish' influenza
- ^ Dennis Shanks G, Mackenzie A, Waller M, Brundage JF (July 2012). "Relationship between "purulent bronchitis" in military populations in Europe prior to 1918 and the 1918–1919 influenza pandemic". Influenza and Other Respiratory Viruses. 6 (4): 235–239. doi:10.1111/j.1750-2659.2011.00309.x. PMC 5779808. PMID 22118532.
- ^ Storey, C (21 September 2020). "OLD NEWS: Influenza was an old foe long before 1918". Arkansas Democrat-Gazette. Opinion. Archived from the original on 7 August 2021. Retrieved 7 August 2021.
I came across this seemingly astute analysis in the Dec. 21, 1913, Gazette.... It was a 'Special Cable to the Gazette Through the International News Service.' Grip Is a Disease Without a Country; All Nations Repudiate Malady: Each Blaming Other Kingdoms, London, Dec. 20. — The grip is a disease without a country, according to a new book just issued which is devoted to the malady. Every country tries to make it out a native of another land.... Eighteenth-century Italian writers say Dr. Hopkirk spoke of "una influenza di freddo" (influence of cold), and English physicians, mistaking the word influenza for the name of the disease itself, used it. The same term is also used in Germany, where a host of dialect names still prevail, such as lightning catarrh and fog plague.
- ^ "Grippe is not new". Los Angeles Herald. 14 January 1899. p. 6 col. 6. Archived from the original on 7 August 2021. Retrieved 7 August 2021 – via California Digital Newspaper Collection.
French doctors gave it the name of "la grippe," which is now anglicized into "the grip" ... It is known all over the world, and there is a disposition in every nation to shift the odium of it upon some other country. Then the Russians call it the Chinese catarrh, the Germans often call it the Russian pest, the Italians name it the German disease, and the French call it sometimes the Italian fever and sometimes the Spanish catarrh.
- ^ Davis 2013, p. 27: "On May 21, 1918, El Liberal published a scant article titled 'Can One Live? The Fashionable Illness.' Likely the first account in Spain of the Spanish flu, it begins: 'For several days, Madrid has been affected by an epidemic, which fortunately is mild; but which, from what it appears, intends to kill doctors from overwork'.... The following day, El Sol and ABC published their first stories about the epidemic: 'What is the Cause? An Epidemic in Madrid' and 'Benign Epidemic. The Sickbay in Madrid,' respectively."
- ^ Alfeirán X (7 December 2015). "La fiebre de los tres días" [The three-day fever]. La Voz de Galicia (in Spanish). A Coruña. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
Las primeras noticias aparecieron en la prensa de Madrid el 21 de mayo de 1918.
[The first news appeared in the press of Madrid on 21 May 1918.] - ^ McClure J (1 May 2009). "Spanish flu of 1918 no three-day fever. Try 365-day worldwide plague". York Daily Record. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
- ^ Calleja S (29 July 2021). "La fiebre de los tres días" [The three-day fever]. Diario de León (in Spanish). León, Spain. Archived from the original on 29 July 2021. Retrieved 21 October 2018.
- ^ "Impacto de la gripe de 1918 en España". Comité Asesor de Vacunas de la Asociación Española de Pediatría [Vaccine Advisory Committee of the Spanish Association of Pediatrics] (in Spanish). Archived from the original on 29 July 2021. Retrieved 29 July 2021.
En España, se le llamó al principio 'la fiebre de los tres días', atendiendo a la creencia, como en otros países, de que la gripe era una enfermedad leve. Las primeras noticias sobre la gripe, llamando la atención sobre que algo distinto estaba ocurriendo, aparecieron en la prensa a finales de mayo. Por ej. en el diario ABC el 22 de mayo mediante una escueta nota en la página 24: 'Los médicos han comprobado, en Madrid, la existencia de una epidemia de índole gripal, muy propagada, pero, por fortuna, de carácter leve'.
[In Spain, it was initially called 'the three-day fever', based on the belief, as in other countries, that the flu was a mild illness. The first reports about the flu, drawing attention to the fact that something different was happening, appeared in the press at the end of May. For example, in the newspaper ABC on 22 May, through a brief note on page 24: "The doctors have verified, in Madrid, the existence of an epidemic of a influenza nature, very widespread, but, fortunately, of a mild nature.'] - ^ Killingray D, Phillips H (2003). The Spanish Influenza Pandemic of 1918–1919: New Perspectives. Routledge. ISBN 978-1-134-56640-2. Archived from the original on 1 October 2024. Retrieved 3 May 2020.
In the popular mind calamities often need to have their origin and cause identified and other countries or peoples credited with blame. This xenophobic response has been common in Europe, that impulse to blame others or the silent places of the Asian heartlands for the source of disease.
- ^ a b Hoppe T (November 2018). ""Spanish Flu": When Infectious Disease Names Blur Origins and Stigmatize Those Infected". American Journal of Public Health. 108 (11): 1462–1464. doi:10.2105/AJPH.2018.304645. PMC 6187801. PMID 30252513.
- ^ a b c Bax D (2020). "Pandemie – Welt im Fieber" [Pandemic – World in Fever]. Der Freitag (in German) (13). Archived from the original on 31 July 2021. Retrieved 31 July 2021.
In Großbritannien wurde die Krankheit dagegen als 'Flandrische Grippe' bezeichnet, weil sich viele —en in den Schützengräben von Flandern ansteckten.
[In Britain, on the other hand, the disease was called the 'Flanders flu' because many soldiers became infected in the trenches of Flanders.] - ^ Reynolds JR (1866). A System of Medicine. Vol. 1. Macmillan. pp. 30–31. Archived from the original on 1 October 2024. Retrieved 24 August 2021.
One is, that every epidemic owns one unknown source, whence it spreads; each nation, in turn, attributing to its neighbour from whom it derived the disease, the unenviable honour of originating it. Thus the Italians have termed it the German disease; the Germans, the Russian pest; the Russians, the Chinese Catarrh;
- ^ Harrison MA, Claiborne JH Jr, eds. (January 1890). "Editorials: La Grippe". Gaillard's Medical Journal. 50: 217. Archived from the original on 1 October 2024. Retrieved 3 August 2021.
The outbreak immediately preceding the present one was in 1879, and has been well described by Da Costa. Like every other disease about whose pathology we know very little, this malady has not a scientifically correct name, but at different times and in different countries has received names which are almost purely local; thus the Russians have called it the Chinese catarrh, because it has often invaded Russia from China. The Germans call it the Russian pest, while the Italians in turn call it the German disease.
- ^ a b c Davis KC (2018). More deadly than war : the hidden history of the Spanish flu and the First World War (First ed.). New York: Henry Holt and Company. ISBN 978-1-250-14512-3. OCLC 1034984776.
The Russians called it the Chinese flu. In Japan, it was wrestler's fever. In South Africa, it was known as either the white man's sickness or kaffersiekte blacks' disease. Soldiers fighting in the Great War called it the three-day fever—a highly inaccurate description—and when it first struck in the spring of 1918, German soldiers called it Flanders fever, after one of the war's most notorious and deadly battlefields
- ^ Porras-Gallo & Davis 2014, p. 51.
- ^ Galvin 2007.
- ^ "The Spanish Epidemic". The Times. London. 2 June 1918. Archived from the original on 21 January 2025. Retrieved 29 July 2021.
The unknown disease which appeared in Madrid a fortnight ago spread with remarkable rapidity.... It is reported that there are well over 100,000 victims in Madrid alone.... Although the disease is clearly of a gripal character...
- ^ "The Spanish Influenza; A Sufferer's Symptoms". The Times. London. 25 June 1918. Archived from the original on 21 January 2025. Retrieved 29 July 2021.
- ^ "Public Notice; 'Spanish Influenza' Epidemic". The Times. London. 28 June 1918. Retrieved 11 August 2021.
- ^ Arnold C (13 September 2018). "Eat More Onions!". Lapham's Quarterly. Archived from the original on 12 August 2021. Retrieved 12 August 2021.
daily newspapers carried an increasing number of advertisements for influenza-related remedies as drug companies played on the anxieties of readers and reaped the benefits. From the Times of London to the Washington Post, page after page was filled with dozens of advertisements for preventive measures and over-the-counter remedies. 'Influenza!' proclaimed an advert extolling the virtues of Formamint lozenges.
- ^ Daniels R (29 April 2018) [1998 Mill Hill Essays]. "In Search of an Enigma: The "Spanish Lady"". NIMR History. Archived from the original on 29 April 2018. Retrieved 9 August 2021 – via web.archive.org.
In turn, when Russia reported on the situation in Moscow, Pravda printed "Ispanka (The Spanish Lady) is in town" and the name has stuck.
- ^ a b Trilla A, Trilla G, Daer C (September 2008). "The 1918 "Spanish flu" in Spain". Clinical Infectious Diseases. 47 (5): 668–673. doi:10.1086/590567. PMID 18652556.
- ^ "Spanish flu facts". Channel 4 News. Archived from the original on 27 January 2010.
- ^ Anderson S (29 August 2006). "Analysis of Spanish flu cases in 1918–1920 suggests transfusions might help in bird flu pandemic". American College of Physicians. Archived from the original on 25 November 2011. Retrieved 2 October 2011.
- ^ Barry 2004, p. 171.
- ^ Spinney L (29 September 2018b). "The centenary of the 20th century's worst catastrophe". The Economist. ISSN 0013-0613. Archived from the original on 3 August 2021. Retrieved 3 August 2021.
On June 29th 1918 Martín Salazar, Spain's inspector-general of health, stood up in front of the Royal Academy of Medicine in Madrid. He declared, not without embarrassment, that the disease which was ravaging his country was to be found nowhere else in Europe. In fact, that was not true. The illness in question, influenza, had been sowing misery in France and Britain for weeks, and in America for longer, but Salazar did not know this because the governments of those countries, a group then at war with Germany and its allies, had made strenuous efforts to suppress such potentially morale-damaging news.
- ^ "Madrid Letter". Journal of the American Medical Association. 71 (19): 1594. 9 November 1918. Archived from the original on 1 October 2024. Retrieved 24 August 2021.
- ^ a b c Vázquez-Espinosa E, Laganà C, Vázquez F (October 2020). "The Spanish flu and the fiction literature". Revista Espanola de Quimioterapia. 33 (5): 296–312. doi:10.37201/req/049.2020. PMC 7528412. PMID 32633114.
French journalists had, initially, called it the 'American flu'; but the fact that the American soldiers were his allies in the warlike conflict advised not to assign such a link to them.... Another most popular name in Madrid, was the 'Soldado de Nápoles' (Naples soldier), a popular song in the zarzuela (popular musical genre or 'género chico' in Spain) called La canción del olvido (The forgotten song) due both, were 'highly contagious'. Today, there are many authors who avoid such a name (the Spanish flu) and they aptly refer to it as the '1918- 1819[sic] influenza pandemic'
- ^ Müller S (2020). "Spanische Grippe" [The Spanish Flu]. www.fes.de (in German). Bonn: Friedrich Ebert Foundation. Archived from the original on 31 July 2021. Retrieved 31 July 2021.
In Europa wurde die Spanischer Grippe auch als 'Blitzkatarrh', als 'Flandern-Fieber', 'flandische Grippe', bei Engländern und Amerikanern als 'three-day'- oder 'knock-me-down'-Fieber, und in Frankreich als 'la grippe', als 'bronchite purulente' (eitrige Bronchitis) oder beim französische Militärärzte als 'Krankheit 11' (maladie onze) bezeichnet. Die Benennung von Krankheiten und insbesondere Seuchen nach ihrem vermuteten Ursprungsort ist nichts Ungewöhnliches. Es ist der Versuch, einem Geschehen auf die Spur zu kommen. Zugleich werden auf diese Weise Krankheiten als etwas Äußerliches gekennzeichnet, als etwas Fremdes, das eingedrungen ist oder eingeschleppt wurde.
[In Europe, the Spanish flu was also referred to as 'Blitzkatarrh', as 'Flanders fever', 'Flanders flu', in English and Americans as 'three-day' or 'knock-me-down' fever, and in France as 'la flu', as 'bronchite purulente' (purulent bronchitis) or by French military doctors as 'disease 11' (maladie onze). The naming of diseases and especially epidemics according to their presumed place of origin is nothing unusual. It is an attempt to track down what is happening. At the same time, in this way, diseases are marked as something external, as something foreign that has invaded or been introduced.] - ^ a b Cotter C (23 April 2020). "From the 'Spanish Flu' to COVID-19: lessons from the 1918 pandemic and First World War". Humanitarian Law & Policy. International Committee of the Red Cross. Archived from the original on 7 August 2021. Retrieved 7 August 2021.
Many other nicknames were given to the pandemic, many based on nationality or race: 'Spanish Lady', 'French Flu', 'Naples Soldier', 'Purple Death', 'War Plague', 'Flanders Grippe', 'Kirghiz Disease', 'Black Man's Disease', 'Hun Flu', 'German Plague', 'Bolshevik Disease' or even the 'Turco-Germanic bacterium criminal entreprise'. These discriminatory epithets reflect the many rumors and theories that quickly spread about the origins of the pathology.
- ^ a b Spinney 2018, p. 58.
- ^ Davis 2013, pp. 103–36.
- ^ Landgrebe P (29 December 2018). "100 Years After: The Name of Death". History Campus. Archived from the original on 16 August 2020. Retrieved 16 August 2020.
- ^ Rodríguez LP, Palomba AL (5 March 2019). "How is the adjective 'Spanish' used in other languages?". El País (English ed.). Madrid. Archived from the original on 1 August 2021. Retrieved 1 August 2021.
The use of 'Spanish' can often have negative connotations, with the adjective often unfairly used to describe unwelcome events and problems. The most obvious example is the so-called 'Spanish flu,' a reference to the 1918 influenza pandemic...
- ^ O'Reilly T (13 May 2020). "How the Spanish Flu wasn't Spanish at all". CBC Radio. Under the Influence. Archived from the original on 1 August 2021. Retrieved 1 August 2021.
Medical professionals and officials in Spain protested. They said the Spanish people were being falsely stigmatized.... If you've ever wondered about the staying power of a brand, the 'Spanish Flu' is a case in point. A full 100 years later, the 'Spanish Flu' is still referenced — and still remains a source of irritation in Spain.
- ^ Dionne & Turkmen 2020.
- ^ Ristić D, Marinković D (14 November 2022). "Biopolitics of othering during the COVID-19 pandemic". Humanities and Social Sciences Communications. 9 (1). Palgrave: 409. doi:10.1057/s41599-022-01435-7. ISSN 2662-9992. PMC 9662131. PMID 36406151.
- ^ Takon L (7 April 2020). "Fighting words: how war metaphors can trigger racism". The Lighthouse. Macquarie University. Archived from the original on 8 August 2021. Retrieved 8 August 2021.
the names given to this disease in different parts of the world reflected prevailing concerns about certain ethnic groups and ideologies. The disease was called the 'Singapore fever' in Penang and the Bolshevik disease in Poland.
- ^ Phillips H (1990). Black October: The Impact of the Spanish Influenza Epidemic of 1918 on South Africa. Government Printer, South Africa. ISBN 978-0-7970-1580-7. Archived from the original on 1 October 2024. Retrieved 12 August 2021.
In one area where Blacks were the first victims, the accusatory term 'Kaffersiekte' was coined; in another district, where the position was reversed, Blacks returned the compliment with, White man's sickness'
- ^ 余録: 「春のさきぶれ」といえば何か聞こえが良いが... [Speaking of "spring sekibu", something sounds good...]. 毎日新聞 =毎日新聞 [Mainichi Shimbun morning newspaper] (in Japanese). 15 May 2020. Archived from the original on 8 August 2021. Retrieved 8 August 2021. 翌月の東京の夏場所は高熱などによる全休力士が相次いだ.世間はこれを「相撲風邪」「力士風邪」と呼んだが、実はこの謎の感染症こそが同年初めから米国で流行の始まった「スペイン風邪」とみられている. [The following month, a number of sumo wrestlers were absent from the summer tournament in Tokyo due to high fevers. People called it the "sumo flu" or "wrestler flu," but in fact, this mysterious infection is believed to be the Spanish flu, which began spreading in the United States early that year.]
- ^ "How the Spanish flu of 1918-20 ravaged Japan". Japan Today. 6 May 2020. Archived from the original on 8 August 2021. Retrieved 8 August 2021.
The first patients in Japan, reported Shukan Gendai (May 2–9), began showing symptoms around April 1918. Initially the disease was referred to as the "Sumo Kaze" (sumo cold) because a contingent of sumo wrestlers contracted it while on a tour of Taiwan. Three well known grapplers, Masagoishi, Choshunada and Wakagiyama, died before they could return from Taiwan. As the contagion spread, the summer sumo tournament, which would have been held on the grounds of Yasukuni shrine, was cancelled.
- ^ Dionne & Turkmen 2020, pp. 213–230.
- ^ "WHO issues best practices for naming new human infectious diseases". www.who.int. 8 May 2015. Archived from the original on 31 July 2021. Retrieved 4 August 2021.
- ^ "Pandemic influenza: an evolving challenge". World Health Organization. 22 May 2018. Archived from the original on 20 March 2020. Retrieved 20 March 2020.
- ^ "Influenza pandemic of 1918–19". Encyclopaedia Britannica. 4 March 2020. Archived from the original on 20 March 2020. Retrieved 20 March 2020.
- ^ Chodosh S (18 March 2020). "What the 1918 flu pandemic can teach us about COVID-19, in four charts". PopSci. Archived from the original on 24 December 2020. Retrieved 20 March 2020.
- ^ Mueller JW (1998). What's in a name. Spanish Influenza in sub-Saharan Africa and what local names say about the perception of this pandemic. Spanish 'Flu 1918–1998: Reflections on the Influenza Pandemic of 1918 after 80 Years. Cape Town, South Africa. Archived from the original on 30 November 2021. Retrieved 13 August 2021.
- ^ Phillips H (8 October 2014). "Influenza Pandemic (Africa)". encyclopedia.1914-1918-online.net. International Encyclopedia of the First World War (WW1). Archived from the original on 13 August 2021. Retrieved 13 August 2021.
- ^ Great Britain Ministry of Health (1920). Report on the Pandemic of Influenza, 1918–19. H.M. Stationery Office. Archived from the original on 1 October 2024. Retrieved 24 August 2021.
There was a high west wind at the time and this was thought to be the carrying agent so that the affliction was called 'the disease of the wind.'
- ^ Afkhami AA (5 February 2019). A Modern Contagion: Imperialism and Public Health in Iran's Age of Cholera. JHU Press. ISBN 978-1-4214-2722-5. Archived from the original on 1 October 2024. Retrieved 24 August 2021.
