Recent from talks
Nothing was collected or created yet.
| Bone | |
|---|---|
 A bone dating from the Pleistocene Ice Age of an extinct species of elephant | |
 A scanning electronic micrograph of bone at 10,000× magnification | |
| Identifiers | |
| MeSH | D001842 |
| TA98 | A02.0.00.000 |
| TA2 | 366, 377 |
| TH | H3.01.00.0.00001 |
| FMA | 5018 |
| Anatomical terminology | |
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals.[1] Bones protect the organs of the body, produce red and white blood cells, store minerals, help regulate acid-base homeostasis, provide structure and support for the body, and enable mobility and hearing. Bones come in a variety of shapes and sizes and have complex internal and external structures.[2]
Bone tissue (also known as osseous tissue or bone in the uncountable) is a form of hard tissue, specialised connective tissue that is mineralized and has an intercellular honeycomb-like matrix,[3] which helps to give the bone rigidity. Bone tissue is made up of different types of bone cells: osteoblasts and osteocytes (bone formation and mineralisation); osteoclasts (bone resorption); modified or flattened osteoblasts (lining cells that form a protective layer on the bone surface). The mineralised matrix of bone tissue has an organic component of mainly ossein, a form of collagen, and an inorganic component of bone mineral, made up of various salts. Bone tissue comprises cortical bone and cancellous bone, although bones may also contain other kinds of tissue including bone marrow, endosteum, periosteum, nerves, blood vessels, and cartilage.
In the human body at birth, approximately 300 bones are present. Many of these fuse together during development, leaving a total of 206 separate bones in the adult, not counting numerous small sesamoid bones.[4][5] The largest bone in the body is the femur or thigh-bone, and the smallest is the stapes in the middle ear.
The Ancient Greek word for bone is ὀστέον ("osteon"). In anatomical terminology, including in the Terminologia Anatomica, the word for a bone is os (for example, os breve, os longum, os sesamoideum).
Gross anatomy
[edit]Five types of bones are found in the human body: long, short, flat, irregular, and sesamoid.[6]

- Long bones are characterized by a shaft, the diaphysis, that is much longer than its width; and by an epiphysis, a rounded head at each end of the shaft. They are made up mostly of compact bone, with lesser amounts of marrow, located within the medullary cavity, and areas of spongy, cancellous bone at the ends of the bones.[7]
- Most bones of the limbs, including those of the fingers and toes, are long bones. The exceptions are the eight carpal bones of the wrist, the seven articulating tarsal bones of the ankle and the sesamoid bone of the kneecap. Long bones such as the clavicle, that have a differently shaped shaft or ends are also called modified long bones.
- Short bones are roughly cube-shaped, and have only a thin layer of compact bone surrounding a spongy interior. Short bones provide stability and support as well as some limited motion.[8]
- The bones of the wrist and ankle are short bones.
- Flat bones are thin and generally curved, with two parallel layers of compact bone sandwiching a layer of spongy bone.
- Sesamoid bones are bones embedded in tendons. Since they act to hold the tendon further away from the joint, the angle of the tendon is increased and thus the leverage of the muscle is increased.
- Irregular bones do not fit into the above categories. They consist of thin layers of compact bone surrounding a spongy interior. As implied by the name, their shapes are irregular and complicated. Often this irregular shape is due to their many centers of ossification or because they contain bony sinuses.
Terminology
[edit]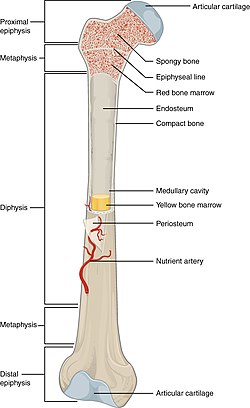
Anatomists use a number of anatomical terms to describe the appearance, shape and function of bones. Like other anatomical terms, many of these derive from Latin and Greek. Some anatomists still use Latin to refer to bones. The term "osseous", and the prefix "osteo-", referring to things related to bone, are still used commonly today.
Some examples of terms used to describe bones include the term "foramen" to describe a hole through which something passes, and a "canal" or "meatus" to describe a tunnel-like structure. A protrusion from a bone can be called a number of terms, including a "condyle", "crest", "spine", "eminence", "tubercle" or "tuberosity", depending on the protrusion's shape and location. In general, long bones are said to have a "head", "neck", and "body".
When two bones join, they are said to "articulate". If the two bones have a fibrous connection and are relatively immobile, then the joint is called a "suture".
Functions
[edit]Mechanical
[edit]Bones serve a variety of mechanical functions. Together the bones in the body form the skeleton. They provide a frame to keep the body supported, and an attachment point for skeletal muscles, tendons, ligaments and joints, which function together to generate and transfer forces so that individual body parts or the whole body can be manipulated in three-dimensional space (the interaction between bone and muscle is studied in biomechanics).
Bones protect internal organs, such as the skull protecting the brain or the ribs protecting the heart and lungs. Because of the way that bone is formed, bone has a high compressive strength of about 170 MPa (1,700 kgf/cm2),[12] poor tensile strength of 104–121 MPa, and a very low shear stress strength (51.6 MPa).[13][14] This means that bone resists pushing (compressional) stress well, resist pulling (tensional) stress less well, but only poorly resists shear stress (such as due to torsional loads). While bone is essentially brittle, bone does have a significant degree of elasticity, contributed chiefly by collagen.
Mechanically, bones also have a special role in hearing. The ossicles are three small bones in the middle ear which are involved in sound transduction.
Synthetic
[edit]The cancellous part of bones contain bone marrow. Bone marrow produces blood cells in a process called hematopoiesis.[15] Blood cells that are created in bone marrow include red blood cells, platelets and white blood cells.[16] Progenitor cells such as the hematopoietic stem cell divide in a process called mitosis to produce precursor cells. These include precursors which eventually give rise to white blood cells, and erythroblasts which give rise to red blood cells.[17] Unlike red and white blood cells, created by mitosis, platelets are shed from very large cells called megakaryocytes.[18] This process of progressive differentiation occurs within the bone marrow. After the cells are matured, they enter the circulation.[19] Every day, over 2.5 billion red blood cells and platelets, and 50–100 billion granulocytes are produced in this way.[20]
As well as creating cells, bone marrow is also one of the major sites where defective or aged red blood cells are destroyed.[20]
Metabolic
[edit]- Mineral storage – bones act as reserves of minerals important for the body, most notably calcium and phosphorus.[21][22][23]
Determined by the species, age, and the type of bone, bone cells make up to 15 percent of the bone. Growth factor storage—mineralized bone matrix stores important growth factors such as insulin-like growth factors, transforming growth factor, bone morphogenetic proteins and others.[24]
- Fat storage – marrow adipose tissue (MAT) acts as a storage reserve of fatty acids.[25]
- Acid-base balance – bone buffers the blood against excessive pH changes by absorbing or releasing alkaline salts.[26]
- Detoxification – bone tissues can also store heavy metals and other foreign elements, removing them from the blood and reducing their effects on other tissues. These can later be gradually released for excretion.[27]
- Endocrine organ – bone controls phosphate metabolism by releasing fibroblast growth factor 23 (FGF-23), which acts on kidneys to reduce phosphate reabsorption. Bone cells also release a hormone called osteocalcin, which contributes to the regulation of blood sugar (glucose) and fat deposition. Osteocalcin increases both the insulin secretion and sensitivity, in addition to boosting the number of insulin-producing cells and reducing stores of fat.[28]
- Calcium balance – the process of bone resorption by the osteoclasts releases stored calcium into the systemic circulation and is an important process in regulating calcium balance. As bone formation actively fixes circulating calcium in its mineral form, removing it from the bloodstream, resorption actively unfixes it thereby increasing circulating calcium levels. These processes occur in tandem at site-specific locations.[29]

Tissue
[edit]Bone is not uniformly solid, but consists of a flexible matrix (about 30%) and bound minerals (about 70%), which are intricately woven and continuously remodeled by a group of specialized bone cells. Their unique composition and design allows bones to be relatively hard and strong, while remaining lightweight. Bone matrix is 90 to 95% composed of elastic collagen fibers, also known as ossein,[30] and the remainder is ground substance.[31] The elasticity of collagen improves fracture resistance.[12] The matrix is hardened by the binding of inorganic mineral salt, calcium phosphate, in a chemical arrangement known as bone mineral, a form of calcium apatite.[32][33] It is the mineralization that gives bones rigidity.
Within any single bone, the tissue is woven into two main patterns: cortical and cancellous bone, each with distinct appearances and characteristics. Bone is actively constructed and remodeled throughout life by specialized bone cells known as osteoblasts and osteoclasts.
Cortex
[edit]
The hard outer layer of bones is composed of cortical bone, which is also called compact bone as it is much denser than cancellous bone. It forms the hard exterior (cortex) of bones. The cortical bone gives bone its smooth, white, and solid appearance, and accounts for 80% of the total bone mass of an adult human skeleton.[34] It facilitates bone's main functions—to support the whole body, to protect organs, to provide levers for movement, and to store and release chemical elements, mainly calcium. It consists of multiple microscopic columns, each called an osteon or Haversian system. Each column is multiple layers of osteoblasts and osteocytes around a central canal called the osteonic canal. Volkmann's canals at right angles connect the osteons together. The columns are metabolically active, and as bone is reabsorbed and created the nature and location of the cells within the osteon will change. Cortical bone is covered by a periosteum on its outer surface, and an endosteum on its inner surface. The endosteum is the boundary between the cortical bone and the cancellous bone.[35] The primary anatomical and functional unit of cortical bone is the osteon.
Trabeculae
[edit]
Cancellous bone or spongy bone,[36][35] also known as trabecular bone, is the internal tissue of the skeletal bone and is an open cell porous network that follows the material properties of biofoams.[37][38] Cancellous bone has a higher surface-area-to-volume ratio than cortical bone and it is less dense. This makes it weaker and more flexible. The greater surface area also makes it suitable for metabolic activities such as the exchange of calcium ions. Cancellous bone is typically found at the ends of long bones, near joints, and in the interior of vertebrae. Cancellous bone is highly vascular and often contains red bone marrow where hematopoiesis, the production of blood cells, occurs. The primary anatomical and functional unit of cancellous bone is the trabecula. The trabeculae are aligned towards the mechanical load distribution that a bone experiences within long bones such as the femur. As far as short bones are concerned, trabecular alignment has been studied in the vertebral pedicle.[39] Thin formations of osteoblasts covered in endosteum create an irregular network of spaces,[40] known as trabeculae. Within these spaces are bone marrow and hematopoietic stem cells that give rise to platelets, red blood cells and white blood cells.[40] Trabecular marrow is composed of a network of rod- and plate-like elements that make the overall organ lighter and allow room for blood vessels and marrow. Trabecular bone accounts for the remaining 20% of total bone mass but has nearly ten times the surface area of compact bone.[41]
The words cancellous and trabecular refer to the tiny lattice-shaped units (trabeculae) that form the tissue. It was first illustrated accurately in the engravings of Crisóstomo Martinez.[42]
Marrow
[edit]Bone marrow, also known as myeloid tissue in red bone marrow, can be found in almost any bone that holds cancellous tissue. In newborns, all such bones are filled exclusively with red marrow or hematopoietic marrow, but as the child ages the hematopoietic fraction decreases in quantity and the fatty/ yellow fraction called marrow adipose tissue (MAT) increases in quantity. In adults, red marrow is mostly found in the bone marrow of the femur, the ribs, the vertebrae and pelvic bones.[43]
Vascular supply
[edit]Bone receives about 10% of cardiac output.[44] Blood enters the endosteum, flows through the marrow, and exits through small vessels in the cortex.[44] In humans, blood oxygen tension in bone marrow is about 6.6%, compared to about 12% in arterial blood, and 5% in venous and capillary blood.[44]
Histology and physiology
[edit]
Bone is metabolically active tissue composed of several types of cells. These cells include osteoblasts, which are involved in the creation and mineralization of bone tissue, osteocytes, and osteoclasts, which are involved in the reabsorption of bone tissue. Osteoblasts and osteocytes are derived from osteoprogenitor cells, but osteoclasts are derived from the same cells that differentiate to form macrophages and monocytes.[45] Within the marrow of the bone there are also hematopoietic stem cells. These cells give rise to other cells, including white blood cells, red blood cells, and platelets.[20]
Osteoblast
[edit]
Osteoblasts are mononucleate bone-forming cells. They are located on the surface of osteon seams and make a protein mixture known as osteoid, which mineralizes to become bone.[46] The osteoid seam is a narrow region of a newly formed organic matrix, not yet mineralized, located on the surface of a bone. Osteoid is primarily composed of Type I collagen. Osteoblasts also manufacture hormones, such as prostaglandins, to act on the bone itself. The osteoblast creates and repairs new bone by actually building around itself. First, the osteoblast puts up collagen fibers. These collagen fibers are used as a framework for the osteoblasts' work. The osteoblast then deposits calcium phosphate which is hardened by hydroxide and bicarbonate ions. The brand-new bone created by the osteoblast is called osteoid.[47] Once the osteoblast is finished working it is actually trapped inside the bone once it hardens. When the osteoblast becomes trapped, it becomes known as an osteocyte. Other osteoblasts remain on the top of the new bone and are used to protect the underlying bone, these become known as bone lining cells.[48]
Osteocyte
[edit]Osteocytes are cells of mesenchymal origin and originate from osteoblasts that have migrated into and become trapped and surrounded by a bone matrix that they themselves produced.[35] The spaces the cell body of osteocytes occupy within the mineralized collagen type I matrix are known as lacunae, while the osteocyte cell processes occupy channels called canaliculi. The many processes of osteocytes reach out to meet osteoblasts, osteoclasts, bone lining cells, and other osteocytes probably for the purposes of communication.[49] Osteocytes remain in contact with other osteocytes in the bone through gap junctions—coupled cell processes which pass through the canalicular channels.