In Tehran, the disease was called the "illness of the wind" (nakhushi-yi bad) due to its initial occurrence during a strong westerly wind burst and its rapid spread.
- ^ Archives of Internal Medicine. Vol. 24. Chicago: American Medical Association. 1919. Archived from the original on 1 October 2024. Retrieved 24 August 2021.
As this included the period of the great influenza epidemic when Type IV infections were usually prevalent, it will be seen that the proportion of fixed types among carriers and among cases is not far from the same.
- ^ United States Surgeon-General's Office (1920). Report of the Surgeon-General of the Army to the Secretary of War for the Fiscal Year Ending June 30, 1919. War Department Annual Reports. Vol. I. U.S. Government Printing Office. Archived from the original on 1 October 2024. Retrieved 24 August 2021.
On September 13, 1918, the first cases of the great influenza epidemic were admitted, and during the next 10 weeks over 4,100 patients were admitted.
- ^ a b c d e f Barry 2004b.
- ^ Spinney 2018, p. 64: "In ... Rhodesia (Zimbabwe) ... officials labelled the new affliction 'influenza (vera)', adding the Latin word vera, meaning 'true' ... German doctors ... called it 'pseudo-influenza'"
- ^ Cross A (29 March 2020). "A look at art and music created in times of pandemic". Global News (Canada). Archived from the original on 10 August 2021. Retrieved 9 August 2021.
Another rhyme with deadly origins appeared during a worldwide influenza pandemic in 1889–1890. Certain the disease could be stopped by sealing up the home from the poisoned air outside, this safety tip emerged in schools: There was a little girl, and she had a little bird; And she called it by the pretty name of Enza; But one day it flew away, but it didn't go to stay; For when she raised the window, in-flu-Enza
- ^ University Libraries. "WWI Exhibit: Influenza Pandemic". content.lib.washington.edu. University of Washington. Archived from the original on 9 August 2021. Retrieved 9 August 2021.
- ^ Hakim J (2002). War, Peace, and All that Jazz. Oxford University Press. ISBN 978-0-19-515335-4. Archived from the original on 1 October 2024. Retrieved 24 August 2021.
In flew Enza—say it fast and it becomes 'influenza.' It was a catchy little rhyme, and boys and girls skipped rope to it.
- ^ Honigsbaum M (2016). Living with Enza: The Forgotten Story of Britain and the Great Flu Pandemic of 1918. Springer. ISBN 978-0-230-23921-0. Archived from the original on 1 October 2024. Retrieved 24 August 2021.
A popular playground skipping rhyme caught the ease with which this 'new disease' was transmitted: I had a little bird; Its name was Enza; I opened the window; And in-flu-enza. As influenza spread from Glasgow to Aberdeen, and from Liverpool to London's Mile End, it was not long before playgrounds throughout the country echoed to the chant.
- ^ March PC (4 September 1932). "General March's Narrative: Glimpses of Woodrow Wilson". The New York Times. p. XX3, Special Features section. Archived from the original on 10 August 2021. Retrieved 10 August 2021.
- ^ "The 1918 Flu Pandemic Killed Hundreds of Thousands of Americans. The White House Never Said a Word About It". Time. Archived from the original on 10 August 2021. Retrieved 10 August 2021.
Crosby's America's Forgotten Pandemic: The Influenza of 1918, Wilson asked Army Chief of Staff General Peyton March in October 1918 if he had heard of the popular jump rope rhyme parodying the virus, and recited part of it.
- ^ a b Crosby 2003.
- ^ a b Worobey M, Cox J, Gill D (2019). "The origins of the great pandemic". Evolution, Medicine, and Public Health. 2019 (1): 18–25. doi:10.1093/emph/eoz001. PMC 6381288. PMID 30805187. S2CID 67863937.
- ^ Oxford JS (December 2001). "The so-called Great Spanish Influenza Pandemic of 1918 may have originated in France in 1916". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 356 (1416). Royal Society: 1857–1859. doi:10.1098/rstb.2001.1012. PMC 1088561. PMID 11779384.
- ^ a b Connor S (8 January 2000). "Flu epidemic traced to Great War transit camp". The Guardian. UK. Archived from the original on 8 August 2009. Retrieved 9 May 2009.
- ^ a b Oxford JS, Lambkin R, Sefton A, Daniels R, Elliot A, Brown R, et al. (January 2005). "A hypothesis: the conjunction of soldiers, gas, pigs, ducks, geese and horses in northern France during the Great War provided the conditions for the emergence of the "Spanish" influenza pandemic of 1918–1919" (PDF). Vaccine. 23 (7): 940–945. doi:10.1016/j.vaccine.2004.06.035. PMID 15603896. Archived from the original (PDF) on 12 March 2020. Retrieved 12 March 2020.
- ^ a b c Shanks GD (January 2016). "No evidence of 1918 influenza pandemic origin in Chinese laborers/soldiers in France". Journal of the Chinese Medical Association. 79 (1): 46–48. doi:10.1016/j.jcma.2015.08.009. PMID 26542935.
- ^ a b Price-Smith 2008.
- ^ a b c d Ansart S, Pelat C, Boelle PY, Carrat F, Flahault A, Valleron AJ (May 2009). "Mortality burden of the 1918–1919 influenza pandemic in Europe". Influenza and Other Respiratory Viruses. 3 (3): 99–106. doi:10.1111/j.1750-2659.2009.00080.x. PMC 4634693. PMID 19453486.
- ^ a b Hannoun C (1993). "La Grippe". Documents de la Conférence de l'Institut Pasteur. La Grippe Espagnole de 1918. Ed Techniques Encyclopédie Médico-Chirurgicale (EMC), Maladies infectieuses. Vol. 8-069-A-10.
- ^ Humphries 2014.
- ^ Vergano D (24 January 2014). "1918 Flu Pandemic That Killed 50 Million Originated in China, Historians Say". National Geographic. Archived from the original on 26 January 2014. Retrieved 4 November 2016.
- ^ a b Spinney 2018, p. 143.
- ^ a b Killingray D, Phillips H (2003). The Spanish Influenza Pandemic of 1918–1919: New Perspectives. Routledge. ISBN 978-1-134-56640-2. Archived from the original on 9 May 2021. Retrieved 9 November 2020.
- ^ Langford C (2005). "Did the 1918–19 Influenza Pandemic Originate in China?". Population and Development Review. 31 (3): 473–505. doi:10.1111/j.1728-4457.2005.00080.x. JSTOR 3401475.
- ^ a b c d e Cheng KF, Leung PC (July 2007). "What happened in China during the 1918 influenza pandemic?". International Journal of Infectious Diseases. 11 (4): 360–364. doi:10.1016/j.ijid.2006.07.009. PMID 17379558.
- ^ Vergano D. "1918 Flu Pandemic That Killed 50 Million Originated in China, Historians Say". National Geographic. Archived from the original on 3 March 2020. Retrieved 5 March 2020.
- ^ a b c d Saunders-Hastings PR, Krewski D (December 2016). "Reviewing the History of Pandemic Influenza: Understanding Patterns of Emergence and Transmission". Pathogens. 5 (4): 66. doi:10.3390/pathogens5040066. PMC 5198166. PMID 27929449.
- ^ "The History of Pandemics in Chicago". Chicago Tribune. Archived from the original on 25 March 2025. Retrieved 17 March 2025.
- ^ a b c Spinney 2018, p. 36.
- ^ "1918 Flu (Spanish flu epidemic)". Avian Bird Flu. Archived from the original on 21 May 2008.
- ^ Sheidlower N (17 March 2020). "How NYC Survived the 1918 Spanish Flu Pandemic". Untapped New York. Archived from the original on 29 September 2020. Retrieved 8 October 2020.
- ^ Billings 1997.
- ^ "The Memoirs of Herbert Hoover: Years of Adventure, 1874–1920. (New York: Macmillan Company. 1951. pp. xi, 496.) and Herbert Hoover and the Russian Prisoners of World War I: A Study in Diplomacy and Relief, 1918–1919. By Edward F. Willis. (Stanford: Stanford University Press. 1951. pp. viii, 67.)". The American Historical Review: 12. 2011. doi:10.1086/ahr/57.3.709. ISSN 1937-5239.
- ^ a b Spinney 2018, p. 37.
- ^ "Queer Epidemic Sweeps North China; Banks and Silk Stores in Peking Closed – Another Loan Sought from Japan". The New York Times. 1 June 1918. ISSN 0362-4331. Archived from the original on 24 June 2020. Retrieved 22 June 2020.
- ^ a b c d e Patterson & Pyle 1991.
- ^ a b "Mortality Statistics 1918: Nineteenth Annual Report" (PDF). United States Census Bureau. 1920. p. 28. Archived (PDF) from the original on 11 July 2020. Retrieved 29 May 2020.
- ^ a b Erkoreka A (March 2010). "The Spanish influenza pandemic in occidental Europe (1918–1920) and victim age". Influenza and Other Respiratory Viruses. 4 (2): 81–89. doi:10.1111/j.1750-2659.2009.00125.x. PMC 5779284. PMID 20167048.
- ^ a b Spinney 2018, p. 38.
- ^ Byerly CR (April 2010). "The U.S. military and the influenza pandemic of 1918–1919". Public Health Reports. 125 Suppl 3 (Suppl 3): 82–91. doi:10.1177/00333549101250S311. PMC 2862337. PMID 20568570.
- ^ a b Spinney 2018, p. 39.
- ^ a b "Atatürk işgalcilerden önce İspanyol Gribini yenmişti". sozcu.com.tr (in Turkish). 23 April 2020. Archived from the original on 8 November 2020. Retrieved 2 November 2020.
- ^ Spinney 2018, p. 40.
- ^ Barry JM (2005). The Great Influenza. United States: Penguin Books. p. 270. ISBN 0-670-89473-7.
On September 15, New York City's first influenza death occurred.
- ^ Flynn M (12 March 2020). "What happens if parades aren't canceled during pandemics? Philadelphia found out in 1918, with disastrous results". The Washington Post. Archived from the original on 4 July 2020. Retrieved 9 July 2020.
- ^ Spinney 2018, p. 41.
- ^ Spinney 2018, p. 42.
- ^ Kenner R (18 January 2010). "Influenza 1918". American Experience. Season 10. Episode 5. PBS. WGBH. Archived from the original on 27 May 2020. Transcript. Retrieved 27 May 2020.
- ^ "Epidemic Influenza". p. 93. Archived from the original on 4 July 2020. Retrieved 4 July 2020.
- ^ Arnold D (2019). "Death and the Modern Empire: The 1918–19 Influenza Epidemic in India". Transactions of the Royal Historical Society. 29: 181–200. doi:10.1017/S0080440119000082. S2CID 211656275.
- ^ a b c d Chandra S, Christensen J, Likhtman S (6 November 2020). "Connectivity and seasonality: the 1918 influenza and COVID-19 pandemics in global perspective". Journal of Global History. 15 (3): 408–420. doi:10.1017/S1740022820000261. S2CID 228988279.
- ^ a b Bootsma MC, Ferguson NM (May 2007). "The effect of public health measures on the 1918 influenza pandemic in U.S. cities". Proceedings of the National Academy of Sciences of the United States of America. 104 (18): 7588–7593. Bibcode:2007PNAS..104.7588B. doi:10.1073/pnas.0611071104. PMC 1849868. PMID 17416677.
- ^ "Public Health Weekly Reports for NOVEMBER 29, 1918". Public Health Reports. 33 (48): 2093–2152. November 1918. PMC 1999834. PMID 19314645.
- ^ a b "Public Health Weekly Reports for DECEMBER 6, 1918". Public Health Reports. 33 (49): 2153–2209. December 1918. PMC 1999845. PMID 19314646.
- ^ Chandra S, Christensen J, Chandra M, Paneth N (March 2021). "Pandemic Reemergence and Four Waves of Excess Mortality Coinciding With the 1918 Influenza Pandemic in Michigan: Insights for COVID-19". American Journal of Public Health. 111 (3): 430–437. doi:10.2105/AJPH.2020.305969. PMC 7893337. PMID 33566641.
- ^ a b c d e f g Jordan EO (1927). Epidemic Influenza: A Survey. Chicago: American Medical Association. Archived from the original on 17 March 2023. Retrieved 17 March 2023.
- ^ "Here are Exact Facts About the Influenza and Its Toll in City, State, Nation, world". Los Angeles Times. 9 February 1919. Archived from the original on 24 July 2020. Retrieved 10 May 2020.
- ^ "WATCH THIS S.F. FLU CHART". San Francisco Call. 18 January 1919. Retrieved 3 July 2025.
, Volume 105, Number 13,
- ^ a b Vaughan WT (July 1921). "Influenza: An Epidemiologic Study". American Journal of Hygiene. Archived from the original on 24 July 2020.
- ^ "Public Health Weekly Reports for February 21, 1919". Public Health Reports. 34 (8): 313–376. February 1919. PMC 1996943. PMID 19314657.
- ^ "Mortality Statistics 1919: Twentieth Annual Report" (PDF). United States Census Bureau. 1921. p. 30. Archived (PDF) from the original on 12 July 2020. Retrieved 11 May 2020.
- ^ Pearce DC, Pallaghy PK, McCaw JM, McVernon J, Mathews JD (March 2011). "Understanding mortality in the 1918–1919 influenza pandemic in England and Wales". Influenza and Other Respiratory Viruses. 5 (2): 89–98. doi:10.1111/j.1750-2659.2010.00186.x. PMC 4954464. PMID 21306572.
- ^ Chowell G, Erkoreka A, Viboud C, Echeverri-Dávila B (July 2014). "Spatial-temporal excess mortality patterns of the 1918–1919 influenza pandemic in Spain". BMC Infectious Diseases. 14 (371) 371. doi:10.1186/1471-2334-14-371. PMC 4094406. PMID 24996457.
- ^ Nunes B, Silva S, Rodrigues A, Roquette R, Batista I, Rebelo-de-Andrade H (December 2018). "The 1918–1919 Influenza Pandemic in Portugal: A Regional Analysis of Death Impact". American Journal of Epidemiology. 187 (12): 2541–2549. doi:10.1093/aje/kwy164. PMC 7314274. PMID 30099487.
- ^ a b c Holbrook C (August 2022). "Public Health in a Federation: Lessons from the Spanish Influenza in Australia". Social History of Medicine. 35 (3): 818–846. doi:10.1093/shm/hkac005. PMC 9383459. PMID 36046217.
- ^ a b c d Shanks GD. "Australia's Experience of the 1918–19 Influenza Pandemic: Lessons Learned" (PDF). University of Otago. Archived (PDF) from the original on 19 September 2020. Retrieved 17 March 2023.
- ^ a b Curson P, McCracken K (2006). "An Australian perspective of the 1918–1919 influenza pandemic". New South Wales Public Health Bulletin. 17 (7–8): 103–107. doi:10.1071/NB06025. PMID 17136138.
- ^ "Public Health Weekly Reports for AUGUST 22, 1919". Public Health Reports. 34 (34): 1927–1968. August 1919. PMC 1996930. PMID 19314683.
- ^ Ho PL, Chow KH (November 2009). "Mortality burden of the 1918–1920 influenza pandemic in Hong Kong". Influenza and Other Respiratory Viruses. 3 (6): 261–263. doi:10.1111/j.1750-2659.2009.00105.x. PMC 4941390. PMID 19903207.
- ^ "Public Health Weekly Reports for MAY 30, 1919". Public Health Reports. 34 (22): 1171–1241. May 1919. PMC 1996920. PMID 19314671.
- ^ "Public Health Weekly Reports for AUGUST 1, 1919". Public Health Reports. 34 (31): 1673–1742. August 1919. PMC 1996928. PMID 19314680.
- ^ "Public Health Weekly Reports for AUGUST 29, 1919". Public Health Reports. 34 (35): 1969–2012. August 1919. PMC 1996931. PMID 19314684.
- ^ "Public Health Weekly Reports for AUGUST 15, 1919". Public Health Reports. 34 (33): 1823–1926. August 1919. PMC 1996932. PMID 19314682.
- ^ a b c Chowell G, Simonsen L, Flores J, Miller MA, Viboud C (November 2014). "Death patterns during the 1918 influenza pandemic in Chile". Emerging Infectious Diseases. 20 (11): 1803–1811. doi:10.3201/eid2011.130632. PMC 4214284. PMID 25341056.
- ^ Cristina J, Pollero R, Pellegrino A (May 2019). "The 1918 influenza pandemic in Montevideo: The southernmost capital city in the Americas". Influenza and Other Respiratory Viruses. 13 (3): 219–225. doi:10.1111/irv.12619. PMC 6468140. PMID 30422393.
- ^ Najera RF (2 January 2019). "Influenza in 1919 and 100 Years Later". College of Physicians of Philadelphia. Archived from the original on 24 July 2020. Retrieved 23 April 2020.
- ^ "Public Health Weekly Reports for AUGUST 15, 1919". Public Health Reports. 34 (33): 1823–1926. August 1919. doi:10.2307/4575271. JSTOR 4575271. PMC 1996932. PMID 19314682.
- ^ "WARNS OF INFLUENZA". The New York Times. 15 August 1919. p. 6. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "AT ISSUE WITH COPELAND". The New York Times. 27 August 1919. p. 9. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "INFLUENZA RETURN EXPECTED BY BLUE". The New York Times. 14 September 1919. p. 4. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "PARIS FEARS RETURN OF "FLU" EPIDEMIC". The Sun. 19 August 1919. p. 4. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "British Prepare for Influenza". The New York Times. 30 October 1919. p. 17. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "GRIP IS EPIDEMIC IN JAPAN". The New York Times. 28 December 1919. p. 6. Archived from the original on 17 March 2023. Retrieved 17 March 2023.
- ^ a b "Influenza Gradually Spreading In Japan". The Honolulu Advertiser. 10 January 1920. p. 3. Archived from the original on 17 March 2023. Retrieved 17 March 2023.
- ^ a b "INFLUENZA IN JAPAN". The Sydney Morning Herald. 28 January 1920. p. 10. Archived from the original on 21 March 2023. Retrieved 17 March 2023.
- ^ "INFLUENZA". The Age. 26 January 1920. p. 5. Archived from the original on 21 March 2023. Retrieved 17 March 2023.
- ^ a b Richard SA, Sugaya N, Simonsen L, Miller MA, Viboud C (August 2009). "A comparative study of the 1918–1920 influenza pandemic in Japan, USA and UK: mortality impact and implications for pandemic planning". Epidemiology and Infection. 137 (8): 1062–1072. doi:10.1017/S0950268809002088. PMC 2704924. PMID 19215637.
- ^ a b Vaughan WT (July 1921). "Influenza: An Epidemologic Study". The American Journal of Hygiene. p. 91. Archived from the original on 6 October 2020. Retrieved 13 August 2020.