Osteoclast
[edit]Osteoclasts are very large multinucleate cells that are responsible for the breakdown of bones by the process of bone resorption. New bone is then formed by the osteoblasts. Bone is constantly remodeled by the resorption of osteoclasts and created by osteoblasts.[45] Osteoclasts are large cells with multiple nuclei located on bone surfaces in what are called Howship's lacunae (or resorption pits). These lacunae are the result of surrounding bone tissue that has been reabsorbed.[50] Because the osteoclasts are derived from a monocyte stem-cell lineage, they are equipped with phagocytic-like mechanisms similar to circulating macrophages.[45] Osteoclasts mature and/or migrate to discrete bone surfaces. Upon arrival, active enzymes, such as tartrate-resistant acid phosphatase, are secreted against the mineral substrate.[citation needed] The reabsorption of bone by osteoclasts also plays a role in calcium homeostasis.[50]
Composition
[edit]Bones consist of living cells (osteoblasts and osteocytes) embedded in a mineralized organic matrix. The primary inorganic component of human bone is hydroxyapatite, the dominant bone mineral, having the nominal composition of Ca10(PO4)6(OH)2.[51] The organic components of this matrix consist mainly of type I collagen—"organic" referring to materials produced as a result of the human body—and inorganic components, which alongside the dominant hydroxyapatite phase, include other compounds of calcium and phosphate including salts. Approximately 30% of the acellular component of bone consists of organic matter, while roughly 70% by mass is attributed to the inorganic phase.[52] The collagen fibers give bone its tensile strength, and the interspersed crystals of hydroxyapatite give bone its compressive strength. These effects are synergistic.[52] The exact composition of the matrix may be subject to change over time due to nutrition and biomineralization, with the ratio of calcium to phosphate varying between 1.3 and 2.0 (per weight), and trace minerals such as magnesium, sodium, potassium and carbonate also be found.[52]
Type I collagen composes 90–95% of the organic matrix, with the remainder of the matrix being a homogenous liquid called ground substance consisting of proteoglycans such as hyaluronic acid and chondroitin sulfate,[52] as well as non-collagenous proteins such as osteocalcin, osteopontin or bone sialoprotein. Collagen consists of strands of repeating units, which give bone tensile strength, and are arranged in an overlapping fashion that prevents shear stress. The function of ground substance is not fully known.[52] Two types of bone can be identified microscopically according to the arrangement of collagen: woven and lamellar.
- Woven bone (also known as fibrous bone), which is characterized by a haphazard organization of collagen fibers and is mechanically weak.[53]
- Lamellar bone, which has a regular parallel alignment of collagen into sheets ("lamellae") and is mechanically strong.[38][53]
Woven bone is produced when osteoblasts produce osteoid rapidly, which occurs initially in all fetal bones, but is later replaced by more resilient lamellar bone. In adults, woven bone is created after fractures or in Paget's disease. Woven bone is weaker, with a smaller number of randomly oriented collagen fibers, but forms quickly; it is for this appearance of the fibrous matrix that the bone is termed woven. It is soon replaced by lamellar bone, which is highly organized in concentric sheets with a much lower proportion of osteocytes to surrounding tissue. Lamellar bone, which makes its first appearance in humans in the fetus during the third trimester,[54] is stronger and filled with many collagen fibers parallel to other fibers in the same layer (these parallel columns are called osteons). In cross-section, the fibers run in opposite directions in alternating layers, much like in plywood, assisting in the bone's ability to resist torsion forces. After a fracture, woven bone forms initially and is gradually replaced by lamellar bone during a process known as "bony substitution". Compared to woven bone, lamellar bone formation takes place more slowly. The orderly deposition of collagen fibers restricts the formation of osteoid to about 1 to 2 μm per day. Lamellar bone also requires a relatively flat surface to lay the collagen fibers in parallel or concentric layers.[55]
Deposition
[edit]The extracellular matrix of bone is laid down by osteoblasts, which secrete both collagen and ground substance. These cells synthesise collagen alpha polypeptide chains and then secrete collagen molecules. The collagen molecules associate with their neighbors and crosslink via lysyl oxidase to form collagen fibrils. At this stage, they are not yet mineralized, and this zone of unmineralized collagen fibrils is called "osteoid". Around and inside collagen fibrils calcium and phosphate eventually precipitate within days to weeks becoming then fully mineralized bone with an overall carbonate substituted hydroxyapatite inorganic phase.[56][52]
In order to mineralise the bone, the osteoblasts secrete alkaline phosphatase, some of which is carried by vesicles. This cleaves the inhibitory pyrophosphate and simultaneously generates free phosphate ions for mineralization, acting as the foci for calcium and phosphate deposition. Vesicles may initiate some of the early mineralization events by rupturing and acting as a centre for crystals to grow on. Bone mineral may be formed from globular and plate structures, and via initially amorphous phases.[57][58]
Development
[edit]

The formation of bone is called ossification. During the fetal stage of development this occurs by two processes: intramembranous ossification and endochondral ossification.[59] Intramembranous ossification involves the formation of bone from connective tissue whereas endochondral ossification involves the formation of bone from cartilage.
Intramembranous ossification mainly occurs during formation of the flat bones of the skull but also the mandible, maxilla, and clavicles; the bone is formed from connective tissue such as mesenchyme tissue rather than from cartilage. The process includes: the development of the ossification center, calcification, trabeculae formation and the development of the periosteum.[60]
Endochondral ossification occurs in long bones and most other bones in the body; it involves the development of bone from cartilage. This process includes the development of a cartilage model, its growth and development, development of the primary and secondary ossification centers, and the formation of articular cartilage and the epiphyseal plates.[61]
Endochondral ossification begins with points in the cartilage called "primary ossification centers". They mostly appear during fetal development, though a few short bones begin their primary ossification after birth. They are responsible for the formation of the diaphyses of long bones, short bones and certain parts of irregular bones. Secondary ossification occurs after birth and forms the epiphyses of long bones and the extremities of irregular and flat bones. The diaphysis and both epiphyses of a long bone are separated by a growing zone of cartilage (the epiphyseal plate). At skeletal maturity (18 to 25 years of age), all of the cartilage is replaced by bone, fusing the diaphysis and both epiphyses together (epiphyseal closure).[62] In the upper limbs, only the diaphyses of the long bones and scapula are ossified. The epiphyses, carpal bones, coracoid process, medial border of the scapula, and acromion are still cartilaginous.[63]
The following steps are followed in the conversion of cartilage to bone:
- Zone of reserve cartilage. This region, farthest from the marrow cavity, consists of typical hyaline cartilage that as yet shows no sign of transforming into bone.[64]
- Zone of cell proliferation. A little closer to the marrow cavity, chondrocytes multiply and arrange themselves into longitudinal columns of flattened lacunae.[64]
- Zone of cell hypertrophy. Next, the chondrocytes cease to divide and begin to hypertrophy (enlarge), much like they do in the primary ossification center of the fetus. The walls of the matrix between lacunae become very thin.[64]
- Zone of calcification. Minerals are deposited in the matrix between the columns of lacunae and calcify the cartilage. These are not the permanent mineral deposits of bone, but only a temporary support for the cartilage that would otherwise soon be weakened by the breakdown of the enlarged lacunae.[64]
- Zone of bone deposition. Within each column, the walls between the lacunae break down and the chondrocytes die. This converts each column into a longitudinal channel, which is immediately invaded by blood vessels and marrow from the marrow cavity. Osteoblasts line up along the walls of these channels and begin depositing concentric lamellae of matrix, while osteoclasts dissolve the temporarily calcified cartilage.[64]
Bone development in youth is extremely important in preventing future complications of the skeletal system. Regular exercise during childhood and adolescence can help improve bone architecture, making bones more resilient and less prone to fractures in adulthood. Physical activity, specifically resistance training, stimulates growth of bones by increasing both bone density and strength. Studies have shown a positive correlation between the adaptations of resistance training and bone density.[65] While nutritional and pharmacological approaches may also improve bone health, the strength and balance adaptations from resistance training are a substantial added benefit.[65] Weight-bearing exercise may assist in osteoblast (bone-forming cells) formation and help to increase bone mineral content. High-impact sports, which involve quick changes in direction, jumping, and running, are particularly effective with stimulating bone growth in the youth.[66] Sports such as soccer, basketball, and tennis have shown to have positive effects on bone mineral density as well as bone mineral content in teenagers.[66] Engaging in physical activity during childhood years, particularly in these high-impact osteogenic sports, can help to positively influence bone mineral density in adulthood.[67] Children and adolescents who participate in regular physical activity will place the groundwork for bone health later in life, reducing the risk of bone-related conditions such as osteoporosis.[67]
Remodeling
[edit]Bone is constantly being created and replaced in a process known as remodeling. This ongoing turnover of bone is a process of resorption followed by replacement of bone with little change in shape. This is accomplished through osteoblasts and osteoclasts. Cells are stimulated by a variety of signals, and together referred to as a remodeling unit. Approximately 10% of the skeletal mass of an adult is remodelled each year.[68] The purpose of remodeling is to regulate calcium homeostasis, repair microdamaged bones from everyday stress, and to shape the skeleton during growth.[69] Repeated stress, such as weight-bearing exercise or bone healing, results in the bone thickening at the points of maximum stress (Wolff's law). It has been hypothesized that this is a result of bone's piezoelectric properties, which cause bone to generate small electrical potentials under stress.[70]
The action of osteoblasts and osteoclasts are controlled by a number of chemical enzymes that either promote or inhibit the activity of the bone remodeling cells, controlling the rate at which bone is made, destroyed, or changed in shape. The cells also use paracrine signalling to control the activity of each other.[26][71] For example, the rate at which osteoclasts resorb bone is inhibited by calcitonin and osteoprotegerin. Calcitonin is produced by parafollicular cells in the thyroid gland, and can bind to receptors on osteoclasts to directly inhibit osteoclast activity. Osteoprotegerin is secreted by osteoblasts and is able to bind RANK-L, inhibiting osteoclast stimulation.[72]
Osteoblasts can also be stimulated to increase bone mass through increased secretion of osteoid and by inhibiting the ability of osteoclasts to break down osseous tissue.[citation needed] Increased secretion of osteoid is stimulated by the secretion of growth hormone by the pituitary, thyroid hormone and the sex hormones (estrogens and androgens). These hormones also promote increased secretion of osteoprotegerin.[72] Osteoblasts can also be induced to secrete a number of cytokines that promote reabsorption of bone by stimulating osteoclast activity and differentiation from progenitor cells. Vitamin D, parathyroid hormone and stimulation from osteocytes induce osteoblasts to increase secretion of RANK-ligand and interleukin 6, which cytokines then stimulate increased reabsorption of bone by osteoclasts. These same compounds also increase secretion of macrophage colony-stimulating factor by osteoblasts, which promotes the differentiation of progenitor cells into osteoclasts, and decrease secretion of osteoprotegerin.[citation needed]
Volume
[edit]Bone volume is determined by the rates of bone formation and bone resorption. Certain growth factors may work to locally alter bone formation by increasing osteoblast activity. Numerous bone-derived growth factors have been isolated and classified via bone cultures. These factors include insulin-like growth factors I and II, transforming growth factor-beta, fibroblast growth factor, platelet-derived growth factor, and bone morphogenetic proteins.[73] Evidence suggests that bone cells produce growth factors for extracellular storage in the bone matrix. The release of these growth factors from the bone matrix could cause the proliferation of osteoblast precursors. Essentially, bone growth factors may act as potential determinants of local bone formation.[73] Cancellous bone volume in postmenopausal osteoporosis may be determined by the relationship between the total bone forming surface and the percent of surface resorption.[74]
Clinical significance
[edit]A number of diseases can affect bone, including arthritis, fractures, infections, osteoporosis and tumors. Conditions relating to bone can be managed by a variety of doctors, including rheumatologists for joints, and orthopedic surgeons, who may conduct surgery to fix broken bones. Other doctors, such as rehabilitation specialists may be involved in recovery, radiologists in interpreting the findings on imaging, and pathologists in investigating the cause of the disease, and family doctors may play a role in preventing complications of bone disease such as osteoporosis.
When a doctor sees a patient, a history and exam will be taken. Bones are then often imaged, called radiography. This might include ultrasound X-ray, CT scan, MRI scan and other imaging such as a Bone scan, which may be used to investigate cancer.[75] Other tests such as a blood test for autoimmune markers may be taken, or a synovial fluid aspirate may be taken.[75]
Fractures
[edit]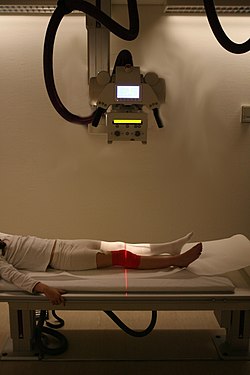
In normal bone, fractures occur when there is significant force applied or repetitive trauma over a long time. Fractures can also occur when a bone is weakened, such as with osteoporosis, or when there is a structural problem, such as when the bone remodels excessively (such as Paget's disease) or is the site of the growth of cancer.[76] Common fractures include wrist fractures and hip fractures, associated with osteoporosis, vertebral fractures associated with high-energy trauma and cancer, and fractures of long-bones. Not all fractures are painful.[76] When serious, depending on the fractures type and location, complications may include flail chest, compartment syndromes or fat embolism. Compound fractures involve the bone's penetration through the skin. Some complex fractures can be treated by the use of bone grafting procedures that replace missing bone portions.
Fractures and their underlying causes can be investigated by X-rays, CT scans and MRIs.[76] Fractures are described by their location and shape, and several classification systems exist, depending on the location of the fracture. A common long bone fracture in children is a Salter–Harris fracture.[77] When fractures are managed, pain relief is often given, and the fractured area is often immobilised. This is to promote bone healing. In addition, surgical measures such as internal fixation may be used. Because of the immobilisation, people with fractures are often advised to undergo rehabilitation.[76]
Tumors
[edit]Tumor that can affect bone in several ways. Examples of benign bone tumors include osteoma, osteoid osteoma, osteochondroma, osteoblastoma, enchondroma, giant-cell tumor of bone, and aneurysmal bone cyst.[78]
Cancer
[edit]Cancer can arise in bone tissue, and bones are also a common site for other cancers to spread (metastasise) to.[79] Cancers that arise in bone are called "primary" cancers, although such cancers are rare.[79] Metastases within bone are "secondary" cancers, with the most common being breast cancer, lung cancer, prostate cancer, thyroid cancer, and kidney cancer.[79] Secondary cancers that affect bone can either destroy bone (called a "lytic" cancer) or create bone (a "sclerotic" cancer). Cancers of the bone marrow inside the bone can also affect bone tissue, examples including leukemia and multiple myeloma. Bone may also be affected by cancers in other parts of the body. Cancers in other parts of the body may release parathyroid hormone or parathyroid hormone-related peptide. This increases bone reabsorption, and can lead to bone fractures.
Bone tissue that is destroyed or altered as a result of cancers is distorted, weakened, and more prone to fracture. This may lead to compression of the spinal cord, destruction of the marrow resulting in bruising, bleeding and immunosuppression, and is one cause of bone pain. If the cancer is metastatic, then there might be other symptoms depending on the site of the original cancer. Some bone cancers can also be felt.
Cancers of the bone are managed according to their type, their stage, prognosis, and what symptoms they cause. Many primary cancers of bone are treated with radiotherapy. Cancers of bone marrow may be treated with chemotherapy, and other forms of targeted therapy such as immunotherapy may be used.[80] Palliative care, which focuses on maximising a person's quality of life, may play a role in management, particularly if the likelihood of survival within five years is poor.
Diabetes
[edit]Type 1 diabetes is an autoimmune disease in which the body attacks the insulin-producing pancreas cells causing the body to not make enough insulin.[81] In contrast type 2 diabetes in which the body creates enough Insulin, but becomes resistant to it over time.[81]
Children makeup approximately 85% of Type 1 Diabetes cases and in America there was an average 22% rise in cases[82] over the first 24 months of the COVID-19 Pandemic. With the increase of developing some form of diabetes across all ranges continually growing the health impacts on bone development and bone health in these populations are still being researched. Most evidence suggests that diabetes, either Type 1 and Type 2, inhibits osteoblastic activity[83] and causes both lower BMD and BMC in both adults and children. The weakening of these developmental aspects is thought to lead to an increased risk of developing many diseases such as osteoarthritis, osteoporosis, osteopenia and fractures.[84] Development of any of these diseases is thought to be correlated with a decrease in ability to perform in athletic environments and activities of daily living.
Focusing on therapies that target molecules like osteocalcin or AGEs could provide new ways to improve bone health and help manage the complications of diabetes more effectively.[85]
Other painful conditions
[edit]- Osteomyelitis is inflammation of the bone or bone marrow due to bacterial infection.[86]
- Osteomalacia is a painful softening of adult bone caused by severe vitamin D deficiency.[87]
- Osteogenesis imperfecta[88]
- Osteochondritis dissecans[89]
- Ankylosing spondylitis[90]
- Skeletal fluorosis is a bone disease caused by an excessive accumulation of fluoride in the bones. In advanced cases, skeletal fluorosis damages bones and joints and is painful.[91]
Osteoporosis
[edit]
Osteoporosis is a disease of bone where there is reduced bone mineral density, increasing the likelihood of fractures.[92] Osteoporosis is defined in women by the World Health Organization as a bone mineral density of 2.5 standard deviations below peak bone mass, relative to the age and sex-matched average. This density is measured using dual energy X-ray absorptiometry (DEXA), with the term "established osteoporosis" including the presence of a fragility fracture.[93] Osteoporosis is most common in women after menopause, when it is called "postmenopausal osteoporosis", but may develop in men and premenopausal women in the presence of particular hormonal disorders and other chronic diseases or as a result of smoking and medications, specifically glucocorticoids.[92] Osteoporosis usually has no symptoms until a fracture occurs.[92] For this reason, DEXA scans are often done in people with one or more risk factors, who have developed osteoporosis and are at risk of fracture.[92]
One of the most important risk factors for osteoporosis is advanced age. Accumulation of oxidative DNA damage in osteoblastic and osteoclastic cells appears to be a key factor in age-related osteoporosis.[94]
Osteoporosis treatment includes advice to stop smoking, decrease alcohol consumption, exercise regularly, and have a healthy diet. Calcium and trace mineral supplements may also be advised, as may Vitamin D. When medication is used, it may include bisphosphonates, Strontium ranelate, and hormone replacement therapy.[95]
Bone health
[edit]Without strong healthy bones, humans are more at risk for different chronic diseases and fractures, with day-to-day function being more difficult with poor bone health. It is estimated that diet and exercise during childhood can impact peak bone mass as an adult nearly 20–40%.[96] One study done on children with developmental coordination disorder found an increase in bone mass up to 4% and 5% in the cortical areas of the tibia alone from a 13-week training period.[97] Peak bone mass occurs between the second and third decade of most people's lives.[98] Studies have shown that increasing calcium stores in childhood via food intake result in significant improvements in bone-mass density and overall health, even into adulthood.[99][100][101]
Osteology
[edit]
The study of bones and teeth is referred to as osteology. It is frequently used in anthropology, archeology and forensic science for a variety of tasks. This can include determining the nutritional, health, age or injury status of the individual the bones were taken from. Preparing fleshed bones for these types of studies can involve the process of maceration.[citation needed]
Typically anthropologists and archeologists study bone tools made by Homo sapiens and Homo neanderthalensis. Bones can serve a number of uses such as projectile points or artistic pigments, and can also be made from external bones such as antlers.[citation needed]
Other animals
[edit]

Bird skeletons are very lightweight. Their bones are smaller and thinner than those of mammals, to aid flight. Among mammals, bats come closest to birds in terms of bone density, suggesting that small dense bones are a flight adaptation. Many bird bones have little marrow due to them being hollow.[102] A bird's beak is primarily made of bone as projections of the mandibles which are covered in keratin.
Some bones, primarily formed separately in subcutaneous tissues, include headgears (such as bony core of horns, antlers, ossicones), osteoderm, and os penis/os clitoris.[103] A deer's antlers are composed of bone which is an unusual example of bone being outside the skin of the animal once the velvet is shed.[104]
The extinct predatory fish Dunkleosteus had sharp edges of hard exposed bone along its jaws.[105][106]
The proportion of cortical bone that is 80% in the human skeleton may be much lower in other animals, especially in marine mammals and marine turtles, or in various Mesozoic marine reptiles, such as ichthyosaurs,[107] among others.[108] This proportion can vary quickly in evolution; it often increases in early stages of returns to an aquatic lifestyle, as seen in early whales and pinnipeds, among others. It subsequently decreases in pelagic taxa, which typically acquire spongy bone, but aquatic taxa that live in shallow water can retain very thick, pachyostotic,[109] osteosclerotic, or pachyosteosclerotic[110] bones, especially if they move slowly, like sea cows. In some cases, even marine taxa that had acquired spongy bone can revert to thicker, compact bones if they become adapted to live in shallow water, or in hypersaline (denser) water.[111][112][113]
Many animals, particularly herbivores, practice osteophagy—the eating of bones. This is presumably carried out in order to replenish lacking phosphate.
Many bone diseases that affect humans also affect other vertebrates—an example of one disorder is skeletal fluorosis.
Society and culture
[edit]
Bones from slaughtered animals have a number of uses:
- In prehistoric times, they have been used for making bone tools.[114] They have further been used in bone carving, already important in prehistoric art, and also in modern time as crafting materials for buttons, beads, handles, bobbins, calculation aids, head nuts, dice, poker chips, pick-up sticks, arrows, scrimshaw, and ornaments.
- Bone glue can be made by prolonged boiling of ground or cracked bones, followed by filtering and evaporation to thicken the resulting fluid. Historically once important, bone glue and other animal glues today have only a few specialized uses, such as in antiques restoration. Essentially the same process, with further refinement, thickening and drying, is used to make gelatin.
- Broth is made by simmering several ingredients for a long time, traditionally including bones.
- Bone char, a porous, black, granular material primarily used for filtration and also as a black pigment, is produced by charring mammal bones.
- Oracle bone script was a writing system used in ancient China based on inscriptions in bones. Its name originates from oracle bones, which were mainly ox clavicle. The Ancient Chinese (mainly in the Shang dynasty), would write their questions on the oracle bone, and burn the bone, and where the bone cracked would be the answer for the questions.
- The wishbones of fowl have been used for divination, and are still customarily used in a tradition to determine which one of two people pulling on either prong of the bone may make a wish.
To point the bone at someone is considered bad luck in some cultures, such as Australian aborigines, such as by the Kurdaitcha.
Various cultures throughout history have adopted the custom of shaping an infant's head by the practice of artificial cranial deformation. A widely practised custom in China was that of foot binding to limit the normal growth of the foot.
Additional images
[edit]-
Cells in bone marrow
-
Scanning electron microscope of bone at 100× magnification
-
Structure detail of an animal bone
See also
[edit]References
[edit]- ^ Lee C (January 2001). The Bone Organ System: Form and Function. Academic Press. pp. 3–20. doi:10.1016/B978-012470862-4/50002-7. ISBN 978-0-12-470862-4. Retrieved 30 January 2022 – via Science Direct.
- ^ de Buffrénil V, de Ricqlès AJ, Zylberberg L, Padian K, Laurin M, Quilhac A (2021). Vertebrate skeletal histology and paleohistology (First ed.). Boca Raton, FL: CRC Press. pp. xii + 825. ISBN 978-1-351-18957-6.
- ^ Langley, Natalie, Tersigni-Terrant, Maria-Teresa, eds. (2017). Forensic Anthropology: A Comprehensive Approach (2nd ed.). CRC Press. p. 82. ISBN 978-1-315-30003-0.
- ^ Steele DG, Bramblett CA (1988). The Anatomy and Biology of the Human Skeleton. Texas A&M University Press. p. 4. ISBN 978-0-89096-300-5.
- ^ Mammal anatomy: an illustrated guide. New York: Marshall Cavendish. 2010. p. 129. ISBN 978-0-7614-7882-9.
- ^ "Types of bone". mananatomy.com. Retrieved 6 February 2016.
- ^ "DoITPoMS – TLP Library Structure of bone and implant materials – Structure and composition of bone". Dissemination of IT for the Promotion of Materials Science (DoITPoMS). Cambridge, UK: University of Cambridge.
- ^
 This article incorporates text available under the CC BY 4.0 license. Betts JG, Desaix P, Johnson E, Johnson JE, Korol O, Kruse D, et al. (8 June 2023). Anatomy & Physiology. Houston: OpenStax CNX. 6.2 Bone classification. ISBN 978-1-947172-04-3.
This article incorporates text available under the CC BY 4.0 license. Betts JG, Desaix P, Johnson E, Johnson JE, Korol O, Kruse D, et al. (8 June 2023). Anatomy & Physiology. Houston: OpenStax CNX. 6.2 Bone classification. ISBN 978-1-947172-04-3.
- ^ Clarke B (2008), "Normal Bone Anatomy and Physiology", Clinical Journal of the American Society of Nephrology, 3 (Suppl 3): S131 – S139, doi:10.2215/CJN.04151206, PMC 3152283, PMID 18988698
- ^ Jerez A, Mangione S, Abdala V (2010), "Occurrence and distribution of sesamoid bones in squamates: a comparative approach", Acta Zoologica, 91 (3): 295–305, doi:10.1111/j.1463-6395.2009.00408.x, hdl:11336/74304
- ^ Pratt R. "Bone as an Organ". AnatomyOne. Amirsys, Inc. Archived from the original on 30 October 2019. Retrieved 28 September 2012.
- ^ a b Schmidt-Nielsen K (1984). Scaling: Why Is Animal Size So Important?. Cambridge: Cambridge University Press. p. 6. ISBN 978-0-521-31987-4.
- ^ Vincent K. "Topic 3: Structure and Mechanical Properties of Bone". BENG 112A Biomechanics, Winter Quarter, 2013. Department of Bioengineering, University of California. Archived from the original on 28 May 2018. Retrieved 24 March 2015.
- ^ Turner CH, Wang T, Burr DB (December 2001). "Shear strength and fatigue properties of human cortical bone determined from pure shear tests". Calcified Tissue International. 69 (6): 373–378. doi:10.1007/s00223-001-1006-1. PMID 11800235. S2CID 30348345.
- ^ Fernández KS, de Alarcón PA (December 2013). "Development of the hematopoietic system and disorders of hematopoiesis that present during infancy and early childhood". Pediatric Clinics of North America. 60 (6): 1273–1289. doi:10.1016/j.pcl.2013.08.002. PMID 24237971.
- ^ Young 2006, pp. 60–61.
- ^ Young 2006, p. 60.
- ^ Young 2006, p. 57.
- ^ Young 2006, p. 46.
- ^ a b c Young 2006, p. 58.
- ^ Doyle ME, Jan de Beur SM (December 2008). "The skeleton: endocrine regulator of phosphate homeostasis". Current Osteoporosis Reports. 6 (4): 134–141. doi:10.1007/s11914-008-0024-6. PMID 19032923. S2CID 23298442.
- ^ "Bone Health In Depth". Linus Pauling Institute. 7 November 2016. Retrieved 13 September 2022.
- ^ Walker K. "Bone". Encyclopedia Britannica. Retrieved 5 October 2017.
- ^ Hauschka PV, Chen TL, Mavrakos AE (1988). "Polypeptide Growth Factors in Bone Matrix". Ciba Foundation Symposium 136 - Cell and Molecular Biology of Vertebrate Hard Tissues. Novartis Foundation Symposia. Vol. 136. pp. 207–225. doi:10.1002/9780470513637.ch13. ISBN 978-0-470-51363-7. PMID 3068010.
- ^ Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. (August 2017). "Exercise Decreases Marrow Adipose Tissue Through β-Oxidation in Obese Running Mice". Journal of Bone and Mineral Research. 32 (8): 1692–1702. doi:10.1002/jbmr.3159. PMC 5550355. PMID 28436105.
- ^ a b Fogelman I, Gnanasegaran G, van der Wall H (2013). Radionuclide and Hybrid Bone Imaging. Springer. ISBN 978-3-642-02400-9.
- ^ Lerro E (2007). "Bone". flipper.diff.org.
- ^ Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. (August 2007). "Endocrine regulation of energy metabolism by the skeleton". Cell. 130 (3): 456–469. doi:10.1016/j.cell.2007.05.047. PMC 2013746. PMID 17693256.
- ^ Wilkin D, Gray-Wilson N (8 February 2024). "Bones". CK-12 Foundation.
- ^ "Ossein". The Free Dictionary.
- ^ Hall J (2011). Textbook of Medical Physiology (12th ed.). Philadelphia: Elsevier. pp. 957–960. ISBN 978-08089-2400-5.
- ^ Wopenka B, Pasteris JD (2005). "A mineralogical perspective on the apatite in bone". Materials Science and Engineering: C. 25 (2): 131–143. doi:10.1016/j.msec.2005.01.008.
- ^ Wang B, Zhang Z, Pan H (February 2023). "Bone Apatite Nanocrystal: Crystalline Structure, Chemical Composition, and Architecture". Biomimetics. 8 (1): 90. doi:10.3390/biomimetics8010090. PMC 10046636. PMID 36975320.
- ^ "Structure of Bone". flexbooks.ck12.org. CK12-Foundation. Retrieved 28 May 2020.
- ^ a b c Young 2006, p. 192.
- ^ "Structure of Bone Tissue". SEER Training. Surveillance, Epidemiology, and End Results Program (SEER) U.S. National Cancer Institute. Retrieved 25 January 2023.
- ^ Meyers MA, Chen PY, Lin AY, Seki Y (January 2008). "Biological materials: Structure and mechanical properties". Progress in Materials Science. 53 (1): 1–206. doi:10.1016/j.pmatsci.2007.05.002. ISSN 0079-6425.
- ^ a b Buss DJ, Kröger R, McKee MD, Reznikov N (2022). "Hierarchical organization of bone in three dimensions: A twist of twists". Journal of Structural Biology. 6 100057. doi:10.1016/j.yjsbx.2021.100057. PMC 8762463. PMID 35072054.
- ^ Gdyczynski CM, Manbachi A, Hashemi S, Lashkari B, Cobbold RS (December 2014). "On estimating the directionality distribution in pedicle trabecular bone from micro-CT images". Physiological Measurement. 35 (12): 2415–2428. Bibcode:2014PhyM...35.2415G. doi:10.1088/0967-3334/35/12/2415. PMID 25391037. S2CID 206078730.
- ^ a b Young 2006, p. 195.
- ^ Hall SJ (2007). Basic Biomechanics with OLC (5th ed.). Burr Ridge: McGraw-Hill Higher Education. p. 88. ISBN 978-0-07-126041-1.