- ^ "300 "FLU" CASES REPORTED". The New York Times. 28 September 1919. p. 5. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "Influenza Spreads in Chicago". The New York Times. 15 January 1920. p. 1. Archived from the original on 22 March 2023. Retrieved 12 April 2022.
- ^ "525 NEW CASES IN CHICAGO". The New York Times. 17 January 1920. p. 11. Retrieved 12 April 2022.
- ^ "Health Service Reports Influenza Under Control in All Parts of the Country". The Evening Star. 21 January 1920. pp. A2. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "CHICAGO INFLUENZA EPIDEMIC SPREADS". The New York Times. 18 January 1920. p. 14. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "INFLUENZA CASES INCREASE IN CHICAGO". The New York Times. 21 January 1920. p. 32. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "INFLUENZA SHOWS A LARGE INCREASE". The New York Times. 21 January 1920. p. 32. Archived from the original on 22 March 2023. Retrieved 12 April 2022.
- ^ "SPREADS IN OTHER CITIES". The New York Times. 23 January 1920. p. 2. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ "TOPICS OF THE TIMES". The New York Times. 22 January 1920. p. 16. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ a b "INFLUENZA CASES GROW THROUGHOUT COUNTRY". The New York Times. 25 January 1920. p. 2. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ a b "COPELAND EXPECTS RISE IN INFLUENZA". The New York Times. 27 January 1920. p. 1. Archived from the original on 22 March 2023. Retrieved 12 April 2022.
- ^ "Influenza Checked in 25 States". The New York Times. 8 February 1920. p. 15. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ Mortality statistics, 1920. Twenty-First Annual Report (PDF). US Bureau of the Census (Report). 1922. Archived (PDF) from the original on 16 March 2023. Retrieved 13 April 2022 – via CDC.
- ^ Schmitt RC, Nordyke EC (1999). "Influenza Deaths in Hawai'i, 1918—1920". Hawaiian Journal of History. 33: 101–117. Archived from the original on 5 July 2022. Retrieved 13 April 2022 – via eVols.
- ^ Grabowski ML, Kosińska B, Knap JP, Brydak LB (October 2017). "The Lethal Spanish Influenza Pandemic in Poland". Medical Science Monitor. 23: 4880–4884. doi:10.12659/MSM.906280. PMC 5649514. PMID 29021518.
- ^ Chowell G, Viboud C, Simonsen L, Miller MA, Hurtado J, Soto G, et al. (July 2011). "The 1918–1920 influenza pandemic in Peru". Vaccine. 29 Suppl 2 (Suppl 2): B21 – B26. doi:10.1016/j.vaccine.2011.02.048. PMC 3144394. PMID 21757099.
- ^ Kim T (2017). "The 1918 Influenza Pandemic and Japanese Government-General of Korea's Preventive Measures against Epidemics". Journal of Humanities, Seoul National University (in Korean). 74 (1): 203–207. Archived from the original on 17 March 2023. Retrieved 17 March 2023 – via Korea Journal Central.
- ^ Hsieh YH (October 2009). "Excess deaths and immunoprotection during 1918–1920 influenza pandemic, Taiwan". Emerging Infectious Diseases. 15 (10): 1617–1619. doi:10.3201/eid1510.080811. PMC 2866376. PMID 19861055.
- ^ Nichols CM, Ewing ET, Gaston KH, Marinari M, Lessoff A, Huyssen D (April 2022). "What Came Next?: Reflections on the Aftermath(s) of the 1918–19 Flu Pandemic in the Age of COVID". The Journal of the Gilded Age and Progressive Era. 21 (2): 126. Archived from the original on 27 February 2023. Retrieved 17 March 2023 – via Cambridge Core.
- ^ Linnanmäki E (2005). Espanjantauti Suomessa — Influenssapandemia 1918–1920 (in Finnish). Helsinki: Suomalaisen Kirjallisuuden Seura. p. 69. ISBN 9789517467162.
- ^ a b c Taubenberger JK, Morens DM (April 2010). "Influenza: the once and future pandemic". Public Health Reports. 125 Suppl 3 (Suppl 3): 16–26. doi:10.1177/00333549101250S305. PMC 2862331. PMID 20568566.
- ^ Morens DM, Taubenberger JK, Fauci AS (July 2009). "The persistent legacy of the 1918 influenza virus". The New England Journal of Medicine. 361 (3): 225–229. doi:10.1056/NEJMp0904819. PMC 2749954. PMID 19564629.
- ^ a b Taubenberger JK, Morens DM (April 2009). "Pandemic influenza – including a risk assessment of H5N1". Revue Scientifique et Technique. 28 (1): 187–202. doi:10.20506/rst.28.1.1879. PMC 2720801. PMID 19618626.
- ^ a b c Rozo M, Gronvall GK (August 2015). "The Reemergent 1977 H1N1 Strain and the Gain-of-Function Debate". mBio. 6 (4) e01013-15. doi:10.1128/mBio.01013-15. PMC 4542197. PMID 26286690.
- ^ Kung HC, Jen KF, Yuan WC, Tien SF, Chu CM (1978). "Influenza in China in 1977: recurrence of influenzavirus A subtype H1N1". Bulletin of the World Health Organization. 56 (6): 913–918. hdl:10665/261795. PMC 2395678. PMID 310732.
- ^ Mills CE, Robins JM, Lipsitch M (December 2004). "Transmissibility of 1918 pandemic influenza". Nature. 432 (7019): 904–906. Bibcode:2004Natur.432..904M. doi:10.1038/nature03063. PMC 7095078. PMID 15602562.
- ^ Qureshi 2016, p. 42.
- ^ Ewald 1994.
- ^ "The Spanish Flu pandemic of 1918". The History Press. Archived from the original on 13 January 2021. Retrieved 20 February 2021.
- ^ Illing S (20 March 2020). "The most important lesson of the 1918 influenza pandemic: Tell the damn truth". Vox. Archived from the original on 25 March 2020.
John M. Barry : The government lied. They lied about everything. We were at war and they lied because they didn't want to upend the war effort. You had public health leaders telling people this was just the ordinary flu by another name. They simply didn't tell the people the truth about what was happening.
- ^ Gladwell 1997, p. 55.
- ^ Gladwell 1997, p. 63.
- ^ Fogarty International Center. "Summer Flu Outbreak of 1918 May Have Provided Partial Protection Against Lethal Fall Pandemic". Fic.nih.gov. Archived from the original on 27 July 2011. Retrieved 19 May 2012.
- ^ Joyce G (March 2020). "The 1919 Stanley Cup Final and modern-history's deadliest pandemic". Sportsnet. Archived from the original on 31 March 2020. Retrieved 23 March 2023.
- ^ a b c d e Spinney 2018, pp. 45–47.
- ^ a b Knobler 2005.
- ^ Morris DE, Cleary DW, Clarke SC (2017). "Secondary Bacterial Infections Associated with Influenza Pandemics". Frontiers in Microbiology. 8 1041. doi:10.3389/fmicb.2017.01041. PMC 5481322. PMID 28690590.
- ^ "Bacterial Pneumonia Caused Most Deaths in 1918 Influenza Pandemic". National Institutes of Health. 23 September 2015. Archived from the original on 22 April 2016. Retrieved 17 April 2016.
- ^ a b Taubenberger et al. 2001, pp. 1829–39.
- ^ Morens DM, Taubenberger JK, Fauci AS (October 2008). "Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness". The Journal of Infectious Diseases. 198 (7): 962–970. doi:10.1086/591708. PMC 2599911. PMID 18710327.
- ^ Barry 2004.
- ^ a b Spinney 2018, p. 61.
- ^ "Washington State Board of Health pessimistic about influenza pandemic in a report to the governor on January 1, 1919". historylink.org. Archived from the original on 21 July 2020. Retrieved 21 July 2020.
- ^ a b c Spinney 2018, p. 62.
- ^ Foxman EF, Storer JA, Fitzgerald ME, Wasik BR, Hou L, Zhao H, et al. (January 2015). "Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells". Proceedings of the National Academy of Sciences of the United States of America. 112 (3): 827–832. Bibcode:2015PNAS..112..827F. doi:10.1073/pnas.1411030112. PMC 4311828. PMID 25561542.
- ^ Lowen AC, Steel J (July 2014). "Roles of humidity and temperature in shaping influenza seasonality". Journal of Virology. 88 (14): 7692–7695. doi:10.1128/JVI.03544-13. PMC 4097773. PMID 24789791.
- ^ Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE (April 2009). "Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature". Veterinary Microbiology. 136 (1–2): 20–26. doi:10.1016/j.vetmic.2008.10.027. PMID 19081209.
- ^ Foxman EF, Storer JA, Vanaja K, Levchenko A, Iwasaki A (July 2016). "Two interferon-independent double-stranded RNA-induced host defense strategies suppress the common cold virus at warm temperature". Proceedings of the National Academy of Sciences of the United States of America. 113 (30): 8496–8501. Bibcode:2016PNAS..113.8496F. doi:10.1073/pnas.1601942113. PMC 4968739. PMID 27402752.
- ^ Klugman KP, Chien YW, Madhi SA (August 2009). "Pneumococcal pneumonia and influenza: a deadly combination". Vaccine. 27 Suppl 3 (s3): C9 – C14. doi:10.1016/j.vaccine.2009.06.007. PMID 19683658.
- ^ Bengtsson D, Safi K, Avril A, Fiedler W, Wikelski M, Gunnarsson G, et al. (February 2016). "Does influenza A virus infection affect movement behaviour during stopover in its wild reservoir host?". Royal Society Open Science. 3 (2) 150633. Bibcode:2016RSOS....350633B. doi:10.1098/rsos.150633. PMC 4785985. PMID 26998334.
- ^ Tolf C, Bengtsson D, Rodrigues D, Latorre-Margalef N, Wille M, Figueiredo ME, et al. (2012). "Birds and viruses at a crossroad – surveillance of influenza A virus in Portuguese waterfowl". PLOS ONE. 7 (11) e49002. Bibcode:2012PLoSO...749002T. doi:10.1371/journal.pone.0049002. PMC 3492218. PMID 23145046.
- ^ Tucker MA, Böhning-Gaese K, Fagan WF, Fryxell JM, Van Moorter B, Alberts SC, et al. (January 2018). "Moving in the Anthropocene: Global reductions in terrestrial mammalian movements". Science. 359 (6374): 466–469. Bibcode:2018Sci...359..466T. doi:10.1126/science.aam9712. hdl:10174/25640. PMID 29371471.
- ^ Blakemore E (3 October 2020). "Catastrophic effect of 1918 flu may have been aided by peculiar influx of cold air into Europe during WWI". The Washington Post. Archived from the original on 11 December 2020. Retrieved 29 November 2020.
- ^ Kent L (28 September 2020). "How environmental conditions like cold and wet weather can affect pandemics, and what that means for COVID-19". CNN. Archived from the original on 2 December 2020. Retrieved 29 November 2020.
- ^ Powell A (5 October 2020). "Six-year deluge linked to Spanish flu, World War I deaths". Harvard Gazette. Archived from the original on 27 November 2020. Retrieved 29 November 2020.
- ^ "Gauze Mask to Halt Spread of Plague". The Washington Times. 27 September 1918. p. 3. Archived from the original on 8 October 2020. Retrieved 5 October 2020.
- ^ a b Hauck G, Gellis K (22 November 2020). "We're celebrating Thanksgiving amid a pandemic. Here's how we did it in 1918 – and what happened next". USA Today. Archived from the original on 21 November 2020.
- ^ a b c d Spinney 2018, pp. 83–84.
- ^ a b c Spinney 2018, pp. 87–88.
- ^ Segrave K (2024). Masking America, 1918–1919: Efforts to Control the Great Influenza Pandemic. Jefferson, NC: McFarland and Co. ISBN 978-1-4766-9449-8. Archived from the original on 1 September 2024. Retrieved 1 September 2024.
- ^ Spinney 2018, p. 91.
- ^ "How NYC beat deadly 1918 flu without vaccines, lockdowns, mask mandates or school closures". silive. 20 September 2021. Archived from the original on 21 September 2021. Retrieved 23 September 2021.
- ^ Bootsma MC, Ferguson NM (May 2007). "The effect of public health measures on the 1918 influenza pandemic in U.S. cities". Proceedings of the National Academy of Sciences of the United States of America. 104 (18): 7588–7593. Bibcode:2007PNAS..104.7588B. doi:10.1073/pnas.0611071104. PMC 1849868. PMID 17416677. S2CID 11280273.
- ^ Spinney 2018, pp. 109–10.
- ^ Spinney 2018, pp. 111–12.
- ^ Flecknoe D, Charles Wakefield B, Simmons A (June 2018). "Plagues & wars: the 'Spanish Flu' pandemic as a lesson from history". Medicine, Conflict and Survival. 34 (2): 61–68. doi:10.1080/13623699.2018.1472892. PMID 29764189. S2CID 21694687.
- ^ a b Spinney 2018, p. 92.
- ^ Spinney 2018, p. 69.
- ^ Spinney 2018, p. 70.
- ^ Taubenberger & Morens 2006, p. 19.
- ^ Taubenberger & Morens 2006, p. 17.
- ^ World Health O (December 2005). "Ten things you need to know about pandemic influenza (update of 14 October 2005)" (PDF). Relevé Épidémiologique Hebdomadaire. 80 (49–50): 428–431. PMID 16372665. Archived (PDF) from the original on 27 July 2021. Retrieved 11 March 2020.
- ^ Jilani TN, Jamil RT, Siddiqui AH (14 December 2019). "H1N1 Influenza (Swine Flu)". StatPearls. Treasure Island, FL: StatPearls. PMID 30020613. Archived from the original on 12 March 2020. Retrieved 11 March 2020 – via NCBI.
- ^ Johnson & Mueller 2002.
- ^ Chandra S, Christensen J (July 2019). "Re: "Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic"". American Journal of Epidemiology. 188 (7): 1404–1406. doi:10.1093/aje/kwz044. PMID 30824934. and response Spreeuwenberg P, Kroneman M, Paget J (July 2019). "The Authors Reply" (PDF). American Journal of Epidemiology. 188 (7): 1405–1406. doi:10.1093/aje/kwz041. PMID 30824908. Archived from the original (PDF) on 12 March 2020. Retrieved 12 March 2020.
- ^ "Historical Estimates of World Population". Archived from the original on 9 July 2012. Retrieved 29 March 2013.
- ^ Mayor S (October 2000). "Flu experts warn of need for pandemic plans". BMJ. 321 (7265): 852. doi:10.1136/bmj.321.7265.852. PMC 1118673. PMID 11021855.
- ^ Chandra, Kuljanin & Wray 2012.
- ^ Arnold D (December 2019). "Death and the Modern Empire: The 1918–19 Influenza Epidemic in India". Transactions of the Royal Historical Society. 29: 181–200. doi:10.1017/S0080440119000082.
- ^ Sreevatsan A (12 March 2020). "Why 1918 matters in India's corona war". mint. Archived from the original on 18 April 2020. Retrieved 7 June 2020.
- ^ "What the history of pandemics tells us about coronavirus". Hindustan Times. 15 April 2020. Archived from the original on 21 April 2020. Retrieved 7 June 2020.
- ^ a b Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH (December 2006). "Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918-20 pandemic: a quantitative analysis". Lancet. 368 (9554). Elsevier: 2211–2218. doi:10.1016/S0140-6736(06)69895-4. PMID 17189032. S2CID 22787011. Archived from the original on 27 September 2020. Retrieved 30 September 2020.
- ^ "Historiallisia Papereita 1". historiallinenyhdistys.fi. ISSN 1456-8055. Archived from the original on 6 February 2020. Retrieved 24 June 2020.
- ^ Åman M (1990). Spanska sjukan: den svenska epidemin 1918–1920 och dess internationella bakgrund (in Swedish). Uppsala; Stockholm: Ubsaliensis Academiae; Distributor, Almqvist & Wiksell International. ISBN 978-91-554-2587-6. OCLC 22451542.
- ^ Fitri E (28 October 2009). "Looking Through Indonesia's History For Answers to Swine Flu". The Jakarta Globe. Archived from the original on 2 November 2009. Retrieved 28 March 2017.
- ^ Kohn 2007.
- ^ a b "İSPANYOL GRİBİNİN DÜNYA VE OSMANLI DEVLETİ ÜZERİNDEKİ ETKİLERİ" (in Turkish). Archived from the original on 8 November 2020. Retrieved 2 November 2020.
- ^ "1918 flu centenary: How to survive a pandemic". Archived from the original on 9 July 2019. Retrieved 19 June 2019.
- ^ Rice G, Bryder L. Black November: the 1918 influenza pandemic in New Zealand (Second, revised and enlarged ed.). Christchurch, NZ. ISBN 978-1-927145-91-3. OCLC 960210402.
- ^ Radusin M (October 2012). "The Spanish flu – part II: the second and third wave". Vojnosanitetski Pregled. 69 (10): 917–927. PMID 23155616. Archived from the original on 9 July 2020. Retrieved 23 April 2020.
- ^ a b "Epidemics and pandemics in Victoria: Historical perspectives". Parliament of Victoria. 23 April 2020. Archived from the original on 14 September 2022. Retrieved 14 September 2022.
- ^ "Influenza pandemic". National Museum of Australia. Archived from the original on 14 September 2022. Retrieved 14 September 2022.
- ^ "1918–1920 – Influenza and Pneumonia Pandemic – Nationwide ~820,000–850,000 – Deadliest American Disasters and Large-Loss-of-Life Events". January 1918. Archived from the original on 30 May 2020. Retrieved 22 May 2020.
- ^ The Great Pandemic: The United States in 1918–1919, U.S. Department of Health & Human Services.
- ^ "The silent invader – Digital Collections – National Library of Medicine". Archived from the original on 24 July 2020. Retrieved 15 April 2020.
- ^ "Flu Epidemic Hit Utah Hard in 1918, 1919". Deseret News. 28 March 1995. Archived from the original on 30 November 2012. Retrieved 7 July 2012.
- ^ "The Great Pandemic of 1918: State by State". 5 March 2018. Archived from the original on 6 May 2009. Retrieved 4 May 2009.
- ^ "A deadly virus rages throughout Canada at the end of the First World War". CBC History.
- ^ "A gripe espanhola no Brasil – Elísio Augusto de Medeiros e Silva, empresário, escritor e membro da AEILIJ" (in Portuguese). Jornal de Hoje. Archived from the original on 2 February 2014. Retrieved 22 January 2014.
- ^ "The "bird flu" that killed 40 million". BBC News. 19 October 2005. Archived from the original on 7 November 2017. Retrieved 26 April 2009.
- ^ Hays 1998.
- ^ Marcus H (1996). Haile Sellassie I: The formative years, 1892–1936. Trenton, NJ: Red Sea Press. pp. 36ff.
- ^ Pankhurst 1991, pp. 48f.
- ^ Pankhurst 1991, p. 63.
- ^ a b c Spinney 2018, pp. 167–69.
- ^ "Viruses of mass destruction". Fortune. CNN. 1 November 2004. Archived from the original on 6 May 2009. Retrieved 30 April 2009.
- ^ Spinney 2018, p. 134.
- ^ "The Flu Pandemic hit Nenana 100 Years Ago". nps.gov. 27 October 2021. Archived from the original on 13 April 2022. Retrieved 12 April 2022.
- ^ a b Denoon 2004.
- ^ Wishart S (July–August 2018). "How the 1918 flu spread". New Zealand Geographic (152): 23. Archived from the original on 3 August 2018. Retrieved 3 August 2018.
- ^ Afkhami 2003; Afkhami 2012.
- ^ FitzGerald J. "Coronavirus: forgotten lessons of the Spanish flu pandemic". The Irish Times. Archived from the original on 5 May 2020. Retrieved 14 June 2020.
Yet the "Spanish" flu epidemic of 1918–19 resulted in about 25,000 extra deaths in Ireland, many of them young adults. This was almost as many deaths as occurred among the Irish fighting in the First World War.
- ^ "Official Ireland Statistics Office". Central Statistics Office Ireland. Archived from the original on 14 June 2020. Retrieved 14 June 2020.
Total deaths in Ireland 1916: 50,627
- ^ Phillips H (10 March 2020). "South Africa bungled the Spanish flu in 1918. History mustn't repeat itself for COVID-19". The Conversation. Archived from the original on 13 June 2020. Retrieved 26 May 2020.
- ^ Spinney 2018, p. 72.
- ^ Pankhurst 1991, pp. 51ff.
- ^ Bell D, Nicoll A, Fukuda K, Horby P, Monto A, Hayden F, et al. (January 2006). "Non-pharmaceutical interventions for pandemic influenza, international measures". Emerging Infectious Diseases. 12 (1): 81–87. doi:10.3201/eid1201.051370. PMC 3291414. PMID 16494722.
- ^ a b Shanks GD, Brundage JF (February 2013). "Pacific islands which escaped the 1918–1919 influenza pandemic and their subsequent mortality experiences". Epidemiology and Infection. 141 (2): 353–356. doi:10.1017/S0950268812000866. PMC 9152047. PMID 22564320. S2CID 23794327.
- ^ Ryan J, ed. (2009). Pandemic Influenza: Emergency planning and community preparedness. Boca Raton, FL: CRC Press. p. 24.
- ^ "Colonial Annual Report", 1919
- ^ a b Iijima W (2003). "Spanish influenza in China, 1918–1920: a preliminary probe". In Phillips H, Killingray D (eds.). The Spanish Flu Pandemic of 1918: New Perspectives. London and New York: Routledge. pp. 101–09.
- ^ Killingray D, Phillips H (2003). The Spanish Influenza Pandemic of 1918–1919: New Perspectives. Routledge. ISBN 978-1-134-56640-2. Archived from the original on 27 July 2021. Retrieved 9 November 2020.
- ^ Killingray D, Phillips H (2003). The Spanish Influenza Pandemic of 1918–1919: New Perspectives. Routledge. ISBN 978-1-134-56640-2. Archived from the original on 27 July 2021. Retrieved 9 November 2020.
- ^ Iijima W (1998). The Spanish influenza in China, 1918–1920. OCLC 46987588.
- ^ Iijima W (1998). The Spanish influenza in China, 1918–1920. Spanish 'Flu 1918–1998: Reflections on the Influenza Pandemic of 1918 after 80 Years. Cape Town, South Africa. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ^ a b c d e f g h Killingray D, Phillips H (2003). The Spanish Influenza Pandemic of 1918–1919: New Perspectives. Routledge. p. 105. ISBN 978-1-134-56640-2. Archived from the original on 27 July 2021. Retrieved 9 November 2020.
- ^ Alita XP, Ling T, Bin ZH, Xiao-ting LI (2010). "Epidemic analysisi of 1918 Spanish influenza in China based on literatures". Journal of Medical Informatics. 31 (1): 47–50. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
- ^ Hong NH (2015). "Epidemic Analysis of 1918 Influenza in China – Research on Huolu County in Zhili Province". Historical Research in Auhui.
- ^ a b c Simonsen et al. 1998.
- ^ Hanssen O (1923). Undersøkelser over influenzaens optræden specielt i Bergen 1918–1922 [Investigations into the appearance of influenza, especially in Bergen 1918–1922)]. Skrifter utgit av Klaus Hansens fond, nr. III. Bergen: Haukeland Sykehus, 1923 (Writings published by the Klaus Hansen Foundation, No. III, Haukeland Hospital Bergen (in Norwegian). Grieg. p. 66.
- ^ Knobler SL, Mack A, Mahmoud A, Lemon SM. "Institute of Medicine (US) Forum on Microbial Threats". NCBI. Archived from the original on 23 December 2020. Retrieved 17 August 2021.
- ^ Barry 2004, p. 239.
- ^ "Key Facts about Swine Influenza". U.S. Centers for Disease Control and Prevention. Archived from the original on 5 May 2009. Retrieved 30 April 2009.
- ^ a b c d e f Spinney 2018, pp. 180–82.
- ^ a b c d Spinney 2018, pp. 183–84.
- ^ Mamelund SE (February 2006). "A socially neutral disease? Individual social class, household wealth and mortality from Spanish influenza in two socially contrasting parishes in Kristiania 1918–19". Social Science & Medicine. 62 (4): 923–940. doi:10.1016/j.socscimed.2005.06.051. PMID 16084634.
- ^ He et al. 2011.
- ^ He et al. 2013.
- ^ "The 'Spanish' Influenza pandemic and its relation to World War I". World War I Centenary. University of Oxford JISC. Archived from the original on 5 August 2020. Retrieved 11 August 2020.
- ^ Garret 2007.
- ^ Lindley R (6 June 2012). "The Forgotten American Pandemic: Historian Dr. Nancy K. Bristow on the Influenza Epidemic of 1918". hnn.us. Archived from the original on 8 August 2014. Retrieved 4 August 2014.
- ^ "What can the Spanish Flu teach us about the COVID-19 pandemic?". World Economic Forum. 2 April 2020. Archived from the original on 9 April 2020. Retrieved 8 April 2020.
- ^ Correia S, Luck S, Verner E (2020). "Pandemics Depress the Economy, Public Health Interventions Do Not: Evidence from the 1918 Flu". SSRN Working Paper Series. arXiv:2207.11636. doi:10.2139/ssrn.3561560. ISSN 1556-5068. S2CID 216202133. Archived from the original on 27 July 2021. Retrieved 14 April 2020.
- ^ Kemp J (7 April 2020). "RPT-COLUMN-Coronavirus lockdowns: can we learn from the 1918 influenza pandemic? Kemp". Reuters. Archived from the original on 17 August 2021. Retrieved 17 August 2021.
- ^ Almond D (1 August 2006). "Is the 1918 Influenza Pandemic Over? Long-Term Effects of In Utero Influenza Exposure in the Post-1940 U.S. Population". Journal of Political Economy. 114 (4): 672–712. doi:10.1086/507154. ISSN 0022-3808. S2CID 20055129.
- ^ Beach B, Ferrie JP, Saavedra MH (2018). "Fetal shock or selection? The 1918 influenza pandemic and human capital development". nber.org. Working Paper Series. doi:10.3386/w24725. Archived from the original on 18 June 2018. Retrieved 18 June 2018.
- ^ Vilensky, Foley & Gilman 2007.
- ^ Fottrell Q (25 August 2020). "Another warning from the 1918 flu for COVID-19: 'Survival does not mean that individuals fully recovered'". MarketWatch. Archived from the original on 24 August 2020.
- ^ Honigsbaum 2008.
- ^ a b Beiner G (2021). "Rediscovering the Great Flu between Pre-Forgetting and Post-Forgetting". In Beiner G (ed.). Pandemic Re-Awakenings: The Forgotten and Unforgotten 'Spanish' Flu of 1918–1919. Oxford University Press. pp. 346–376. ISBN 9780192843739.
- ^ Morrisey 1986.
- ^ Benedict & Braithwaite 2000, p. 38.
- ^ Crosby 2003, pp. 320–322.
- ^ a b "Why Did So Few Novels Tackle the 1918 Pandemic?". Smithsonian Magazine. Archived from the original on 6 June 2023. Retrieved 6 June 2023.
- ^ Crosby 2003, p. 315.
- ^ Hansen L (24 September 2006). "Mullen's View from 'The Last Town on Earth'". NPR. Retrieved 11 June 2023.
- ^ Corrigan M (20 July 2020). "1918 Flu Inspired Donoghue's 'Pull Of The Stars' — A Disquieting Pandemic Novel". NPR. Archived from the original on 6 June 2023. Retrieved 6 June 2023.
- ^ Hilleman MR (August 2002). "Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control". Vaccine. 20 (25–26): 3068–87. doi:10.1016/S0264-410X(02)00254-2. PMID 12163258.
- ^ Potter CW (October 2001). "A history of influenza". Journal of Applied Microbiology. 91 (4): 572–9. doi:10.1046/j.1365-2672.2001.01492.x. PMID 11576290. S2CID 26392163.
- ^ Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L (September 2014). "Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature". BMC Infectious Diseases. 14 (1): 480. doi:10.1186/1471-2334-14-480. PMC 4169819. PMID 25186370.
- ^ Mills CE, Robins JM, Lipsitch M (December 2004). "Transmissibility of 1918 pandemic influenza". Nature. 432 (7019): 904–6. Bibcode:2004Natur.432..904M. doi:10.1038/nature03063. PMC 7095078. PMID 15602562.
- ^ Taubenberger JK, Morens DM (January 2006). "1918 Influenza: the mother of all pandemics". Emerging Infectious Diseases. 12 (1): 15–22. doi:10.3201/eid1201.050979. PMC 3291398. PMID 16494711.
- ^ a b c d e f g h "Report of the Review Committee on the Functioning of the International Health Regulations (2005) in relation to Pandemic (H1N1) 2009" (PDF). 5 May 2011. p. 37. Archived (PDF) from the original on 14 May 2015. Retrieved 1 March 2015.
- ^ Spreeuwenberg P, Kroneman M, Paget J (December 2018). "Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic". American Journal of Epidemiology. 187 (12): 2561–2567. doi:10.1093/aje/kwy191. PMC 7314216. PMID 30202996.
- ^ Morens DM, Fauci AS (April 2007). "The 1918 influenza pandemic: insights for the 21st century". The Journal of Infectious Diseases. 195 (7): 1018–28. doi:10.1086/511989. PMID 17330793.
- ^ Johnson NP, Mueller J (2002). "Updating the accounts: global mortality of the 1918-1920 "Spanish" influenza pandemic". Bulletin of the History of Medicine. 76 (1): 105–15. doi:10.1353/bhm.2002.0022. PMID 11875246. S2CID 22974230.
- ^ Lin II RG, Karlamangla S (6 March 2020). "Why the coronavirus outbreak isn't likely to be a repeat of the 1918 Spanish flu". Los Angeles Times.
- ^ Schwarzmann SW, Adler JL, Sullivan RJ, Marine WM (June 1971). "Bacterial pneumonia during the Hong Kong influenza epidemic of 1968-1969". Archives of Internal Medicine. 127 (6): 1037–41. doi:10.1001/archinte.1971.00310180053006. PMID 5578560.
- ^ Michaelis M, Doerr HW, Cinatl J (August 2009). "Novel swine-origin influenza A virus in humans: another pandemic knocking at the door". Medical Microbiology and Immunology. 198 (3): 175–83. doi:10.1007/s00430-009-0118-5. PMID 19543913. S2CID 20496301.
- ^ Donaldson LJ, Rutter PD, Ellis BM, Greaves FE, Mytton OT, Pebody RG, et al. (December 2009). "Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study". BMJ. 339 b5213. doi:10.1136/bmj.b5213. PMC 2791802. PMID 20007665.
- ^ "First Global Estimates of 2009 H1N1 Pandemic Mortality Released by CDC-Led Collaboration". Centers for Disease Control and Prevention (CDC). 25 June 2012. Retrieved 7 July 2012.
- ^ Kelly H, Peck HA, Laurie KL, Wu P, Nishiura H, Cowling BJ (5 August 2011). "The age-specific cumulative incidence of infection with pandemic influenza H1N1 2009 was similar in various countries prior to vaccination". PLOS ONE. 6 (8) e21828. Bibcode:2011PLoSO...621828K. doi:10.1371/journal.pone.0021828. PMC 3151238. PMID 21850217.
- ^ Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, et al. (September 2012). "Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study". The Lancet. Infectious Diseases. 12 (9): 687–95. doi:10.1016/S1473-3099(12)70121-4. PMID 22738893.
- ^ Riley S, Kwok KO, Wu KM, Ning DY, Cowling BJ, Wu JT, et al. (June 2011). "Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a longitudinal community cohort study". PLOS Medicine. 8 (6) e1000442. doi:10.1371/journal.pmed.1000442. PMC 3119689. PMID 21713000.
- ^ Wong JY, Kelly H, Ip DK, Wu JT, Leung GM, Cowling BJ (November 2013). "Case fatality risk of influenza A (H1N1pdm09): a systematic review". Epidemiology. 24 (6): 830–41. doi:10.1097/EDE.0b013e3182a67448. PMC 3809029. PMID 24045719.
- ^ "WHO Europe – Influenza". World Health Organization (WHO). June 2009. Archived from the original on 17 June 2009. Retrieved 12 June 2009.
- ^ "Key Facts About Influenza (Flu)". Centers for Disease Control and Prevention. 28 October 2019. Retrieved 10 March 2020.
- ^ Tokars JI, Olsen SJ, Reed C (May 2018). "Seasonal Incidence of Symptomatic Influenza in the United States". Clinical Infectious Diseases. 66 (10): 1511–1518. doi:10.1093/cid/cix1060. PMC 5934309. PMID 29206909.
- ^ "Influenza: Fact sheet". World Health Organization (WHO). 6 November 2018. Archived from the original on 17 December 2019. Retrieved 25 January 2020.
- ^ "H1N1 fatality rates comparable to seasonal flu". The Malaysian Insider. Washington, D.C., US. Reuters. 17 September 2009. Archived from the original on 20 October 2009. Retrieved 26 September 2009.
- ^ Harder TC, Ortrud W. "Chapter Two: Avian Influenza". Influenza Report 2006. published online. Archived from the original on 9 August 2017. Retrieved 26 October 2007.
Sometimes a virus contains both avian-adapted genes and human-adapted genes. Both the H2N2 and H3N2 pandemic strains contained avian flu virus RNA segments. "While the pandemic human influenza viruses of 1957 (H2N2) and 1968 (H3N2) arose through reassortment between human and avian viruses, the influenza virus causing the 'Spanish flu' in 1918 appears to be entirely derived from an avian source (Belshe 2005).
- ^ a b c Taubenberger et al. 2005.
- ^ Vana & Westover 2008.
- ^ a b dos Reis, Hay & Goldstein 2009.
- ^ "Researchers reconstruct 1918 pandemic influenza virus; effort designed to advance preparedness". Center for Disease Control. Archived from the original on 19 October 2011. Retrieved 2 September 2009.
- ^ "Closing In On a Killer: Scientists Unlock Clues to the Spanish Influenz Virus". National Museum of Health and Medicine. Archived from the original on 2 May 2020. Retrieved 23 March 2020.
- ^ Kolata G (16 February 1999). "Scientists Uncover Clues To Flu Epidemic of 1918". The New York Times. Archived from the original on 22 March 2020. Retrieved 23 March 2020.
- ^ Jordan D, Tumpey T, Jester B. "The Deadliest Flu: The Complete Story of the Discovery and Reconstruction of the 1918 Pandemic Virus". archive.cdc.gov. Archived from the original on 15 July 2020. Retrieved 22 June 2024.
- ^ Taubenberger JK, Baltimore D, Doherty PC, Markel H, Morens DM, Webster RG, et al. (November 2012). "Reconstruction of the 1918 influenza virus: unexpected rewards from the past". mBio. 3 (5) e00201-12. doi:10.1128/mBio.00201-12. PMC 3448162. PMID 22967978.
- ^ Kobasa & et al. 2007.
- ^ "Research on monkeys finds resurrected 1918 flu killed by turning the body against itself". USA Today. Archived from the original on 5 March 2009. Retrieved 14 August 2008.
- ^ Fox 2008.
- ^ Yu X, Tsibane T, McGraw PA, House FS, Keefer CJ, Hicar MD, et al. (September 2008). "Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors". Nature. 455 (7212): 532–536. Bibcode:2008Natur.455..532Y. doi:10.1038/nature07231. PMC 2848880. PMID 18716625.
- ^ Fox 2010.
- ^ Madhav 2013.
- ^ Branswell H (5 December 2018). "A shot-in-the-dark e-mail leads to a century-old family treasure – and hope of cracking a deadly flu's secret". Stat. Archived from the original on 5 December 2018. Retrieved 5 December 2018.
- ^ Hammond JA, Rolland W, Shore TH (14 July 1917). "Purulent bronchitis: A study of cases occurring amongst the British troops at a base in France" (PDF). The Lancet. 190 (4898): 41–45. doi:10.1016/S0140-6736(01)56229-7. Archived from the original (PDF) on 26 August 2019.
- ^ "The Influenza Pandemic of 1918". World War 1 Centenary. Oxford, England: University of Oxford. Archived from the original on 9 December 2018. Retrieved 8 December 2018.
Bibliography
[edit]- Antonovics J, Hood ME, Baker CH (April 2006). "Molecular virology: was the 1918 flu avian in origin?". Nature. 440 (7088): E9, discussion E9-E9, discussion 10. Bibcode:2006Natur.440E...9A. doi:10.1038/nature04824. PMID 16641950. S2CID 4382489.
- Afkhami A (2003). "Compromised constitutions: the Iranian experience with the 1918 influenza pandemic". Bulletin of the History of Medicine. 77 (2): 367–392. doi:10.1353/bhm.2003.0049. PMID 12955964. S2CID 37523219. Archived from the original on 31 March 2020. Retrieved 3 October 2017. – Open access material by the Psychiatry and Behavioral Sciences at Health Sciences Research Commons.
- Afkhami A (29 March 2012) [15 December 2004]. "Influenza". In Yarshater E (ed.). Encyclopædia Iranica. Fasc. 2. Vol. XIII (Online ed.). New York City: Bibliotheca Persica Press. pp. 140–43. Archived from the original on 27 November 2020. Retrieved 3 October 2017.