- ^ Gomez S (February 2002). "Crisóstomo Martinez, 1638-1694: the discoverer of trabecular bone". Endocrine. 17 (1): 3–4. doi:10.1385/ENDO:17:1:03. PMID 12014701. S2CID 46340228.
- ^ Barnes-Svarney PL, Svarney TE (2016). The Handy Anatomy Answer Book: Includes Physiology. Detroit: Visible Ink Press. pp. 90–91. ISBN 978-1-57859-542-6.
- ^ a b c Marenzana M, Arnett TR (September 2013). "The Key Role of the Blood Supply to Bone". Bone Research. 1 (3): 203–215. doi:10.4248/BR201303001. PMC 4472103. PMID 26273504.
- ^ a b c Young 2006, p. 189.
- ^ Young 2006, pp. 189–190.
- ^ "The O' Cells". Bone Cells. The University of Washington. 3 April 2013. Archived from the original on 7 August 2011.
- ^ Wein MN (28 April 2017). "Bone Lining Cells: Normal Physiology and Role in Response to Anabolic Osteoporosis Treatments". Current Molecular Biology Reports. 3 (2): 79–84. doi:10.1007/s40610-017-0062-x. S2CID 36473110.
- ^ Sims NA, Vrahnas C (November 2014). "Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts". Archives of Biochemistry and Biophysics. 561: 22–28. doi:10.1016/j.abb.2014.05.015. PMID 24875146.
- ^ a b Young 2006, p. 190.
- ^ Enhancement of Hydroxyapatite Dissolution Journal of Materials Science & Technology,38, 148-158
- ^ a b c d e f Hall 2005, p. 981.
- ^ a b Currey, John D. (2002). "The Structure of Bone Tissue" Archived 25 April 2017 at the Wayback Machine, pp. 12–14 in Bones: Structure and Mechanics. Princeton University Press. Princeton, NJ. ISBN 978-1-4008-4950-5
- ^ Salentijn, L. Biology of Mineralized Tissues: Cartilage and Bone, Columbia University College of Dental Medicine post-graduate dental lecture series, 2007
- ^ Royce PM, Steinmann B (14 April 2003). Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects. John Wiley & Sons. ISBN 978-0-471-46117-3.
- ^ Buss DJ, Reznikov N, McKee MD (November 2020). "Crossfibrillar mineral tessellation in normal and Hyp mouse bone as revealed by 3D FIB-SEM microscopy". Journal of Structural Biology. 212 (2) 107603. doi:10.1016/j.jsb.2020.107603. PMID 32805412. S2CID 221164596.
- ^ Bertazzo S, Bertran CA (2006). "Morphological and dimensional characteristics of bone mineral crystals". Key Engineering Materials. 309–311: 3–6. doi:10.4028/www.scientific.net/kem.309-311.3. S2CID 136883011.
- ^ Bertazzo S, Bertran C, Camilli J (2006). "Morphological Characterization of Femur and Parietal Bone Mineral of Rats at Different Ages". Key Engineering Materials. 309–311: 11–14. doi:10.4028/www.scientific.net/kem.309-311.11. S2CID 135813389.
- ^ Betts JG, Young KA, Wise JA, Johnson E, Poe B, Kruse DH, et al. (25 April 2013). "6.4 Bone Formation and Development". Anatomy and Physiology. OpenStax. Retrieved 26 February 2016.
- ^ "Bone Growth and Development". Biology for Majors II. lumenlearning.com. Retrieved 28 May 2020.
- ^ Tortora GJ, Derrickson BH (2018). Principles of Anatomy and Physiology. John Wiley & Sons. ISBN 978-1-119-44445-9.
- ^ "6.4B: Postnatal Bone Growth". Medicine LibreTexts. 19 July 2018. Retrieved 28 May 2020.
- ^ Agur A (2009). Grant's Atlas of Anatomy. Philadelphia: Lippincott Williams & Wilkins. p. 598. ISBN 978-0-7817-7055-2.
- ^ a b c d e Saladin K (2012). Anatomy and Physiology: The Unity of Form and Function. New York: McGraw-Hill. p. 217. ISBN 978-0-07-337825-1.
- ^ a b Layne JE, Nelson ME (January 1999). "The effects of progressive resistance training on bone density: a review". Medicine and Science in Sports and Exercise. 31 (1): 25–30. doi:10.1097/00005768-199901000-00006. PMID 9927006.
- ^ a b López-García R, Cruz-Castruita R, Morales-Corral P, Banda-Sauceda N, Lagunés-Carrasco J (16 December 2019). "Evaluación del mineral óseo con la dexa en futbolistas juveniles". Revista Internacional de Medicina y Ciencias de la Actividad Física y del Deporte. 19 (76): 617–626. doi:10.15366/rimcafd2019.76.004 (inactive 4 September 2025). hdl:10486/689625. ISSN 1577-0354.
{{cite journal}}: CS1 maint: DOI inactive as of September 2025 (link) - ^ a b Van Langendonck L, Lefevre J, Claessens AL, Thomis M, Philippaerts R, Delvaux K, et al. (September 2003). "Influence of participation in high-impact sports during adolescence and adulthood on bone mineral density in middle-aged men: a 27-year follow-up study". American Journal of Epidemiology. 158 (6): 525–533. doi:10.1093/aje/kwg170. PMID 12965878.
- ^ Manolagas SC (April 2000). "Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis". Endocrine Reviews. 21 (2): 115–137. doi:10.1210/edrv.21.2.0395. PMID 10782361.
- ^ Hadjidakis DJ, Androulakis II (December 2006). "Bone remodeling". Annals of the New York Academy of Sciences. 1092 (1): 385–396. Bibcode:2006NYASA1092..385H. doi:10.1196/annals.1365.035. PMID 17308163. S2CID 39878618.
- ^ Woodburne RT, ed. (1999). Anatomy, physiology, and metabolic disorders (5th ed.). Summit, N.J.: Novartis Pharmaceutical Corp. pp. 187–189. ISBN 978-0-914168-88-1.
- ^ "Introduction to cell signaling (article)". Khan Academy. Retrieved 24 December 2020.
- ^ a b Boulpaep EL, Boron WF (2005). Medical physiology: a cellular and molecular approach. Philadelphia: Saunders. pp. 1089–1091. ISBN 978-1-4160-2328-9.
- ^ a b Mohan S, Baylink DJ (February 1991). "Bone growth factors". Clinical Orthopaedics and Related Research. 263 (263): 30–48. doi:10.1097/00003086-199102000-00004. PMID 1993386.
- ^ Nordin BE, Aaron J, Speed R, Crilly RG (August 1981). "Bone formation and resorption as the determinants of trabecular bone volume in postmenopausal osteoporosis". Lancet. 2 (8241): 277–279. doi:10.1016/S0140-6736(81)90526-2. PMID 6114324. S2CID 29646037.
- ^ a b Davidson 2010, pp. 1059–1062.
- ^ a b c d Davidson 2010, p. 1068.
- ^ Salter RB, Harris WR (1963). "Injuries Involving the Epiphyseal Plate". J Bone Joint Surg Am. 45 (3): 587–622. doi:10.2106/00004623-196345030-00019. S2CID 73292249. Archived from the original on 2 December 2016. Retrieved 2 December 2016.
- ^ "Benign Bone Tumours". Cleveland Clinic. 2017. Retrieved 29 March 2017.
- ^ a b c Davidson 2010, p. 1125.
- ^ Davidson 2010, p. 1032.
- ^ a b Ndisang JF, Vannacci A, Rastogi S (2017). "Insulin Resistance, Type 1 and Type 2 Diabetes, and Related Complications 2017". Journal of Diabetes Research. 2017 1478294. doi:10.1155/2017/1478294. PMC 5723935. PMID 29279853.
- ^ Kamrath C, Holl RW, Rosenbauer J (June 2023). "Elucidating the Underlying Mechanisms of the Marked Increase in Childhood Type 1 Diabetes During the COVID-19 Pandemic-The Diabetes Pandemic". JAMA Network Open. 6 (6): e2321231. doi:10.1001/jamanetworkopen.2023.21231. PMID 37389881.
- ^ Loxton P, Narayan K, Munns CF, Craig ME (August 2021). "Bone Mineral Density and Type 1 Diabetes in Children and Adolescents: A Meta-analysis". Diabetes Care. 44 (8): 1898–1905. doi:10.2337/dc20-3128. PMC 8385468. PMID 34285100.
- ^ de Araújo IM, Moreira ML, de Paula FJ (November 2022). "Diabetes and bone". Archives of Endocrinology and Metabolism. 66 (5): 633–641. doi:10.20945/2359-3997000000552. PMC 10118819. PMID 36382752.
- ^ Booth SL, Centi A, Smith SR, Gundberg C (January 2013). "The role of osteocalcin in human glucose metabolism: marker or mediator?". Nature Reviews. Endocrinology. 9 (1): 43–55. doi:10.1038/nrendo.2012.201. PMC 4441272. PMID 23147574.
- ^ "Osteomyelitis". The Lecturio Medical Concept Library. Retrieved 26 August 2021.
- ^ "Osteomalacia and Rickets". The Lecturio Medical Concept Library. Retrieved 26 August 2021.
- ^ "Osteogenesis Imperfecta". The Lecturio Medical Concept Library. Retrieved 26 August 2021.
- ^ "Osteochondritis Dissecans". The Lecturio Medical Concept Library. Retrieved 26 August 2021.
- ^ "Ankylosing Spondylitis". The Lecturio Medical Concept Library. Retrieved 26 August 2021.
- ^ Whitford GM (June 1994). "Intake and metabolism of fluoride". Advances in Dental Research. 8 (1): 5–14. doi:10.1177/08959374940080011001. PMID 7993560. S2CID 21763028.
- ^ a b c d Davidson 2010, pp. 1116–1121.
- ^ WHO (1994). "Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group". World Health Organization Technical Report Series. 843: 1–129. PMID 7941614.
- ^ Chen Q, Liu K, Robinson AR, et al. DNA damage drives accelerated bone aging via an NF-κB-dependent mechanism. J Bone Miner Res. 2013;28(5):1214-1228. doi:10.1002/jbmr.1851
- ^ Davidson 2010, pp. 1116–1121
- ^ Faienza MF, Urbano F, Chiarito M, Lassandro G, Giordano P (2023). "Musculoskeletal health in children and adolescents". Frontiers in Pediatrics. 11 1226524. doi:10.3389/fped.2023.1226524. PMC 10754974. PMID 38161439.
- ^ Tan JL, Siafarikas A, Rantalainen T, Hart NH, McIntyre F, Hands B, et al. (December 2020). "Impact of a multimodal exercise program on tibial bone health in adolescents with Development Coordination Disorder: an examination of feasibility and potential efficacy". Journal of Musculoskeletal & Neuronal Interactions. 20 (4): 445–471. PMC 7716678. PMID 33265073.
- ^ Baronio F, Baptista F (2022). "Editorial: Bone health and development in children and adolescents". Frontiers in Endocrinology. 13 1101403. doi:10.3389/fendo.2022.1101403. PMC 9791941. PMID 36578952.
- ^ Pan K, Zhang C, Yao X, Zhu Z (January 2020). "Association between dietary calcium intake and BMD in children and adolescents". Endocrine Connections. 9 (3): 194–200. doi:10.1530/EC-19-0534. PMC 7040863. PMID 31990673.
- ^ Closa-Monasterolo R, Zaragoza-Jordana M, Ferré N, Luque V, Grote V, Koletzko B, et al. (June 2018). "Adequate calcium intake during long periods improves bone mineral density in healthy children. Data from the Childhood Obesity Project". Clinical Nutrition. 37 (3): 890–896. doi:10.1016/j.clnu.2017.03.011. PMID 28351509.
- ^ Gordon RJ, Misra M, Mitchell DM (2000). "Osteoporosis and Bone Fragility in Children". In Feingold KR, Anawalt B, Blackman MR, Boyce A (eds.). Endotext. South Dartmouth (MA): MDText.com, Inc. PMID 37490575. Retrieved 15 November 2024.
- ^ Dumont ER (July 2010). "Bone density and the lightweight skeletons of birds". Proceedings. Biological Sciences. 277 (1691): 2193–2198. doi:10.1098/rspb.2010.0117. PMC 2880151. PMID 20236981.
- ^ Nasoori A (August 2020). "Formation, structure, and function of extra-skeletal bones in mammals". Biological Reviews of the Cambridge Philosophical Society. 95 (4): 986–1019. doi:10.1111/brv.12597. PMID 32338826. S2CID 216556342.
- ^ Rolf HJ, Enderle A (May 1999). "Hard fallow deer antler: a living bone till antler casting?". The Anatomical Record. 255 (1): 69–77. doi:10.1002/(SICI)1097-0185(19990501)255:1<69::AID-AR8>3.0.CO;2-R. PMID 10321994.
- ^ "Dunkleosteus". American Museum of Natural History.
- ^ "My, What a Big Mouth You Have | Cleveland Museum of Natural History".
- ^ de Buffrénil V, Mazin JM (1990). "Bone histology of the ichthyosaurs: comparative data and functional interpretation". Paleobiology. 16 (4): 435–447. Bibcode:1990Pbio...16..435D. doi:10.1017/S0094837300010174. JSTOR 2400968. S2CID 88171648.
- ^ Laurin M, Canoville A, Germain D (2011). "Bone microanatomy and lifestyle: a descriptive approach". Comptes Rendus Palevol. 10 (5–6): 381–402. doi:10.1016/j.crpv.2011.02.003.
- ^ Houssaye A, De Buffrenil V, Rage JC, Bardet N (12 September 2008). "An analysis of vertebral 'pachyostosis' in Carentonosaurus mineaui (Mosasauroidea, Squamata) from the Cenomanian (early Late Cretaceous) of France, with comments on its phylogenetic and functional significance". Journal of Vertebrate Paleontology. 28 (3): 685–691. doi:10.1671/0272-4634(2008)28[685:AAOVPI]2.0.CO;2. ISSN 0272-4634. S2CID 129670238.
- ^ de Buffrénil V, Canoville A, D'Anastasio R, Domning DP (June 2010). "Evolution of Sirenian Pachyosteosclerosis, a Model-case for the Study of Bone Structure in Aquatic Tetrapods". Journal of Mammalian Evolution. 17 (2): 101–120. doi:10.1007/s10914-010-9130-1. S2CID 39169019.
- ^ Dewaele L, Lambert O, Laurin M, De Kock T, Louwye S, de Buffrénil V (December 2019). "Generalized Osteosclerotic Condition in the Skeleton of Nanophoca vitulinoides, a Dwarf Seal from the Miocene of Belgium" (PDF). Journal of Mammalian Evolution. 26 (4): 517–543. doi:10.1007/s10914-018-9438-9. S2CID 20885865.