- Barry JM (2004). The Great Influenza: The Epic Story of the Greatest Plague in History. Viking Penguin. ISBN 978-0-670-89473-4.
- Barry JM (January 2004b). "The site of origin of the 1918 influenza pandemic and its public health implications". Journal of Translational Medicine. 2 (1) 3. doi:10.1186/1479-5876-2-3. PMC 340389. PMID 14733617.
- Beiner G, ed. (2022). Pandemic Re-Awakenings: The Forgotten and Unforgotten 'Spanish' Flu of 1918–1919. Oxford and New York: Oxford University Press. ISBN 9780192843739.
- Benedict ML, Braithwaite M (2000). "The Year of the Killer Flu". In the Face of Disaster: True Stories of Canadian Heroes from the Archives of Maclean's. New York: Viking. p. 38. ISBN 978-0-670-88883-2.
- Billings M (1997). "The 1918 Influenza Pandemic". Virology at Stanford University. Archived from the original on 27 June 2009. Retrieved 1 May 2009.
- Bristow NK (2012). American Pandemic: The Lost Worlds of the 1918 Influenza Epidemic. New York: OUP. ISBN 978-0-19-023855-1. OCLC 1231722653.
- Chandra S, Kuljanin G, Wray J (August 2012). "Mortality from the influenza pandemic of 1918–1919: the case of India". Demography. 49 (3): 857–865. doi:10.1007/s13524-012-0116-x. PMID 22661303. S2CID 39247719.
- Crosby AW (1976). Epidemic and Peace, 1918. Westport, CT: Greenwood Press. ISBN 978-0-8371-8376-3.
- Crosby AW (2003). America's Forgotten Pandemic: The Influenza of 1918 (2nd ed.). Cambridge University Press. ISBN 978-0-521-54175-6. Archived from the original on 3 June 2020. Retrieved 3 May 2020 – via Google Books.
- Davis RA (2013). The Spanish Flu: Narrative and Cultural Identity in Spain, 1918. Palgrave Macmillan. ISBN 978-1-137-33921-8. Archived from the original on 27 July 2021. Retrieved 9 November 2020 – via Google Books.
- Denoon D (2004). "New Economic Orders: Land, Labour and Dependency". In Denoon D (ed.). The Cambridge History of the Pacific Islanders. CUP. p. 247. ISBN 978-0-521-00354-4.
- Dionne KY, Turkmen FF (2020). "The Politics of Pandemic Othering: Putting COVID-19 in Global and Historical Context". International Organization. 74 (S1): E213 – E230. doi:10.1017/S0020818320000405. S2CID 230561266.
- dos Reis M, Hay AJ, Goldstein RA (October 2009). "Using non-homogeneous models of nucleotide substitution to identify host shift events: application to the origin of the 1918 'Spanish' influenza pandemic virus". Journal of Molecular Evolution. 69 (4). Springer Science and Business Media LLC: 333–345. Bibcode:2009JMolE..69..333D. doi:10.1007/s00239-009-9282-x. PMC 2772961. PMID 19787384.
- Ewald PW (1994). Evolution of infectious disease. OUP. ISBN 978-0-19-506058-4.
- Gagnon A, Miller MS, Hallman SA, Bourbeau R, Herring DA, Earn DJ, et al. (2013). "Age-specific mortality during the 1918 influenza pandemic: unravelling the mystery of high young adult mortality". PLOS ONE. 8 (8) e69586. Bibcode:2013PLoSO...869586G. doi:10.1371/journal.pone.0069586. PMC 3734171. PMID 23940526.
- Fox M (29 December 2008). "Researchers unlock secrets of 1918 flu pandemic". Reuters. Archived from the original on 9 November 2020. Retrieved 2 September 2009.
- Fox M (16 June 2010). "Swine flu shot protects against 1918 flu: study". Reuters. Archived from the original on 18 June 2010. Retrieved 30 June 2017.
- Galvin J (31 July 2007). "Spanish Flu Pandemic: 1918". Popular Mechanics. Archived from the original on 20 September 2011. Retrieved 2 October 2011.
- Garret TA (2007). Economic Effects of the 1918 Influenza Pandemic: Implications for a Modern-day Pandemic (PDF). Archived (PDF) from the original on 22 September 2020. Retrieved 22 November 2016.
- Gladwell M (29 September 1997). "The Dead Zone". The New Yorker. Archived from the original on 15 December 2020. Retrieved 16 March 2020.
- Hays JN (1998). The Burdens of Disease: Epidemics and Human Response in Western History. Rutgers University Press. p. 274. ISBN 978-0-8135-2528-0. Archived from the original on 27 July 2021. Retrieved 3 May 2020 – via Google Books.
- He D, Dushoff J, Day T, Ma J, Earn DJ (2011). "Mechanistic modelling of the three waves of the 1918 influenza pandemic". Theoretical Ecology. 4 (2): 283–88. Bibcode:2011ThEco...4..283H. doi:10.1007/s12080-011-0123-3. ISSN 1874-1738. S2CID 2010776.
- He D, Dushoff J, Day T, Ma J, Earn DJ (September 2013). "Inferring the causes of the three waves of the 1918 influenza pandemic in England and Wales". Proceedings. Biological Sciences. 280 (1766) 20131345. doi:10.1098/rspb.2013.1345. PMC 3730600. PMID 23843396.
- Honigsbaum M (2008). Living with Enza: The Forgotten Story of Britain and the Great Flu Pandemic of 1918. Palgrave Macmillan. ISBN 978-0-230-21774-4.
- Humphries MO (2014). "Paths of Infection: The First World War and the Origins of the 1918 Influenza Pandemic". War in History. 21 (1): 55–81. doi:10.1177/0968344513504525. S2CID 159563767.
- Johnson NP, Mueller J (2002). "Updating the accounts: global mortality of the 1918–1920 "Spanish" influenza pandemic". Bulletin of the History of Medicine. 76 (1): 105–115. doi:10.1353/bhm.2002.0022. PMID 11875246. S2CID 22974230.
- Knobler S, Mack A, Mahmoud A, Lemon S, eds. (2005). "1: The Story of Influenza". The Threat of Pandemic Influenza: Are We Ready? Workshop Summary (2005). Washington, DC: The National Academies Press. pp. 60–61. Archived from the original on 23 December 2020. Retrieved 20 August 2019.
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, et al. (January 2007). "Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus". Nature. 445 (7125): 319–323. Bibcode:2007Natur.445..319K. doi:10.1038/nature05495. PMID 17230189. S2CID 4431644.
- Kohn GC (2007). Encyclopedia of plague and pestilence: from ancient times to the present (3rd ed.). Infobase Publishing. p. 363. ISBN 978-0-8160-6935-4. Archived from the original on 1 January 2016. Retrieved 29 October 2015 – via Google Books.
- Madhav N (February 2013). "Modeling a Modern Day Spanish Flu Pandemic" (PDF). AIR Worldwide. Archived from the original on 26 February 2020. Retrieved 5 May 2020.
- More AF, Loveluck CP, Clifford H, Handley MJ, Korotkikh EV, Kurbatov AV, et al. (September 2020). "The Impact of a Six-Year Climate Anomaly on the "Spanish Flu" Pandemic and WWI". GeoHealth. 4 (9) e2020GH000277. Bibcode:2020GHeal...4..277M. doi:10.1029/2020GH000277. ISSN 2471-1403. PMC 7513628. PMID 33005839.
- Morrisey CR (1986). "The Influenza Epidemic of 1918". Navy Medicine. 77 (3): 11–17.
- Noymer A, Carreon D, Johnson N (April 2010). "Questioning the salicylates and influenza pandemic mortality hypothesis in 1918–1919". Clinical Infectious Diseases. 50 (8): 1203, author reply 1203. doi:10.1086/651472. PMID 20233050.
- Pankhurst R (1991). An Introduction to the Medical History of Ethiopia. Trenton: Red Sea Press. ISBN 978-0-932415-45-5.
- Patterson KD, Pyle GF (1991). "The geography and mortality of the 1918 influenza pandemic". Bulletin of the History of Medicine. 65 (1): 4–21. PMID 2021692.
- Porras-Gallo M, Davis RA, eds. (2014). "The Spanish Influenza Pandemic of 1918–1919: Perspectives from the Iberian Peninsula and the Americas". Rochester Studies in Medical History. Vol. 30. University of Rochester Press. ISBN 978-1-58046-496-3. Archived from the original on 22 January 2021. Retrieved 9 November 2020 – via Google Books.
- Price-Smith AT (2008). Contagion and Chaos. Cambridge, MA: MIT Press. ISBN 978-0-262-66203-1.
- Qureshi AI (2016). Ebola Virus Disease: From Origin to Outbreak. Academic Press. ISBN 978-0-12-804242-7 – via Google Books.
- Rice GW (2005). Black November; the 1918 Influenza Pandemic in New Zealand (2nd ed.). University of Canterbury Press. ISBN 978-1-877257-35-3.
- Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K (July 1998). "Pandemic versus epidemic influenza mortality: a pattern of changing age distribution". The Journal of Infectious Diseases. 178 (1): 53–60. CiteSeerX 10.1.1.327.2581. doi:10.1086/515616. JSTOR 30114117. PMID 9652423.
- Spinney L (2018). Pale rider: the Spanish flu of 1918 and how it changed the world. London: Vintage. ISBN 978-1-78470-240-3. OCLC 1090305029.
- Starko KM (November 2009). "Salicylates and pandemic influenza mortality, 1918–1919 pharmacology, pathology, and historic evidence". Clinical Infectious Diseases. 49 (9): 1405–1410. doi:10.1086/606060. PMID 19788357. Lay summary in: Infectious Diseases Society of America (3 October 2009). "Aspirin Misuse May Have Made 1918 Flu Pandemic Worse". ScienceDaily. Archived from the original on 9 January 2021.
- Zhuang W, Liu J, Li W (2010). "hsa-miR-33-5p as a Therapeutic Target Promotes Apoptosis of Breast Cancer Cells via Selenoprotein T". Frontiers in Medicine. 50 (8) 651473. doi:10.1086/651473. PMC 8110722. PMID 33987194.
- Taubenberger JK, Reid AH, Janczewski TA, Fanning TG (December 2001). "Integrating historical, clinical and molecular genetic data in order to explain the origin and virulence of the 1918 Spanish influenza virus". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 356 (1416): 1829–1839. doi:10.1098/rstb.2001.1020. PMC 1088558. PMID 11779381.
- Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG (October 2005). "Characterization of the 1918 influenza virus polymerase genes". Nature. 437 (7060): 889–893. Bibcode:2005Natur.437..889T. doi:10.1038/nature04230. PMID 16208372. S2CID 4405787.
- Taubenberger JK, Morens DM (January 2006). "1918 Influenza: the mother of all pandemics". Emerging Infectious Diseases. 12 (1): 15–22. doi:10.3201/eid1201.050979. PMC 3291398. PMID 16494711.
- Vana G, Westover KM (June 2008). "Origin of the 1918 Spanish influenza virus: a comparative genomic analysis". Molecular Phylogenetics and Evolution. 47 (3): 1100–1110. Bibcode:2008MolPE..47.1100V. doi:10.1016/j.ympev.2008.02.003. PMID 18353690.
- Vilensky JA, Foley P, Gilman S (August 2007). "Children and encephalitis lethargica: a historical review". Pediatric Neurology. 37 (2): 79–84. doi:10.1016/j.pediatrneurol.2007.04.012. PMID 17675021. Archived from the original on 17 April 2020. Retrieved 22 November 2016.
Further reading
[edit]- Beiner G (2006). "Out in the Cold and Back: New-Found Interest in the Great Flu". Cultural and Social History. 3 (4): 496–505. doi:10.1191/1478003806cs070ra (inactive 12 July 2025). Archived from the original on 24 July 2020. Retrieved 29 May 2020.
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - Beiner G, ed. (2022), Pandemic Re-Awakenings: The Forgotten and Unforgotten 'Spanish' Flu of 1918–1919, Oxford and New York: Oxford University Press, ISBN 9780192843739
- Brown J (2018). Influenza: The Hundred Year Hunt to Cure the Deadliest Disease in History. New York: Atria. ISBN 978-1-5011-8124-5.
- Byerly CR (2010). "The U.S. military and the influenza pandemic of 1918–1919". Public Health Reports (Washington, D.C.: 1974). 125 Suppl 3 (Suppl 3): 82–91. ISSN 0033-3549. PMC 2862337. PMID 20568570.
- Byerly CR (2005). Fever of War: The Influenza Epidemic in the U.S. Army during World War I. New York: New York University Press. ISBN 0814799248.
- Chandra S, Christensen J, Likhtman S (November 2020). "Connectivity and seasonality: the 1918 influenza and COVID-19 pandemics in global perspective". Journal of Global History. 15 (3): 408–20. doi:10.1017/S1740022820000261. S2CID 228988279.
- Cimino E (Winter 2023–2024). "The Supervisors are Carrying the Bag: The Nurses' Emergency Council, Settlement Houses, and the 1918 Influenza Pandemic in New York City". New York History. 104 (2): 296–314. doi:10.1353/nyh.2023.a918265. Archived from the original on 19 January 2025. Retrieved 16 November 2024.
- Collier R (1974). The Plague of the Spanish Lady – The Influenza Pandemic of 1918–19. Atheneum. ISBN 978-0-689-10592-0.
- Davis KC (2018). More Deadly Than War: The Hidden History of the Spanish Flu and the First World War. Henry Holt and Co. ISBN 978-1250145123.
- Dorratoltaj N (29 March 2018). Carrell H (ed.). "What the 1918 Flu Pandemic Can Teach Today's Insurers". AIR Research and Modeling Group. Archived from the original on 7 November 2020. Retrieved 28 May 2019.
- Duncan K (2003). Hunting the 1918 flu: one scientist's search for a killer virus. University of Toronto Press. ISBN 978-0-8020-8748-5. Archived from the original on 24 July 2020. Retrieved 3 May 2020.
- Humphries MO (2013). The Last Plague: Spanish Influenza and the Politics of Public Health in Canada. University of Toronto Press. ISBN 978-1-4426-1044-6.
- Johnson N (2006). Britain and the 1918–19 Influenza Pandemic: A Dark Epilogue. London and New York: Routledge. ISBN 978-0-415-36560-4.
- Johnson N (2003). "Measuring a pandemic: Mortality, demography and geography". Popolazione e Storia. 4 (2): 31–52. Archived from the original on 25 December 2022. Retrieved 29 July 2021.
- Johnson N (2003). "Scottish 'flu – The Scottish mortality experience of the 'Spanish flu'". Scottish Historical Review. 83 (2): 216–26. doi:10.3366/shr.2004.83.2.216.
- Kolata G (1999). Flu: The Story of the Great Influenza Pandemic of 1918 and the Search for the Virus That Caused It. New York: Farrar, Straus and Giroux. ISBN 978-0-374-15706-7.
- Little J (2007). If I Die Before I Wake: The Flu Epidemic Diary of Fiona Macgregor, Toronto, Ontario, 1918. Dear Canada. Markham, ON: Scholastic Canada. ISBN 978-0-439-98837-7.
- Noymer A, Garenne M (2000). "The 1918 influenza epidemic's effects on sex differentials in mortality in the United States". Population and Development Review. 26 (3): 565–581. doi:10.1111/j.1728-4457.2000.00565.x. PMC 2740912. PMID 19530360.
- Oxford JS, Sefton A, Jackson R, Innes W, Daniels RS, Johnson NP (February 2002). "World War I may have allowed the emergence of "Spanish" influenza". The Lancet. Infectious Diseases. 2 (2): 111–114. doi:10.1016/S1473-3099(02)00185-8. PMID 11901642.
- Oxford JS, Sefton A, Jackson R, Johnson NP, Daniels RS (December 1999). "Who's that lady?". Nature Medicine. 5 (12): 1351–1352. doi:10.1038/70913. PMID 10581070. S2CID 5065916.
- Pettit D, Janice B (2008). A Cruel Wind: Pandemic Flu in America, 1918–1920. Murfreesboro, TN: Timberlane Books. ISBN 978-0-9715428-2-2.
- Philips H (2010). "The re-appearing shadow of 1918: trends in the historiography of the 1918–19 influenza pandemic". Canadian Bulletin of Medical History. 21 (1): 121–134. doi:10.3138/cbmh.21.1.121. PMID 15202430.
- Phillips H (1 September 2014). "The Recent Wave of 'Spanish' Flu Historiography". Social History of Medicine. 27 (4): 789–808. doi:10.1093/shm/hku066.
- Phillips H, Killingray D, eds. (2003). The Spanish Flu Pandemic of 1918: New Perspectives. London and New York: Routledge.
- Phillips H (November 2020). "'17,'18,'19: religion and science in three pandemics, 1817, 1918, and 2019". Journal of Global History. 15 (3): 434–43. doi:10.1017/S1740022820000315. S2CID 228999483.
- Potter CW (October 2001). "A history of influenza". Journal of Applied Microbiology. 91 (4): 572–579. doi:10.1046/j.1365-2672.2001.01492.x. PMID 11576290. S2CID 26392163.
- Birge JR, Kärtner FX (May 2007). "Efficient optimization of multilayer coatings for ultrafast optics using analytic gradients of dispersion". Applied Optics. 46 (14): 2656–2662. doi:10.2307/132645. JSTOR 132645. PMID 17446914.
- Tuckel P, Sassler S, Maisel R, Leykam A (2006). "The Diffusion of the Influenza Pandemic of 1918 in Hartford, Connecticut". Social Science History. 30 (2): 167–196. doi:10.1017/S0145553200013432. JSTOR 40267904.
- Tumpey TM, García-Sastre A, Mikulasova A, Taubenberger JK, Swayne DE, Palese P, et al. (October 2002). "Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus". Proceedings of the National Academy of Sciences of the United States of America. 99 (21): 13849–13854. Bibcode:2002PNAS...9913849T. doi:10.1073/pnas.212519699. PMC 129786. PMID 12368467.
Videos
[edit]- We Heard the Bells: The Influenza of 1918 – NIH interviews with Spanish Flu survivors.
- Presentation by Nancy Bristow on the Influenza Pandemic and World War I, November 1, 2019, C-SPAN
- "Spanish Flu: a warning from history". Cambridge University. 30 November 2018. Archived from the original on 27 October 2021.
- Dr. Wise on Influenza (1919) – An 18-minute, dramatised public information film (silent) produced by Britain's Local Government Board, the predecessor of the Ministry of Health. Via the British Film Institute National Archive.
External links
[edit]- The American Influenza Epidemic of 1918–1919: A Digital Encyclopedia – largest digital collection of newspapers, archival manuscripts, and interpretive essays exploring the impact of the epidemic on 50 U.S. cities (University of Michigan).
Spanish flu
View on GrokipediaThe 1918–1920 influenza pandemic, known as the Spanish flu, was a worldwide outbreak caused by an H1N1 subtype of influenza A virus with avian genetic origins.[1][2] It infected roughly 500 million people—about one-third of the global population—and resulted in estimates of 50–100 million deaths, marking it as the deadliest pandemic in recorded history.[3][4][5] The name "Spanish flu" arose not from its origin but because Spain, neutral in World War I, reported the epidemic freely without wartime censorship, unlike Allied and Central Powers nations that minimized coverage to preserve public morale.[6] The pandemic unfolded in three waves: a relatively mild first wave in spring 1918, a far deadlier second wave in fall 1918 that accounted for most fatalities, and a third wave in early 1919.[7] Distinct from typical seasonal influenza, which primarily kills the very young and elderly, the Spanish flu exhibited an atypical W-shaped mortality curve, with disproportionate deaths among healthy young adults aged 20–40, attributed to hyperactive immune responses triggering cytokine storms and frequent secondary bacterial pneumonias in the absence of antibiotics.[8][9] The virus's precise zoonotic emergence remains debated, with genetic evidence pointing to a novel reassortment shortly before 1918, possibly in the United States or Europe, though early cases appeared in military camps like Camp Funston, Kansas.[10][11] Lacking vaccines or antivirals, responses relied on non-pharmaceutical interventions such as quarantines, school closures, and mask mandates, which varied in enforcement and effectiveness, highlighting early challenges in pandemic management.[7] The event's scale overwhelmed healthcare systems, exacerbated wartime conditions, and left lasting demographic impacts, including population declines and shifts in global influenza dynamics.[3]
Naming and Etymology
Origins of the Term "Spanish Flu"
The term "Spanish flu" arose during World War I due to wartime censorship in Allied nations, which suppressed reports of influenza outbreaks to preserve troop morale and public support for the war effort, while neutral Spain freely published accounts of the disease's impact.[12][13] Belligerent countries like the United States, France, and the United Kingdom minimized or delayed disclosures of cases in military camps and civilian populations, fearing it would signal weakness or disrupt mobilization.[12] In contrast, Spanish newspapers provided detailed coverage without such restrictions, leading foreign observers to associate the pandemic prominently with Spain despite its global emergence.[6] A pivotal event amplifying this perception occurred in early May 1918, when King Alfonso XIII contracted severe influenza, nearly succumbing to the illness; his high-profile case received extensive uncensored reporting in the Spanish press, including headlines in outlets like El Sol on May 28, which highlighted the epidemic's toll.[14][15] This royal affliction, combined with Spain's neutrality, fostered the misconception that the outbreak originated there, as Allied media increasingly referenced "Spanish" cases to deflect scrutiny from domestic or military sources.[16] Empirical records contradict the nomenclature's implication of Spanish origins, documenting the earliest confirmed outbreak on March 11, 1918, at Camp Funston in Fort Riley, Kansas, where over 100 U.S. soldiers reported flu-like symptoms amid crowded training conditions.[17][6] By late March, the illness had spread to other American military bases, predating widespread Spanish reporting by nearly two months and underscoring how propaganda-driven labeling obscured the pandemic's North American genesis in public discourse.[18]Alternative and Local Names
The 1918 influenza pandemic elicited a range of alternative and local designations that mirrored regional symptom interpretations, epidemiological observations, and socio-political contexts. In the United States and parts of Europe, early mild cases prompted the term "three-day fever," highlighting the rapid resolution in non-severe instances, as noted in contemporary public health reports and Madrid's El Sol newspaper on 28 May 1918 reporting over 80,000 infections in the city.[6][19] In Germany, "Blitzkatarrh" (lightning catarrh) captured the explosive onset and catarrhal symptoms akin to a sudden cold, evoking wartime imagery of swift attacks among soldiers.[20][21] France referred to it as "purulent bronchitis" emphasizing suppurative respiratory complications, while Italy used "sandfly fever" drawing parallels to vector-borne illnesses despite viral etiology.[6] Severe manifestations inspired evocative sobriquets like "purple death," alluding to the heliotrope cyanosis—bluish-purple skin discoloration from lung fluid accumulation and hypoxia—in terminal patients, particularly documented in military camps.[22] Other locale-specific terms included "Flanders grippe" in Britain linking to Western Front trenches, "Naples soldier" in Spain possibly referencing troop movements, "Bolshevik fever" in Poland amid revolutionary unrest, and "wrestler's fever" in Japan connoting physical prostration.[21][23] These varied monikers, often avoiding national stigma or aligning with immediate experiences, precluded a singular contemporaneous label; post hoc virological analysis identifies the pathogen as influenza A(H1N1), favoring empirical subtype nomenclature over descriptive or geographic proxies.[2]Virological and Pathological Characteristics
Identification and Genetic Reconstruction
The identification of the 1918 influenza virus as an H1N1 subtype began with the extraction of viral RNA from formalin-fixed lung tissues preserved from autopsy samples of victims who died in the autumn of 1918.[25] In 1995, a team at the Armed Forces Institute of Pathology (AFIP), led by molecular pathologist Jeffery Taubenberger, identified suitable archival materials from U.S. military personnel and civilians, initiating a multi-year effort to sequence fragmented viral genetic material using reverse transcription polymerase chain reaction (RT-PCR) techniques.[26] Initial progress included the full sequencing of the hemagglutinin (HA) gene by 1997, revealing phylogenetic divergence from known human influenza strains and closer affinity to avian influenza A viruses, indicating a novel introduction into human populations rather than reassortment with circulating human viruses.[27][28] Subsequent sequencing efforts, involving collaborators Ann Reid and others, expanded to the neuraminidase (NA) and other internal genes between 1998 and 2004, overcoming challenges posed by RNA degradation through multiple amplification rounds and comparison with partial sequences from permafrost-preserved Alaskan Eskimo tissues.[29][26] The complete genome was achieved in 2005 through a collaborative project at the Centers for Disease Control and Prevention (CDC), where Taubenberger's AFIP data were integrated with synthetic reverse genetics to reconstruct infectious virus clones under biosafety level 3 conditions.[27] This reconstruction confirmed the virus's avian genetic roots, with all eight segments deriving from a purely avian-like ancestor that underwent adaptive mutations for human host replication efficiency prior to or during 1918.[28][30] Key genetic features identified included specific mutations in the HA gene, such as substitutions at positions enabling preferential binding to human-type α-2,6-linked sialic acid receptors while retaining avidity for deeper alveolar epithelial cells, a tropism pattern distinct from modern seasonal H1N1 strains that primarily target upper airways.[31][32] These HA alterations, absent in avian progenitors, facilitated enhanced replication in mammalian lung tissue, as demonstrated in vitro and in ferret models using the reconstructed virus.[33] The full genomic data also enabled functional studies revealing the virus's capacity to induce hyperinflammatory responses, including elevated cytokine production in infected cells, underpinning its exceptional pathogenicity without reliance on bacterial superinfections.[27][31]Key Viral Features Contributing to Virulence
The 1918 H1N1 influenza virus demonstrated a pronounced tropism for the lower respiratory tract, replicating extensively in alveolar type II pneumocytes and macrophages, which precipitated primary viral pneumonia rather than the secondary bacterial infections typical of milder strains.[34] This deep-lung replication pattern, confirmed through reconstruction and animal model studies, resulted in rapid viral dissemination, epithelial necrosis, and diffuse alveolar damage, distinguishing it from seasonal influenzas confined largely to upper airways.[28][27] The hemagglutinin (HA) glycoprotein played a pivotal role in this enhanced virulence by facilitating receptor binding in alveolar cells and promoting fusion with host membranes under conditions favoring lower respiratory infection.[35] Genetic analyses and reverse engineering experiments swapping the 1918 HA into modern strains induced severe lung pathology, including marked inflammation and cytokine dysregulation, underscoring HA's contribution to tissue tropism and immune hyperactivation.[36] Complementing this, the neuraminidase (NA) and polymerase basic 1 (PB1) genes supported efficient viral release and replication kinetics in mammalian lungs.[37] The PB2 subunit of the viral RNA polymerase further amplified lethality by optimizing cap-snatching and transcription efficiency in human cells at physiological temperatures, leading to elevated viral loads and exacerbated proinflammatory signaling.[38] Unlike avian-adapted polymerases, the 1918 PB2 configuration enabled sustained replication without reliance on specific mammalian mutations like E627K, yet drove dysregulated host responses, including impaired lung repair via Wnt pathway interference.[39][36] An antigenic shift introduced novel HA and NA subtypes absent in human circulation since the late 19th century, engendering near-universal susceptibility across age groups, particularly young adults lacking cross-protective immunity from prior H3-dominant epidemics.[40] This immunological naivety, coupled with the virus's evasion of innate defenses through polymerase-mediated nuclear trafficking, permitted unchecked proliferation before adaptive responses could mitigate damage.[41]Transmission Mechanisms and Mutations
The 1918 influenza A (H1N1) virus primarily transmitted through respiratory droplets and aerosols expelled during coughing, sneezing, talking, or breathing by infected individuals, facilitating close-range person-to-person spread in crowded settings such as military camps and urban areas.[42][43] This mode was amplified by World War I troop mobilizations, which concentrated susceptible populations and enabled rapid dissemination across continents via rail, ship, and overland routes.[32] Experimental studies using reconstructed 1918 virus in animal models, including ferrets, confirmed efficient respiratory droplet transmission, underscoring the dominance of airborne routes over alternatives.[44] Fomite-mediated transmission via contaminated surfaces played a minor role, as evidenced by the virus's environmental persistence being insufficient for significant indirect spread under typical conditions, with contact-tracing data from contemporaneous outbreaks prioritizing droplet exposure.[45][43] Genetic analyses of archived 1918 viral sequences reveal mutations in the hemagglutinin (HA) gene that enhanced binding affinity to human α2,6-linked sialic acid receptors, promoting efficient upper respiratory tract infection and thereby boosting transmissibility compared to avian-adapted precursors.[32][46] These HA receptor-binding site variants, including substitutions like Glu190Asp, emerged early in the pandemic and were conserved across isolates, enabling sustained human-to-human propagation without requiring extensive reassortment.[47][48] Subsequent evolution during serial passages in human hosts yielded second-wave strains with further polymerase (PB1) and neuraminidase adaptations that optimized replication and shedding, as reconstructed from lung tissue genomes showing increased viral fitness.[49][37] Epidemiological models derived from city-level outbreak data estimate the basic reproduction number (R0) for the 1918 virus at 1.4–2.8, exceeding that of seasonal influenza (typically ~1.3) due to prolonged infectious periods facilitated by asymptomatic and pre-symptomatic shedding, particularly in young adults whose immune responses delayed overt symptoms while allowing viral dissemination.[50] Contact-tracing from military bases and genomic phylogenies indicate local chains of transmission interspersed with long-distance seeding events, consistent with droplet-driven propagation rather than sustained fomite or vector roles.[52] No single mutation universally accounted for heightened transmissibility, but cumulative HA and internal gene changes collectively lowered the transmission threshold in naive populations.[32][53]Origins and Emergence
Evidence for North American Origin
The earliest documented outbreak of the 1918 influenza occurred in Haskell County, Kansas, in January 1918, where local physician Dr. Loring Miner observed an unusually severe form of influenza causing rapid deterioration and deaths among patients.[54] Miner reported these cases to U.S. Public Health Service authorities, marking the first known alert of atypical influenza severity worldwide.[11] This rural, isolated county's epidemic preceded similar reports elsewhere, with affected individuals including recruits who soon traveled to nearby Camp Funston at Fort Riley.[55] On March 4, 1918, the first confirmed military case emerged at Camp Funston, a major U.S. Army training base with over 26,000 troops, shortly after Haskell County residents enlisted there.[55] The camp's crowded conditions facilitated rapid spread, sickening hundreds within days and resulting in dozens of deaths from secondary pneumonia.[56] Infected soldiers were mobilized overseas, with troop ships carrying the virus to Europe by late March, aligning temporally with early outbreaks in Brest, France, among arriving American Expeditionary Forces.[56] This pattern of dissemination via U.S. military movements provides epidemiological linkage absent in alternative origin claims.[11] Virological evidence from the reconstructed 1918 H1N1 genome supports mammalian adaptation consistent with North American precursors, including concurrent respiratory outbreaks in U.S. swine populations that shared genetic markers with the human pandemic strain.[57] Phylogenetic analyses position the 1918 hemagglutinin genes as mammalian-like, basal to modern human and classical swine H1 clades, which originated post-1918 in North America rather than aligning closely with pre-existing Asian avian or swine strains.[58] Genomic continuity between preserved 1918 U.S. samples and early European isolates further corroborates transatlantic seeding from American sources over independent Eurasian emergence.[28] These findings, derived from fixed tissue sequencing, underscore the Kansas region's role as the empirical epicenter, prioritizing direct outbreak records over speculative zoonotic jumps elsewhere lacking contemporaneous data.[11]Competing Hypotheses from Europe and Asia
One hypothesis for a European origin centers on outbreaks at the British military camp in Étaples, France, during the winter of 1916–1917, where a respiratory illness labeled "purulent bronchitis" infected thousands of soldiers amid overcrowding, animal proximity (including ducks and pigs), and potential irritants from wartime chemical agents.[59] [60] Pathologists from Étaples and Aldershot barracks retrospectively linked these events to the 1918 pandemic, proposing the camp's conditions facilitated viral adaptation from avian or swine sources to humans.[61] However, no genomic sequencing from Etaples samples exists to confirm identity with the reconstructed 1918 H1N1 virus, and diagnoses likely conflated influenza-like illnesses with concurrent wartime pathogens such as Bartonella quintana (trench fever) or secondary bacterial infections, given diagnostic limitations and incomplete records.[62] Wartime reporting biases, including suppression of health data to maintain morale, further undermine retrospective attributions.[63] An Asian origin theory invokes northern China, where respiratory outbreaks reportedly struck laborer recruitment areas in November 1917, potentially spreading via over 140,000 Chinese workers shipped to France and other European sites by mid-1918 for war support roles.[64] Advocates point to shipment timings aligning with early European cases and archival mentions of flu-like illnesses in Guangdong and northern provinces.[11] Contradictorily, serological antibody surveys from the era reveal no elevated pre-1918 influenza immunity clusters in those regions, inconsistent with an epicenter of emergence, while China's comparatively low pandemic mortality—estimated at under 1% versus global averages—suggests either effective containment, population genetics, or absence of the virulent strain's initial adaptation there.[65] [63] Direct tracing fails to connect laborer movements to the specific H1N1 genomic signature, with analyses concluding insufficient evidence for this vector.[66] These theories share vulnerabilities from World War I-era underreporting and censorship, which delayed or obscured notifications across military fronts, complicating precedence claims; no hypothesis yields a uniquely verifiable pre-1918 viral isolate, highlighting reliance on symptomatic correlations over molecular proof.[11] [63]Factors Influencing Initial Emergence
The 1918 H1N1 influenza virus likely emerged via zoonotic spillover from avian reservoirs, with genetic evidence indicating an avian-like progenitor that adapted to mammals through minimal genetic changes. Reconstruction and sequencing of the virus from preserved human tissues reveal that its eight genome segments exhibit avian influenza characteristics, distinct from contemporary human strains, supporting a direct or indirect jump from birds without requiring extensive reassortment.[28] [32] Swine served as a probable intermediate host, as concurrent respiratory outbreaks in U.S. pigs mirrored early human cases, facilitating viral adaptation via a "mixing vessel" mechanism where avian and mammalian viruses co-circulate.[57] Agricultural practices in the early 20th-century United States, characterized by integrated farming of poultry, swine, and humans, heightened interspecies contact and thus opportunities for spillover. Proximity of livestock to human populations, including on military bases and rural farms, created environmental preconditions for cross-species transmission, though direct causation remains inferred from phylogenetic patterns rather than contemporaneous records.[67] Animal model experiments confirm the virus's efficient mammalian adaptation, with only 11 key coding mutations—such as PB2 E627K—enabling high replication in human cells and virulence in ferrets and mice without further serial passage.[68] [69] World War I-era conditions further lowered barriers to viral establishment by inducing malnutrition and immune suppression across populations, reducing innate defenses against novel pathogens. Studies in malnourished animal models demonstrate enhanced influenza replication and severity, mirroring how wartime food shortages and stress could have facilitated initial human infections by impairing cytokine responses and epithelial barriers.[70] No empirical or genetic evidence supports laboratory origins or intentional release; all reconstructed sequences align with natural evolutionary trajectories from avian sources, predating modern gain-of-function research.[71][32]Pandemic Timeline
Antecedent Waves and Early Detection
Epidemiological analysis of mortality data from New York City reveals an antecedent wave during the 1917/1918 influenza season, marked by a pronounced shift in the age distribution of excess deaths. Prior to 1918, excess mortality primarily affected the elderly, but from February to April 1918, it shifted toward younger adults aged 25-29, indicating possible circulation of the pandemic strain at sub-pandemic intensity levels.[72] [73] This pattern, while not reaching the lethality of later waves, provided early signals of viral adaptation distinct from seasonal influenza norms.[74] Global serological evidence further supports limited pre-1918 circulation of H1N1-related viruses, as 1930s studies detected neutralizing antibodies in individuals born before 1918 against swine H1N1 subtypes, suggesting prior human exposure without widespread pandemics.[75] However, the precursor virus likely achieved broader human transmission only shortly before the recognized outbreak, evading detection amid seasonal flu variability.[28] Early detection faced significant barriers due to contemporary diagnostic limitations; influenza's viral etiology was unknown until the 1930s, with cases routinely misdiagnosed as bacterial pneumonias based on postmortem findings of secondary infections like Streptococcus pneumoniae.[76] [77] The 1918 H1N1 strain's identity was confirmed decades later through genetic reconstruction from preserved lung tissues in 2005, highlighting how reliance on bacterial culturing obscured the primary viral driver.[28] U.S. military training camps functioned as effective sentinels owing to mandated health surveillance, reporting influenza outbreaks in 14 major facilities from March to May 1918.[56] The earliest documented cluster emerged at Camp Funston, Fort Riley, Kansas, on March 4, 1918, among recruits exhibiting acute respiratory symptoms that later analysis linked to the H1N1 subtype.[78] [79] These confined, monitored populations accelerated recognition of the pathogen's spread, though initial interpretations framed it as routine "three-day fever" rather than a novel threat.[80]First Wave: Spring and Summer 1918