- ^ Dewaele L, Gol'din P, Marx FG, Lambert O, Laurin M, Obadă T, et al. (January 2022). "Hypersalinity drives convergent bone mass increases in Miocene marine mammals from the Paratethys". Current Biology. 32 (1): 248–255.e2. Bibcode:2022CBio...32E.248D. doi:10.1016/j.cub.2021.10.065. PMID 34813730. S2CID 244485732.
- ^ Houssaye A (January 2022). "Evolution: Back to heavy bones in salty seas" (PDF). Current Biology. 32 (1): R42 – R44. Bibcode:2022CBio...32..R42H. doi:10.1016/j.cub.2021.11.049. PMID 35015995. S2CID 245879886. Archived (PDF) from the original on 23 November 2022.
- ^ Laszlovszky J, Szabo P (January 2003). People and Nature in Historical Perspective. Central European University Press. ISBN 978-963-9241-86-2.
Sources
[edit]- Davidson S (2010). Colledge NR, Walker BR, Ralston SH (eds.). Davidson's Principles and Practice of Medicine. Illustrated by Robert Britton (21st ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 978-0-7020-3085-7.
- Hall AC, Guyton JE (2005). Textbook of Medical Physiology (11th ed.). Philadelphia: W.B. Saunders. ISBN 978-0-7216-0240-0.
- Young B, Lowe JS, Stevens A, Heath JW (2006). Wheater's Functional Histology: a text and colour atlas (5th ed.). London: Churchill Livingstone/Elsevier. ISBN 978-0-443-068-508.
Further reading
[edit]- Derrickson BH, Tortora GJ (2005). Principles of anatomy and physiology. New York: Wiley. ISBN 978-0-471-68934-8.
- Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al. (2008). Harrison's principles of internal medicine (17th ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-147692-8.
- Hoehn K, Marieb EN (2007). Human Anatomy & Physiology (7th ed.). San Francisco: Benjamin Cummings. ISBN 978-0-8053-5909-1.
- Kini U, Nandeesh BN (3 January 2013). "Ch 2: Physiology of Bone Formation, Remodeling, and Metabolism" (PDF). In Fogelman I, Gnanasegaran G, van der Wall H (eds.). Radionuclide and hybrid bone imaging. Berlin: Springer. pp. 29–57. ISBN 978-3-642-02399-6. Archived from the original (PDF) on 6 November 2020. Retrieved 28 August 2017.
External links
[edit]Structure and Composition
Macroscopic Structure
Bone tissue at the macroscopic level is organized into distinct layers and compartments that provide structural integrity, support metabolic functions, and facilitate nutrient exchange. Compact bone, also known as cortical bone, forms the dense outer layer of most bones and constitutes approximately 80% of the total skeletal mass in adults.[3] This layer offers primary mechanical strength and protection against external forces, appearing solid and smooth to the naked eye.[4] In contrast, cancellous bone, or trabecular bone, comprises the porous inner network within bones, characterized by a lattice of interconnected struts and plates that create a spongy architecture.[5] This structure is optimized for metabolic exchange due to its high surface area-to-volume ratio, while remaining lightweight to reduce overall skeletal weight.[4] The interior of bones contains marrow cavities that house different types of bone marrow depending on location and age. Red marrow, which is actively involved in hematopoiesis, is primarily found in the cavities of flat bones such as the pelvis and sternum, as well as in the epiphyses of long bones.[6] Yellow marrow, consisting mainly of adipose tissue for fat storage, occupies the medullary cavities of the diaphyses in long bones, particularly in adults where it replaces much of the red marrow over time.[7] These cavities are lined by a thin membrane that separates the marrow from the surrounding bone tissue. Vascular supply to bone is essential for its nourishment and maintenance, entering through specific anatomical openings. Nutrient arteries, the primary blood supply for the inner bone, penetrate the cortical layer via nutrient foramina—small openings typically located on the diaphysis of long bones—and branch into the medullary cavity to irrigate cancellous bone and marrow.[1] Periosteal vessels, arising from the outer surface, provide additional blood flow to the compact bone layer through a network of capillaries and anastomoses.[8] Venous drainage parallels this arterial system, exiting via similar foramina to return deoxygenated blood to the systemic circulation.[9] The outer and inner surfaces of bone are covered by specialized connective tissue layers that contribute to its overall organization. The periosteum is a dense, fibrous membrane enveloping the external surface of bones, excluding areas of articulation, and serves as the site for attachment of tendons, ligaments, and muscles while housing blood vessels and nerves.[8] Internally, the endosteum lines the marrow cavities, trabecular surfaces, and vascular canals within compact bone, forming a thin layer that interfaces with the bone matrix.[10]Microscopic Structure
Bone tissue exhibits distinct microscopic architectures that vary between cortical and trabecular regions, enabling specialized mechanical and metabolic functions. Cortical bone, also known as compact bone, consists of densely packed cylindrical units called osteons or Haversian systems, each featuring a central Haversian canal that houses blood vessels and nerves running parallel to the bone's long axis.[5] These canals are surrounded by concentric lamellae, which are layered sheets of mineralized matrix approximately 3-7 micrometers thick, providing structural integrity through organized deposition.[11] Between osteons, interstitial lamellae fill the spaces, formed from remnants of older osteons and contributing to the overall solidity of the tissue.[5] In contrast, trabecular bone, or spongy bone, displays a porous, lattice-like structure composed of interconnected trabeculae that form rods and plates, creating an open network of irregular cavities filled with bone marrow.[12] Unlike cortical bone, trabecular bone lacks organized osteons; instead, its trabeculae align along principal stress lines to optimize strength while minimizing mass, with a porosity often exceeding 50-90%.[5] Nutrient diffusion occurs through canaliculi connecting to adjacent marrow spaces and vascular elements, supporting the metabolic demands of this highly vascularized tissue.[12] The bone matrix, which constitutes about 90% of bone tissue by volume, is organized into lamellae where type I collagen fibers are arranged in parallel bundles, conferring tensile strength and flexibility to withstand mechanical loads.[13] Hydroxyapatite mineral crystals, primarily plate-like and 20-100 nanometers in length, align along the collagen fibrils within these lamellae, enhancing compressive resistance through a composite structure that mimics fiber-reinforced materials.[14] The ground substance interspersed among these fibers includes proteoglycans and glycoproteins, which bind water to maintain hydration, facilitate nutrient diffusion, and regulate matrix assembly.[15] Bone types differ microscopically in maturity and organization: woven bone, an immature form, features randomly oriented collagen fibers in a disorganized, basket-weave pattern, resulting in lower mechanical strength and higher remodeling rates.[16] Lamellar bone, the mature variant predominant in adults, exhibits highly ordered, layered collagen arrangements in concentric or parallel patterns, providing superior durability and resistance to fracture.[17] Osteocytes reside within lacunae embedded in the matrix, connected via canaliculi to vascular canals for nutrient exchange.[5]Cellular Components
Bone tissue is maintained by a dynamic population of specialized cells that orchestrate its formation, resorption, and homeostasis. The primary cellular components include osteoblasts, osteocytes, osteoclasts, bone-lining cells, and progenitor stem cells, each derived from distinct lineages and contributing uniquely to bone integrity. These cells interact within the bone microenvironment to ensure structural support and metabolic balance.[18] Osteoblasts are the bone-forming cells responsible for synthesizing and mineralizing the organic bone matrix. Derived from mesenchymal stem cells, they originate from osteoprogenitor precursors and differentiate under the influence of factors such as bone morphogenetic proteins (BMPs), RUNX2, and Osterix.[18][19] These mononuclear cells feature prominent Golgi apparatus and rough endoplasmic reticulum, enabling robust protein synthesis. Osteoblasts secrete osteoid, an unmineralized matrix primarily composed of type I collagen (about 90%) along with non-collagenous proteins, which they subsequently mineralize by depositing hydroxyapatite crystals via matrix vesicles containing alkaline phosphatase.[18][19] Upon completion of matrix deposition, osteoblasts may undergo apoptosis, flatten into bone-lining cells, or become embedded in the matrix as osteocytes.[18] Osteocytes represent the most abundant cell type in mature bone, comprising over 90-95% of all bone cells, and serve as mature, terminally differentiated osteoblasts entrapped within the mineralized matrix. They reside in lacunae and extend dendritic processes through a network of canaliculi, forming gap junctions that facilitate intercellular communication, nutrient diffusion, and mechanotransduction.[19][20] Osteocytes act as mechanosensors, detecting mechanical loading and regulating mineral homeostasis by secreting factors like fibroblast growth factor 23 (FGF-23) and sclerostin to modulate bone remodeling and phosphate levels.[18] With a lifespan of up to 25 years in humans, osteocytes are among the longest-lived cells in the body, enabling sustained oversight of bone tissue integrity.[21][20] Osteoclasts are multinucleated giant cells specialized for bone resorption, essential for calcium mobilization and skeletal remodeling. They derive from the monocyte-macrophage lineage of hematopoietic stem cells, where precursor monocytes fuse to form these large cells containing 5-20 nuclei.[18][19] During resorption, osteoclasts attach to the bone surface via a sealing zone, forming a ruffled border that creates an isolated resorption compartment. They acidify this space using vacuolar H+-ATPase proton pumps to dissolve hydroxyapatite and secrete lysosomal enzymes, notably cathepsin K, to degrade the organic matrix.[18][19] Degraded products are transcytosed across the cell and released at the functional secretory domain, preventing intracellular accumulation.[18] Bone-lining cells are flattened, quiescent osteoblasts that cover inactive bone surfaces, comprising a thin layer that modulates ion exchange between bone and extracellular fluid without active matrix production. Derived from osteoblasts that have ceased formation activity, they prevent direct contact between osteoclasts and the mineralized matrix during periods of low remodeling.[18][19] These cells express receptors for hormones and growth factors, enabling rapid activation into osteoblasts when bone formation is required.[19] Mesenchymal stem cells (MSCs) serve as multipotent progenitors for the osteoblast lineage within the bone marrow stroma and other connective tissues. These self-renewing cells differentiate into osteoblasts, adipocytes, chondrocytes, and other mesenchymal derivatives under specific signaling cues like BMPs and Wnt pathways.[18][19] In bone, MSCs commit to the osteoblastic pathway via osteoprogenitors, providing a renewable source for ongoing tissue maintenance and repair.[18]Chemical Composition
Bone tissue comprises an organic matrix, an inorganic mineral phase, and water, which together determine its structural integrity and biomechanical performance. By dry weight, the organic components constitute approximately 30-35% of bone mass, providing flexibility and resilience, while the inorganic phase accounts for 65-70%, imparting rigidity and hardness.[22][23] Water makes up 10-20% of the total bone volume, facilitating molecular diffusion, nutrient transport, and the plastic deformation necessary for absorbing mechanical stress without fracture.[23] The organic matrix is dominated by type I collagen, which forms about 90% of the total protein content and assembles into fibrils that confer elasticity and tensile strength to bone. These collagen fibrils serve as a scaffold, with their periodic banding pattern directing the oriented deposition of mineral crystals. Non-collagenous proteins, comprising the remaining 10% of the matrix proteins, include osteocalcin and bone sialoprotein, which regulate mineralization by binding calcium ions and initiating crystal nucleation at specific sites along collagen fibers. Glycosaminoglycans, such as chondroitin sulfate, are minor constituents that enhance matrix hydration, modulate collagen fibril assembly, and contribute to bone toughness by influencing mineral distribution and preventing excessive brittleness.[22][24][25] The inorganic phase primarily consists of hydroxyapatite crystals, with the chemical formula , which embed within the organic matrix to provide compressive strength and overall stiffness. These nanoscale platelets, approximately 50-100 nm long and 20-50 nm wide, align parallel to collagen fibrils, optimizing load transfer. Trace elements, including magnesium and fluoride, substitute for calcium in the hydroxyapatite lattice or adsorb onto crystal surfaces, thereby influencing crystal size, solubility, and growth kinetics; for instance, magnesium inhibits excessive crystal perfection to maintain some solubility for remodeling, while fluoride promotes denser crystal formation but can reduce mechanical toughness at high levels.[26][27] The interplay of these components yields distinct biomechanical properties, such as a Young's modulus of 10-20 GPa for cortical bone, reflecting its stiffness under elastic deformation. Bone exhibits anisotropic behavior, with compressive strength (up to 170 MPa) exceeding tensile strength (about 120 MPa) due to the composite architecture, where mineral reinforces collagen against buckling while the organic phase resists crack propagation.[28] Mineral deposition in bone follows a tightly regulated process beginning with nucleation on collagen fibrils, particularly at hole zones within the fibril structure, where non-collagenous proteins like bone sialoprotein concentrate ions to form initial amorphous calcium phosphate clusters. This is followed by epitaxial growth, where crystals expand along the collagen axis, transforming into mature hydroxyapatite plates. Alkaline phosphatase, an enzyme secreted by osteoblasts, plays a crucial role by hydrolyzing inorganic pyrophosphate—a potent mineralization inhibitor—thereby elevating local phosphate levels to drive crystal formation and prevent pathological calcification elsewhere.[29][30]Development and Growth
Embryonic Development
The embryonic skeleton originates from distinct mesodermal populations that establish the basic body plan. The axial skeleton, including vertebrae and ribs, derives from the paraxial mesoderm, which segments into somites during early gastrulation around the third week of development.[31] In contrast, the appendicular skeleton, comprising the limbs and girdles, arises from the lateral plate mesoderm, which migrates to form limb buds.[32] Hox genes, a family of homeobox transcription factors, play a crucial role in regulating segment identity along the anterior-posterior axis, ensuring proper patterning of both axial and appendicular elements by specifying regional identities in these mesodermal derivatives.[33] Following patterning, mesenchymal precursor cells from these mesodermal sources undergo condensation, aggregating into dense clusters that serve as templates for future skeletal elements. This process is orchestrated by signaling molecules such as fibroblast growth factors (FGFs), which promote cell proliferation and migration to initiate aggregation, and bone morphogenetic proteins (BMPs), which induce differentiation within the condensates.[34][35] These condensations typically occur between weeks 5 and 7, forming the foundational anlagen for most bones. Early differentiation of these mesenchymal condensates primarily follows the path of chondrogenesis, where cells commit to the chondrocyte lineage under the control of the transcription factor Sox9, which is essential for activating cartilage-specific genes like those for type II collagen and aggrecan.[36] This results in the formation of hyaline cartilage templates, or models, that outline the prospective long bones, vertebrae, and other endochondral elements. Exceptions include the cranial vault bones, such as the parietal and frontal, which differentiate directly into bone via intramembranous pathways without a cartilaginous intermediate, relying instead on neural crest-derived mesenchyme.[37] In the developing limbs, patterning of the appendicular skeleton involves interactions between the apical ectodermal ridge (AER), a thickened epithelium at the limb bud distal margin that drives proximodistal outgrowth through FGF signaling, and the zone of polarizing activity (ZPA) in the posterior mesenchyme, which establishes anteroposterior polarity via Sonic hedgehog (Shh) gradients.[38][39] Skeletal anlagen become visible by the end of week 5 in human embryos, with limb bud condensations and early vertebral precursors emerging around Carnegie stage 18. By birth, the fetal skeleton consists of approximately 275 distinct cartilaginous and membranous precursors, many of which fuse postnatally to form the 206 bones of the adult skeleton.[40] This embryonic phase sets the stage for subsequent ossification, where cartilage templates begin to mineralize.Ossification Processes
Ossification is the process by which bone tissue is formed, primarily through two distinct mechanisms: intramembranous ossification and endochondral ossification. These processes transform mesenchymal precursors derived from embryonic development into mature bone structures, enabling the skeletal system's mechanical support and growth. Intramembranous ossification occurs directly within mesenchymal condensations without a cartilaginous intermediate, while endochondral ossification involves the replacement of a hyaline cartilage model by bone tissue.[41] Intramembranous ossification is the direct differentiation of mesenchymal cells into osteoblasts, forming flat bones such as those of the skull, clavicle, and mandible. Mesenchymal progenitor cells cluster into ossification centers, where they proliferate and differentiate into osteoblasts that secrete osteoid, which subsequently mineralizes into woven bone. This process begins around the sixth to seventh week of embryonic development and progresses radially from the ossification centers, with osteoblasts organizing into trabeculae that mature into compact and spongy bone layers. Vascular invasion supports the recruitment of additional osteoprogenitor cells and osteoclasts, facilitating bone remodeling within these sites.[41][42][43] Endochondral ossification, the predominant mechanism for forming long bones like the femur and humerus, replaces a preformed cartilage model with bone through a multistep process. It initiates in the embryonic cartilage anlage, where chondrocytes in the diaphysis hypertrophy, promoting matrix calcification and attracting vascular invasion from the periosteum. This vascular ingrowth delivers osteoprogenitor cells and osteoclasts, forming the primary ossification center in the diaphysis, where calcified cartilage is resorbed and replaced by woven bone deposited by osteoblasts. Secondary ossification centers emerge in the epiphyses after birth, following a similar sequence but leaving the epiphyseal growth plate intact for longitudinal growth.[44][41][45] The epiphyseal growth plate, or metaphysis, orchestrates longitudinal bone elongation through distinct zones of chondrocyte activity: resting, proliferative, hypertrophic, and calcifying. In the proliferative zone, chondrocytes divide and elongate the cartilage template; in the hypertrophic zone, they swell and secrete factors that induce matrix mineralization. A critical negative feedback loop involving Indian hedgehog (Ihh) from prehypertrophic and hypertrophic chondrocytes and parathyroid hormone-related protein (PTHrP) from periarticular cells regulates this progression, maintaining a balance between proliferation and differentiation to sustain controlled growth. Ihh stimulates PTHrP expression, which in turn inhibits hypertrophic differentiation, ensuring a steady pool of proliferative chondrocytes.[46][47][48] Angiogenesis plays an indispensable role in both ossification types by supplying oxygen, nutrients, and cells essential for bone formation. In endochondral ossification, hypertrophic chondrocytes express vascular endothelial growth factor (VEGF), which recruits blood vessels into the calcified matrix, enabling the invasion of osteoprogenitor cells from the perichondrium and the activity of osteoclasts for cartilage resorption. Similarly, in intramembranous ossification, vascularization within mesenchymal condensations supports osteoblast differentiation and matrix deposition. Without adequate vascular support, ossification stalls, as seen in conditions disrupting VEGF signaling.[49][50][51] Defects in endochondral ossification, such as achondroplasia—the most common form of dwarfism—arise from gain-of-function mutations in the fibroblast growth factor receptor 3 (FGFR3) gene, which hyperactivate inhibitory signaling in chondrocytes. These mutations, most frequently G380R, disrupt the growth plate by accelerating hypertrophic differentiation and reducing proliferative zone expansion, leading to shortened long bones while sparing intramembranous bones like the skull. This highlights FGFR3's role as a negative regulator of endochondral growth, with impaired Ihh-PTHrP feedback contributing to the phenotype.[52][53][54]Postnatal Growth and Maturation
Postnatal bone growth involves two primary mechanisms: longitudinal elongation and appositional expansion, which together contribute to the achievement of skeletal maturity. Longitudinal growth occurs through endochondral ossification at the epiphyseal growth plates of long bones, where chondrocytes proliferate, hypertrophy, and are replaced by bone tissue.[55] This process is primarily regulated by growth hormone (GH) and insulin-like growth factor-1 (IGF-1), which stimulate chondrocyte activity and overall bone lengthening during childhood and adolescence.[55] Growth continues until the growth plates close, typically between ages 18 and 25, with females generally completing closure earlier (by around age 19) than males (by age 21), marking the end of linear expansion.[56] Epiphyseal fusion events, such as the union of the distal femur epiphysis, occur around ages 16 to 18 in males and slightly earlier in females, permanently halting further elongation at those sites.[57] Appositional growth enables circumferential expansion of bones, primarily through the activity of osteoblasts on the periosteal surface, which deposit new bone layers outward, increasing bone diameter and strength.[58] This outward addition is balanced by endosteal resorption on the inner surface, preventing excessive thickening and allowing for marrow cavity development.[58] Bone modeling, distinct from later adult remodeling, involves uncoupled resorption and formation processes that shape bone contours during growth; for instance, differential endosteal resorption widens the marrow cavity while periosteal formation maintains structural integrity.[3][59] The culmination of these processes is the attainment of peak bone mass, with approximately 90% accrued by age 18 to 20, after which gains plateau into early adulthood.[60] Genetics play a dominant role, accounting for 50% to 80% of variability in peak bone mass through heritability of bone mineral density.[61] Pubertal timing significantly influences accrual, as earlier onset may shorten the window for bone building, while sex differences result in males achieving higher peak bone density due to prolonged growth and greater overall skeletal mass.[62][63]Classification of Bones
By Shape
Bones are classified by their gross morphology into five main categories: long, short, flat, irregular, and sesamoid, each adapted to specific mechanical demands in the body.[64][65] This classification emphasizes external shape and overall form rather than internal tissue composition, reflecting their primary roles in support, protection, and movement.[66] Long bones are elongated structures longer than they are wide, typically featuring a cylindrical shaft called the diaphysis surrounded by two broader ends known as epiphyses, connected by the metaphysis.[64][65] Examples include the femur in the thigh, humerus in the upper arm, and bones of the forearm and lower leg such as the radius, ulna, tibia, and fibula.[66] These bones primarily provide leverage and support for body weight, facilitating movement through attachments for muscles and ligaments.[65] Short bones exhibit a compact, cube-like shape with roughly equal dimensions in length, width, and thickness, designed for stability and some shock absorption.[64][66] Representative examples are the carpals of the wrist and tarsals of the ankle.[65] Their morphology allows for gliding movements while maintaining structural integrity in areas subject to compression.[66] Flat bones are thin, broad, and often slightly curved plates that serve protective functions and provide extensive surfaces for muscle attachment.[64][65] Common examples include the bones of the skull such as the parietal and frontal, the scapulae, sternum, and ribs.[66] This shape enables them to shield vital organs like the brain and thoracic contents while accommodating red bone marrow in their internal spaces.[64] Irregular bones possess complex, asymmetrical shapes that do not fit neatly into the long, short, or flat categories, tailored to specialized roles such as articulation and support.[65][66] Examples encompass the vertebrae of the spine, certain facial bones like the mandible and maxilla, and the hip bones (pelvis).[64] Their intricate forms allow for protection of structures like the spinal cord and facilitation of joint movements.[66] Sesamoid bones are small, rounded structures embedded within tendons, resembling sesame seeds, which develop to reduce friction and alter the angle of tendon pull during movement.[65][66] The patella, or kneecap, is the largest and most prominent example, located anterior to the knee joint.[64] These bones vary in presence and number among individuals, often forming through endochondral ossification in response to mechanical stress.[65][66][67]By Structure and Density
Bones are classified by their internal structure and density into primary types—compact (cortical) bone and spongy (trabecular or cancellous) bone—each exhibiting distinct architectural organizations that influence mechanical properties and physiological roles. Compact bone predominates in regions requiring high mechanical strength, such as the diaphyses of long bones and the outer tables of flat bones, where its dense matrix provides robust load-bearing capacity.[68][69] This tissue features an apparent density of 1.8–2.0 g/cm³ and low porosity of less than 10%, enabling it to withstand compressive and tensile forces effectively while minimizing weight.[70][71] In contrast, spongy bone is prevalent in the epiphyses of long bones, the interiors of short and irregular bones, and the inner tables of flat bones, where its porous lattice supports metabolic activities such as hematopoiesis and mineral storage with reduced mass.[68][69] Characterized by an apparent density ranging from 0.2 to 1.0 g/cm³ and porosity of 50–90%, spongy bone's trabecular network distributes loads across a larger surface area, optimizing lightweight structural support.[72][73] Within these categories, bone tissue subtypes differ in organization and maturity: woven bone, an immature form with disorganized collagen fibers and high cellular turnover, forms rapidly during early development or healing and exhibits lower mineral density compared to mature forms.[74][16] Lamellar bone, the stable mature subtype, features orderly layered collagen and mineral deposition, providing greater strength and reduced remodeling rates.[74][16] Bone density and structure display heterogeneity across regions, with gradients often higher at sites experiencing tensile stresses to enhance resistance, as seen in the varying mineral content from periosteum to endosteum.[75] Regional adaptations further illustrate this, such as pneumatic bones in birds, where air-filled cavities reduce overall density for weight minimization while preserving structural integrity.[76][77] Bone mineral density (BMD) is quantitatively assessed using dual-energy X-ray absorptiometry (DXA), a non-invasive technique that measures areal BMD in g/cm² to evaluate structural integrity and density variations.[78][79]Functions
Mechanical Functions
Bone serves as the primary structural framework of the vertebrate body, bearing the weight of the organism and maintaining posture against gravitational forces. In the axial skeleton, such as the vertebrae and long bones of the legs, cortical and trabecular bone distribute compressive loads efficiently, with trabecular architecture aligning along principal stress trajectories to optimize load-bearing capacity. This adaptation follows Wolff's law, which posits that bone remodels in response to mechanical usage, increasing density and strength in areas of high stress while resorbing in low-stress regions, thereby enhancing overall structural integrity.[80][81] In addition to support, bone provides critical protection for vital organs by encasing them within rigid enclosures. The cranium, composed of flat bones fused into a vault, safeguards the brain from traumatic impacts, while the ribcage—formed by 12 pairs of curved ribs articulating with the thoracic vertebrae and sternum—shields the heart and lungs from external forces. These protective functions are particularly evident in flat bones, which possess broad, thin structures that dissipate energy from blows, reducing the risk of internal injury.[82][83] Bone also enables locomotion and manipulation by functioning as rigid levers that amplify muscle forces. Long bones, such as the femur and humerus, serve as attachment sites for skeletal muscles, with their elongated shafts providing mechanical advantage during contraction to produce movement at synovial joints. Articulations between bones, reinforced by ligaments, allow controlled rotation, flexion, and extension, while sesamoid bones—small, rounded nodules embedded within tendons, like the patella in the quadriceps tendon—optimize force transmission by reducing friction and altering tendon angles for efficient pull.[84] The biomechanical prowess of bone arises from its hierarchical, composite structure, exhibiting anisotropic properties that confer direction-dependent strength. Bone is significantly stronger in compression (withstanding up to 170 MPa in cortical regions) than in tension (around 125 MPa), reflecting the oriented arrangement of collagen fibers and hydroxyapatite crystals along the longitudinal axis. This material demonstrates viscoelastic behavior, where time-dependent deformation under load provides energy dissipation and fatigue resistance, preventing crack propagation during cyclic stresses like walking. Furthermore, the collagen-mineral nanocomposite imparts fracture toughness, with collagen offering ductility to bridge microcracks and minerals providing stiffness, resulting in a toughness value of approximately 2-10 MPa·m^{1/2} that rivals engineering composites.[72][85][86] Bone's ability to adapt mechanically involves piezoelectric effects, where deformation generates electric potentials across the tissue, influencing cellular activity and directing remodeling. This phenomenon, originating from the oriented collagen fibrils, produces streaming potentials under stress that stimulate osteoblasts and osteoclasts, promoting bone deposition in loaded areas as per Wolff's adaptive principles. First demonstrated in bone specimens, these bioelectric signals underscore bone's dynamic responsiveness to mechanical environments.[87][88]Metabolic Functions
Bone serves as the primary reservoir for calcium in the human body, storing over 99% of total body calcium, which amounts to approximately 1-2 kg in adults, primarily in the form of hydroxyapatite crystals within the skeletal matrix.[89] During periods of hypocalcemia, osteoclast-mediated bone resorption releases calcium ions into the bloodstream to restore serum levels and support essential physiological processes such as muscle contraction, nerve signaling, and blood clotting.[90] In addition to calcium, bone functions as a major storage site for phosphate, containing about 85% of the body's phosphate reserves integrated into hydroxyapatite structures.[91] This storage enables bone to buffer systemic phosphate concentrations, ensuring availability for critical cellular functions including energy metabolism via ATP synthesis, nucleic acid formation, and cellular signaling.[92] Bone also contributes to acid-base homeostasis through its carbonate ions, which are mobilized during bone resorption to neutralize excess acids in conditions of metabolic acidosis, thereby helping to maintain blood pH balance.[93] Beyond mineral storage, bone exhibits endocrine functions; osteoblasts secrete osteocalcin, a hormone that enhances insulin sensitivity in peripheral tissues and promotes testosterone biosynthesis in Leydig cells, linking skeletal metabolism to glucose regulation and male reproductive health.[94] Osteocytes produce fibroblast growth factor 23 (FGF23), which acts on the kidneys to inhibit phosphate reabsorption in the proximal tubules, thereby preventing hyperphosphatemia and supporting mineral balance.[95] These metabolic roles integrate with systemic regulation: parathyroid hormone (PTH) stimulates osteoclast activity to promote bone resorption and calcium release, while calcitonin inhibits this process to limit calcium mobilization; active vitamin D (calcitriol) synergizes by enhancing intestinal calcium absorption to complement bone-derived supplies.[90][96][97]Hematopoietic Functions