The first documented outbreak of the 1918 influenza pandemic occurred on March 4, 1918, at Camp Funston in Fort Riley, Kansas, where U.S. Army cook Albert Gitchell reported symptoms shortly before breakfast, followed by over 100 soldiers falling ill by noon.[78] Cases rapidly increased, with more than 100 soldiers affected within days at this training camp housing over 50,000 troops.[17] The illness presented as typical influenza symptoms, resembling a severe cold, and spread quickly through overcrowded military environments.[78] From Kansas, the virus disseminated to other U.S. military installations, including Camps Hancock, Lewis, Sherman, and Fremont, as well as civilian sites like San Quentin prison in California.[78] Troop movements facilitated transatlantic transmission; approximately 84,000 U.S. soldiers shipped to Europe in March and 118,000 in April carried the pathogen across the Atlantic, with early cases noted among the 15th U.S. Cavalry en route, recording 36 illnesses and 6 deaths.[78] By April, outbreaks appeared among American Expeditionary Forces in ports like Brest, France, marking the virus's arrival in Europe.[41] Sporadic spread continued unevenly through the United States, Europe, and possibly Asia during spring and summer.[41] This initial wave was characterized by high illness rates but mortality comparable to seasonal influenza, with death rates not exceeding normal levels in most areas.[41] Case fatality remained low, estimated below 1% in affected populations, contrasting sharply with subsequent waves.[17] Wartime censorship and prioritization of military efforts led to underreporting, as Allied and Central Powers governments minimized public disclosure of non-combat illnesses to preserve morale and operational secrecy.[12] By June, Britain reported 31,000 cases, affecting a small fraction of the population—around 1% in some regions—yet the mild nature and suppressed information allowed undetected viral circulation, potentially enabling antigenic shifts.[78]Second Wave: Autumn 1918 Peak Mortality
The second wave of the 1918 influenza pandemic escalated rapidly from late August through December, with peak mortality concentrated in September to November across multiple hemispheres, exhibiting unusual global synchronicity facilitated by intensified troop movements and maritime shipping during World War I.[81] In the United States, this phase accounted for the majority of the pandemic's estimated 500,000 to 675,000 excess deaths, far exceeding prior years' influenza baselines by factors of 10 or more.[82] Mortality patterns deviated markedly from typical seasonal flu, with disproportionate fatalities among healthy young adults aged 20 to 40—comprising nearly half of influenza-related deaths—due to the virus's capacity to trigger cytokine storms and subsequent lung damage in previously unexposed or partially immune individuals.[28][83] Urban crowding exacerbated transmission independent of seasonal weather variations, as evidenced by synchronized outbreaks in temperate, tropical, and southern regions linked to military camps and ports rather than cold temperatures. A stark example occurred in Philadelphia on October 5, 1918, when city officials proceeded with a Liberty Loan parade attended by over 200,000 people despite early influenza cases and medical warnings, resulting in an explosive local surge: approximately 45,000 infections and 12,000 deaths within weeks, overwhelming hospitals and morgues.[84][85] Similar dynamics played out in military bases and troop ships, where confined quarters accelerated spread; for instance, U.S. Army camps reported infection rates exceeding 30% in days, with secondary waves hitting "seasoned" personnel despite prior mild exposures from the spring phase.[86] Pathologically, the wave's lethality stemmed from the H1N1 virus predisposing hosts to rampant bacterial superinfections, particularly pneumococcal and streptococcal pneumonias, which infiltrated damaged lungs in crowded, unsanitary environments. Autopsies from the period revealed that while primary viral pneumonitis caused rapid deterioration in some cases, most fatalities involved bacterial coinvasion exploiting flu-induced immunosuppression, with neutrophil influx failing to clear pathogens effectively.[87][88] This interplay, amplified by wartime malnutrition and delayed medical access, drove case-fatality rates above 2.5%—orders of magnitude higher than non-pandemic influenzas—and underscored crowding's role over climatic factors in the wave's ferocity.[28]Third and Fourth Waves: 1919–1920
The third wave of the 1918 influenza pandemic began in early 1919, manifesting with reduced lethality relative to the preceding autumn surge, though it persisted in causing elevated mortality across multiple regions. In the United States, this wave intensified during the winter months, extending the death toll from the prior outbreak and affecting urban centers with renewed hospital burdens. Globally, the wave's spread highlighted incomplete population-level immunity from earlier exposures, allowing residual transmission despite prior infections.[7][89] Australia, isolated by stringent maritime quarantines until late 1918, encountered the virus primarily during this third wave starting in January 1919, following the arrival of infected passengers on ships from overseas; the outbreak resulted in an estimated 12,000 to 15,000 deaths, disproportionately impacting young adults and indigenous communities. This delayed introduction underscores how geographic barriers temporarily mitigated but ultimately deferred the epidemic's impact, with the wave's milder case-fatality rate still yielding significant excess deaths in a previously unexposed population.[90][91] A fragmented fourth wave emerged from December 1919 into spring 1920, blending with endemic influenza circulation and exhibiting variable intensity by locality, such as severe localized mortality spikes in parts of the United States and Europe. Across the pandemic's waves, cumulative global fatalities ranged from 50 to 100 million, reflecting the virus's sustained transmissibility amid demographic vulnerabilities. The waning of these later waves aligned with the buildup of herd immunity from extensive prior infections, as epidemiological and econometric analyses indicate that achieved immunity thresholds, rather than non-pharmaceutical interventions in isolation, primarily constrained resurgence and facilitated subsidence by mid-1920.[92][93][94][28]Clinical and Epidemiological Features
Primary Symptoms and Disease Progression
The 1918 influenza presented with abrupt onset, often striking individuals with dizziness, weakness, and pain during daily activities.[95] Initial symptoms included high fever ranging from 100°F to 104°F, chills, severe headache, myalgia affecting the back, legs, and arms, and profound prostration leading to immobilization.[95] Respiratory manifestations followed, featuring an unproductive cough, harsh breathing, reddened mucous membranes, sneezing, and occasionally bloody nasal discharge.[95] In mild cases, symptoms resolved within a few days, with fever subsiding and recovery ensuing without complications.[95] Severe cases progressed rapidly to pneumonia within 2–3 days, marked by dyspnea, irregular pyrexia, toxemia, vasomotor depression, and cyanosis indicating poor prognosis.[95] [96] The average interval from onset to death in fatal instances was approximately 9 days, with ongoing viral replication evident at autopsy.[96] This swift deterioration to primary viral pneumonia contrasted with the typically milder, self-limited course of seasonal influenza.[97] Autopsy examinations of fatal cases revealed histopathological hallmarks of primary influenza viral pneumonia, including diffuse alveolar damage (DAD) in 62% of reviewed lungs, characterized by hyaline membranes and desquamated epithelial cells.[96] [97] Pulmonary edema affected 60% of cases, filling alveolar spaces with fluid, fibrin, and inflammatory cells, while acute alveolar hemorrhage occurred in 40%.[96] These findings underscored the virus's direct cytopathic effects on lung epithelium and vasculature, distinguishing the pathology from predominant bacterial pneumonias through the primacy of viral-induced damage.[97]
Atypical Fatality Patterns Among Young Adults
The 1918 influenza pandemic deviated markedly from the typical age distribution of influenza mortality, which usually follows a U-shaped curve with peaks among infants and the elderly due to vulnerabilities in immature or waning immune systems. Instead, excess death rates formed a distinctive W-shaped pattern, featuring an additional peak among young adults aged approximately 20–40 years, where mortality was disproportionately high relative to other age groups.[28][98] In the United States, this translated to death rates in the 20–40 age bracket exceeding pre-pandemic levels by over 20 times, accounting for roughly half of the total 675,000 influenza-associated fatalities despite comprising a smaller proportion of the population.[28][99] This anomaly contrasted sharply with subsequent pandemics, such as the 1957 H2N2 and 1968 H3N2 events, where excess mortality aligned more closely with seasonal influenza patterns, disproportionately affecting those over 65 years and showing lower representation of deaths under age 65 (36% in 1957 and 48% in 1968).[28] The 1918 pattern's emphasis on young adults—peaking around ages 25–34—challenged assumptions of inherent frailty in extremes of age, as working-age individuals, often previously healthy, succumbed at rates far exceeding expectations for respiratory pathogens.[98][100] Causally, the elevated young adult mortality has been linked to an overexuberant immune response, termed a cytokine storm, in which vigorous T-cell and cytokine production by fit, unexposed immune systems damaged pulmonary tissues more severely than the virus itself.[83][8] Reconstruction of the 1918 H1N1 virus and its testing in animal models, including ferrets and nonhuman primates, replicated this pathology: young animals exhibited hypercytokinemia, diffuse alveolar damage, and high lethality mirroring human autopsy findings, whereas older models showed milder responses, supporting the role of age-related immune vigor in driving fatalities.[8] This mechanism explains the inversion of standard vulnerability without invoking confounding factors like bacterial superinfections, though direct viral virulence remained essential.[101]Role of Secondary Bacterial Infections
Historical analyses of autopsy records from the 1918–1919 pandemic indicate that secondary bacterial pneumonias accounted for the majority of fatalities, with over 90% of deaths involving superinfections by pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus.[76][102] These bacteria exploited damage to the respiratory epithelium caused by the influenza virus, leading to rapid progression from viral tracheobronchitis to lobar pneumonia and sepsis.[87][103] Contemporary clinicians and pathologists frequently isolated these organisms from lung tissues, blood, and pleural fluids of deceased patients, underscoring their direct causal role in mortality absent effective antibacterial therapies.[104] The influenza virus's cytopathic effects, including desquamation of ciliated epithelial cells and impaired mucociliary clearance, created an environment conducive to bacterial colonization and invasion, amplifying lethality beyond primary viral pathology.[105][106] Pre-antibiotic era diagnostics often conflated viral and bacterial components, with bronchial lavage and sputum cultures revealing mixed infections in nearly half of severe cases examined.[107] This predisposition was evident in military camps, where overcrowding and poor sanitation facilitated bacterial dissemination among virus-compromised hosts.[103] Reconstruction of the 1918 H1N1 virus and subsequent animal modeling confirm its independent virulence—inducing severe pneumonia and death in ferrets and mice without bacterial involvement—but highlight synergistic enhancement in coinfection scenarios mirroring human outcomes.[108] In murine models, sequential inoculation with the reconstructed virus followed by S. pneumoniae shortened survival times and increased lung bacterial loads compared to either pathogen alone, demonstrating how viral suppression of innate immunity facilitates bacterial proliferation.[108] These findings align with historical data, indicating that while the virus initiated tissue destruction, unchecked secondary infections drove the pandemic's exceptional mortality, particularly among young adults with robust but dysregulated immune responses.[96][107]Public Health Interventions and Medical Treatments
Non-Pharmaceutical Measures and Their Implementation
In response to the escalating influenza outbreak in 1918, public health authorities in the United States implemented a range of non-pharmaceutical interventions, including closures of schools, churches, theaters, and other public venues, as well as prohibitions on mass gatherings such as parades, fairs, and sporting events.[109] These measures varied significantly by locality; for instance, cities like St. Louis enacted early and comprehensive bans on public assemblies starting in late September 1918, while others, such as Philadelphia, delayed restrictions despite rising cases, allowing events like the Liberty Loan parade on September 28 that drew over 200,000 participants.[82] Bans often extended to funerals, limiting attendance to immediate family and requiring closed caskets to minimize exposure risks.[110] Mask-wearing mandates emerged as a prominent intervention in densely populated areas, particularly on public transportation and in indoor spaces. In San Francisco, the Board of Supervisors passed an ordinance on October 18, 1918, requiring gauze masks in streetcars, theaters, and crowded areas, which was enforced through fines up to $100 or jail time; this was lifted after the World War I armistice on November 11 but reinstated on January 17, 1919, amid a resurgence.[111] Similar requirements appeared in cities like Seattle, where police officers patrolled in masks to model compliance, and Oakland, where violations led to arrests.[112] Isolation of the ill and quarantine of contacts were standard protocols, often involving home confinement or designated facilities, though enforcement relied on voluntary reporting in many jurisdictions.[113] Within U.S. military camps, which housed hundreds of thousands of troops amid wartime mobilization, commanders imposed quarantines to contain outbreaks, such as segregating barracks and restricting inter-camp travel following initial detections at Camp Funston, Kansas, in March 1918.[56] These efforts included halting troop movements and screening arrivals, temporarily limiting spread within isolated bases, though lapses occurred due to overcrowding and rapid personnel turnover.[114] Internationally, some nations enacted travel restrictions; Australia, for example, closed ports to infected ships and imposed a maritime quarantine that postponed the pandemic's arrival until January 1919.[115] In Europe and the U.S., wartime censorship and troop transports complicated full closures, resulting in partial curbs like ship inspections that delayed but did not avert transoceanic dissemination.[116] Compliance with these measures proved uneven, marked by public defiance and organized resistance. In San Francisco, the second mask ordinance sparked the formation of the Anti-Mask League in January 1919, which petitioned for repeal citing discomfort and inefficacy, leading to widespread non-adherence and arrests of "slackers."[117] Store owners in Denver openly violated closure orders by remaining open, while clergy in multiple cities protested bans on religious services, arguing hypocrisy when saloons stayed operational amid Prohibition debates.[118] Political opposition framed restrictions as overreach, with critics decrying them as tyrannical impositions on liberty, particularly during armistice celebrations that prompted unauthorized gatherings in defiance of lingering prohibitions.[119] Black markets for exemptions and informal networks evading quarantines further undermined enforcement in urban centers.[118]Empirical Evidence on Intervention Effectiveness
Analyses of excess mortality data from 43 U.S. cities during the 1918-1919 influenza pandemic indicate that early and layered implementation of nonpharmaceutical interventions, such as school closures, bans on public gatherings, and isolation measures, was associated with reductions in peak death rates by 48% in cities like St. Louis compared to later or less comprehensive efforts.[109] These findings, derived from contemporaneous death certificate records and newspaper reports on intervention timing, suggest that interventions implemented two weeks before a city's epidemic peak lowered transmission rates by up to 30-50% in responsive locales like San Francisco and Milwaukee.[94] However, overall mortality reductions were more modest, averaging 10-30%, as interventions were often temporary and did not eliminate subsequent waves.[94] Timing of interventions proved more critical than their duration or stringency, with cities acting preemptively—before local case surges—achieving disproportionate benefits, while prolonged measures in non-compliant areas yielded diminishing returns.[109] Confounders in these assessments include variable enforcement, seasonal weather patterns favoring viral decline, and differences in baseline population immunity or bacterial co-infections, which could independently suppress or exacerbate mortality independent of interventions.[94] Retrospective modeling corroborates moderate transmission reductions but highlights data limitations from inconsistent vital statistics reporting amid wartime disruptions.[120] Critiques of intervention efficacy note widespread evasion and non-compliance, such as anti-mask campaigns in San Francisco and illegal gatherings, which eroded public adherence and potentially amplified spread in overreaching jurisdictions.[121] Wartime censorship in Allied nations suppressed reporting of intervention failures or high mortality in military camps, potentially inflating perceptions of uniform success by obscuring regional disparities and undercounting deaths.[12] Econometric reviews of city-level data further indicate that while pandemics themselves depressed short-term activity, interventions did not cause sustained economic harm, with recoveries offsetting initial disruptions, though such analyses control for mortality as a primary driver rather than policy alone.[122]Treatment Approaches and Limitations
Supportive care formed the cornerstone of treatment for influenza patients during the 1918–1919 pandemic, emphasizing bed rest, hydration, nutrition, and nursing interventions such as sponge baths and clean bedding to manage symptoms like fever and weakness.[123][124] Physicians prioritized these measures because the viral etiology of influenza remained unknown, with many attributing the disease to bacteria like Haemophilus influenzae (then called Pfeiffer's bacillus).[89] Without mechanical ventilation or intensive care units, outcomes depended heavily on addressing dehydration and secondary complications through fluids and rest, though overwhelmed medical systems limited their consistent application.[27] Pharmacological interventions were largely empirical and ineffective against the virus itself. Aspirin, newly available since 1917, was widely prescribed in high doses—often 975–1,300 mg per administration, totaling up to 8 grams daily—to combat fever and pain, exceeding modern safe limits of about 4 grams per day.[125] These dosages, recommended in medical journals and public health guidelines, could induce salicylate toxicity, manifesting as pulmonary edema, hemorrhages, and exacerbated viral pathology, potentially contributing to mortality rates; pathologist Karen Starko hypothesized this link based on contemporaneous dosing regimens and autopsy findings of wet lungs and bleeding.[126][127] Quinine, administered in doses of 5 grains two to four times daily, was used symptomatically for its antipyretic effects but offered no antiviral benefit.[128] Over-the-counter remedies, including cough syrups and whiskey, supplemented these but lacked efficacy beyond palliation.[124] Vaccine development efforts targeted presumed bacterial causes, yielding polyvalent preparations against H. influenzae and other pathogens, administered to soldiers and civilians in campaigns by pharmaceutical firms and military labs.[129] These vaccines, produced rapidly from autopsied lung cultures, showed mixed results in trials—some reported reduced pneumonia incidence, but overall they failed to prevent or mitigate viral infection due to erroneous etiology assumptions, with medical literature documenting contradictory efficacy claims.[129][130] By late 1919, convalescent plasma transfusions from recovered individuals demonstrated promise in lowering mortality for severe cases, but logistical constraints like blood typing limitations and scarcity restricted widespread use.[131] Therapeutic limitations stemmed from diagnostic gaps and the absence of targeted antivirals or broad-spectrum antibiotics, leaving secondary bacterial pneumonias untreated effectively despite some experimental sera.[132] Global research accelerated post-first wave, yet the pandemic's rapid waves outpaced breakthroughs, as the virus receded before viable interventions emerged; this reactive paradigm highlighted medicine's dependence on etiological understanding, which only advanced decades later with viral isolation in the 1930s.[6][130] High mortality, particularly from cytokine storms in young adults, underscored supportive care's insufficiency against the H1N1 strain's virulence without modern immunomodulators.[133]Global Mortality and Demographic Impacts
Total Death Toll Estimates and Uncertainties
Estimates of the global death toll from the 1918-1920 influenza pandemic, commonly known as the Spanish flu, vary due to incomplete vital registration systems, wartime disruptions, and inconsistent diagnostic criteria, but scholarly reassessments place the figure at approximately 50 million deaths.[134] This estimate, derived by Niall Johnson and Jürgen Mueller in 2002 through compilation of country-level data and adjustments for underreporting, revises earlier projections of 24.7-39.3 million upward, accounting for gaps in records from regions with sparse documentation.[134] Some analyses suggest the total could approach 100 million, particularly when incorporating indirect effects like exacerbated famine or reduced births, though such higher bounds remain speculative without uniform corroboration across datasets.[135] In the United States, official counts recorded 675,000 influenza-related deaths, a figure adjusted higher in some excess mortality studies to reflect unverified cases amid overwhelmed reporting.[136] Uncertainties stem primarily from the coincidence with World War I, which suppressed public disclosure of civilian mortality in combatant nations to maintain morale, while neutral countries like Spain faced no such censorship but still suffered from limited infrastructure for tracking deaths.[41] In British India, where the pandemic struck hardest proportionally, estimates hover around 18 million deaths, but these rely on extrapolations from partial provincial reports prone to undercounting due to rural inaccessibility and reliance on local enumerators without standardized verification.[134] African data are even more fragmentary, with colonial records capturing only urban or mission-station fatalities, leaving vast rural and nomadic populations unaccounted for and inviting systematic underestimation.[137] Standard methodologies calculate tolls via excess all-cause mortality—comparing observed deaths in 1918-1920 against pre-pandemic baselines—yet this approach introduces confounders, as wartime conditions like malnutrition, troop movements, and bacterial co-infections elevated baselines unpredictably, potentially overstating or masking influenza-attributable deaths.[138] Even in well-documented areas, misattribution between influenza, pneumonia, and other respiratory illnesses compounded errors, given the absence of viral testing until decades later.[5] Contemporary refinements using statistical models and genomic reconstructions of the H1N1 strain offer indirect validation but cannot retroactively resolve archival voids, underscoring persistent debates over true scale.[139]Geographic Disparities in Mortality
Mortality from the 1918 influenza pandemic exhibited stark geographic variations, influenced by factors such as population density, prior exposure conferring partial immunity, baseline health conditions, and the timing and efficacy of public health responses. Isolated communities with limited prior contact faced catastrophic losses upon introduction of the virus due to the absence of herd immunity, while denser urban areas experienced amplified transmission through crowding. Conversely, regions with effective early isolation or quarantine measures, or potentially higher pre-existing immunity from earlier influenza strains, recorded comparatively lower rates. These disparities underscore the role of local epidemiological and socioeconomic conditions in modulating pandemic impact, independent of viral virulence alone.[140][141] In the Pacific islands, mortality reached extreme levels in areas with delayed but unchecked spread; Western Samoa reported 19%–22% of its population dying within two months of the virus's arrival in November 1918, totaling approximately 8,500 deaths from a pre-epidemic population of around 38,000, attributable to rapid person-to-person transmission in close-knit communities lacking immunity. Neighboring American Samoa averted disaster through stringent maritime quarantine enforced from September 1918, resulting in zero influenza deaths among its 8,000 residents. Such contrasts highlight how geographic isolation could either shield populations entirely or exacerbate devastation upon breach.[142][143] Within the United States, urban centers suffered higher per capita mortality than rural areas, with studies indicating elevated influenza-related death rates in cities due to intensified transmission in crowded settings; for instance, analyses of England, Wales, and analogous U.S. patterns showed urban excess mortality surpassing rural by factors linked to density. Indigenous populations, particularly Alaska Natives, faced disproportionate tolls, with regional mortality reaching up to 38% in some areas and overall territory excess deaths at 1,672 per 100,000—eight times the rate for non-Natives—reflecting vulnerabilities from malnutrition, inadequate housing, and limited medical access rather than inherent susceptibility.[141][144] In Europe, Sweden experienced relatively low mortality of approximately 35,000 deaths—or about 0.6% of its 5.8 million population—during the pandemic's three waves, potentially due to a combination of neutral wartime status enabling open reporting and implementation of measures like school closures, alongside possible cross-immunity from milder early strains. Asian regions showed varied patterns, with Japan recording 257,000–481,000 deaths (0.5%–0.9% of ~55 million population) amid high absolute numbers elsewhere like India, where estimates suggest 12–17 million fatalities (~4%–6% of ~300 million), though per capita rates were moderated in some areas by rural dispersion and underreporting; overall, lower urban-rural gradients in parts of Asia contrasted with Western patterns, tied to differing immunity profiles and response capacities.[145][146]Long-Term Population and Health Consequences
The 1918 influenza pandemic led to a notable decline in fertility rates, particularly evident in the United States where birth rates dropped by 5% to 15% in the months following peak mortality, with the nadir occurring approximately 6 to 7 months after the epidemic's height, consistent with increased miscarriages among infected pregnant women.[147] This natality depression contributed to a "lost generation" effect, as U.S. census data indicate deficits in the 1919 birth cohort, partly attributed to conception postponement during the crisis and fetal losses, though some analyses suggest the 1920 uptick was not a compensatory boom but rather a return to trend amid broader post-war fertility declines.[148][149] Cohorts exposed in utero during the pandemic exhibited long-term health impairments, including elevated rates of cardiovascular disease in later adulthood; survivors aged 60 to 82 showed at least 20% excess cardiovascular risk linked to prenatal influenza exposure.[150] Empirical analyses of U.S. Census data from 1960 to 1980 reveal that these fetal-exposed groups faced reduced educational attainment and 20% higher disability rates by age 61, supporting fetal origins mechanisms rather than selection effects from immediate mortality.[151] However, evidence for genetic scarring—such as transgenerational inheritance of vulnerabilities—is lacking, with effects primarily attributable to developmental programming during gestation.[152] Among young adult survivors of active infection, studies indicate modest reductions in life expectancy, with exposed cohorts showing at most a 20-day decrement overall, though likely smaller after accounting for incidence variations; no consistent postnatal effects on longevity were found beyond early life.[153] A temporal spike in encephalitis lethargica cases from 1919 to the 1930s coincided with the pandemic, prompting hypotheses of influenza-triggered neurological sequelae or post-viral autoimmunity, but direct causal links remain unproven due to absent virological confirmation and failure to isolate influenza from affected brains.[154][155] Survivor immunity to subsequent H1N1 strains was partial, as prior exposure could induce dysregulated responses in reinfections, though population-level weakening was not empirically dominant compared to age-specific priming effects.[98]Societal, Economic, and Political Effects
Influence on World War I and Wartime Censorship
The 1918 influenza pandemic significantly impaired military operations during the final months of World War I, particularly affecting Allied forces. In the American Expeditionary Forces (AEF), approximately 340,000 soldiers were hospitalized due to influenza, exceeding battle casualties in some periods.[156] The U.S. Army recorded over one million influenza cases among troops in training camps and Europe, with infection rates reaching 25% in the Army and 40% in the Navy.[157] [158] On the Western Front, attack rates among soldiers approximated 40%, contributing to delays in Allied offensives such as the Meuse-Argonne campaign, where troop morbidity reduced combat effectiveness.[79] [156] Wartime censorship in belligerent nations suppressed reporting on the pandemic's scale to preserve morale and operational secrecy, distorting public awareness and hindering coordinated responses. Governments in the United States, Britain, France, and Germany minimized flu coverage, with U.S. authorities invoking the Espionage Act of 1917 and the Sedition Act of 1918 to prosecute perceived "alarmism" that could undermine the war effort, resulting in thousands of cases broadly targeting dissent.[12] [159] [160] This suppression fostered distrust when outbreaks became undeniable, as empirical evidence of widespread illness eroded confidence in official narratives.[12] Neutral Spain's uncensored press freely reported the pandemic, including King Alfonso XIII's illness in May 1918, leading to the ironic misnomer "Spanish flu" despite the virus's likely origins elsewhere, such as U.S. military camps.[161] [16] The pandemic's toll on troop strength and morale likely hastened the armistice on November 11, 1918, as weakened armies on both sides reduced capacity for prolonged fighting, with some historians attributing the war's abrupt end partly to influenza's debilitating effects.[12] [156]Economic Disruptions and Recovery Patterns
The 1918 influenza pandemic induced short-term labor shortages across various sectors, particularly in manufacturing and mining, due to widespread illness and mortality among working-age adults, which temporarily elevated wages in affected U.S. industries as employers competed for reduced labor supplies.[162][163] In the coal sector, a key indicator of industrial activity, labor supply disruptions were sharp but brief, with production recovering rapidly without significant long-term spillovers to other economic areas.[164] City-level analyses in the U.S. indicate that mortality-driven absenteeism contributed to output dips, yet aggregate GDP contracted by only about 1.5 percent nationally, far less than in many other countries, with non-pharmaceutical interventions imposing minimal additional economic costs relative to lives saved.[165][164] Agricultural production faced disruptions from elevated rural mortality rates, which reduced the farm labor force by an estimated 8 percent in some regions, though overall output held steady due to prior mechanization trends and adaptive reallocations.[166] In developing areas like Java, influenza deaths correlated with sharp declines in cash crop yields, such as sugar, where mortality spikes exceeded 30 per thousand in peak months, compounding seasonal vulnerabilities.[167] Globally, trade volumes dipped amid port closures and shipping delays, but disentangling flu-specific effects from the concurrent Armistice of November 1918 and World War I's logistical strains remains challenging, as wartime blockades had already suppressed pre-pandemic commerce.[168] Economic recovery accelerated in 1919, with U.S. real per capita GDP rebounding above pre-pandemic trends and stock market indices like the Dow Jones rising 30.5 percent that year, reflecting resilient market adjustments such as labor reallocation and pent-up demand rather than fiscal stimuli.[169] Unlike narratives of severe downturns, the pandemic did not precipitate a depression; post-war recessions in 1919–1920 stemmed more from demobilization and inflation than influenza, underscoring the economy's capacity for self-correction without modern-style lockdowns.[170][169]Social Behaviors and Public Compliance Challenges
Public compliance with non-pharmaceutical interventions during the 1918-1919 influenza pandemic varied widely, with significant defiance undermining official efforts in multiple cities. In San Francisco, mask mandates enacted in late October 1918 faced immediate resistance, leading to the formation of the Anti-Mask League, which argued that enforced wearing infringed on personal liberties and lacked scientific backing.[112][117] Noncompliance, termed "slackerism," prompted police enforcement, including arrests for refusing masks on streetcars and public spaces, yet vigilante-like public scolding by citizens supplemented official measures.[171][172] Religious gatherings often persisted despite closures, correlating with elevated local mortality. In Philadelphia, churches defied bans in September 1918, contributing to over 12,000 deaths in that city alone during the October wave, as congregational transmission accelerated spread among healthy adults.[173] Similar patterns emerged elsewhere, where spiritual resilience prioritized communal worship over isolation, empirically linking higher attendance to excess deaths in affected parishes.[118] Stigma toward sufferers manifested in social avoidance and quarantine enforcement, reducing casual contacts but exacerbating isolation. Families predominantly provided care at home, with estimates indicating over 80% of cases managed domestically to avert hospital overload, as institutional capacity collapsed under patient surges.[174][175] This grassroots approach, while risky for caregivers, distributed burden and preserved systemic function amid scarce professional resources.[176] Following the major waves, denialism surged, with public celebrations like San Francisco's November 1919 mask bonfires symbolizing rejection of prolonged restrictions.[117] Criticisms of mandates as governmental overreach proliferated, fostering long-term skepticism toward centralized public health directives, evidenced by a decade-long decline in vaccination compliance post-pandemic.[118][177] Such reactions highlighted resilience against top-down controls but also perpetuated risks from unheeded empirical lessons on transmission dynamics.[178]Legacy, Comparisons, and Contemporary Research
Cultural Representations and Historical Memory
Literature from the era and shortly after captured the personal devastation of the Spanish flu, often intertwining it with broader themes of isolation and mortality. Katherine Anne Porter's 1939 novella Pale Horse, Pale Rider draws from her own near-fatal illness during the pandemic, portraying a young woman's delirium and loss amid wartime reporting in Denver, emphasizing individual fragility over collective chaos.[179] Earlier works, such as E.E. Cummings' The Enormous Room (1922), reference the flu's intrusion into prisoner-of-war experiences, highlighting its indiscriminate disruption.[180] These fictional accounts prioritize anecdotal human suffering, contrasting with factual records of widespread societal adaptation. Visual arts provided stark, contemporaneous depictions, as seen in Edvard Munch's 1919 self-portrait showing his emaciated figure post-infection, symbolizing the flu's physical toll on artists and intellectuals.[181] Spanish satirical cartoons from November 1918 likened the pandemic to a "tragic game of football" between war and influenza, personifying the dual afflictions devastating Earth.[182] Films rarely addressed the flu directly during its peak due to censorship and production halts; British silent cinema, for instance, features only one surviving informational reel, while later portrayals like the 1985 film 1918 romanticized community resilience in Texas towns, focusing on endurance rather than horror.[182][183] Historical memory of the Spanish flu remains selective and subdued, with public memorials scarce until the early 21st century; a 2018 granite marker in Sullivan, Missouri, commemorates over 50 million global deaths, one of few dedicated sites amid thousands of war monuments.[184] This oversight stems partly from the pandemic's eclipse by World War I's narrative dominance and the absence of tangible, enduring symbols like battlefields, leading to cultural forgetting despite its higher death toll compared to later events.[185] In contrast to COVID-19's pervasive media documentation, the 1918 event lacks similar archival visibility, fostering a gap between factual records of rapid societal recovery and romanticized victimhood in retrospective lore.[186] Oral histories from survivors underscore community solidarity, revealing acts of neighborly aid that sustained isolated families, as in Idaho accounts where unaffected residents delivered food and care during household outbreaks.[187] Alabama interviews from the 1970s-2000s describe mutual support networks forming organically, countering emphases on helplessness in some cultural retellings by evidencing pragmatic resilience without reliance on medical interventions.[188] Library of Congress ethnographic collections preserve folk narratives of everyday heroism, such as volunteers nursing strangers, which align with empirical data on localized compliance and recovery patterns rather than pervasive despair.[189] These firsthand accounts highlight a factual legacy of adaptive human agency, often downplayed in favor of war-centric historical focus.[190]Parallels and Differences with Modern Pandemics
The basic reproductive number (R0) for the 1918 H1N1 influenza virus has been estimated at 1.4 to 2.8, comparable to the original SARS-CoV-2 strain's R0 of approximately 2 to 3, indicating similar inherent transmissibility in the absence of interventions.[191][192] Both pathogens spread primarily via respiratory droplets and aerosols, leading to rapid global dissemination, though 1918 occurred amid World War I troop movements that accelerated transmission without modern air travel equivalents.[192] Mortality patterns diverged sharply by age: the Spanish flu exhibited a W-shaped distribution, with excess deaths among young adults aged 20-40 years—often healthy individuals succumbing to a hyperinflammatory "cytokine storm" response—contrasting COVID-19's J-shaped curve concentrated in those over 70, driven by comorbidities and immunosenescence rather than robust immune overreactions in youth.[193][194] Pathogenetically, secondary bacterial coinfections, such as with Streptococcus pneumoniae or Haemophilus influenzae, contributed to over 90% of 1918 fatalities via superimposed pneumonia on viral damage, whereas COVID-19 deaths more often stemmed from direct SARS-CoV-2 endothelial and alveolar injury with less frequent bacterial superinfections, occurring in under 10% of severe cases.[192][195] Non-pharmaceutical interventions (NPIs) like school closures, public gathering bans, and mask mandates proved effective short-term in both pandemics, reducing peak transmission by up to 50% in 1918 U.S. cities implementing layered measures early; analogous COVID-19 analyses confirmed NPIs delayed waves and lowered case rates, though adherence waned over time in both eras.[196][82] Unlike COVID-19's prolonged lockdowns extending months or years in some regions, 1918 responses emphasized transient NPIs lasting 2-8 weeks per wave, correlating with faster societal resumption and minimal long-term economic scarring—U.S. GDP dipped modestly in 1918-1919 before rebounding by 1920, versus COVID-19's sharper contractions exceeding 10% in major economies with slower recoveries tied to extended restrictions.[82][169] The 1918 pandemic lacked global coordination, relying on fragmented national or local efforts without organizations like the WHO, whereas modern preparedness incorporates 1918-derived lessons such as rapid viral sequencing—enabled by post-1918 virology advances—and stockpiled antivirals, though critiques highlight overreliance on predictive models that underestimated 1918's bacterial cofactors and overestimated uniform NPI scalability amid varying compliance.[197][198]Advances in Virology and Preparedness Lessons
The reconstruction of the 1918 H1N1 influenza virus genome marked a pivotal advance in virology, beginning with efforts in 1996 by Jeffrey Taubenberger and colleagues at the Armed Forces Institute of Pathology, who extracted RNA from preserved lung tissues of victims.[199] By 2005, the full genome sequence was completed, revealing unique adaptations such as mutations in the hemagglutinin (HA) and polymerase genes that enhanced replication efficiency and triggered excessive cytokine responses, contributing to the virus's lethality.[38] This avian-origin strain's characterization via reverse genetics systems enabled safe laboratory recreation of the virus for controlled studies, elucidating mechanisms like the PB1-F2 protein's role in secondary bacterial infections and immune dysregulation.[27] These insights directly informed vaccine development, as recombinant vaccines incorporating the 1918 HA protein demonstrated protection in animal models against lethal challenge, informing strategies for universal influenza vaccines targeting conserved epitopes across H1N1 variants.[133] Antiviral testing confirmed oseltamivir's efficacy; in macaque models, the drug reduced viral loads and prevented severe disease from the reconstructed 1918 strain, though resistance mutations could emerge under selective pressure, underscoring the need for stockpiling and combination therapies.[200] Such facsimile-based research has accelerated preclinical evaluation of broad-spectrum antivirals and adjuvanted vaccines, enhancing preparedness for zoonotic influenza threats. Key preparedness lessons from the 1918 pandemic emphasize robust global surveillance of influenza viruses in animal reservoirs and human populations to detect antigenic shifts early, as coordinated monitoring informs risk assessment and prepandemic vaccine production.[201] While international cooperation through networks like the WHO's Global Influenza Surveillance and Response System is essential for data sharing and strain distribution, historical and recent politicization—such as delayed reporting or origin disputes—has eroded trust, highlighting the causal importance of transparent, apolitical mechanisms to avoid amplifying transmission via withheld information.[202] Empirical analyses of 1918 responses affirm the mortality benefits of non-pharmaceutical interventions (NPIs) like school closures, public gathering bans, and quarantine when implemented early and in layers, as evidenced by lower peak death rates in U.S. cities such as St. Louis compared to Philadelphia.[203] Recent 2020s studies, revisiting these data, indicate NPIs flattened mortality curves without imposing lasting economic penalties; cities with stringent measures experienced similar medium-term recoveries to those without, countering claims of inevitable trade-offs and supporting evidence-based calibration over indiscriminate shutdowns or panic-driven policies.[204][205] This underscores causal realism: pandemics inherently depress output via workforce morbidity, but targeted NPIs mitigate deaths while preserving resilience through adaptive enforcement.References
- https://www.[ancestry.com](/page/Ancestry.com)/c/ancestry-blog/the-killer-flu-how-did-the-1918-pandemic-affect-your-family
- https://www.[researchgate](/page/ResearchGate).net/publication/267908581_Estimate_of_the_Reproductive_Number_for_the_1918_Influenza_Pandemic