The bone marrow, housed within the spongy bone of the skeleton, serves as the primary site for hematopoiesis, the process of blood cell formation, integrating anatomical structure with physiological function to sustain blood production throughout life.[98] In adults, active red bone marrow is predominantly located in the axial skeleton, including the vertebrae, pelvis, sternum, ribs, and skull, as well as the proximal ends of long bones such as the femurs and humeri.[99] This distribution supports efficient hematopoiesis while minimizing interference with mechanical bone functions. During postnatal development, hematopoietic activity shifts from peripheral sites, such as the distal long bones and limbs, to more central axial locations, accompanied by the gradual conversion of red marrow to inactive yellow marrow in peripheral regions.[100] Hematopoiesis occurs through hematopoietic stem cells (HSCs) residing in specialized marrow niches, which differentiate into erythrocytes, leukocytes, and platelets under the influence of cytokines; for instance, erythropoietin stimulates red blood cell production from erythroid progenitors.[101] Stromal cells, including osteoblasts and endothelial cells, form these niches: the endosteal niche near the bone surface maintains quiescent HSCs via factors like CXCL12, while the vascular niche promotes HSC proliferation and mobilization through interactions with sinusoidal endothelium.[102] In adults, much of the marrow converts to yellow marrow, characterized by adipocyte accumulation that replaces hematopoietic tissue, though this process is reversible under physiological stress such as chronic anemia, allowing reconversion to red marrow to meet increased demand.[103] The total bone marrow volume in adults is approximately 2.6 liters, generating around 500 billion blood cells daily to maintain homeostasis.[104] Clinically, bone marrow biopsies are commonly performed at the posterior iliac crest to assess hematopoietic function, providing samples for microscopic evaluation of cell morphology and composition.[105] This marrow activity also links to broader metabolic roles, as released platelets require calcium ions—stored in the bone matrix—for effective blood clotting.[98]Remodeling and Homeostasis
Remodeling Process
Bone remodeling is a lifelong process that maintains skeletal integrity by replacing old or damaged bone tissue with new bone, primarily through the coordinated activity of osteoclasts and osteoblasts within temporary anatomical structures known as basic multicellular units (BMUs).[106] These BMUs consist of osteoclasts leading the resorption front, followed by reversal cells and osteoblasts at the formation tail, all encased in a bone-remodeling compartment formed by overlying canopy cells.[106] The process ensures calcium homeostasis and adaptation to mechanical stresses without net change in bone mass during adulthood under normal conditions.[107] The remodeling cycle unfolds in five sequential phases: activation, resorption, reversal, formation, and quiescence. In the activation phase, mechanical loading or microdamage sensed by osteocytes triggers signaling pathways that recruit pre-osteoclasts to the bone surface via factors such as receptor activator of nuclear factor kappa-B ligand (RANKL).[106] This is followed by the resorption phase, where mature osteoclasts attach to the bone surface, secrete acid and enzymes to dissolve the mineral and organic matrix, creating characteristic Howship's lacunae; this phase lasts approximately 2-4 weeks.[106] The reversal phase then couples resorption to formation, with mononuclear cells clearing debris and releasing coupling signals like RANKL and osteoprotegerin (OPG) to prepare the site for osteoblasts.[106] During the formation phase, osteoblasts deposit unmineralized osteoid matrix, which subsequently mineralizes into mature lamellar bone over 2-3 months, restoring the resorbed volume.[106] Finally, the quiescence phase (or termination) sees the site return to a resting state, with excess osteoblasts becoming osteocytes or bone-lining cells, until the next cycle begins.[106] On average, about 10% of the adult skeleton undergoes remodeling annually, with rates varying by bone type: trabecular bone turns over at 25-28% per year due to its higher surface area, while cortical bone remodels more slowly at 3-5%.[108][109] This turnover occurs at specific sites, including intracortical (Haversian) remodeling within osteons for repairing fatigue damage, trabecular remodeling along plate-like surfaces for metabolic adaptation, and envelope-specific activity on periosteal (outer) and endosteal (inner) surfaces for shape maintenance.[110][111] Remodeling activity peaks during childhood and adolescence to support rapid skeletal growth, with high rates of both resorption and formation contributing to bone elongation and strengthening.[108] After peak bone mass is achieved around age 30, remodeling intensity declines progressively, leading to a negative balance where resorption outpaces formation and results in gradual net bone loss.[108]Regulation of Remodeling
Bone remodeling is tightly regulated by a balance of hormonal, mechanical, and molecular signals that coordinate osteoclast-mediated resorption and osteoblast-driven formation to maintain skeletal homeostasis. Intermittent pulses of parathyroid hormone (PTH) promote bone formation by activating osteoblasts through Wnt signaling and inhibiting sclerostin, while continuous PTH exposure enhances resorption via increased osteoclast activity.[112] Estrogen inhibits osteoclast differentiation and activity primarily by suppressing RANKL expression in osteoblasts and stromal cells, thereby reducing bone resorption and preserving density.[113] Growth hormone (GH) and insulin-like growth factor-1 (IGF-1) stimulate osteoblast proliferation and differentiation via the PI3K/AKT/mTOR pathway, supporting longitudinal bone growth and remodeling balance.[114] Mechanical loading plays a crucial role in regulating remodeling through osteocyte-mediated sensing of strain, which activates anabolic pathways to favor bone formation. Osteocytes detect mechanical stress and respond by downregulating sclerostin expression, thereby enhancing Wnt/β-catenin signaling to promote osteoblast activity and inhibit resorption.[115] Exercise-induced loading suppresses sclerostin release from osteocytes, leading to increased bone formation rates and adaptation to physical demands.[116] At the molecular level, the RANKL/RANK/OPG system is central to osteoclast regulation, where RANKL binding to RANK on osteoclast precursors drives differentiation and activation, while osteoprotegerin (OPG) acts as a decoy receptor to block this interaction and maintain remodeling equilibrium.[117] The Wnt/β-catenin pathway supports osteoblast differentiation by stabilizing β-catenin, which translocates to the nucleus to upregulate osteogenic genes and OPG, countering resorption.[118] Transforming growth factor-β (TGF-β), released from the bone matrix during resorption, couples the processes by recruiting mesenchymal stem cells to resorptive sites and stimulating osteoblast proliferation via SMAD signaling.[119] Systemic factors further modulate remodeling dynamics. Active vitamin D (1,25-dihydroxyvitamin D) enhances intestinal calcium absorption and directly boosts osteoblast activity while upregulating RANKL to fine-tune osteoclastogenesis.[117] Pro-inflammatory cytokines like interleukin-6 (IL-6) promote resorption by activating JAK/STAT signaling in osteoclast precursors, increasing RANKL sensitivity during inflammatory states.[117] Feedback mechanisms ensure precise control, including the calcium-sensing receptor (CaSR) on osteoclasts, which detects elevated extracellular calcium from resorption and inhibits further osteoclast activity to prevent excessive bone loss.[120] Circadian rhythms regulate remodeling temporally, with osteoblast and osteoclast activities peaking at distinct times of day, influenced by clock genes that modulate RANKL/OPG expression and hormonal pulses.[121]Clinical Significance
Fractures and Injuries
Bone fractures occur when the mechanical loading on a bone exceeds its capacity to absorb energy, resulting in a break in the continuity of the bone tissue. These injuries are classified based on several criteria, including the relationship to the skin, the extent of the break, and the pattern of the fracture line. A closed fracture, also known as a simple fracture, involves a break in the bone without disruption of the overlying skin, whereas an open fracture, or compound fracture, occurs when the broken bone pierces the skin, increasing the risk of infection.[122][123] Complete fractures extend through the entire bone, separating it into distinct segments, while incomplete fractures involve only partial disruption, such as in greenstick fractures common in children where the bone bends and cracks but does not fully separate.[124][125] Fracture patterns further describe the morphology and often indicate the mechanism of injury, particularly in long bones like the femur or tibia. Transverse fractures result from forces perpendicular to the bone's long axis, typically from direct impact, creating a horizontal break across the bone. Oblique fractures arise from angled forces combining compression and shear, producing a diagonal fracture line. Comminuted fractures involve the bone shattering into three or more fragments, usually from high-energy trauma such as motor vehicle accidents. Spiral fractures are caused by twisting or torsional forces, resulting in a helical pattern along the bone, often seen in sports injuries or assaults. Stress fractures, distinct from acute traumatic breaks, develop from repetitive low-level loading that accumulates microdamage over time, leading to insufficiency fractures in weakened bone or fatigue fractures in normal bone under overuse, such as in runners.[124][126][123] Biomechanically, long bones are primarily subjected to axial compression, bending moments, and torsional loads during daily activities and trauma. In bending, the bone's convex side experiences tension while the concave side undergoes compression, with failure often initiating at the tensile surface where bone is weaker; this explains transverse or oblique patterns in falls or impacts. Torsional forces generate shear stresses that propagate along the bone's length, leading to spiral fractures when the applied torque exceeds the bone's shear strength. Energy absorption at the fracture site is limited by the bone's material properties, with cortical bone dissipating energy through microcracking before macroscopic failure, but high-velocity impacts can overwhelm this capacity, resulting in comminuted injuries.[127][128][129] The healing of bone fractures follows a well-orchestrated biological process involving overlapping stages that restore structural integrity. Immediately after injury (days 1-5), hematoma formation occurs as disrupted blood vessels release blood that clots at the fracture site, providing a provisional scaffold rich in growth factors and recruiting mesenchymal stem cells. This transitions into the inflammatory phase (days 5-14), where granulation tissue forms through angiogenesis and influx of inflammatory cells, stabilizing the site and initiating fibrocartilage production. By weeks 2-6, a soft callus of fibrocartilage and hyaline cartilage bridges the gap, offering initial mechanical stability. The hard callus stage (weeks 6-12) involves endochondral ossification, where the soft callus mineralizes into woven bone, bridging the fracture. Finally, remodeling (months to years) reshapes the callus into organized lamellar bone through osteoclastic resorption and osteoblastic deposition, adapting to mechanical stresses.[130][131][132] Several factors influence the rate and success of fracture healing, with complications like non-union (failure to form bridging callus) or malunion (healing in a misaligned position) occurring in 5-10% of cases. Advanced age slows healing due to reduced cellular activity and vascularity, often prolonging the process by weeks to months in the elderly. Nutritional deficiencies impair collagen synthesis and mineralization; for instance, vitamin C is essential for hydroxylation in collagen formation, while vitamin D and calcium support ossification. Smoking delays healing by vasoconstriction and reduced oxygen delivery, increasing non-union risk by up to 2-4 times through impaired angiogenesis and osteoblast function. Other contributors include poor blood supply, infection, and excessive motion at the site, all of which disrupt the inflammatory and callus formation stages.[130][133][134] Initial management of fractures prioritizes stabilization to promote healing and prevent further damage. First aid involves immobilizing the injured limb using splints or slings to minimize movement, applying ice to reduce swelling, and elevating the area while seeking immediate medical evaluation. Non-surgical immobilization with casts—made of plaster or fiberglass—maintains alignment for stable fractures, allowing natural healing over 6-8 weeks in adults. For unstable or displaced fractures, open reduction and internal fixation (ORIF) is employed, where surgery realigns the bone fragments under direct visualization and secures them with plates, screws, or intramedullary nails to provide rigid stability and facilitate early mobilization.[126][135][136]Bone Diseases and Disorders
Bone diseases and disorders encompass metabolic, infectious, and inflammatory conditions that compromise bone integrity without involving acute trauma or neoplasia, often resulting from imbalances in remodeling, nutrient deficiencies, infections, or autoimmune processes. These disorders lead to structural weakening, pain, deformities, and heightened susceptibility to complications, with diagnosis typically relying on clinical evaluation, imaging, and biochemical markers. Osteoporosis is defined by reduced bone mass and microarchitectural deterioration, which increases bone fragility and the risk of low-trauma fractures. The condition is diagnosed via dual-energy X-ray absorptiometry (DXA) when the T-score falls below -2.5 at key sites such as the lumbar spine, hip, or distal forearm. Primary osteoporosis manifests as postmenopausal type (driven by estrogen deficiency in women) or senile type (age-related in both sexes over 70 years), whereas secondary forms arise from underlying causes like long-term glucocorticoid therapy, which suppresses osteoblast activity and promotes resorption. This distinction guides targeted screening, with postmenopausal cases accelerating after menopause due to hormonal shifts. Osteomalacia and rickets represent disorders of impaired bone mineralization, predominantly stemming from vitamin D deficiency that disrupts calcium and phosphate homeostasis. In adults, osteomalacia causes bone softening, muscle weakness, and diffuse pain, while in growing children, rickets leads to skeletal deformities such as bowing of the legs (genu varum), widened growth plates, and delayed walking. These conditions arise from inadequate sunlight exposure, dietary insufficiency, or malabsorption, with biochemical hallmarks including low serum 25-hydroxyvitamin D levels and elevated parathyroid hormone. Paget's disease of bone, synonymous with osteitis deformans, features focal excessive bone remodeling where hyperactive osteoclasts resorb bone, followed by disorganized osteoblast-driven replacement, yielding a characteristic mosaic pattern of woven and lamellar bone under microscopy. This results in enlarged, deformed, and weakened bones, often in the pelvis, skull, or long bones, with elevated serum alkaline phosphatase reflecting heightened turnover. A severe complication occurs in about 1% of cases, involving sarcomatous transformation of pagetic bone, which carries poor prognosis. Osteomyelitis constitutes a key infectious bone disorder, typically bacterial and dominated by Staphylococcus aureus as the causative pathogen in both hematogenous and contiguous forms. Acute osteomyelitis presents with fever, localized pain, and swelling within weeks of onset, progressing to chronic stages if untreated, marked by persistent drainage, sequestrum formation, and bone necrosis. Spread occurs hematogenously from distant sites like skin infections or directly via trauma, surgery, or adjacent soft tissue involvement, necessitating prompt antimicrobial therapy to prevent systemic spread. Inflammatory bone diseases include ankylosing spondylitis, an autoimmune spondyloarthropathy strongly linked to HLA-B27, which targets the sacroiliac joints and axial skeleton through chronic enthesitis and synovitis. This leads to erosions, sclerosis, and eventual ankylosis (fusion) of the sacroiliac joints and spine, causing stiffness, pain, and kyphotic deformity. Autoimmune mechanisms involve T-cell driven inflammation and cytokine dysregulation, distinguishing it from infectious or metabolic etiologies. Diabetes mellitus exerts a detrimental effect on bone health, where chronic hyperglycemia accelerates osteoclast-mediated resorption and impairs collagen quality via advanced glycation end products, thereby elevating fracture risk by 2- to 4-fold relative to non-diabetic populations despite sometimes normal bone density. This risk stems from disrupted remodeling balance and vascular complications, with type 2 diabetes showing particularly pronounced effects due to insulin resistance and prolonged exposure.Bone Tumors and Cancer
Bone tumors encompass a range of neoplasms originating from bone tissue or metastatic spread to bone, classified as benign or malignant, primary or secondary. Primary bone tumors arise directly within the skeletal system, while metastatic tumors represent secondary involvement from distant primary cancers. Benign tumors generally do not metastasize but can cause local complications, whereas malignant ones exhibit aggressive growth, invasion, and potential for distant spread.[137] Benign bone tumors include osteomas, which are characterized by compact bone overgrowth typically occurring on the skull or facial bones through subperiosteal ossification.[138] Osteochondromas represent the most common benign bone tumor, comprising 20-50% of cases, and manifest as cartilage-capped bony exostoses primarily on the surface of long bones such as the femur or tibia.[137] Enchondromas are intramedullary benign tumors of hyaline cartilage origin, often asymptomatic and located within the medullary cavity of small tubular bones like those in the hands and feet.[139] Primary malignant bone tumors are rare but aggressive, with osteosarcoma being the most common, predominantly affecting adolescents and originating in the metaphyses of long bones where it produces malignant osteoid matrix.[140] The five-year survival rate for localized osteosarcoma is approximately 60-70%, influenced by factors such as tumor stage and response to neoadjuvant therapy.[141] Chondrosarcoma typically occurs in adults, frequently involving the pelvis or proximal femur, and is notable for its resistance to chemotherapy due to low vascularity and sparse dividing cells.[142] Ewing sarcoma primarily affects children and adolescents, arising in the diaphyses of long bones and characterized histologically by uniform small round blue cells with high nuclear-to-cytoplasmic ratios.[143] Metastatic bone cancer commonly originates from primary sites such as breast, prostate, or lung, accounting for the majority of bone malignancies and exhibiting either lytic patterns (bone destruction, as in breast and lung cancers) or blastic patterns (excessive bone formation, as in prostate cancer).[144] Osteolytic metastases from these primaries can lead to hypercalcemia through excessive bone resorption and release of calcium into the bloodstream.[145] The pathophysiology of bone tumors involves genetic mutations that drive uncontrolled proliferation, such as TP53 alterations in osteosarcoma, which impair tumor suppression and promote genomic instability.[146] In Ewing sarcoma, the EWSR1-FLI1 gene fusion, resulting from t(11;22) translocation, acts as an oncogenic driver by dysregulating transcription and cell cycle control.[147] Tumor progression is further facilitated by angiogenesis, enabling nutrient supply to growing masses, and degradation of the extracellular bone matrix via enzymes like matrix metalloproteinases (MMPs).[148] These processes often hijack normal bone remodeling mechanisms, leading to pathological bone resorption or formation.[149] Diagnosis of bone tumors relies on a multidisciplinary approach, beginning with imaging such as MRI for soft tissue extension and CT for bony details, followed by biopsy to confirm histology and molecular features.[150] Treatment for primary malignant tumors typically involves neoadjuvant chemotherapy, surgical resection with wide margins, and adjuvant radiation, particularly for Ewing sarcoma.[143] For metastatic disease, systemic therapies targeting the primary cancer are combined with localized interventions like radiation for pain control, and bisphosphonates to inhibit osteoclast activity and prevent skeletal-related events.[151]Regenerative Medicine
Regenerative medicine in bone repair has advanced significantly in recent years, focusing on innovative therapies to enhance osteogenesis and address critical defects that natural healing cannot fully resolve. Mesenchymal stem cells (MSCs) play a central role in these approaches due to their ability to differentiate into osteoblasts and promote bone formation. A 2025 study from Northwestern University demonstrated that deforming the nuclei of MSCs using microstructured scaffolds triggers regenerative signals, improving bone healing efficiency in preclinical models by modulating mechanotransduction pathways.[152] Functionalization strategies, such as gene editing or biomaterial coatings, further enhance MSC therapeutic potential by increasing their survival and osteogenic differentiation in vivo.[153] Three-dimensional (3D) bioprinting has emerged as a key technology for creating scaffolds that mimic the extracellular matrix (ECM) of bone, incorporating bioinks composed of hydroxyapatite and growth factors like bone morphogenetic protein-2 (BMP-2). These constructs support cell adhesion, proliferation, and vascularization, making them suitable for repairing critical-sized bone defects where traditional grafts fail. Progress in 2024-2025 includes the development of pre-vascularized 3D-printed scaffolds using biodegradable polymers, which integrate endothelial cells and osteogenic factors to promote angiogenesis-osteogenesis coupling and accelerate integration with host tissue.[154][155] Bone organoids, derived from induced pluripotent stem cells (iPSCs), offer advanced 3D models for studying bone pathophysiology and screening regenerative therapies. These organoids recapitulate multicellular interactions in bone tissue, enabling disease modeling for conditions like osteoporosis and high-throughput drug testing. Recent 2025 research highlights the synergistic activation of BMP and Wnt signaling pathways to mature iPSC-derived bone organoids, enhancing mineralization and structural complexity for more accurate in vitro simulations.[156][157] Novel discoveries in skeletal progenitor cells have expanded regenerative potential. In 2025, researchers at the University of California, Irvine identified a new skeletal tissue termed "lipocartilage," which exhibits hybrid properties of lipid storage and cartilage resilience, offering promise for engineered grafts in load-bearing repairs. Additionally, fibro-adipogenic progenitors (FAPs), particularly the Prg4+ subset, have been shown to critically support endochondral bone repair by transitioning from muscle to skeletal lineages, enhancing regeneration in injury models.[158][159] Gene editing via CRISPR-Cas9 targeting RUNX2, a master regulator of osteoblast differentiation, has enabled precise modulation of osteogenic pathways, with activation strategies increasing bone formation markers in stem cell cultures.[160] Clinical translation of these advances is progressing through trials and approved therapies. Recombinant human BMP-2 (rhBMP-2) received FDA approval in 2002 for spinal fusions and continues to demonstrate superior fusion rates in anterior lumbar interbody fusion procedures, with ongoing multicenter trials confirming its efficacy at low doses for up to 100% radiographic fusion by 12 months. Emerging AI-optimized implants, leveraging machine learning for patient-specific design, improve fit and biocompatibility, potentially reducing rejection risks by minimizing mismatch-induced inflammation in orthopedic applications.[161][162][163]Bone Health and Maintenance
Nutritional Factors
Calcium is a primary mineral essential for bone formation and maintenance, constituting approximately 99% of the body's calcium stores in the form of hydroxyapatite crystals within the bone matrix.[164] The recommended dietary allowance (RDA) for calcium is 1,000 mg per day for adults aged 19–50 years and 1,200 mg per day for women over 51 years and men over 70 years.[164] Dietary sources include dairy products such as milk and yogurt, as well as leafy green vegetables like kale and broccoli.[164] Calcium absorption in the intestines occurs primarily through vitamin D-dependent active transport at lower intake levels, with parathyroid hormone regulating serum calcium levels by mobilizing bone reserves when dietary intake is insufficient.[164] Deficiency in calcium can lead to secondary hyperparathyroidism, where elevated parathyroid hormone promotes bone resorption to maintain blood calcium homeostasis, ultimately weakening bone structure.[164] Vitamin D plays a crucial role in bone health by facilitating calcium and phosphate absorption and supporting mineralization processes.[165] The RDA for vitamin D is 600 IU (15 mcg) per day for adults aged 19–70 years and 800 IU (20 mcg) per day for those over 70 years.[165] It is synthesized in the skin upon exposure to UVB sunlight or obtained from dietary sources such as fatty fish like salmon and fortified foods.[165] The active form, 1,25-dihydroxyvitamin D (calcitriol), enhances intestinal uptake of calcium and phosphate while promoting osteoblast activity for bone formation.[165] Other micronutrients contribute to bone integrity through various mechanisms. Phosphorus, with an RDA of 700 mg per day for adults, forms hydroxyapatite alongside calcium and is sourced from dairy products and meats.[91] Magnesium, required at 310–320 mg per day for adult women and 400–420 mg for men, supports bone quality and is found in nuts, seeds, and leafy greens.[166] Vitamin K, particularly phylloquinone (vitamin K1), has an adequate intake of 90 mcg per day for women and 120 mcg for men; it serves as a cofactor for the gamma-carboxylation of osteocalcin, a bone protein that binds calcium to promote mineralization, with sources including green leafy vegetables.[167] Vitamin C, with an RDA of 75 mg per day for women and 90 mg for men, is vital for collagen synthesis in the bone matrix and is obtained from fruits and vegetables.[168] Protein provides amino acids necessary for the organic matrix of bone, including collagen, and adequate intake supports bone mineral density. The RDA is 0.8 g per kg of body weight per day, though intakes of 1.0–1.2 g/kg may benefit bone health, particularly from high-quality animal sources like meat and dairy.[169] Nutrient interactions can influence bone health outcomes. Oxalates in spinach and phytates in grains inhibit calcium absorption by forming insoluble complexes in the gut.[170] Excessive alcohol consumption impairs osteoblast function and disrupts bone remodeling, leading to reduced bone density.[171]Lifestyle and Prevention
Regular physical activity plays a pivotal role in optimizing bone mass and reducing fracture risk through non-dietary means. Weight-bearing exercises, such as brisk walking for at least 30 minutes per day, along with resistance training, apply mechanical loads to the skeleton that stimulate bone formation via mechanotransduction, where osteocytes sense and respond to these stresses to promote osteogenesis.[172] These interventions have strong evidence for preserving bone density and reducing fracture risk in older adults when performed consistently.[173] Avoiding harmful behaviors further supports bone health. Smoking cessation can mitigate the dose-dependent bone loss associated with tobacco use, with former smokers showing improved bone mineral density compared to current smokers.[174] Limiting alcohol intake to fewer than two standard drinks per day prevents the bone resorption linked to chronic heavy consumption, which otherwise elevates fracture risk.[175] In the elderly, balance training programs, such as tai chi or targeted exercises performed several times weekly, significantly lower fall rates and subsequent fracture incidence by improving postural stability.[176] For high-risk individuals, pharmacological prevention is an option alongside lifestyle measures. Bisphosphonates, including alendronate, are first-line agents for postmenopausal women at elevated fracture risk, as they inhibit osteoclast activity to maintain bone density and reduce vertebral and non-vertebral fractures.[177] Denosumab, a monoclonal antibody that inhibits RANKL to suppress bone resorption, is indicated for severe osteoporosis cases in postmenopausal women and men to prevent fractures.[178] Screening facilitates early intervention to preserve bone health. Dual-energy X-ray absorptiometry (DXA) scans are recommended starting at age 65 for women and 70 for men, or earlier for those with risk factors like prior fractures or glucocorticoid use, to assess bone mineral density.[179] The FRAX tool integrates clinical risk factors and femoral neck BMD to estimate 10-year probability of major osteoporotic or hip fracture, guiding decisions on preventive therapy.[180] Public health initiatives underscore these strategies for broader impact. The World Health Organization promotes lifestyle approaches, including physical activity and risk avoidance, as core elements of osteoporosis prevention to curb fragility fractures globally.[181] Disparities in access to screening and preventive care, however, disproportionately affect low-income groups, resulting in lower DXA utilization and higher untreated fracture risks among these populations.[182]Comparative and Evolutionary Aspects
Bone in Other Animals
In teleost fish, bone is characteristically acellular, lacking osteocytes embedded within the matrix, a feature that distinguishes it from cellular bone in other vertebrates and is thought to facilitate mineral homeostasis through alternative mechanisms like periosteal regulation.[183][184] This acellular structure supports the lightweight endoskeleton necessary for aquatic buoyancy, allowing efficient movement in water. In contrast, chondrichthyans exhibit hypermineralized tissues in their jaws; for example, holocephalans such as chimaeras have pleromin layers in tooth plates that enhance durability for crushing prey without relying on a fully bony skeleton.[185] Some amphibians, such as certain anurans, exhibit porous cortical bone similar to that in reptiles, which facilitates extensive remodeling to accommodate growth and environmental stresses.[186] In crocodilians, a secondary bony palate formed by fused palatal bones provides additional structural reinforcement to the skull, aiding in powerful biting and aquatic lifestyles.[187] Birds have evolved pneumatic bones, which are permeated by extensions of the respiratory air sacs, significantly reducing skeletal mass to facilitate flight; the humerus, for instance, contains foramina that connect to these sacs for efficient gas exchange and weight minimization.[188] Additionally, avian bones undergo rapid calcium turnover, with medullary bone deposits resorbed to supply up to 40% of the calcium needed for eggshell formation during reproduction.[189] Rodents exhibit continuous bone growth in structures like incisors, which serve as key models for studying odontogenesis and regeneration due to their persistent eruption.[190] In elephants, tusks represent modified incisors that are largely avascular in their erupted portions and dominated by dentin, providing tools for foraging and defense while minimizing vascular demands.[191] Invertebrates lack true bone, instead relying on analogs such as the chitinous exoskeletons of arthropods, which offer external support and protection but differ fundamentally in composition and cellular embedding from vertebrate bone.[192]Evolutionary Development
The evolutionary origins of bone tissue date to approximately 500 million years ago in the Cambrian period, when agnathans—jawless fish—developed acellular dermal bone as a lightweight, protective exoskeleton lacking embedded cells. This innovation provided structural defense without the metabolic cost of cellular maintenance, forming the basis for subsequent skeletal complexity. Ostracoderms, an early group of extinct agnathans from the Ordovician to Devonian periods, exhibited elaborate calcified armor composed of this bone type, which covered their bodies and heads in plated structures adapted to predatory pressures in ancient aquatic environments.[193][194] The advent of jawed vertebrates around 420 million years ago marked a major diversification, with placoderms pioneering an endoskeleton that integrated bony elements into cartilaginous frameworks, facilitating greater mobility and jaw function essential for predation. This shift from purely cartilaginous supports to ossified structures was orchestrated by Runx transcription factors, which initially regulated cartilage formation and later drove its endochondral replacement by bone in gnathostomes, enhancing durability and load-bearing capacity.[195][196] In post-Devonian tetrapods, emerging around 360 million years ago, limb bones evolved specialized adaptations for terrestrial weight-bearing, such as thickened cortices and reinforced articulations to withstand gravitational stresses during the fish-to-amphibian transition. The evolution of endothermy around 300 million years ago in the common ancestors of mammals, birds, and crocodylians elevated bone remodeling rates through heightened vascularization and osteoblast-osteoclast activity, supporting rapid skeletal adjustments to elevated metabolic demands.[197][198] Critical innovations further refined bone's functionality: osteocytes, specialized cells for mechanosensing and mineral regulation, appeared by about 400 million years ago in early osteichthyans, allowing bone to dynamically respond to mechanical loads as evidenced by fossilized cellular structures with modern-like metabolic traits. Hematopoietic marrow, enabling intraskeletal blood cell production, originated in synapsids around 300 million years ago during the Carboniferous-Permian transition, linking skeletal evolution to advanced immune and oxygen transport systems in mammalian ancestors.[199][200] These developments underscore profound genetic conservation across metazoans, exemplified by bone morphogenetic protein (BMP) homologs such as decapentaplegic (dpp) in Drosophila melanogaster, which governs embryonic patterning in ways analogous to BMP's role in vertebrate skeletogenesis. Recent studies (as of 2025) indicate that the evolution of skeletal cell types, such as osteoblasts and hypertrophic chondrocytes, follows trends of co-opting key regulatory genes across vertebrates.[201][202] Fossil records illuminate these shifts, as seen in Australopithecus afarensis specimen "Lucy" (dated to 3.2 million years ago), whose lower limb bones display elevated trabecular density and robusticity adapted for habitual bipedalism, reflecting selective pressures for efficient terrestrial weight distribution.[203]References
- https://wikimsk.org/wiki/Bone_Biomechanics